Abstract
Prematurity is the leading cause of neonatal deaths and high economic costs; it depends on numerous biological and social factors, and is highly prevalent in males. Several factors can affect the metabolome of premature infants. Accordingly, the aim of the present study was to analyze the role played by gestational age (GA), parenteral nutrition (PN), and caffeine treatment in sex-related differences of blood metabolome of premature neonates through a MS/MS-based targeted metabolomic approach for the detection of amino acids and acylcarnitines in dried blood spots. GA affected the blood metabolome of premature neonates: male and female very premature infants (VPI) diverged in amino acids but not in acylcarnitines, whereas the opposite was observed in moderate or late preterm infants (MLPI). Moreover, an important reduction of metabolites was observed in female VPI fed with PN, suggesting that PN might not satisfy an infant’s nutritional needs. Caffeine showed the highest significant impact on metabolite levels of male MLPI. This study proves the presence of a sex-dependent metabolome in premature infants, which is affected by GA and pharmacological treatment (e.g., caffeine). Furthermore, it describes an integrated relationship among several features of physiology and health.
1. Introduction
Prematurity, defined as childbirth before 37 weeks of gestation, annually affects 9.6% of pregnancies [1,2], being the 84% of preterm births of the so-called moderate or late preterm infants (MLPI) [3]. Relevantly, prematurity may have negative consequences for the family and the infants, during both child and adult life, and the healthcare system. Prematurity is, in fact, the leading cause of neonatal deaths and high economic costs [4]. Prematurity depends on numerous biological and social factors, such as gender, age, and smoking [5]. Notably, its incidence is higher in male neonates [6].
Several factors, including gestational age (GA) at birth, can affect qualitatively and quantitatively the metabolites measured in premature infants [7]. It can be associated with lowered maturation of metabolic pathways, elevation of catabolic stress, and other numerous confounding variables (e.g., feeding) [7,8,9]. Preterm infants can have feeding difficulties and then weight loss and growth difficulties [3]; consequently, early parenteral nutrition (PN) is recommended. A typical PN includes carbohydrates (mostly glucose), proteins (both essential and non-essential amino acids), lipids, and vitamins [10].
Moreover, preterm infants often present respiratory symptoms (apnea, intermittent hypoxemia, and extubation in mechanically ventilated premature newborns [11]) which are treated with caffeine. This methylxanthine increases respiratory rate and minute volume, stimulates respiratory centers, and increases pulmonary blood flow and the sensitivity of central medullary areas to hypercapnia, acting as a competitive inhibitor of A1 and A2A receptors of adenosine [12]. In addition, caffeine and its metabolites (especially paraxanthine) may enhance the inhibition of systemic enzymes, which modulate lipid and glucose metabolisms [13,14]. In adults, the predominant enzyme in caffeine metabolism is CYP1A2, which is more active in men [15]; however, caffeine metabolism does not seem to present a sexual dimorphism [16].
Recently, gender and sex differences have been highlighted in healthy and ill individuals [17,18]. Gender mainly refers to the socially constructed identities of individuals, whereas sex refers to the fundamental biological disparities between males and females [15,19]. Gender and sex differences start in utero and Clarke reported sex differences in birth outcomes since 1786 [20]. Moreover, they are influenced by GA at birth [7,21]. However, the influence of sex and gender is still neglected in many studies [7,22]. Although Gucciardi and colleagues did not find sex differences in acylcarnitines in neonates after different GA [23], others found sex differences in blood amino acids and acylcarnitines and urinary organic acids of at-term neonates [24,25,26].
As a matter of fact, the metabolome of biological fluids of preterm infants collected during the first days after birth shows a specific urinary and blood pattern, with high variability in amino acids, organic acids, and other metabolites [27,28,29].
Thus, the aim of the present study was to assess the role played by GA in the occurrence of sex-related differences in the blood metabolome of premature neonates through a targeted metabolomic approach for the detection of amino acids and acylcarnitines in dried blood spots (DBS). In addition, it was evaluated whether the sex effect was influenced by feeding condition (PN vs no parenteral nutrition (NPN)) and by caffeine treatment.
2. Results
2.1. Populations
The population of preterm infants was stratified according to sex, birth weight (BW), GA, and treatment with caffeine or PN. As expected, both male and female very premature infants (VPI) significantly weighted less than male and female MLPI. Moreover, male MLPI fed with PN had lower BW and GA than those exposed to NPN (Table 1). Caffeine-treated MLPI showed a lower GA than those not treated (Table 1).

Table 1.
Population stratification according to sex, gestational age (GA) (weeks), body weight (BW) (g) and caffeine treatment.
2.2. GA Effect on Blood Metabolome of Premature Female and Male Babies
2.2.1. Intra-Sex Analysis of GA Effect
A total of 53 variables were measured (Supplementary Table S1) from newborns-derived DBS within a platform set to detect metabolism errors. Both female and male VPI had higher levels of C4, C5, C8, and C4OH in comparison with those of MLPI, being C4 and C5 the most different in female and male neonates, respectively (Table 2). Interestingly, sexes were associated with small specific metabolic variations: VPI females had higher Tyr, C5:1, C14:2 when compared with MLPI, whereas VPI males had lower Ala and Glu and higher C3, C3DC, and C10:2 than those measured in MLPI (Table 2). Other metabolites were not statistically different (Supplementary Table S2).

Table 2.
Intra-sex analysis of GA effect in female and male preterm neonates.
Then, partial least squares-discriminant analysis (PLS-DA) was carried out to identify metabolites closely associated with sex-dependent GA according to their variable importance on projection (VIP) score. In the female cohort, Tyr, C12, and C18:1OH differentiated between VPI and MLPI cohorts (VIP > 2.0), whereas in the male cohort, C5, C5OH, and C4 (VIP > 2.0) differentiated between VPI and MLPI (Supplementary Figure S1A,B).
2.2.2. Inter-Sex Analysis of GA Effect
Female and male VPI diverged in their amino acid profile (Figure 1A), with Ala, Tyr, Asp, Glu, Gly, and Cit being significantly higher in female infants. Acylcarnitines and total esterified carnitine did not show sexual dimorphism in VPI (Supplementary Table S3). By contrast, female and male MLPI diverged in acylcarnitine levels (Figure 1B): C2, C6, C4DC, C18:1, C18:2, and total esterified carnitine were higher in males. Instead, amino acid levels did not diverge between sexes in MLPI (Supplementary Table S3).
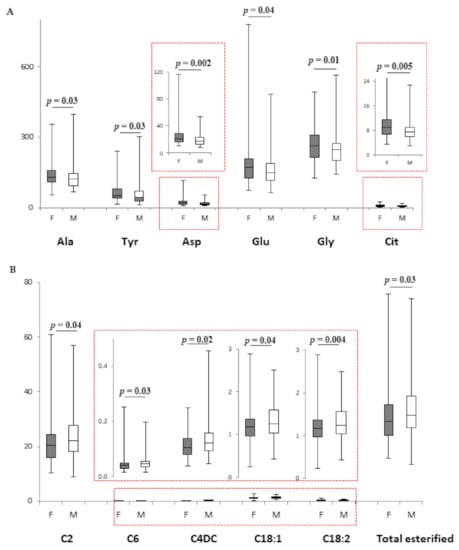
Figure 1.
(A). Box plot of amino acids (µM) that displays the sex difference in female (n = 66; grey bars) and male (n = 81; white bars) VPI. (B). Box plot of acylcarnitines (µM) that displays the sex difference in female (n = 71; grey bars) and male (n = 93; white bars) MLPI. The horizontal line across the box represents the median, and the box comprises the first and the third quartiles. The vertical lines represent the minimum and the maximum values. Red boxes represent an enlargement of the box plots necessary due to the different scale on the y axis.
PLS-DA analysis shows that C12 and Asp (Supplementary Figure S1C) were discriminant between VPI females and males. C2, C18:1, and C10DC differentiated MLPI females and males (Supplementary Figure S1D).
2.3. PN Effect on Blood Metabolome of Female and Male Premature Neonates
2.3.1. Intra-Sex Analysis of PN Effect
In female VPI, PN lowered 9 metabolites (Asp, Glu, Gly, Cit, ArgSuc, C5OH, C4DC, C12:1, and C18OH) and significantly increased C18:2 of ~73% in comparison with that found in the NPN group (Table 3). In female MLPI, PN lowered only Glu levels and increased C5, C12, C6DC, C14:1, C8DC, and C10:2 (Table 3). All the other metabolites were not modified (Supplementary Table S4).

Table 3.
Intra-sex analysis of PN effect in females and males.
In male VPI, PN increased only Phe of ~20%, whereas C2, C8, C10:2, C14-OH, total esterified carnitine, and total esterified/C0 ratio were lowered (Table 3). In male MLPI, PN lowered Asp, Glu, and ArgSuc, and increased C5, C10:1, C10, C12, C6DC, and C14:1 (Table 3). All the other metabolites were not modified (Supplementary Table S5).
PLS-DA showed that ArgSuc, Glu, Orn, and C12 could discriminate between female VPI fed with NPN and PN (Supplementary Figure S2A), whereas C12 and total esterified/C0 discriminated between NPN female MLPI and PN cohorts (Supplementary Figure S2B). C5DC and total esterified/C0 ratio could discriminate between male VPI fed with NPN and PN (Supplementary Figure S2C). C12, Glu, and C14 discriminated between male MLPI fed with NPN and PN (Supplementary Figure S2D).
2.3.2. Inter-Sex Differences of PN Effect
NPN females had higher Gly and Cit concentrations and lower levels of C8, C16, and C18:2 when compared with NPN males (Figure 2). All the other metabolites were not modified (Supplementary Table S6). PLS-DA showed that males and females were differentiated by altered level of discriminant metabolites C18:1OH, C6:1, Val, Orn, and Tyr (Supplementary Figure S2E). No sex-related differences were observed in infants fed with PN (Supplementary Table S6), even if C18:1 and the total esterified/C0 ratio discriminated male and female in the PN group (Supplementary Figure S2F).
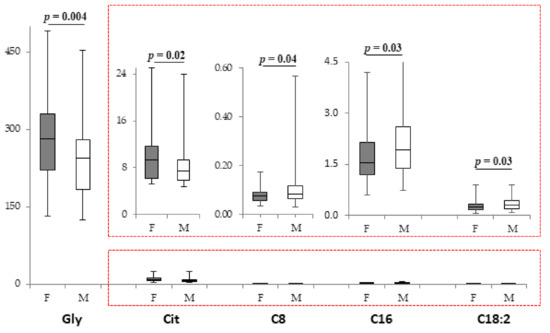
Figure 2.
Box plot of metabolites (µM) that displays the sex difference in female (n = 62; grey bars) and male (n = 79; white bars) infants not fed with PN. The horizontal line across the box represents the median, and the box comprises the first and the third quartiles. The vertical lines represent the minimum and the maximum values. Red boxes represent an enlargement of the box plots necessary due to the different scale on the y axis.
2.4. Caffeine Effect on Blood Metabolome of Female and Male Premature Neonates
2.4.1. Intra-Sex Analysis of Caffeine Effect
Female VPI treated with caffeine were different only for Glu and C5:1, whose levels were higher when compared with those of non-treated individuals (Figure 3A), whereas female caffeine-treated MLPI did not show any significant differences in comparison with non-treated cases (Supplementary Table S7).
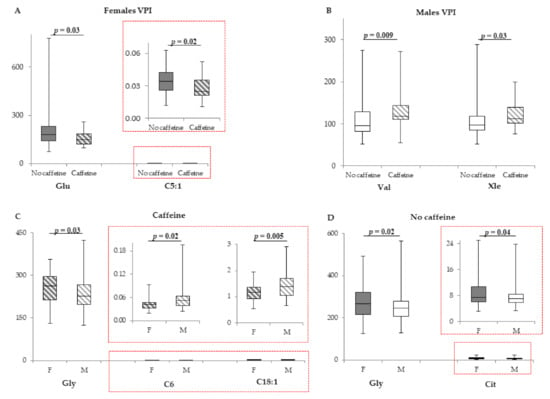
Figure 3.
(A). Box plot of metabolites (µM) that displays the difference in VPI females not treated with caffeine (n = 41; grey bars) and treated with caffeine (n = 25; stripped bars). (B). Box plot of amino acids (µM) that displays the sex difference in VPI males not treated with caffeine (n = 52; white bars) and treated with caffeine (n = 29; stripped bars). (C). Box plot of metabolites (µM) that displays the sex difference in females (n = 41; grey stripped bars) and males (n = 50; white stripped bars) treated with caffeine. (D). Box plot of metabolites (µM) that displays the sex difference in females (n = 96; grey bars) and males (n = 25; white bars) not treated with caffeine. The horizontal line across the box represents the median, and the box comprises the first and the third quartiles. The vertical lines represent the minimum and the maximum values. Red boxes represent an enlargement of the box plots necessary due to the different scale on the y axis.
In male VPI, caffeine significantly increased Val and Xle blood levels (Figure 3B). In MLPI males, the treatment significantly affected 8 parameters: Asp and Gly were lower in the caffeine-treated cohort, whereas the acylcarnitines C3, C5, C5OH, C14:2, C4OH, and C8:1 were significantly higher (Table 4). Differences ranged from −14.3% (Gly) to 36.4% (C5OH). All the other metabolites were not modified (Supplementary Table S8).

Table 4.
Caffeine effect in male MLPI.
PLS-DA showed that VIP and MLPI caffeine-treated females were discriminated by ArgSuc, C6:1, Orn, and by C18:2, C16, respectively (Supplementary Figure S3A,B). VIP and MLPI caffeine-treated males were differentiated by C14:1, C6:1, Asp, C18:2 (Supplementary Figure S3C) and by C4DC when compared with those not treated, respectively (Supplementary Figure S3D).
2.4.2. Inter-Sex Differences of Caffeine Effect
Caffeine-treated females had higher Gly and lower C6 and C18:1 in comparison with those of caffeine-treated males (Figure 3C). In caffeine-free infants, Gly and Cit were significantly higher in females (Figure 3D). All the other metabolites were not modified (Supplementary Table S9).
PLS-DA showed that C5DC and Asp could differentiate male and female babies treated with caffeine (Supplementary Figure S3E), whereas C10DC, C18OH, C14, and C18:1 were key sex discriminants in caffeine-free infants (Supplementary Figure S3F).
2.5. Cluster Analysis of Cohorts
Cluster analysis showed four different clusters: the first one included 57 subjects, whereas the second one included 81 individuals. The third and fourth ones grouped 63 and 109 infants, respectively (Table 5). Cluster 1 included 63.2% of males and grouped almost all subjects with higher values of acylcarnitines.

Table 5.
Clusters analysis of cohorts.
Cluster 2 included VPI infants only (43.2% were males), with the lowest values of BW, GA, and several acylcarnitines (red in Table 5).
Cluster 3 had the largest percentage (66.7%) of males and grouped almost all subjects treated with caffeine, showing that caffeine highly affects males, in agreement with Kruskall–Wallis analysis.
Cluster 4 included most of all late preterm infants (55.1% were males), characterized by the highest values of BW and GA and the lowest values of several acylcarnitines (red in Table 5).
2.6. Correlation Analysis
Correlations between BW, GA, and parameters are reported in Figure 4. In females, 17 metabolites negatively correlated with BW and 10 with GA (C10DC was positively correlated, whereas the others only negatively). In males, 12 metabolites negatively correlated with BW and 16 with GA (6 positively and 10 negatively).
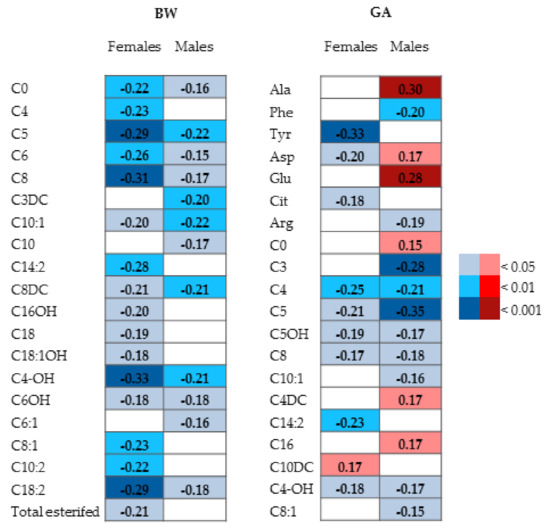
Figure 4.
Heat maps of correlations among BW or GA in female and male neonates. Values represent the Spearman’s correlation coefficients. The red and blue colors of the cells indicate positive and negative statistically significant correlation, respectively.
Furthermore, for both sexes there was a positive correlation between BW and GA (coefficient: 0.468; p-value < 0.001 for males; coefficient: 0.503; p-value < 0.001 for females).
3. Discussion
The present study confirms that different GA impact qualitatively and quantitatively on blood metabolome of premature neonates with corresponding change in several amino acids, free carnitine, and acylcarnitines. However, some main novelties may be elicited: (i) the evidence of quantitative and qualitative sex differences in the blood of premature neonates; (ii) the sex differences are affected by GA, type of nutrition, and caffeine. Prematurity is characterized by sex-specific levels of metabolites: VPI females had higher Tyr, C5:1, and C14:2 when compared with MLPI ones, whereas VPI males had lower Ala and Glu, as well as higher C3, C3DC, and C10:2 than those found in MLPI. Interestingly, GA also affects sex differences, with their onset being closely related to the specific GA. Male and female VPI showed different amino acids levels with the only exception of acylcarnitines; the opposite scenario was observed in MLPI (sex difference found in acylcarnitines but not in amino acids), making infants at different stages of prematurity metabolically distinct, as confirmed by the cluster analysis. This is in agreement with what we previously reported studying term infants: females had higher amino acids concentrations, whereas males had higher acylcarnitines [24]. Previously, Wilson et al. reported that Arg, leucine, Orn, Phe, and Val levels increase as a function of prematurity [7]; however, they do not assess the effects of sex. A recent study showed that 1459 metabolites, including amino acids, carbohydrates, dipeptides, lipids, nucleotides, polyamines, and xenobiotics significantly correlate with GA [30]. However, this study did not evaluate the sex influence on the metabolome. Indeed, our study assesses metabolome changes related to GA that are also influenced by sex. Prematurity may influence analyte levels through several different mechanisms related to an increased catabolic stress, prematurity of metabolic pathways, and/or organ systems (renal and hepatic) [8,31,32].
PN induced qualitative changes in the blood metabolome when the sex of babies and the grade of prematurity was considered. Preterm infants receive PN, which leads to a reduction in the levels of nine metabolites (Asp, Glu, Gly, Cit, ArgSuc, C5OH, C4DC, C12:1, and C18OH), ranging from 8% to 52% in female VPI. The decrease is less relevant in male VPI, ranging from 12% to 20% for six metabolites (C2, C8, C10:2, C14-OH, total esterified carnitine, and total esterified/C0 ratio). The dramatic reduction of metabolites in VPI females fed with PN might suggest that PN could not address a female infant’s nutritional needs, indicating the need of sex specific PN. In female MLPI, PN caused an increase (ranged from 10% to 100%) of six metabolites (C5, C12, C6DC, C14:1, C8DC, and C10:2) and a decrease of Glu. In male MLPI, a significant increase (ranged from 17% to 50%) was observed for six metabolites (C5, C10:1, C10, C12, C6DC, and C14:1), as well as a decrease of Asp, Glu, and ArgSuc. In Asian preterm infants, it has been reported that leucine, Met, tryptophan, histidine, Arg, Glu, Asp, and serine significantly increase after PN, while Phe, Tyr, Gly, and Ala decrease. However, this study did not consider the sex [33]. In addition, comparing VPI versus MLPI it emerges that the metabolism of amino acids and acylcarnitines changes with GA, being that these modifications sex-dependent.
PN administration can lead to serious complications, being inappropriate or unsuitable for infants’ metabolic demand [34,35,36], so that a balance between the demand and intake of nutrients appears critical. As an example, earlier and higher intravenous amino acid and lipid intakes particularly increased the risk of metabolic acidosis in VPI, depending also on GA [37]. The results of our study indicate that sex is another variable that must be considered, highlighting the need to evaluate PN according to GA and sex.
The sex-specificity of the effect exerted by caffeine on the metabolome is an innovative result, at least in prematures. Caffeine causes relevant metabolic effects, modulating lipid and glucose metabolism [13,14]. In particular, Altmaier observed a negative association between caffeine and plasma concentrations of long- and medium-chain acylcarnitines in adults [38]. Caffeine decreases the levels of branched-chain and aromatic amino acids in plasma of male rats or of human adults, whereas it has no significant effects on large neutral amino acids concentrations [39,40]. It is not clear if the metabolic actions of caffeine are affected by sex; however, some adenosine activities are sex-dependent [41,42], and it has been reported that caffeine metabolism is more active in female than male preterm neonates [43]. Nevertheless, the major metabolizing enzyme of caffeine, CYP1A2, is more active in adult men than in women [15]. Caffeine activity showed the highest impact on male MLPI metabolites levels, leading to a reduction in Asp and Gly levels, and increasing C3, C5, C5OH, C14:2, C4OH, and C8:1, and favoring sex differences in Gly, C6, and C18:1. This aspect was also confirmed by cluster analysis, where almost all subjects treated with caffeine were included in Cluster 3, which also contained the largest percentage of males. The optimal dose of caffeine should be carefully chosen in agreement with GA and sex when treating preterm infants to avoid potential metabolism alterations, which could affect growth trajectories and the risk of disease.
The current study presents some limitations associated with the lack of specific information on maternal nutrition and specific data on mothers (age, health conditions, etc.), including the reason of premature birth and the mode of birth (cesarean or spontaneous delivery). Furthermore, PN received by preterm infants, even if based on pediatric protocols, was not identical because PN formulations were supplied by different pharmaceutical companies.
4. Methods
4.1. Populations
The recruited sample included 311 preterm infants (137 females and 174 males). It was divided into 2 groups according to the WHO [43] GA classification, where preterm birth is any birth before 37 completed weeks of gestation, or fewer than 259 days since the first day of the woman’s last menstrual period; VPI, with any birth between 28–31 completed weeks of gestation; and MLPI, with any birth between 32–36 completed weeks of gestation.
Preterm infants were characterized by several pathological conditions, requiring the administration of pharmacological therapies. Due to the high variability of pharmacological treatments in preterm infants, subjects treated with dopamine and glucose solutions were excluded, as well as infants with jaundice, those exposed to blood transfusions, and those fed with carnitine solutions. Characteristics of the cohorts stratified by gender and GA are summarized in Supplementary Table S10.
DBS were collected from hospitals participating in the newborn screening program of Campania Region (Italy). All experiments were performed in compliance with national laws and institutional guidelines, approved by Italian Ministry of Health in law no. 167 (19 August 2016).
4.2. Sample Preparation for Tandem Mass Spectrometry
Blood samples were collected from the heel of newborns between 48 and 72 h of life on a special filter paper, dried overnight at room temperature, and processed for tandem mass spectrometry (MS/MS) analysis, as previously described [24,44,45,46]. Metabolites were extracted in 200 µL of methanol containing labelled amino acids and acylcarnitines standards, (15N,2-13C-Gly; 2H4-Ala; 2H8-Val; 2H3-Leu; 2H3-Met; 13C6-Phe; 13C6-Tyr; 2H3-Asp; 2H3-Glu; 2H2-Orn; 2H2-Cit; 2H4,5-13C-Arg; and 2H9-C0; 2H3-C2; 2H3-C3; 2H3-C4; 2H9-C5; 2H3-C8; 2H9-C14; 2H3-C16) at concentrations of 500–2500 µM and 7.6–152 µM, respectively. Samples were incubated at room temperature for 20 min and dried under nitrogen flow. Metabolites were derivatized using 80 µL n-butanol/3N HCl at 65 °C for 25 min. Samples were dried and resuspended in 300 µL of acetonitrile/water (70:30) containing 0.05% formic acid. A final volume of 40 µL was used for MS analysis.
4.3. Tandem Mass Spectrometry Analysis
Metabolites were identified and quantified by MS/MS using an API 4000 triple quadrupole mass spectrometer (Applied Biosystems-Sciex, Toronto, Canada) coupled with a high performance liquid chromatograph (1200 series; Agilent Technologies, Waldbronn, Germany).
Acylcarnitines and amino acid quantification was performed by precursor ion scan and neutral loss scan, respectively; glycine (Gly), ornithine (Orn), arginine (Arg), and citrulline (Cit) were quantified by multiple reaction monitoring (MRM). Precursor ion scan was performed using the following parameters: precursor ion mass: 85.1 Da; polarity: positive; m/z range (Da): 200–560; declustering potential (DP) range (volts): 55–80; collision energy (CE) range (volts): 34–60. Neutral loss scan was performed according to: neutral loss mass: 102 Da; polarity: positive; m/z range (Da): 130–280; DP (volts): 45; CE (volts): 25. Finally, MRM mode parameters were: positive polarity; Q1/Q3 (m/z): 132.1/76.0 (Gly), 189.1/70.0 (Orn), 231.2/70.0 (Arg), 232.2/113.1 (Cit); DP (volts): 43 (Gly), 33 (Orn), 60 (Arg), 50 (Cit); CE (volts): 14 (Gly), 33 (Orn), 45 (Arg), 28 (Cit).
Quantitative analysis of the data was performed with ChemoView v1.2 software (SCIEX, Framingham, MA, USA) using stable isotope-labeled internal standards to improve matrix correction and compare analyte areas. Quality controls, used to test the mass spectrometry methods’ accuracy and precision, were provided by CDC (Centers for Disease Control and Prevention; Atlanta, GA, USA) and ERNDIM (European Research Network for evaluation and improvement of screening, Diagnosis, and treatment of Inherited disorders of Metabolism; Manchester, UK; www.erndimqa.nl).
4.4. Statistical Analysis
Concentrations are reported as µM, this unit being commonly used by laboratories that perform newborn screening. Qualitative variables were described with absolute and relative frequencies, whereas quantitative variables with means (standard deviations) or medians (interquartile ranges), depending on their normal or non-normal distribution, respectively. Qualitative variables were compared with chi-squared or Fisher exact test, when appropriate. Normal and non-normal quantitative variables were compared with Student t or Mann–Whitney test, respectively. A Spearman correlation was performed. A factor loading of 0.4 was considered to select the variables for the cluster analysis. Visual assessment of the distribution of the clusters was based on a dendrogram. The Gower dissimilarity value was adopted for the identification of the clusters. A p-value lower than 0.05 was considered statistically significant.
All statistical computations were performed with the statistical software STATA version 16 (StatsCorp, College Station, Texas, USA). Multivariate statistical analysis was performed using MetaboAnalyst 4.0 (http://www.metaboanalyst.ca) [47,48]. The dataset was processed to estimate missing values, removing the features with >50% missing values and replacing the remaining ones by using 1/5 of the minimum value of each variable. Data were log (2)-transformed and Pareto scaled.
Partial least squares-discriminant analysis (PLS-DA) was used, and the corresponding variable importance on projection (VIP) was estimated for each differentially abundant metabolite [47].
5. Conclusions
This study confirms that metabolomics analysis is a powerful tool in the early characterization of the sexual specificity of perinatal, pediatric, and adulthood status. In preterm infants, the levels of amino acids and acylcarnitines depend on prematurity, PN and caffeine treatment, and on sex. Sex, in fact, induces qualitative changes in all examined conditions (GA, PN, caffeine treatment). Therefore, sex should be an independent variable in metabolomic studies. Finally, the study shows the importance of intersectionality, considering integrated relationships among the studied parameters.
Supplementary Materials
The following are available online at https://www.mdpi.com/2218-1989/11/3/158/s1, Table S1: List of metabolites measured through a targeted metabolomic approach and their abbreviations; Table S2: Intra-sex analysis of GA effect; Table S3: Inter-sex analysis of GA effect; Table S4: Intra-sex analysis of PN effect in females; Table S5: Intra-sex analysis of PN effect in males; Table S6: Inter-sex analysis of PN effect; Table S7: Intra-sex analysis of caffeine effect in females; Table S8: Intra-sex analysis of caffeine effect in males; Table S9: Inter-sex analysis of caffeine effect; Table S10: Characteristics of the cohorts stratified by sex and GA; Supplementary Figure S1: Metabolites which differentiate VPI and MLPI groups in female and male cohorts; Supplementary Figure S2: Metabolites which differentiate PN and no-PN fed groups; Supplementary Figure S3. Metabolites which differentiate caffeine and no-caffeine groups.
Author Contributions
Conceptualization, I.C., F.F., M.C. (Marianna Caterino), and M.R.; methodology, M.C. (Marianna Caterino), M.C. (Michele Costanzo), L.A., and D.C.; formal analysis, I.C. and M.C. (Marianna Caterino); statistical analysis, G.S. and L.S.; investigation, I.C., F.F., M.C. (Marianna Caterino), and M.R.; data curation, I.C. and M.C. (Marianna Caterino); writing—original draft preparation, I.C., M.C. (Marianna Caterino), F.F., M.R., and A.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of University of Naples Federico II (protocol n° 78/21, 03/03/2021; Marcatori di predisposizione genetica: impatto clinico ed appropriatezza prescritiva).
Informed Consent Statement
Informed consent was obtained from subjects’ parents.
Data Availability Statement
The data presented in this study are available on request from the corresponding authors. The data are not publicly available due to privacy policy.
Acknowledgments
We thank Simone Dore for his precious help in statistical analysis.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Martin, J.A.; Hamilton, B.E.; Osterman, M.J.; Driscoll, A.K.; Mathews, T.J. Births: Final data for 2015. Natl. Vital. Stat. Rep. 2017, 66, 1. [Google Scholar]
- Souza, R.T.; Mayrink, J.; Leite, D.F.; Costa, M.L.; Calderon, I.M.; Rocha Filho, E.A.; Vettorazzi, J.; Feitosa, F.E.; Cecatti, J.G. Metabolomics applied to maternal and perinatal health: A review of new frontiers with a translation potential. Clinics 2019, 74, e894. [Google Scholar] [CrossRef]
- Natarajan, G.; Shankaran, S. Short- and long-term outcomes of moderate and late preterm infants. Am. J. Perinatol. 2016, 33, 305–317. [Google Scholar] [PubMed]
- Blencowe, H.; Cousens, S.; Chou, D.; Oestergaard, M.; Say, L.; Moller, A.B.; Kinney, M.; Lawn, J. Born too soon: The global epidemiology of 15 million preterm births. Reprod. Health 2013, 10 (Suppl. S1), S2. [Google Scholar] [CrossRef] [PubMed]
- Souza, R.T.; Cecatti, J.G. A Comprehensive integrative review of the factors associated with spontaneous preterm birth, its prevention and prediction, including metabolomic markers. Rev. Bras. Ginecol. Obs. 2020, 42, 51–60. [Google Scholar] [CrossRef]
- Weng, Y.H.; Yang, C.Y.; Chiu, Y.W. Neonatal outcomes in relation to sex differences: A national cohort survey in Taiwan. Biol. Sex Differ. 2015, 6, 30. [Google Scholar] [CrossRef]
- Wilson, K.; Hawken, S.; Ducharme, R.; Potter, B.K.; Little, J.; Thebaud, B.; Chakraborty, P. Metabolomics of prematurity: Analysis of patterns of amino acids, enzymes, and endocrine markers by categories of gestational age. Pediatr. Res. 2014, 75, 367–373. [Google Scholar] [CrossRef]
- Kaye, C.I.; Accurso, F.; La Franchi, S.; Lane, P.A.; Northrup, H.; Pang, S.; Schaefer, G.B. Introduction to the newborn screening fact sheets. Pediatrics 2006, 118, 1304–1312. [Google Scholar] [CrossRef]
- Ryckman, K.K.; Berberich, S.L.; Shchelochkov, O.A.; Cook, D.E.; Murray, J.C. Clinical and environmental influences on metabolic biomarkers collected for newborn screening. Clin. Biochem. 2013, 46, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.; Bhatia, J. Total parenteral nutrition for the very low birth weight infant. Semin. Fetal Neonatal Med. 2017, 22, 2–7. [Google Scholar] [CrossRef]
- Abdel-Hady, H.; Nasef, N.; Shabaan, A.E.; Nour, I. Caffeine therapy in preterm infants. World J. Clin. Pediatr. 2015, 4, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Aranda, J.V.; Beharry, K.D. Pharmacokinetics, pharmacodynamics and metabolism of caffeine in newborns. Semin. Fetal Neonatal Med. 2020, 25, 101183. [Google Scholar] [CrossRef]
- Sinha, R.A.; Farah, B.L.; Singh, B.K.; Siddique, M.M.; Li, Y.; Wu, Y.; Ilkayeva, O.R.; Gooding, J.; Ching, J.; Zhou, J.; et al. Caffeine stimulates hepatic lipid metabolism by the autophagy-lysosomal pathway in mice. Hepatology 2014, 59, 1366–1380. [Google Scholar] [CrossRef]
- Yeo, S.E.; Jentjens, R.L.; Wallis, G.A.; Jeukendrup, A.E. Caffeine increases exogenous carbohydrate oxidation during exercise. J. Appl. Physiol. 2005, 99, 844–850. [Google Scholar] [CrossRef] [PubMed]
- Franconi, F.; Campesi, I.; Colombo, D.; Antonini, P. Sex-gender variable: Methodological recommendations for increasing scientific value of clinical studies. Cells 2019, 8, 476. [Google Scholar] [CrossRef]
- Nehlig, A. Interindividual differences in caffeine metabolism and factors driving caffeine consumption. Pharmacol. Rev. 2018, 70, 384–411. [Google Scholar] [CrossRef]
- Rich-Edwards, J.W.; Kaiser, U.B.; Chen, G.L.; Manson, J.E.; Goldstein, J.M. Sex and gender differences research design for basic, clinical, and population studies: Essentials for investigators. Endocr. Rev. 2018, 39, 424–439. [Google Scholar] [CrossRef]
- Campesi, I.; Franconi, F.; Seghieri, G.; Meloni, M. Sex-gender-related therapeutic approaches for cardiovascular complications associated with diabetes. Pharmacol. Res. 2017, 119, 195–207. [Google Scholar] [CrossRef]
- Franconi, F.; Campesi, I. Sex impact on biomarkers, pharmacokinetics and pharmacodynamics. Curr. Med. Chem. 2017, 24, 2561–2575. [Google Scholar] [CrossRef]
- Clarke, J. Observations on some causes of excess mortality of males above that of females. Philos. Trans. R. Soc. Lond. 1786, 76, 349–364. [Google Scholar]
- Oladipo, O.O.; Weindel, A.L.; Saunders, A.N.; Dietzen, D.J. Impact of premature birth and critical illness on neonatal range of plasma amino acid concentrations determined by LC-MS/MS. Mol. Genet. Metab. 2011, 104, 476–479. [Google Scholar] [CrossRef]
- Carter, R.A.; Pan, K.; Harville, E.W.; McRitchie, S.; Sumner, S. Metabolomics to reveal biomarkers and pathways of preterm birth: A systematic review and epidemiologic perspective. Metabolomics 2014, 15, 124. [Google Scholar] [CrossRef] [PubMed]
- Gucciardi, A.; Zaramella, P.; Costa, I.; Pirillo, P.; Nardo, D.; Naturale, M.; Chiandetti, L.; Giordano, G. Analysis and interpretation of acylcarnitine profiles in dried blood spot and plasma of preterm and full-term newborns. Pediatr. Res. 2015, 77, 36–47. [Google Scholar] [CrossRef]
- Ruoppolo, M.; Scolamiero, E.; Caterino, M.; Mirisola, V.; Franconi, F.; Campesi, I. Female and male human babies have distinct blood metabolomic patterns. Mol. Biosyst. 2015, 11, 2483–2492. [Google Scholar] [CrossRef] [PubMed]
- Caterino, M.; Ruoppolo, M.; Villani, G.R.D.; Marchese, E.; Costanzo, M.; Sotgiu, G.; Dore, S.; Franconi, F.; Campesi, I. Influence of sex on urinary organic acids: A cross-sectional study in children. Int. J. Mol. Sci. 2020, 21, 582. [Google Scholar] [CrossRef]
- Lopez-Hernandez, Y.; Oropeza-Valdez, J.J.; Blanco-Sandate, J.O.; Herrera-Van Oostdam, A.S.; Zheng, J.; Chi Guo, A.; Lima-Rogel, V.; Rajabzadeh, R.; Salgado-Bustamante, M.; Adrian-Lopez, J.; et al. The urinary metabolome of healthy newborns. Metabolites 2020, 10, 165. [Google Scholar] [CrossRef]
- Foxall, P.J.; Bewley, S.; Neild, G.H.; Rodeck, C.H.; Nicholson, J.K. Analysis of fetal and neonatal urine using proton nuclear magnetic resonance spectroscopy. Arch. Dis. Child Fetal Neonatal Ed. 1995, 73, F153–F157. [Google Scholar] [CrossRef]
- Atzori, L.; Antonucci, R.; Barberini, L.; Locci, E.; Marincola, F.C.; Scano, P.; Cortesi, P.; Agostiniani, R.; Defraia, R.; Weljie, A.; et al. 1H NMR-based metabolomic analysis of urine from preterm and term neonates. Front. Biosci. 2011, 3, 1005–1012. [Google Scholar] [CrossRef]
- Moltu, S.J.; Sachse, D.; Blakstad, E.W.; Strommen, K.; Nakstad, B.; Almaas, A.N.; Westerberg, A.C.; Ronnestad, A.; Braekke, K.; Veierod, M.B.; et al. Urinary metabolite profiles in premature infants show early postnatal metabolic adaptation and maturation. Nutrients 2014, 6, 1913–1930. [Google Scholar] [CrossRef]
- Ernst, M.; Rogers, S.; Lausten-Thomsen, U.; Björkbom, A.; Laursen, S.S.; Courraud, J.; Børglum, A.; Nordentoft, M.; Werge, T.; Mortensen, P.B.; et al. Gestational age-dependent development of the neonatal metabolome. Pediatr. Res. 2020. [Google Scholar] [CrossRef]
- Slaughter, J.L.; Meinzen-Derr, J.; Rose, S.R.; Leslie, N.D.; Chandrasekar, R.; Linard, S.M.; Akinbi, H.T. The effects of gestational age and birth weight on false-positive newborn-screening rates. Pediatrics 2010, 126, 910–916. [Google Scholar] [CrossRef]
- Clark, R.H.; Kelleher, A.S.; Chace, D.H.; Spitzer, A.R. Gestational age and age at sampling influence metabolic profiles in premature infants. Pediatrics 2014, 134, e37–e46. [Google Scholar] [CrossRef]
- Liu Msc, D.; Wang Msc, L.; Shen Phd, H.; Han Phd, L.; Wang Phd, Y.; He Phd, Z. Dynamic changes in blood amino acid concentrations in preterm infants in different nutritional periods. Asia Pac. J. Clin. Nutr. 2020, 29, 803–812. [Google Scholar] [CrossRef]
- Martin, C.R.; Brown, Y.F.; Ehrenkranz, R.A.; O’Shea, T.M.; Allred, E.N.; Belfort, M.B.; McCormick, M.C.; Leviton, A. Nutritional practices and growth velocity in the first month of life in extremely premature infants. Pediatrics 2009, 124, 649–657. [Google Scholar] [CrossRef]
- Horbar, J.D.; Ehrenkranz, R.A.; Badger, G.J.; Edwards, E.M.; Morrow, K.A.; Soll, R.F.; Buzas, J.S.; Bertino, E.; Gagliardi, L.; Bellu, R. Weight growth velocity and postnatal growth failure in infants 501 to 1500 grams: 2000–2013. Pediatrics 2015, 136, e84–e92. [Google Scholar] [CrossRef]
- Griffin, I.J.; Tancredi, D.J.; Bertino, E.; Lee, H.C.; Profit, J. Postnatal growth failure in very low birthweight infants born between 2005 and 2012. Arch. Dis. Child Fetal Neonatal Ed. 2016, 101, F50–F55. [Google Scholar] [CrossRef]
- Bonsante, F.; Gouyon, J.-B.; Robillard, P.-Y.; Gouyon, B.; Iacobelli, S. Early optimal parenteral nutrition and metabolic acidosis in very preterm infants. PLoS ONE 2017, 12, e0186936. [Google Scholar] [CrossRef]
- Altmaier, E.; Kastenmuller, G.; Romisch-Margl, W.; Thorand, B.; Weinberger, K.M.; Adamski, J.; Illig, T.; Doring, A.; Suhre, K. Variation in the human lipidome associated with coffee consumption as revealed by quantitative targeted metabolomics. Mol. Nutr. Food Res. 2009, 53, 1357–1365. [Google Scholar] [CrossRef]
- Schlosberg, A.J.; Fernstrom, J.D.; Kopczynski, M.C.; Cusack, B.M.; Gillis, M.A. Acute effects of caffeine injection on neutral amino acids and brain monoamine levels in rats. Life Sci. 1981, 29, 173–183. [Google Scholar] [CrossRef]
- Struder, H.K.; Ferrauti, A.; Gotzmann, A.; Weber, K.; Hollmann, W. Effect of carbohydrates and caffeine on plasma amino acids, neuroendocrine responses and performance in tennis. Nutr. Neurosci. 1998, 1, 419–426. [Google Scholar] [CrossRef]
- Simoes-Henriques, C.; Mateus-Pinheiro, M.; Gaspar, R.; Pinheiro, H.; Mendes Duarte, J.; Baptista, F.I.; Canas, P.M.; Fontes-Ribeiro, C.A.; Cunha, R.A.; Ambrosio, A.F.; et al. Microglia cytoarchitecture in the brain of adenosine A2A receptor knockout mice: Brain region and sex specificities. Eur. J. Neurosci. 2020, 51, 1377–1387. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Teng, B.; Mustafa, S.J. Sex difference in coronary endothelial dysfunction in apolipoprotein E knockout mouse: Role of NO and A2A adenosine receptor. Microcirculation 2015, 22, 518–527. [Google Scholar] [CrossRef]
- World Health Organization; March of Dimes; Partnership for Maternal Newborn and Child Health. Save the Children Born Too Soon: The Global Action Report on Preterm Birth; World Health Organization: Geneva, Switzerland, 2012. [Google Scholar]
- Ruoppolo, M.; Caterino, M.; Albano, L.; Pecce, R.; Di Girolamo, M.G.; Crisci, D.; Costanzo, M.; Milella, L.; Franconi, F.; Campesi, I. Targeted metabolomic profiling in rat tissues reveals sex differences. Sci. Rep. 2018, 8. [Google Scholar] [CrossRef]
- Ruoppolo, M.; Campesi, I.; Scolamiero, E.; Pecce, R.; Caterino, M.; Cherchi, S.; Mercuro, G.; Tonolo, G.; Franconi, F. Serum Metabolomic Profiles Suggest Influence of Sex and Oral Contraceptive Use. Am. J. Transl. Res. 2014, 6, 614–624. [Google Scholar] [PubMed]
- Giacco, A.; Paoli, G.D.; Senese, R.; Cioffi, F.; Silvestri, E.; Moreno, M.; Ruoppolo, M.; Caterino, M.; Costanzo, M.; Lombardi, A.; et al. The saturation degree of fatty acids and their derived acylcarnitines determines the direct effect of metabolically active thyroid hormones on insulin sensitivity in skeletal muscle cells. FASEB J. 2019, 33, 1811–1823. [Google Scholar] [CrossRef] [PubMed]
- De Pasquale, V.; Caterino, M.; Costanzo, M.; Fedele, R.; Ruoppolo, M.; Pavone, L.M. Targeted metabolomic analysis of a mucopolysaccharidosis IIIB mouse model reveals an imbalance of branched-chain amino acid and fatty acid metabolism. Int. J. Mol. Sci. 2020, 21, 4211. [Google Scholar] [CrossRef]
- Costanzo, M.; Caterino, M.; Cevenini, A.; Jung, V.; Chhuon, C.; Lipecka, J.; Fedele, R.; Guerrera, I.C.; Ruoppolo, M. Proteomics reveals that methylmalonyl-coa mutase modulates cell architecture and increases susceptibility to stress. Int. J. Mol. Sci. 2020, 21, 4998. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).