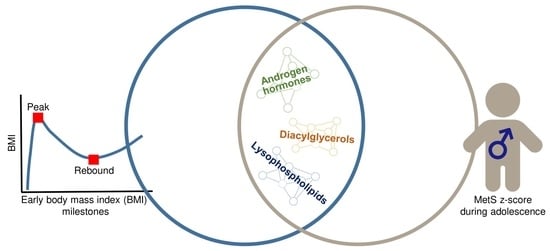
Metabolite Profiles of the Relationship between Body Mass Index (BMI) Milestones and Metabolic Risk during Early Adolescence
Abstract
1. Introduction
2. Results
3. Discussion
3.1. Summary of Main Findings
3.2. Primary Findings (in Boys Only)
3.2.1. ME7: Androgen Hormones (Associated with Age at BMI Rebound and MetS z-Score)
3.2.2. ME8: Lysophospholipids (Associated with Age and Magnitude of BMI at Rebound, and MetS z-Score)
3.2.3. ME11: Diacylglycerols (Associated with Age at BMI Rebound and MetS z-Score)
3.3. Secondary Findings in Boys
3.3.1. ME5: Energetics (Associated with Magnitude of BMI at Rebound)
3.3.2. ME9: Microbial and Aromatic Amino Acid Metabolism (Associated with Age at BMI Rebound)
3.3.3. ME13: Bile Acid Metabolism (Associated with Age at BMI Rebound)
3.4. Secondary Findings in Girls
3.4.1. ME4: Cellular Stress Response (Associated with Infancy BMI Peak)
3.4.2. ME5: Energetics, ME7: Androgen Hormones, and ME8: Lysophospholipids (Associated with Age at BMI Rebound)
3.5. Strengths and Limitations
4. Methods
4.1. Study Population
4.2. Early Growth Milestones: Infancy BMI Peak and Childhood BMI Rebound
4.3. Blood Collection for Metabolomics Assays and Conventional Biomarkers
4.4. Untargeted Metabolomics Profiling
4.5. Metabolic Risk during Early Adolescence
4.6. Covariates and Study Population Characteristics
4.6.1. Child Characteristics at the Early Teen Visit
4.6.2. Perinatal, Sociodemographic, and Background Characteristics
4.7. Statistical Analysis
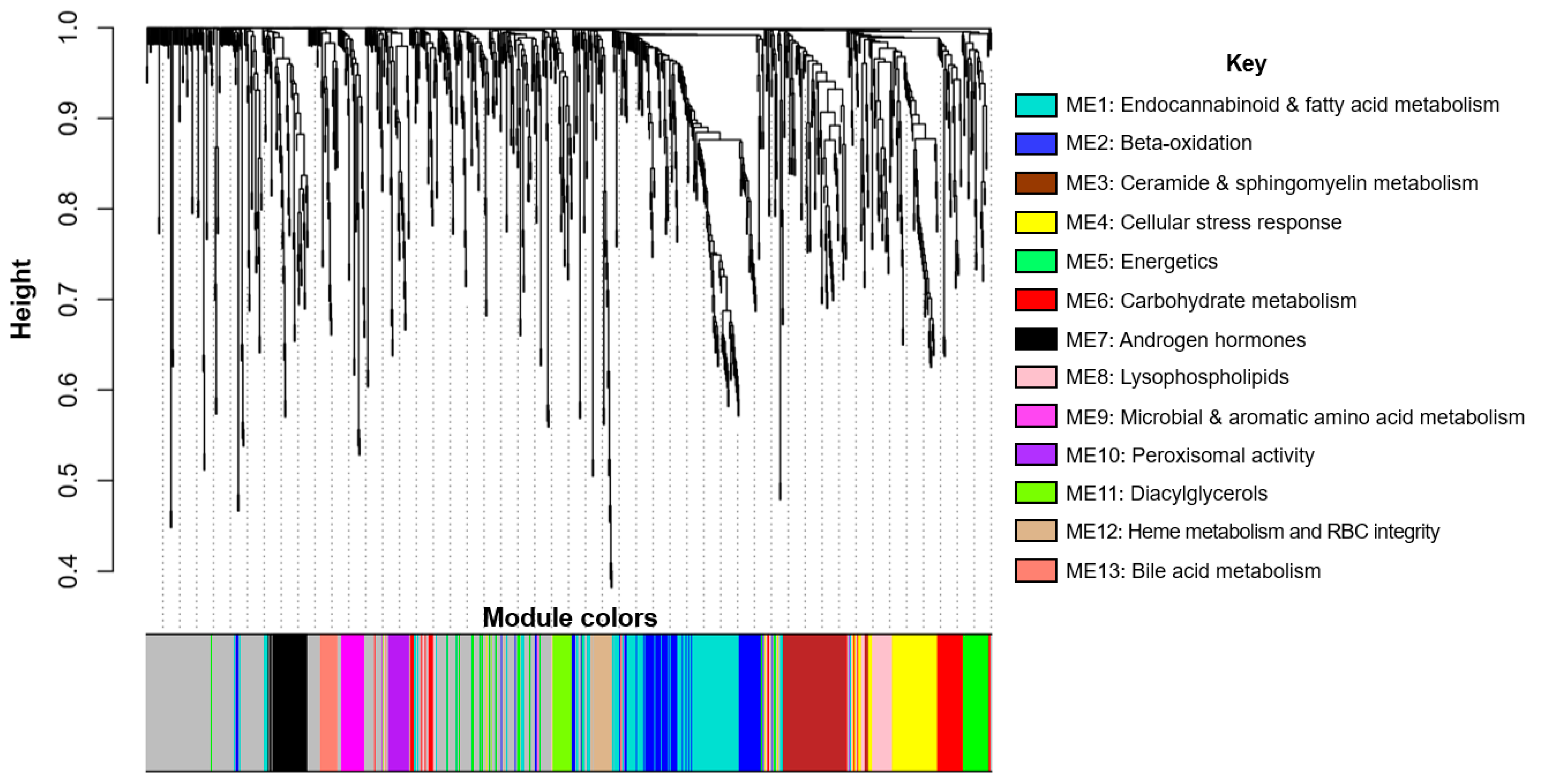
4.7.1. Metabolite Network Analysis
4.7.2. Regression Analysis
4.7.3. Step 1: Identify Growth Milestones Associated with MetS z-Score
4.7.4. Step 2: Identify Metabolite Networks Associated with Growth Milestones
4.7.5. Step 3: Identify Growth-Related Metabolite Networks Associated with MetS z-Score
4.7.6. Sensitivity Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BMI | body mass index |
| FDR | false discovery rate |
| HDL | high-density lipoprotein |
| HOMA-IR | homeostatic model assessment of insulin resistnace |
| ME | metabolite eigenvector |
| MetS z-score | metabolic syndrome z-score |
| SD | standard deviation |
| TOM | topological overlap matrix |
| WGCNA | weighted correlation network analysis |
References
- Taveras, E.M.; Rifas-Shiman, S.L.; Belfort, M.B.; Kleinman, K.P.; Oken, E.; Gillman, M.W. Weight status in the first 6 months of life and obesity at 3 years of age. Pediatrics 2009, 123, 1177–1183. [Google Scholar] [CrossRef]
- Sacco, M.R.; de Castro, N.P.; Euclydes, V.L.; Souza, J.M.; Rondo, P.H. Birth weight, rapid weight gain in infancy and markers of overweight and obesity in childhood. Eur. J. Clin. Nutr. 2013, 67, 1147–1153. [Google Scholar] [CrossRef]
- Min, J.; Li, J.; Li, Z.; Wang, Y. Impacts of infancy rapid weight gain on 5-year childhood overweight development vary by age and sex in China. Pediatric Obes. 2012, 7, 365–373. [Google Scholar] [CrossRef][Green Version]
- Druet, C.; Stettler, N.; Sharp, S.; Simmons, R.K.; Cooper, C.; Smith, G.D.; Ekelund, U.; Levy-Marchal, C.; Jarvelin, M.R.; Kuh, D.; et al. Prediction of childhood obesity by infancy weight gain: An individual-level meta-analysis. Paediatr Perinat Epidemiol. 2012, 26, 19–26. [Google Scholar] [CrossRef]
- Jones-Smith, J.C.; Fernald, L.C.; Neufeld, L.M. Birth size and accelerated growth during infancy are associated with increased odds of childhood overweight in Mexican children. J. Am. Diet. Assoc. 2007, 107, 2061–2069. [Google Scholar] [CrossRef] [PubMed]
- Perng, W.; Hajj, H.; Belfort, M.B.; Rifas-Shiman, S.L.; Kramer, M.S.; Gillman, M.W.; Oken, E. Birth Size, Early Life Weight Gain, and Midchildhood Cardiometabolic Health. J. Pediatrics 2016, 173, 122–130. [Google Scholar] [CrossRef]
- Monteiro, P.O.; Victora, C.G. Rapid growth in infancy and childhood and obesity in later life—a systematic review. Obes. Rev. 2005, 6, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Belfort, M.B.; Rifas-Shiman, S.L.; Rich-Edwards, J.; Kleinman, K.P.; Gillman, M.W. Size at birth, infant growth, and blood pressure at three years of age. J. Pediatrics 2007, 151, 670–674. [Google Scholar] [CrossRef] [PubMed]
- Leunissen, R.W.; Kerkhof, G.F.; Stijnen, T.; Hokken-Koelega, A. Timing and tempo of first-year rapid growth in relation to cardiovascular and metabolic risk profile in early adulthood. JAMA. 2009, 301, 2234–2242. [Google Scholar] [CrossRef]
- Adair, L.S.; Cole, T.J. Rapid child growth raises blood pressure in adolescent boys who were thin at birth. Hypertension 2003, 41, 451–456. [Google Scholar] [CrossRef]
- Perng, W.; Rifas-Shiman, S.L.; Kramer, M.S.; Haugaard, L.K.; Oken, E.; Gillman, M.W.; Belfort, M.B. Early Weight Gain, Linear Growth, and Mid-Childhood Blood Pressure: A Prospective Study in Project Viva. Hypertension 2016, 67, 301–308. [Google Scholar] [CrossRef]
- Bhargava, S.K.; Sachdev, H.S.; Fall, C.H.; Osmond, C.; Lakshmy, R.; Barker, D.J.; Biswas, S.K.; Ramji, S.; Prabhakaran, D.; Reddy, K.S. Relation of serial changes in childhood body-mass index to impaired glucose tolerance in young adulthood. N. Engl. J. Med. 2004, 350, 865–875. [Google Scholar] [CrossRef] [PubMed]
- Chivers, P.; Hands, B.; Parker, H.; Beilin, L.; Kendall, G.; Bulsara, M. Longitudinal modelling of body mass index from birth to 14 years. Obes. Facts 2009, 2, 302–310. [Google Scholar] [CrossRef]
- Wen, X.; Kleinman, K.; Gillman, M.W.; Rifas-Shiman, S.L.; Taveras, E.M. Childhood body mass index trajectories: Modeling, characterizing, pairwise correlations and socio-demographic predictors of trajectory characteristics. BMC Med. Res. Methodol. 2012, 12, 38. [Google Scholar] [CrossRef] [PubMed]
- Perng, W.; Baek, J.; Zhou, C.W.; Cantoral, A.; Tellez-Rojo, M.M.; Song, P.X.K.; Peterson, K.E. Associations of the infancy body mass index peak with anthropometry and cardiometabolic risk in Mexican adolescents. Ann. Hum. Biol. 2018, 45, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Silverwood, R.J.; De Stavola, B.L.; Cole, T.J.; Leon, D.A. BMI peak in infancy as a predictor for later BMI in the Uppsala Family Study. Int. J. Obes. 2009, 33, 929–937. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Nwaru, B.I.; Hua, J.; Li, X.; Wu, Z. Infant BMI peak as a predictor of overweight and obesity at age 2 years in a Chinese community-based cohort. BMJ Open 2017, 7, e015122. [Google Scholar] [CrossRef]
- Hof, M.H.P.; Vrijkotte, T.G.M.; de Hoog, M.L.A.; van Eijsden, M.; Zwinderman, A.H. Association between Infancy BMI Peak and Body Composition and Blood Pressure at Age 5–6 Years. PLoS ONE 2013, 8, e80517. [Google Scholar] [CrossRef]
- Jones-Smith, J.C.; Neufeld, L.M.; Laraia, B.; Ramakrishnan, U.; Garcia-Guerra, A.; Fernald, L.C.H. Early life growth trajectories and future risk for overweight. Nutr. Diabetes 2013, 3, e60. [Google Scholar] [CrossRef]
- Roy, S.M.; Chesi, A.; Mentch, F.; Xiao, R.; Chiavacci, R.; Mitchell, J.A.; Kelly, A.; Hakonarson, H.; Grant, S.F.; Zemel, B.S.; et al. Body mass index (BMI) trajectories in infancy differ by population ancestry and may presage disparities in early childhood obesity. J. Clin. Endocrinol. Metab. 2015, 100, 1551–1560. [Google Scholar] [CrossRef]
- Aris, I.M.; Rifas-Shiman, S.L.; Li, L.-J.; Kleinman, K.P.; Coull, B.A.; Gold, D.R.; Hivert, M.-F.; Kramer, M.S.; Oken, E. Patterns of body mass index milestones in early life and cardiometabolic risk in early adolescence. Int. J. Epidemiol. 2019, 48, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Rolland-Cachera, M.F.; Peneau, S. Growth trajectories associated with adult obesity. World Rev. Nutr. Diet. 2013, 106, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.W.; Grant, A.M.; Goulding, A.; Williams, S.M. Early adiposity rebound: Review of papers linking this to subsequent obesity in children and adults. Curr. Opin. Clin. Nutr. Metab. Care 2005, 8, 607–612. [Google Scholar] [CrossRef]
- Aris, I.M.; Bernard, J.Y.; Chen, L.W.; Tint, M.T.; Pang, W.W.; Lim, W.Y.; Soh, S.E.; Saw, S.M.; Godfrey, K.M.; Gluckman, P.D.; et al. Infant body mass index peak and early childhood cardio-metabolic risk markers in a multi-ethnic Asian birth cohort. Int. J. Epidemiol. 2017, 46, 513–525. [Google Scholar] [CrossRef] [PubMed]
- Whitaker, R.C.; Pepe, M.S.; Wright, J.A.; Seidel, K.D.; Dietz, W.H. Early adiposity rebound and the risk of adult obesity. Pediatrics 1998, 101, E5. [Google Scholar] [CrossRef]
- Williams, S.; Dickson, N. Early growth, menarche, and adiposity rebound. Lancet 2002, 359, 580–581. [Google Scholar] [CrossRef]
- Dietz, W.H. Critical periods in childhood for the development of obesity. Am. J. Clin. Nutr. 1994, 59, 955–959. [Google Scholar] [CrossRef]
- Perng, W.; Gillman, M.W.; Mantzoros, C.S.; Oken, E. A prospective study of maternal prenatal weight and offspring cardiometabolic health in midchildhood. Ann. Epidemiol. 2014, 24, 793–800. [Google Scholar] [CrossRef]
- Regnault, N.; Gillman, M.W.; Rifas-Shiman, S.L.; Eggleston, E.; Oken, E. Sex-Specific Associations of Gestational Glucose Tolerance With Childhood Body Composition. Diabetes Care 2013. [Google Scholar] [CrossRef]
- Perng, W.; Rifas-Shiman, S.L.; Hivert, M.F.; Chavarro, J.E.; Sordillo, J.; Oken, E. Metabolic trajectories across early adolescence: Differences by sex, weight, pubertal status and race/ethnicity. Ann. Hum. Biol. 2019, 46, 205–214. [Google Scholar] [CrossRef]
- Perng, W.; Rifas-Shiman, S.L.; McCulloch, S.; Chatzi, L.; Mantzoros, C.; Hivert, M.-F.; Oken, E. Associations of cord blood metabolites with perinatal characteristics, newborn anthropometry, and cord blood hormones in project viva. Metabolism 2017, 76, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Perng, W.; Rifas-Shiman, S.L.; Sordillo, J.; Hivert, M.-F.; Oken, E. Metabolomic Profiles of Overweight/Obesity Phenotypes During Adolescence: A Cross-Sectional Study in Project Viva. Obesity 2020, 28, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Michaliszyn, S.F.; Sjaarda, L.A.; Mihalik, S.J.; Lee, S.; Bacha, F.; Chace, D.H.; De Jesus, V.R.; Vockley, J.; Arslanian, S.A. Metabolomic profiling of amino acids and beta-cell function relative to insulin sensitivity in youth. J. Clin. Endocrinol. Metab. 2012, 97, E2119–E2124. [Google Scholar] [CrossRef] [PubMed]
- Perng, W.; Gillman, M.W.; Fleisch, A.F.; Michalek, R.D.; Watkins, S.M.; Isganaitis, E.; Patti, M.E.; Oken, E. Metabolomic profiles and childhood obesity. Obesity 2014, 22, 2570–2578. [Google Scholar] [CrossRef]
- Perng, W.; Oken, E.; Roumeliotaki, T.; Sood, D.; Siskos, A.P.; Chalkiadaki, G.; Dermitzaki, E.; Vafeiadi, M.; Kyrtopoulos, S.; Kogevinas, M.; et al. Leptin, acylcarnitine metabolites, and development of adiposity in the Rhea Mother-Child Cohort in Crete, Greece. Obes. Sci. Pract.
- Perng, W.; Hector, E.C.; Song, P.X.K.; Tellez Rojo, M.M.; Raskind, S.; Kachman, M.; Cantoral, A.; Burant, C.F.; Peterson, K.E. Metabolomic Determinants of Metabolic Risk in Mexican Adolescents. Obesity 2017, 25, 1594–1602. [Google Scholar] [CrossRef]
- Mihalik, S.J.; Michaliszyn, S.F.; de las Heras, J.; Bacha, F.; Lee, S.; Chace, D.H.; DeJesus, V.R.; Vockley, J.; Arslanian, S.A. Metabolomic profiling of fatty acid and amino acid metabolism in youth with obesity and type 2 diabetes: Evidence for enhanced mitochondrial oxidation. Diabetes Care 2012, 35, 605–611. [Google Scholar] [CrossRef]
- Butte, N.F.; Liu, Y.; Zakeri, I.F.; Mohney, R.P.; Mehta, N.; Voruganti, V.S.; Goring, H.; Cole, S.A.; Comuzzie, A.G. Global metabolomic profiling targeting childhood obesity in the Hispanic population. Am. J. Clin. Nutr. 2015, 102, 256–267. [Google Scholar] [CrossRef]
- Koyama, S.; Ichikawa, G.; Kojima, M.; Shimura, N.; Sairenchi, T.; Arisaka, O. Adiposity Rebound and the Development of Metabolic Syndrome. Pediatrics 2014, 133, e114. [Google Scholar] [CrossRef]
- González, L.; Corvalán, C.; Pereira, A.; Kain, J.; Garmendia, M.L.; Uauy, R. Early adiposity rebound is associated with metabolic risk in 7-year-old children. Int. J. Obes. 2014, 38, 1299–1304. [Google Scholar] [CrossRef]
- Oken, E.; Kleinman, K.P.; Rich-Edwards, J.; Gillman, M.W. A nearly continuous measure of birth weight for gestational age using a United States national reference. BMC Pediatr. 2003, 3, 6. [Google Scholar] [CrossRef]
- Bonser, A.M.; Garcia-Webb, P. C-peptide measurement: Methods and clinical utility. Crit. Rev. Clin. Lab. Sci. 1984, 19, 297–352. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, T.; Okubo, T.; Sato, K.; Fujita, S.; Goto, K.; Hamaoka, T.; Iemitsu, M. Glycerophosphocholine enhances growth hormone secretion and fat oxidation in young adults. Nutrition 2012, 28, 1122–1126. [Google Scholar] [CrossRef] [PubMed]
- Polewski, M.A.; Burhans, M.S.; Zhao, M.; Colman, R.J.; Shanmuganayagam, D.; Lindstrom, M.J.; Ntambi, J.M.; Anderson, R.M. Plasma diacylglycerol composition is a biomarker of metabolic syndrome onset in rhesus monkeys. J. Lipid Res. 2015, 56, 1461–1470. [Google Scholar] [CrossRef] [PubMed]
- Aris, I.M.; Rifas-Shiman, S.L.; Li, L.J.; Kleinman, K.; Coull, B.A.; Gold, D.R.; Hivert, M.F.; Kramer, M.S.; Oken, E. Pre-, Perinatal, and Parental Predictors of Body Mass Index Trajectory Milestones. J. Pediatrics 2018, 201, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Widén, E.; Silventoinen, K.; Sovio, U.; Ripatti, S.; Cousminer, D.L.; Hartikainen, A.-L.; Laitinen, J.; Pouta, A.; Kaprio, J.; Järvelin, M.-R.; et al. Pubertal timing and growth influences cardiometabolic risk factors in adult males and females. Diabetes Care 2012, 35, 850–856. [Google Scholar] [CrossRef]
- Mihalopoulos, N.L.; Holubkov, R.; Young, P.; Dai, S.; Labarthe, D.R. Expected changes in clinical measures of adiposity during puberty. J. Adolesc Health 2010, 47, 360–366. [Google Scholar] [CrossRef]
- Moran, A.; Jacobs, D.R., Jr.; Steinberger, J.; Hong, C.P.; Prineas, R.; Luepker, R.; Sinaiko, A.R. Insulin resistance during puberty: Results from clamp studies in 357 children. Diabetes 1999, 48, 2039–2044. [Google Scholar] [CrossRef]
- Vaz, J.D.S.; Buffarini, R.; Kac, G.; Bielemann, R.M.; Oliveira, I.; Menezes, A.B.; Assunção, M.C.F. Dietary patterns are associated with blood lipids at 18-year-olds: A cross-sectional analysis nested in the 1993 Pelotas (Brazil) birth cohort. Nutr. J. 2018, 17, 77. [Google Scholar] [CrossRef]
- PubChem Database CID=9543695. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/9543695 (accessed on 23 May 2019).
- Metabolomics Workbench. RefMet Compound Details: DG(18:1_18:2) [ONLINE]. Available online: https://www.metabolomicsworkbench.org/databases/refmet/refmet_details.php?REFMET_NAME=DG%2818%3A1_18%3A2%29 (accessed on 29 July 2020).
- National Library of Medicine. Compound Summary: Dihomo-Gamma-Linolenic acid [ONLINE]. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Dihomo-gamma-linolenic-acid (accessed on 25 May 2020).
- Perng, W.; Villamor, E.; Mora-Plazas, M.; Marin, C.; Baylin, A. Alpha-linolenic acid (ALA) is inversely related to development of adiposity in school-age children. Eur. J. Clin. Nutr. 2015, 69, 167–172. [Google Scholar] [CrossRef][Green Version]
- Kumar, A.; Bachhawat, A.K. Pyroglutamic acid: Throwing light on a lightly studied metabolite. Curr. Sci. 2012, 102, 288–297. [Google Scholar]
- National Library of Medicine. Compound Summary: 9,12-Hexadecadienylcarnitine [ONLINE]. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/9_12-Hexadecadienylcarnitine (accessed on 25 May 2020).
- Minkler, P.E.; Kerner, J.; North, K.N.; Hoppel, C.L. Quantitation of long-chain acylcarnitines by HPLC/fluorescence detection: Application to plasma and tissue specimens from patients with carnitine palmitoyltransferase-II deficiency. Clin. Chim. Acta Int. J. Clin. Chem. 2005, 352, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Human Metabolome Database. Arachidonoylcarnitine (HMDB0006455) [ONLINE]. Available online: https://hmdb.ca/metabolites/HMDB0006455 (accessed on 25 May 2020).
- Human Metabolome Database. p-Cresol Glucuronide (HMDB0011686) [ONLINE]. Available online: https://hmdb.ca/metabolites/HMDB0011686 (accessed on 25 May 2020).
- National Library of Medicine. Compound Summary: Phenyl Acetate [ONLINE]. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Phenyl-acetate (accessed on 25 May 2020).
- Banoglu, E.; Jha, G.G.; King, R.S. Hepatic microsomal metabolism of indole to indoxyl, a precursor of indoxyl sulfate. Eur. J. Drug Metab. Pharmacokinet. 2001, 26, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Tilg, H.; Kaser, A. Gut microbiome, obesity, and metabolic dysfunction. J. Clin. Investig. 2011, 121, 2126–2132. [Google Scholar] [CrossRef] [PubMed]
- National Library of Medicine. PubChem Compound Summary: Glycochenodeoxycholate-3-sulfate [ONLINE]. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Glycochenodeoxycholate-3-sulfate (accessed on 1 June 2020).
- Human Metabolome Database. Glycochlic Acid (HMDB0000138) [ONLINE]. Available online: https://hmdb.ca/metabolites/HMDB0000138 (accessed on 1 June 2020).
- Human Metabolome Database. Taurocholic Acid (HMDB0000036). Available online: https://hmdb.ca/metabolites/HMDB0000036 (accessed on 1 June 2020).
- Kalhan, S.C.; Guo, L.; Edmison, J.; Dasarathy, S.; McCullough, A.J.; Hanson, R.W.; Milburn, M. Plasma metabolomic profile in nonalcoholic fatty liver disease. Metabolism 2011, 60, 404–413. [Google Scholar] [CrossRef]
- Karastergiou, K.; Smith, S.R.; Greenberg, A.S.; Fried, S.K. Sex differences in human adipose tissues-the biology of pear shape. Biol. Sex Differ. 2012, 3, 13. [Google Scholar] [CrossRef]
- Guo, S.S.; Chumlea, W.C.; Roche, A.F.; Siervogel, R.M. Age- and maturity-related changes in body composition during adolescence into adulthood: The Fels Longitudinal Study. Int. J. Obes. Relat. Metab. Disord. 1997, 21, 1167–1175. [Google Scholar] [CrossRef]
- Eissa, M.A.; Dai, S.; Mihalopoulos, N.L.; Day, R.S.; Harrist, R.B.; Labarthe, D.R. Trajectories of fat mass index, fat free-mass index, and waist circumference in children: Project HeartBeat2! Am. J. Prev. Med. 2009, 37, S34–S39. [Google Scholar] [CrossRef]
- Cook, S.; Auinger, P.; Huang, T.T. Growth curves for cardio-metabolic risk factors in children and adolescents. J. Pediatr. 2009, 155, S6.e15–e26. [Google Scholar] [CrossRef]
- Perng, W.; Aslibekyan, S. Find the needle in the haystack, then find it again: Replication and validation in the ’omics era. Metabolites 2020, 10, 286. [Google Scholar] [CrossRef]
- Chadeau-Hyam, M.; Athersuch, T.J.; Keun, H.C.; De Iorio, M.; Ebbels, T.M.; Jenab, M.; Sacerdote, C.; Bruce, S.J.; Holmes, E.; Vineis, P. Meeting-in-the-middle using metabolic profiling—a strategy for the identification of intermediate biomarkers in cohort studies. Biomarkers 2011, 16, 83–88. [Google Scholar] [CrossRef]
- Gluckman, P.D.; Hanson, M.A. Developmental and epigenetic pathways to obesity: An evolutionary-developmental perspective. Int. J. Obes. 2008, 32, S62–S71. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Ma, F.; Lou, H.; Liu, Y. The utility of fat mass index vs. body mass index and percentage of body fat in the screening of metabolic syndrome. BMC Public Health 2013, 13, 629. [Google Scholar] [CrossRef]
- Santos, S.; Severo, M.; Lopes, C.; Oliveira, A. Anthropometric Indices Based on Waist Circumference as Measures of Adiposity in Children. Obesity 2018, 26, 810–813. [Google Scholar] [CrossRef] [PubMed]
- Oken, E.; Baccarelli, A.A.; Gold, D.R.; Kleinman, K.P.; Litonjua, A.A.; De Meo, E.; Rich-Edwards, J.W.; Rifas-Shiman, S.L.; Sagiv, S.; Taveras, E.M.; et al. Cohort profile: Project Viva. Int. J. Epidemiol. 2020, in press. [Google Scholar] [CrossRef] [PubMed]
- Gall, W.E.; Beebe, K.; Lawton, K.A.; Adam, K.P.; Mitchell, M.W.; Nakhle, P.J.; Ryals, J.A.; Milburn, M.V.; Nannipieri, M.; Camastra, S.; et al. alpha-hydroxybutyrate is an early biomarker of insulin resistance and glucose intolerance in a nondiabetic population. PLoS ONE 2010, 5, e10883. [Google Scholar] [CrossRef]
- Shin, S.Y.; Fauman, E.B.; Petersen, A.K.; Krumsiek, J.; Santos, R.; Huang, J.; Arnold, M.; Erte, I.; Forgetta, V.; Yang, T.P.; et al. An atlas of genetic influences on human blood metabolites. Nat. Genet. 2014, 46, 543–550. [Google Scholar] [CrossRef]
- Evans, A.; Bridgetwater, B.; Liu, Q.; Mitchell, M.; Robinson, R.; Dai, H.; Stewart, S.; DeHaven, C.; Miller, L. High resolution mass spectrometry improves data quantity and quality as compared to unit mass resolution mass spectrometry in high-throughput profiling metabolomics. Metabolomics 2014, 4. [Google Scholar] [CrossRef]
- Boeke, C.E.; Oken, E.; Kleinman, K.P.; Rifas-Shiman, S.L.; Taveras, E.M.; Gillman, M.W. Correlations among adiposity measures in school-aged children. BMC Pediatr 2013, 13, 99. [Google Scholar] [CrossRef]
- Janssen, I.; Katzmarzyk, P.T.; Ross, R. Waist circumference and not body mass index explains obesity-related health risk. Am. J. Clin. Nutr. 2004, 79, 379–384. [Google Scholar] [CrossRef]
- Kuk, J.L.; Katzmarzyk, P.T.; Nichaman, M.Z.; Church, T.S.; Blair, S.N.; Ross, R. Visceral fat is an independent predictor of all-cause mortality in men. Obesity 2006, 14, 336–341. [Google Scholar] [CrossRef]
- Levy-Marchal, C.; Arslanian, S.; Cutfield, W.; Sinaiko, A.; Druet, C.; Marcovecchio, M.L.; Chiarelli, F. ESPE-LWPES-ISPAD-APPES-APEG-SLEP-JSPE; the Insulin Resistance in Children Consensus Conference Group. Insulin Resistance in Children: Consensus, Perspective, and Future Directions. J. Clin. Endocrinol. Metab. 2010, 95, 5189–5198. [Google Scholar] [CrossRef] [PubMed]
- Morrison, J.A.; Glueck, C.J.; Horn, P.S.; Schreiber, G.B.; Wang, P. Pre-teen insulin resistance predicts weight gain, impaired fasting glucose, and type 2 diabetes at age 18–19 y: A 10-y prospective study of black and white girls. Am. J. Clin. Nutr. 2008, 88, 778–788. [Google Scholar] [CrossRef]
- Zhang, X.; Li, J.; Zheng, S.; Luo, Q.; Zhou, C.; Wang, C. Fasting insulin, insulin resistance, and risk of cardiovascular or all-cause mortality in non-diabetic adults: A meta-analysis. Biosci. Rep. 2017, 37. [Google Scholar] [CrossRef] [PubMed]
- Webber, L.S.; Srinivasan, S.R.; Wattigney, W.A.; Berenson, G.S. Tracking of serum lipids and lipoproteins from childhood to adulthood. The Bogalusa Heart Study. Am. J. Epidemiol. 1991, 133, 884–899. [Google Scholar] [CrossRef] [PubMed]
- Barker, D.J.; Bagby, S.P. Developmental antecedents of cardiovascular disease: A historical perspective. Journal of the American Society of Nephrology: JASN 2005, 16, 2537–2544. [Google Scholar] [CrossRef]
- Viitasalo, A.; Lakka, T.A.; Laaksonen, D.E.; Savonen, K.; Lakka, H.M.; Hassinen, M.; Komulainen, P.; Tompuri, T.; Kurl, S.; Laukkanen, J.A.; et al. Validation of metabolic syndrome score by confirmatory factor analysis in children and adults and prediction of cardiometabolic outcomes in adults. Diabetologia 2014, 57, 940–949. [Google Scholar] [CrossRef]
- Fryar, C.D.; Gu, Q.; Ogden, C.L.; Flegal, K.M. Anthropometric Reference Data for Children and Adults: United States, 2011-2014; The United States Department of Health & Human Services: Hyattsville, MA, USA, 2016. [Google Scholar]
- Lee, J.M.; Okumura, M.J.; Davis, M.M.; Herman, W.H.; Gurney, J.G. Prevalence and determinants of insulin resistance among U.S. adolescents: A population-based study. Diabetes Care 2006, 29, 2427–2432. [Google Scholar] [CrossRef]
- Flynn, J.T.; Kaelber, D.C.; Baker-Smith, C.M.; Blowey, D.; Carroll, A.E.; Daniels, S.R.; de Ferranti, S.D.; Dionne, J.M.; Falkner, B.; Flinn, S.K.; et al. Subcommittee on Screening and Management of High Blood Pressure in Children. Clinical Practice Guideline for Screening and Management of High Blood Pressure in Children and Adolescents. Pediatrics 2017, 140, e20171904. [Google Scholar] [CrossRef]
- World Health Organization. BMI Classification. Available online: http://apps.who.int/bmi/index.jsp?introPage=intro_3.html (accessed on 21 May 2012).
- Langfelder, P.; Horvath, S. WGCNA: An R package for weighted correlation network analysis. BMC Bioinform. 2008, 9, 559. [Google Scholar] [CrossRef]
- Pemmaraju, S.; Skiena, S. Computational Discrete Mathematics: Combinatorics and Graph Theory with Mathematica, 1st ed.; Cambridge University Press: New York, NY, USA, 2003. [Google Scholar]
- Yip, A.M.; Horvath, S. Gene network interconnectedness and the generalized topological overlap measure. BMC Bioinform. 2007, 8, 22. [Google Scholar] [CrossRef]
- Langfelder, P.; Zhang, B.; Horvath, S. Defining clusters from a hierarchical cluster tree: The Dynamic Tree Cut package for R. Bioinformatics 2008, 24, 719–720. [Google Scholar] [CrossRef] [PubMed]
- Bareinboim, E.; Barbosa, V.C. Descents and nodal load in scale-free networks. Phys. Rev. E. Stat. Nonlin. Soft Matter Phys. 2008, 77, 046111. [Google Scholar] [CrossRef] [PubMed]
- Langfelder, P.; Luo, R.; Oldham, M.C.; Horvath, S. Is my network module preserved and reproducible? PLoS Comput. Biol. 2011, 7, e1001057. [Google Scholar] [CrossRef] [PubMed]
| Term/concept | Explanation |
|---|---|
| Infancy body mass index (BMI) peak | An individual-level BMI trajectory milestone characterized by a vertex that typically occurs between 7 and 9 months of age. Later age and higher magnitude of this milestone has been associated with future obesity and metabolic risk. |
| Childhood BMI rebound | An individual-level BMI trajectory milestone characterized by a nadir that typically occurs between 3 and 6 years of age. Earlier age and higher magnitude of this milestone has been related to future obesity and metabolic risk. |
| False discovery rate (FDR) | A statistical procedure that protects against type 1 error (i.e., false positive findings) when making multiple comparisons. The correction requires an investigator-defined threshold of acceptable type 1 error. In this study, we used an error rate of 5%. |
| Untargeted metabolomics platform | An unbiased laboratory approach to measuring low-molecular-weight compounds (metabolites) in a biosample. This type of platform typically involves mass spectrometry (MS) paired with liquid (LC) and/or gas chromatography (GC) to estimate relative concentrations of all detectable metabolites (from hundreds to thousands) in a biosample. The broad coverage of this type of platform makes it ideal for hypothesis generation and discovery of new biomarkers. |
| Metabolic syndrome (MetS) z-score | A metabolic syndrome risk z-score calculated as the average of externally age- and sex-standardized waist circumference, systolic blood pressure, reversed high-density lipoprotein (HDL), the homeostatic model assessment of insulin resistance (HOMA-IR), and triglycerides. |
| Weighted correlation network analysis (WGCNA) | A correlation-based dimension reduction procedure that constructs latent variables (“consensus networks”) that account for the majority of variability in a high-dimensional dataset. |
| Meeting-in-the-middle | An analytical strategy that involves identifying a features (e.g., metabolites) from a high-dimensional dataset (e.g., untargeted metabolomics data) that are associated with an exposure, and also associated with an outcome. |
| Mean ± SD or % (N) | ||
|---|---|---|
| Boys | Girls | |
| n = 249 | n = 227 | |
| Perinatal and Family Characteristics | ||
| Mother′s age at enrollment (years) | 32.7 ± 5.6 | 32.7 ± 4.9 |
| Annual household income > $70,000 | 63.9% (145) | 62.8% (130) |
| Mother is college graduate | 69.0% (161) | 71.2% (161) |
| Maternal pre-pregnancy BMI (kg/m2) | 24.8 ± 5.2 | 24.9 ± 5.3 |
| Gestational diabetes mellitus diagnosis | 4.4% (11) | 4.0% (9) |
| Mother smoked during pregnancy | 11.2% (28) | 8.9% (20) |
| Birthweight-for-gestational age z-score a | 0.26 ± 1.01 | 0.18 ± 0.97 |
| Growth hallmarks | ||
| Age at infancy BMI peak (months) | 8.0 ± 1.8 | 8.7 ± 3.0 |
| BMI at peak (kg/m2) | 18.4 ± 1.4 | 17.8 ± 1.3 |
| Age at BMI rebound (years) | 5.2 ± 1.8 | 4.8 ± 1.7 |
| BMI at rebound (kg/m2) | 16.0 ± 1.2 | 15.8 ± 1.3 |
| Child characteristics at the early teen visit | ||
| Age (years) | 12.9 ± 0.6 | 13.0 ± 0.7 |
| Race/ethnicity | ||
| Black | 16.1% (40) | 12.8% (29) |
| Hispanic | 4.4% (11) | 4.0% (9) |
| White | 61.8% (154) | 66.4% (150) |
| Asian | 2.8% (7) | 3.1% (7) |
| >1 race/ethnicity | 14.9% (37) | 13.7% (31) |
| Puberty score >2 | 45.2% (112) | 83.6% (189) |
| Waist circumference (cm) | 73.0 ± 11.2 | 72.6 ± 10.4 |
| SBP (mmHg) | 108.7 ± 8.9 | 105.7 ± 8.8 |
| HDL (mg/dL) | 56.2 ± 13.7 | 55.9 ± 12.5 |
| HOMA-IR | 2.75 ± 1.73 | 3.42 ± 2.23 |
| Triglycerides (mg/dL) | 66.8 ± 30.9 | 71.7 ± 29.8 |
| MetS z-scoreb | −0.13 ± 0.43 | −0.20 ± 0.43 |
| Unadjusted | Adjusted b | |||||||
|---|---|---|---|---|---|---|---|---|
| Boys (n = 249) | Girls (n = 227) | Boys (n = 248) | Girls (n = 225) | |||||
| β (95% CI) | P | β (95% CI) | P | β (95% CI) | P | β (95% CI) | P | |
| BMI peak | ||||||||
| Age | 0.05 (0.00, 0.11) | 0.05 | −0.02 (−0.07, 0.04) | 0.51 | 0.05 (0.00, 0.11) | 0.05 | −0.03 (−0.08, 0.03) | 0.36 |
| Magnitude | 0.01 (−0.04, 0.07) | 0.59 | 0.09 (0.03, 0.14) | 0.001 | 0.01 (−0.04, 0.07) | 0.64 | 0.08 (0.02, 0.14) | 0.005 |
| BMI rebound | ||||||||
| Age | −0.19 (−0.24, −0.14) | <0.0001 | −0.10 (−0.16, −0.05) | 0.0002 | −0.20 (−0.25, −0.15) | <0.0001 | −0.09 (−0.15, −0.04) | 0.0008 |
| Magnitude | 0.12 (0.07, 0.17) | <0.0001 | 0.12 (0.07, 0.17) | <0.0001 | 0.12 (0.07, 0.17) | <0.0001 | 0.11 (0.06, 0.17) | <0.0001 |
| Boys | Girls | |||
|---|---|---|---|---|
| Y-Variable: Age at Peak | Y-Variable: Magnitude of BMI at Peak | |||
| X-variable | β (95% CI) per 1.85 mo | P | β (95% CI) per 1.32 kg/m2 | P |
| ME1 | −0.001 (−0.005, 0.003) | 0.51 | 0.000 (−0.008, 0.008) | 0.99 |
| ME2 | −0.0057 (−0.013, 0.002) | 0.12 | 0.004 (−0.004, 0.012) | 0.37 |
| ME3 | −0.006 (−0.013, 0.001) | 0.11 | −0.003 (−0.010, 0.005) | 0.54 |
| ME4 | −0.002 (−0.009, 0.005) | 0.57 | 0.009 (0.001, 0.017) | 0.03 |
| ME5 | 0.001 (−0.007, 0.008) | 0.84 | 0.004 (−0.004, 0.012) | 0.34 |
| ME6 | −0.004 (−0.011, 0.004) | 0.23 | 0.003 (−0.005, 0.010) | 0.52 |
| ME7 | 0.003 (−0.004, 0.011) | 0.36 | 0.003 (−0.005, 0.011) | 0.52 |
| ME8 | −0.004 (−0.011, 0.004) | 0.33 | 0.001 (−0.007, 0.009) | 0.77 |
| ME9 | −0.002 (−0.009, 0.006) | 0.67 | −0.002 (−0.010, 0.006) | 0.67 |
| ME10 | −0.003 (−0.010, 0.004) | 0.43 | 0.003 (−0.005, 0.011) | 0.49 |
| ME11 | 0.005 (−0.002, 0.013) | 0.17 | 0.001 (−0.007, 0.009) | 0.88 |
| ME12 | −0.006 (−0.013, 0.001) | 0.09 | −0.003 (−0.011, 0.005) | 0.43 |
| ME13 | 0.003 (−0.004, 0.010) | 0.44 | 0.003 (−0.006, 0.011) | 0.52 |
| Boys | ||||
|---|---|---|---|---|
| Y-Variable: BMI Rebound | ||||
| Age | Magnitude | |||
| β (95% CI) per 21.4 mo | P | β (95% CI) per 1.21 kg/m2 | P | |
| X-variable | ||||
| ME1 | −0.004 (−0.011, 0.003) | 0.29 | 0.000 (−0.007, 0.008) | 0.90 |
| ME2 | 0.003 (−0.005, 0.010) | 0.50 | −0.003 (−0.011, 0.004) | 0.37 |
| ME3 | −0.002 (−0.009, 0.006) | 0.63 | 0.000 (−0.007, 0.007) | 0.97 |
| ME4 | 0.000 (−0.008, 0.007) | 0.93 | 0.000 (−0.008, 0.007) | 0.99 |
| ME5 | 0.000 (−0.008, 0.008) | 0.99 | −0.010 (−0.017, −0.002) | 0.01 |
| ME6 | 0.007 (0.000, 0.014) | 0.06 | −0.002 (−0.009, 0.005) | 0.62 |
| ME7 | −0.011 (−0.018, −0.004) * | 0.003 * | 0.004 (−0.004, 0.011) | 0.35 |
| ME8 | 0.014 (0.008, 0.021) * | <0.0001 * | −0.011 (−0.018, −0.004) * | 0.002 * |
| ME9 | 0.008 (0.001, 0.016) | 0.03 | −0.005 (−0.013, 0.002) | 0.16 |
| ME10 | 0.007 (−0.001, 0.014) | 0.07 | −0.007 (−0.014, 0.000) | 0.06 |
| ME11 | −0.010 (−0.018, −0.003) | 0.007 * | 0.004 (−0.003, 0.012) | 0.25 |
| ME12 | 0.004 (−0.004, 0.011) | 0.31 | −0.003 (−0.010, 0.005) | 0.45 |
| ME13 | 0.008 (0.000, 0.015) | 0.04 | −0.005 (−0.012, 0.002) | 0.19 |
| Girls | ||||
| Y-Variable: BMI Rebound | ||||
| Age | Magnitude | |||
| β (95% CI) per 20.3 mo | P | β (95% CI) per 1.26 kg/m2 | P | |
| X-variable | ||||
| ME1 | 0.003 (−0.006, 0.011) | 0.55 | 0.003 (−0.004, 0.011) | 0.40 |
| ME2 | 0.000 (−0.008, 0.009) | 0.98 | 0.002 (−0.006, 0.010) | 0.62 |
| ME3 | 0.002 (−0.007, 0.010) | 0.67 | −0.001 (−0.009, 0.006) | 0.74 |
| ME4 | 0.004 (−0.004, 0.013) | 0.32 | 0.006 (−0.003, 0.014) | 0.18 |
| ME5 | 0.010 (0.001, 0.018) | 0.03 | −0.002 (−0.010, 0.006) | 0.63 |
| ME6 | −0.003 (−0.011, 0.005) | 0.47 | 0.004 (−0.003, 0.012) | 0.29 |
| ME7 | −0.013 (−0.021, −0.005) | 0.002 | 0.007 (0.000, 0.015) | 0.06 |
| ME8 | 0.010 (0.002, 0.018) | 0.02 | −0.006 (−0.014, 0.002) | 0.14 |
| ME9 | 0.005 (−0.004, 0.013) | 0.27 | −0.005 (−0.013, 0.003) | 0.22 |
| ME10 | 0.000 (−0.009, 0.008) | 0.93 | 0.004 (−0.003, 0.010) | 0.27 |
| ME11 | 0.003 (−0.005, 0.011) | 0.48 | 0.000 (−0.008, 0.008) | 0.92 |
| ME12 | 0.005 (−0.003, 0.013) | 0.25 | −0.003 (−0.010, 0.005) | 0.44 |
| ME13 | 0.004 (−0.005, 0.012) | 0.38 | −0.001 (−0.009, 0.007) | 0.84 |
| Difference in MetS z-Score per 1 Unit Metabolite Network in Boys | ||||||||
|---|---|---|---|---|---|---|---|---|
| Unadjusted (n = 249) | Model 1 (n = 248) | Model 2 (n = 248) | Model 3 (n = 247) | |||||
| Metabolite Network | β (95% CI) | P | β (95% CI) | P | β (95% CI) | P | β (95% CI) | P |
| ME7 | 1.28 (0.39, 2.17) | 0.005 | 1.43 (0.52, 2.34) | 0.002 | 1.16 (0.19, 2.13) | 0.02 | 1.18 (0.25, 2.12) | 0.01 |
| ME8 | −0.92 (−1.86, 0.02) | 0.06 | −1.01 (−1.96, −0.07) | 0.04 | −0.95 (−1.88, −0.01) | 0.05 | −0.56 (−1.42, 0.30) | 0.20 |
| ME11 | 2.83 (2.01, 3.65) | <0.0001 | 2.88 (2.06, 3.70) | <0.0001 | 2.79 (1.98, 3.61) | <0.0001 | 2.79 (2.00, 3.59) | <0.0001 |
| Waist Circumference z-Score | HOMA-IR z-Score | HDL z-Score | Triglyceride z-Score | SBP z-Score | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| β (95% CI) | P | β (95% CI) | P | β (95% CI) | P | β (95% CI) | P | β (95% CI) | P | |
| Metabolite network | ||||||||||
| ME7 | 2.27 (1.02, 3.52) | 0.0004 | 2.05 (0.25, 3.85) | 0.03 | −1.30 (−2.77, 0.16) | 0.08 | 0.11 (−1.15, 1.37) | 0.86 | 1.40 (−0.35, 3.15) | 0.12 |
| ME8 | −3.76 (−4.99, −2.52) | <0.0001 | 0.14 (−1.73, 2.01) | 0.88 | 2.91 (1.43, 4.38) | 0.0001 | 1.47 (0.19, 2.75) | 0.02 | −0.02 (−1.84, 1.79) | 0.98 |
| ME11 | 1.81 (0.59, 3.02) | 0.004 | 2.24 (0.51, 3.97) | 0.01 | −3.70 (−5.05, −2.35) | <0.0001 | 6.06 (5.11, 7.00) | <0.0001 | 0.59 (−1.10, 2.29) | 0.49 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perng, W.; Rahman, M.L.; Aris, I.M.; Michelotti, G.; Sordillo, J.E.; Chavarro, J.E.; Oken, E.; Hivert, M.-F. Metabolite Profiles of the Relationship between Body Mass Index (BMI) Milestones and Metabolic Risk during Early Adolescence. Metabolites 2020, 10, 316. https://doi.org/10.3390/metabo10080316
Perng W, Rahman ML, Aris IM, Michelotti G, Sordillo JE, Chavarro JE, Oken E, Hivert M-F. Metabolite Profiles of the Relationship between Body Mass Index (BMI) Milestones and Metabolic Risk during Early Adolescence. Metabolites. 2020; 10(8):316. https://doi.org/10.3390/metabo10080316
Chicago/Turabian StylePerng, Wei, Mohammad L. Rahman, Izzuddin M. Aris, Gregory Michelotti, Joanne E. Sordillo, Jorge E. Chavarro, Emily Oken, and Marie-France Hivert. 2020. "Metabolite Profiles of the Relationship between Body Mass Index (BMI) Milestones and Metabolic Risk during Early Adolescence" Metabolites 10, no. 8: 316. https://doi.org/10.3390/metabo10080316
APA StylePerng, W., Rahman, M. L., Aris, I. M., Michelotti, G., Sordillo, J. E., Chavarro, J. E., Oken, E., & Hivert, M.-F. (2020). Metabolite Profiles of the Relationship between Body Mass Index (BMI) Milestones and Metabolic Risk during Early Adolescence. Metabolites, 10(8), 316. https://doi.org/10.3390/metabo10080316