Impact of Sex and Age on the Mevalonate Pathway in the Brain: A Focus on Effects Induced by Maternal Exposure to Exogenous Compounds
Abstract
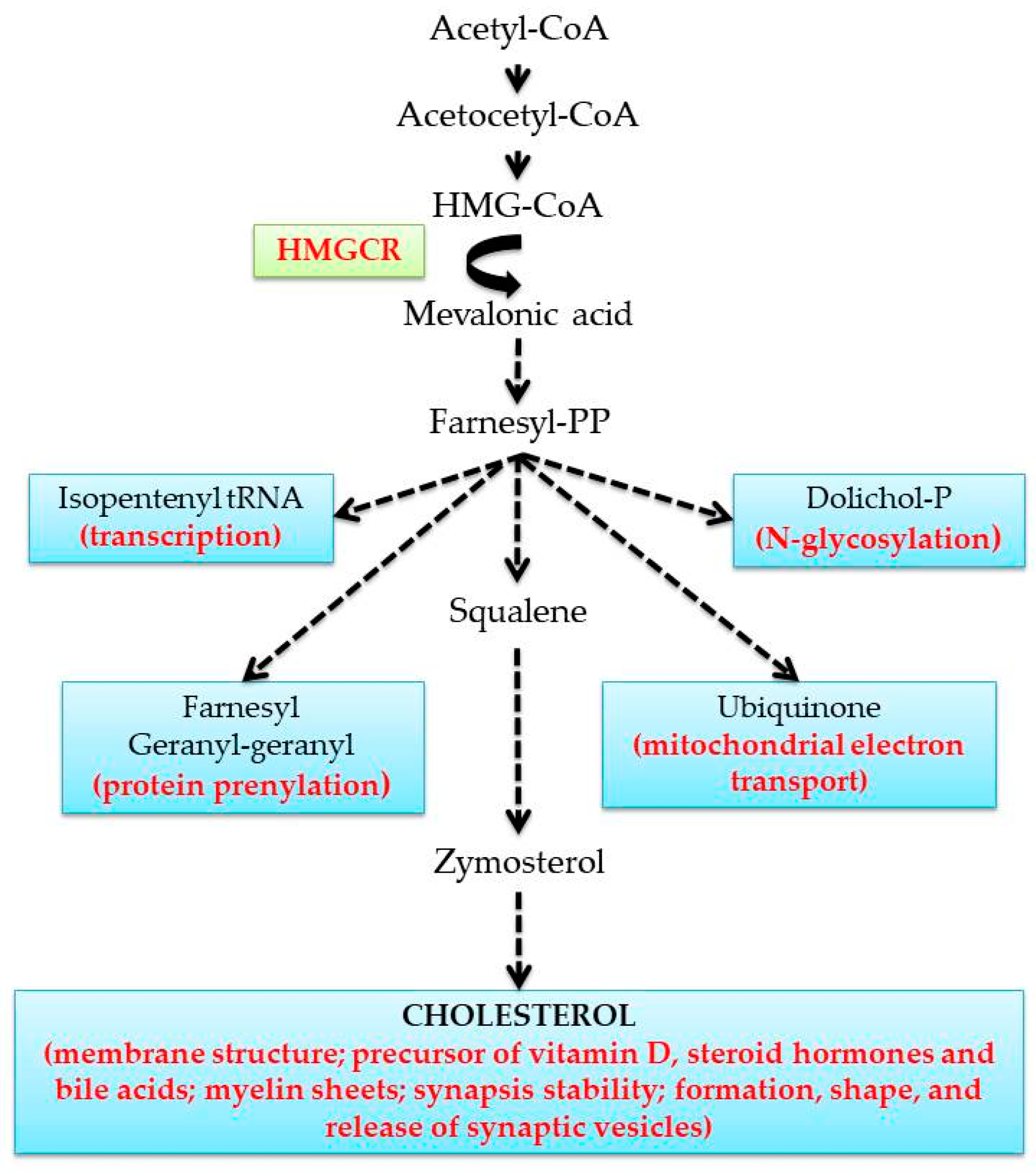
1. Introduction
2. Sex- and Age-Dependent Differences of MVA Pathway in the Liver
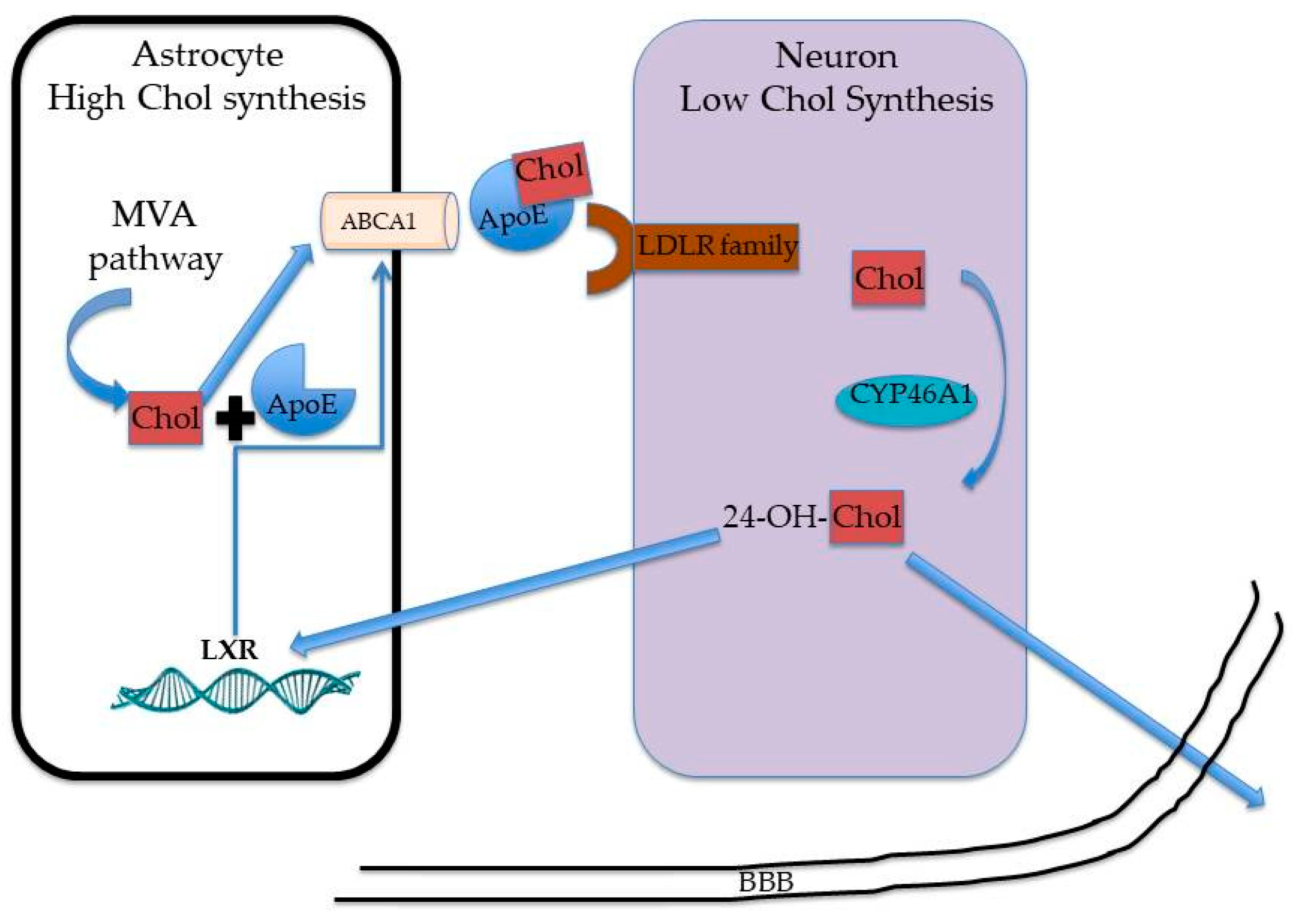
3. Cholesterol Metabolism in the Brain: What about Sex and Age?
4. Modulation of MVA Pathway by Endogenous and Exogenous Compounds
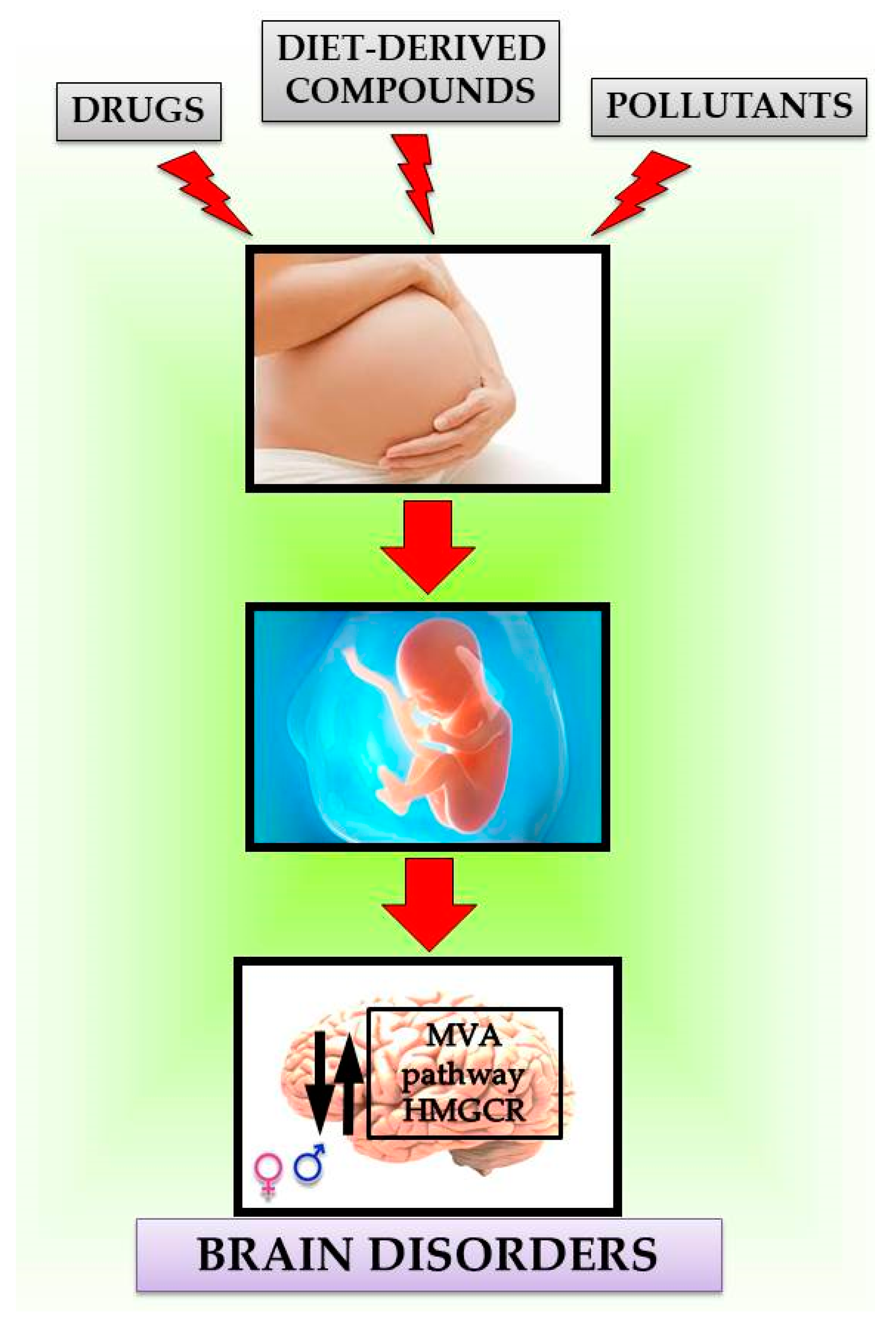
Maternal Exposure Effects of Exogenous Compounds on MVA Pathway in the Brain
5. Conclusions and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ikonen, E. Mechanisms for Cellular Cholesterol Transport: Defects and Human Disease. Physiol. Rev. 2006, 86, 1237–1261. [Google Scholar] [CrossRef] [PubMed]
- Wang, H. Lipid rafts: A signaling platform linking cholesterol metabolism to synaptic deficits in autism spectrum disorders. Front. Behav. Neurosci. 2014, 8, 104. [Google Scholar] [CrossRef] [PubMed]
- Mesa-Herrera, F.; Taoro-González, L.; Valdés-Baizabal, C.; Diaz, M.; Marín, R. Lipid and Lipid Raft Alteration in Aging and Neurodegenerative Diseases: A Window for the Development of New Biomarkers. Int. J. Mol. Sci. 2019, 20, 3810. [Google Scholar] [CrossRef] [PubMed]
- Martini, C.; Pallottini, V. Cholesterol: From feeding to gene regulation. Genes Nutr. 2007, 2, 181–193. [Google Scholar] [CrossRef]
- Saito, K.; Dubreuil, V.; Arai, Y.; Wilsch-Bräuninger, M.; Schwudke, D.; Saher, G.; Miyata, T.; Breier, G.; Thiele, C.; Shevchenko, A.; et al. Ablation of cholesterol biosynthesis in neural stem cells increases their VEGF expression and angiogenesis but causes neuron apoptosis. Proc. Natl. Acad. Sci. USA 2009, 106, 8350–8355. [Google Scholar] [CrossRef]
- Trapani, L.; Segatto, M.; Pallottini, V. Regulation and deregulation of cholesterol homeostasis: The liver as a metabolic “power station. World J. Hepatol. 2012, 4, 184–190. [Google Scholar] [CrossRef]
- Espenshade, P.J.; Hughes, A.L. Regulation of Sterol Synthesis in Eukaryotes. Annu. Rev. Genet. 2007, 41, 401–427. [Google Scholar] [CrossRef]
- Pallottini, V. 3-Hydroxy-3-methylglutaryl-coenzyme A reductase modulator: Toward age- and sex-personalized medicine. Expert Opin. Ther. Patents 2015, 25, 1079–1083. [Google Scholar] [CrossRef]
- Segatto, M.; Trapani, L.; Marino, M.; Pallottini, V. Age- and sex-related differences in extra-hepatic low-density lipoprotein receptor. J. Cell. Physiol. 2011, 226, 2610–2616. [Google Scholar] [CrossRef]
- Segatto, M.; Trapani, L.; Lecis, C.; Pallottini, V. Regulation of cholesterol biosynthetic pathway in different regions of the rat central nervous system. Acta Physiol. 2012, 206, 62–71. [Google Scholar] [CrossRef]
- Segatto, M.; Di Giovanni, A.; Marino, M.; Pallottini, V. Analysis of the protein network of cholesterol homeostasis in different brain regions: An age and sex dependent perspective. J. Cell. Physiol. 2013, 228, 1561–1567. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.G.; Ahmed, T.; Korovaichuk, A.; Venero, C.; Menchón, S.A.; Salas, I.; Munck, S.; Herreras, O.; Balschun, D.; Dotti, C.G. Constitutive hippocampal cholesterol loss underlies poor cognition in old rodents. EMBO Mol. Med. 2014, 6, 902–917. [Google Scholar] [CrossRef] [PubMed]
- Palomer, E.; Martín-Segura, A.; Baliyan, S.; Ahmed, T.; Balschun, D.; Venero, C.; Martin, M.G.; Dotti, C.G. Aging Triggers a Repressive Chromatin State at Bdnf Promoters in Hippocampal Neurons. Cell Rep. 2016, 16, 2889–2900. [Google Scholar] [CrossRef] [PubMed]
- Colin, J.; Gregory-Pauron, L.; Lanhers, M.-C.; Claudepierre, T.; Corbier, C.; Yen, F.T.; Malaplate-Armand, C.; Oster, T. Membrane raft domains and remodeling in aging brain. Biochimie 2016, 130, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Raihan, O.; Brishti, A.; Molla, R.; Li, W.; Zhang, Q.; Xu, P.; Khan, M.I.; Zhang, J.; Liu, Q. The Age-dependent Elevation of miR-335-3p Leads to Reduced Cholesterol and Impaired Memory in Brain. Neuroscice 2018, 390, 160–173. [Google Scholar] [CrossRef] [PubMed]
- Maas, A.H.E.M.; Appelman, Y. Gender differences in coronary heart disease. Neth. Hear. J. 2010, 18, 598–602. [Google Scholar] [CrossRef]
- De Marinis, E.; Martini, C.; Trentalance, A.; Pallottini, V. Sex differences in hepatic regulation of cholesterol homeostasis. J. Endocrinol. 2008, 198, 635–643. [Google Scholar] [CrossRef]
- Henriksson, P.; Einarsson, K.; Eriksson, A.; Kelter, U.; Angelin, B. Estrogen-induced gallstone formation in males. Relation to changes in serum and biliary lipids during hormonal treatment of prostatic carcinoma. J. Clin. Investig. 1989, 84, 811–816. [Google Scholar] [CrossRef]
- Wang, H.H.; Afdhal, N.H.; Wang, D.Q.-H. Estrogen receptor α, but not β, plays a major role in 17β-estradiol-induced murine cholesterol gallstones. Gastroenterology 2004, 127, 239–249. [Google Scholar] [CrossRef]
- Parini, P.; Angelin, B.; Rudling, M. Cholesterol and lipoprotein metabolism in aging: Reversal of hypercholesterolemia by growth hormone treatment in old rats. Arter. Thromb. Vasc. Biol. 1999, 19, 832–839. [Google Scholar] [CrossRef]
- Pallottini, V.; Martini, C.; Bassi, A.M.; Romano, P.; Nanni, G.; Trentalance, A. Rat HMGCoA reductase activation in thioacetamide-induced liver injury is related to an increased reactive oxygen species content. J. Hepatol. 2006, 44, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Pallottini, V.; Martini, C.; Cavallini, G.; Bergamini, E.; Mustard, K.J.; Hardie, D.G.; Trentalance, A. Age-related HMG-CoA reductase deregulation depends on ROS-induced p38 activation. Mech. Ageing Dev. 2007, 128, 688–695. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhang, W.; Li, X.; Han, J.; Chen, Y.; Duan, Y. Impact of age and sex on the development of atherosclerosis and expression of the related genes in apoE deficient mice. Biochem. Biophys. Res. Commun. 2016, 469, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-González, G.L.; Reyes-Castro, L.A.; Bautista, C.J.; Beltrán, A.A.; Ibáñez, C.A.; Vega, C.C.; Lomas-Soria, C.; Castro-Rodríguez, D.C.; Elías-López, A.L.; Nathanielsz, P.W.; et al. Maternal obesity accelerates rat offspring metabolic ageing in a sex-dependent manner. J. Physiol. 2019, 597, 5549–5563. [Google Scholar] [CrossRef] [PubMed]
- Pfrieger, F.W. Role of cholesterol in synapse formation and function. Biochim. Biophys. Acta 2003, 1610, 271–280. [Google Scholar] [CrossRef]
- Martín, M.G.; Pfrieger, F.; Dotti, C.G. Cholesterol in brain disease: Sometimes determinant and frequently implicated. EMBO Rep. 2014, 15, 1036–1052. [Google Scholar] [CrossRef]
- Segatto, M.; Tonini, C.; Pfrieger, F.; Trezza, V.; Pallottini, V. Loss of Mevalonate/Cholesterol Homeostasis in the Brain: A Focus on Autism Spectrum Disorder and Rett Syndrome. Int. J. Mol. Sci. 2019, 20, 3317. [Google Scholar] [CrossRef]
- Cartocci, V.; Servadio, M.; Trezza, V.; Pallottini, V. Can Cholesterol Metabolism Modulation Affect Brain Function and Behavior? J. Cell. Physiol. 2016, 232, 281–286. [Google Scholar] [CrossRef]
- Valenza, M.; Cattaneo, E. Emerging roles for cholesterol in Huntington’s disease. Trends Neurosci. 2011, 34, 474–486. [Google Scholar] [CrossRef]
- Leoni, V.; Caccia, C. The impairment of cholesterol metabolism in Huntington disease. Biochim. Biophys. Acta 2015, 1851, 1095–1105. [Google Scholar] [CrossRef]
- Iuliano, L.; Crick, P.J.; Zerbinati, C.; Tritapepe, L.; Abdel-Khalik, J.; Poirot, M.; Wang, Y.; Griffiths, W.J. Cholesterol metabolites exported from human brain. Steroids 2015, 99, 189–193. [Google Scholar] [CrossRef]
- Mauch, D.H. CNS Synaptogenesis Promoted by Glia-Derived Cholesterol. Science 2001, 294, 1354–1357. [Google Scholar] [CrossRef] [PubMed]
- Pfrieger, F. Outsourcing in the brain: Do neurons depend on cholesterol delivery by astrocytes? Bioessays 2002, 25, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Pfrieger, F.; Ungerer, N. Cholesterol metabolism in neurons and astrocytes. Prog. Lipid Res. 2011, 50, 357–371. [Google Scholar] [CrossRef] [PubMed]
- Shanmugaratnam, J.; Berg, E.; Kimerer, L.; Johnson, R.J.; Amaratunga, A.; Schreiber, B.; Fine, R.E. Retinal Muller glia secrete apolipoproteins E and J which are efficiently assembled into lipoprotein particles. Mol. Brain Res. 1997, 50, 113–120. [Google Scholar] [CrossRef]
- Björkhem, I.; Meaney, S. Brain Cholesterol: Long Secret Life Behind a Barrier. Arter. Thromb. Vasc. Biol. 2004, 24, 806–815. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Ong, W.-Y. Localization of the transcription factor, sterol regulatory element binding protein-2 (SREBP-2) in the normal rat brain and changes after kainate-induced excitotoxic injury. J. Chem. Neuroanat. 2009, 37, 71–77. [Google Scholar] [CrossRef] [PubMed]
- El Hajj, A.; Yen, F.T.; Oster, T.; Malaplate, C.; Pauron, L.; Corbier, C.; Lanhers, M.-C.; Claudepierre, T. Age-related changes in regiospecific expression of Lipolysis Stimulated Receptor (LSR) in mice brain. PLoS ONE 2019, 14, e0218812. [Google Scholar] [CrossRef]
- Quan, G.; Xie, C.; Dietschy, J.M.; Turley, S.D. Ontogenesis and regulation of cholesterol metabolism in the central nervous system of the mouse. Dev. Brain Res. 2003, 146, 87–98. [Google Scholar] [CrossRef]
- Xie, C.; Lund, E.G.; Turley, S.D.; Russell, D.W.; Dietschy, J.M. Quantitation of two pathways for cholesterol excretion from the brain in normal mice and mice with neurodegeneration. J. Lipid Res. 2003, 44, 1780–1789. [Google Scholar] [CrossRef]
- Lund, E.G.; Xie, C.; Kotti, T.; Turley, S.D.; Dietschy, J.M.; Russell, D.W. Knockout of the Cholesterol 24-Hydroxylase Gene in Mice Reveals a Brain-specific Mechanism of Cholesterol Turnover. J. Biol. Chem. 2003, 278, 22980–22988. [Google Scholar] [CrossRef] [PubMed]
- Yutuc, E.; Angelini, R.; Baumert, M.; Mast, N.; Pikuleva, I.; Newton, J.; Clench, M.R.; Skibinski, D.O.F.; Howell, O.W.; Wang, Y.; et al. Localization of sterols and oxysterols in mouse brain reveals distinct spatial cholesterol metabolism. Proc. Natl. Acad. Sci. USA 2020, 117, 5749–5760. [Google Scholar] [CrossRef]
- Kotti, T.J.; Ramirez, D.M.O.; Pfeiffer, B.E.; Huber, K.M.; Russell, D.W. Brain cholesterol turnover required for geranylgeraniol production and learning in mice. Proc. Natl. Acad. Sci. USA 2006, 103, 3869–3874. [Google Scholar] [CrossRef] [PubMed]
- Russell, D.; Halford, R.W.; Ramirez, D.M.O.; Shah, R.; Kotti, T. Cholesterol 24-hydroxylase: An enzyme of cholesterol turnover in the brain. Annu. Rev. Biochem. 2009, 78, 1017–1040. [Google Scholar] [CrossRef] [PubMed]
- Boussicault, L.; Kacher, R.; Lamazière, A.; Vanhoutte, P.; Caboche, J.; Betuing, S.; Potier, M.-C. CYP46A1 protects against NMDA-mediated excitotoxicity in Huntington’s disease: Analysis of lipid raft content. Biochimie 2018, 153, 70–79. [Google Scholar] [CrossRef]
- Segatto, M.; Leboffe, L.; Trapani, L.; Pallottini, V. Cholesterol homeostasis failure in the brain: Implications for synaptic dysfunction and cognitive decline. Curr. Med. Chem. 2014, 21, 2788–2802. [Google Scholar] [CrossRef]
- Adorni, M.P.; Ruscica, M.; Ferri, N.; Bernini, F.; Zimetti, F. Proprotein Convertase Subtilisin/Kexin Type 9, Brain Cholesterol Homeostasis and Potential Implication for Alzheimer’s Disease. Front. Aging Neurosci. 2019, 11, 120. [Google Scholar] [CrossRef]
- Marchi, C.; Adorni, M.P.; Caffarra, P.; Ronda, N.; Spallazzi, M.; Barocco, F.; Galimberti, D.; Bernini, F.; Zimetti, F. ABCA1- and ABCG1-mediated cholesterol efflux capacity of cerebrospinal fluid is impaired in Alzheimer’s disease. J. Lipid Res. 2019, 60, 1449–1456. [Google Scholar] [CrossRef]
- Jin, U.; Park, S.J.; Park, S.M. Cholesterol Metabolism in the Brain and Its Association with Parkinson’s Disease. Exp. Neurobiol. 2019, 28, 554–567. [Google Scholar] [CrossRef]
- Walterfang, M.; Fietz, M.; Abel, L.; Bowman, E.; Mocellin, R.; Velakoulis, D. Gender dimorphism in siblings with schizophrenia-like psychosis due to Niemann-Pick disease type C. J. Inherit. Metab. Dis. 2009, 32, 221–226. [Google Scholar] [CrossRef]
- Cougnoux, A.; Fellmeth, M.; Gu, T.; Davidson, C.D.; Gibson, A.L.; Pavan, W.J.; Porter, F.D. Maternal immune activation modifies the course of Niemann-pick disease, type C1 in a gender specific manner. Mol. Genet. Metab. 2020, 129, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Norton, J.; Carrière, I.; Pérès, K.; Gabelle, A.; Berr, C.; Ritchie, K.; Ancelin, M.-L. Sex-specific depressive symptoms as markers of pre-Alzheimer dementia: Findings from the Three-City cohort study. Transl. Psychiatry 2019, 9, 291. [Google Scholar] [CrossRef] [PubMed]
- Ferretti, M.T.; Martinkova, J.; Biskup, E.; Benke, T.; Gialdini, G.; Nedelska, Z.; Rauen, K.; Mantua, V.; Religa, D.; Hort, J.; et al. Sex and gender differences in Alzheimer’s disease: Current challenges and implications for clinical practice: Position paper of the Dementia and Cognitive Disorders Panel of the European Academy of Neurology. Eur. J. Neurol. 2020, 27, 928–943. [Google Scholar] [CrossRef] [PubMed]
- El Haj, M.; Kapogiannis, D.; Antoine, P. The (fatalistic) present as experienced by individuals with Alzheimer’s disease: A preliminary study. Neurol. Sci. 2019, 41, 427–433. [Google Scholar] [CrossRef]
- Loomes, R.; Hull, L.; Mandy, W. What Is the Male-to-Female Ratio in Autism Spectrum Disorder? A Systematic Review and Meta-Analysis. J. Am. Acad. Child Adolesc. Psychiatry 2017, 56, 466–474. [Google Scholar] [CrossRef]
- Melancia, F.; Schiavi, S.; Servadio, M.; Cartocci, V.; Campolongo, P.; Palmery, M.; Pallottini, V.; Trezza, V. Sex-specific autistic endophenotypes induced by prenatal exposure to valproic acid involve anandamide signalling. Br. J. Pharmacol. 2018, 175, 3699–3712. [Google Scholar] [CrossRef]
- Lai, M.-C.; Szatmari, P. Resilience in autism: Research and practice prospects. Autism 2019, 23, 539–541. [Google Scholar] [CrossRef]
- Meoni, S.; Macerollo, A.; Moro, E. Sex differences in movement disorders. Nat. Rev. Neurol. 2020, 16, 84–96. [Google Scholar] [CrossRef]
- Arito, M.; Horiba, T.; Hachimura, S.; Inoue, J.; Sato, R. Growth Factor-induced Phosphorylation of Sterol Regulatory Element-binding Proteins Inhibits Sumoylation, Thereby Stimulating the Expression of Their Target Genes, Low Density Lipoprotein Uptake, and Lipid Synthesis. J. Biol. Chem. 2008, 283, 15224–15231. [Google Scholar] [CrossRef]
- Suzuki, R.; Lee, K.Y.; Jing, E.; Biddinger, S.B.; McDonald, J.G.; Montine, T.J.; Craft, S.; Kahn, C.R. Diabetes and Insulin in Regulation of Brain Cholesterol Metabolism. Cell Metab. 2010, 12, 567–579. [Google Scholar] [CrossRef]
- Ness, G.C.; Chambers, C.M. Feedback and Hormonal Regulation of Hepatic 3-Hydroxy-3-Methylglutaryl Coenzyme A Reductase: The Concept of Cholesterol Buffering Capacity. Proc. Soc. Exp. Biol. Med. 2000, 224, 8–19. [Google Scholar] [CrossRef] [PubMed]
- More, V.R.; Lao, J.; McLaren, D.G.; Cumiskey, A.-M.; Murphy, B.A.; Chen, Y.; Previs, S.; Stout, S.; Patel, R.; Satapati, S.; et al. Glucagon like receptor 1/ glucagon dual agonist acutely enhanced hepatic lipid clearance and suppressed de novo lipogenesis in mice. PLoS ONE 2017, 12, e0186586. [Google Scholar] [CrossRef] [PubMed]
- Spolitu, S.; Okamoto, H.; Dai, W.; Zadroga, J.A.; Wittchen, E.S.; Gromada, J.; Ozcan, L. Hepatic Glucagon Signaling Regulates PCSK9 and Low-Density Lipoprotein Cholesterol. Circ. Res. 2019, 124, 38–51. [Google Scholar] [CrossRef] [PubMed]
- Sinha, R.A.; Singh, B.K.; Yen, P.M. Direct effects of thyroid hormones on hepatic lipid metabolism. Nat. Rev. Endocrinol. 2018, 14, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Song, Y.; Feng, M.; Zhou, X.; Lu, Y.; Gao, L.; Yu, C.; Jiang, X.; Zhao, J. Thyroid-stimulating hormone decreases HMG-CoA reductase phosphorylation via AMP-activated protein kinase in the liver. J. Lipid Res. 2015, 56, 963–971. [Google Scholar] [CrossRef]
- Marino, M.; Distefano, E.; Pallottini, V.; Caporali, S.; Bruscalupi, G.; Trentalance, A. Activation of IP3 -Protein Kinase C-α Signal Transduction Pathway Precedes the Changes of Plasma Cholesterol, Hepatic Lipid Metabolism and Induction of Low-Density Lipoprotein Receptor Expression in 17-β-Oestradiol-Treated Rats. Exp. Physiol. 2001, 86, 39–45. [Google Scholar] [CrossRef]
- Messa, C.; Notarnicola, M.; Russo, F.; Cavallini, A.; Pallottini, V.; Trentalance, A.; Bifulco, M.; Laezza, C.; Caruso, M.G. Estrogenic regulation of cholesterol biosynthesis and cell growth in DLD-1 human colon cancer cells. Scand. J. Gastroenterol. 2005, 40, 1454–1461. [Google Scholar] [CrossRef]
- Endo, A.; Kuroda, M.; Tsujita, Y. ML-236A, ML-236B, and ML-236C, new inhibitors of cholesterogensis produced by Penicillium citrinum. J. Antibiot. 1976, 29, 1346–1348. [Google Scholar] [CrossRef]
- Alberts, A.W.; Chen, J.; Kuron, G.; Hunt, V.; Huff, J.; Hoffman, C.; Rothrock, J.; Lopez, M.; Joshua, H.; Harris, E.; et al. Mevinolin: A highly potent competitive inhibitor of hydroxymethylglutaryl-coenzyme A reductase and a cholesterol-lowering agent. Proc. Natl. Acad. Sci. USA 1980, 77, 3957–3961. [Google Scholar] [CrossRef]
- Cabral, M.; Delgado, O.; Sampietro, D.A.; Catalan, C.; De Figueroa, L.I.C.; Fariña, J. Antifungal Activity and the Potential Correlation with Statin-Producing Ability: An Optimized Screening Applied to Filamentous Fungi from Las Yungas Subtropical Rainforest. Res. J. Microbiol. 2010, 5, 833–848. [Google Scholar] [CrossRef][Green Version]
- Trapani, L.; Segatto, M.; Ascenzi, P.; Pallottini, V. Potential role of nonstatin cholesterol lowering agents. IUBMB Life 2011, 63, 964–971. [Google Scholar] [CrossRef] [PubMed]
- Fracassi, A.; Marangoni, M.; Rosso, P.; Pallottini, V.; Fioramonti, M.; Siteni, S.; Segatto, M. Statins and the Brain: More than Lipid Lowering Agents? Curr. Neuropharmacol. 2018, 17, 59–83. [Google Scholar] [CrossRef] [PubMed]
- Raparelli, V.; Pannitteri, G.; Todisco, T.; Toriello, F.; Napoleone, L.; Manfredini, R.; Basili, S.; Raparelli, G.P.V. Treatment and Response to Statins: Gender-related Differences. Curr. Med. Chem. 2017, 24, 2628–2638. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, K.M.; Zullig, L.L.; Bastian, L.A.; Bosworth, H. Statin Adherence: Does Gender Matter? Curr. Atheroscler. Rep. 2016, 18, 63. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.; Nie, J.; Sun, P. The impact of particulate matter (PM2.5) on skin barrier revealed by transcriptome analysis: Focusing on cholesterol metabolism. Toxicol. Rep. 2020, 7, 1–9. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, H.; Zou, J.; Mai, H.; Su, D.; Feng, X.; Feng, D. Bisphenol A exposure induces cholesterol synthesis and hepatic steatosis in C57BL/6 mice by down-regulating the DNA methylation levels of SREBP-2. Food Chem. Toxicol. 2019, 133, 110786. [Google Scholar] [CrossRef]
- Soultanov, V.S. New hepatic and neurological clinical implications of long-chain plant polyprenols acting on the mammalian isoprenoid pathway. Exp. Clin. Gastroenterol. 2016, 11, 104–113. [Google Scholar]
- García-Pelayo, M.C.; García-Peregrín, E.; Martínez-Cayuela, M. Modification of phospholipids fatty acid composition in reuber H35 hepatoma cells: Effect on HMG-CoA reductase activity. J. Cell. Biochem. 2003, 90, 586–591. [Google Scholar] [CrossRef]
- Tonini, C.; Schiavi, S.; Macca, F.; Segatto, M.; Trezza, V.; Pallottini, V. Long-lasting impact of perinatal dietary supplementation of omega 3 fatty acids on mevalonate pathway: Potential role on neuron trophism in male offspring hippocampal formation. Nutr. Neurosci. 2020, 9, 1–12. [Google Scholar] [CrossRef]
- Trapani, L.; Segatto, M.; Simeoni, V.; Balducci, V.; Dhawan, A.; Parmar, V.S.; Prasad, A.K.; Saso, L.; Incerpi, S.; Pallottini, V. Short- and long-term regulation of 3-hydroxy 3-methylglutaryl coenzyme A reductase by a 4-methylcoumarin. Biochimie 2011, 93, 1165–1171. [Google Scholar] [CrossRef]
- Rocha, J.; Trapani, L.; Segatto, M.; La Rosa, P.; Nogueira, C.W.; Zeni, G.; Pallottini, V. Molecular Effects of Diphenyl Diselenide on Cholesterol and Glucose Cell Metabolism. Curr. Med. Chem. 2013, 20, 4426–4434. [Google Scholar] [CrossRef] [PubMed]
- Chin, K.-Y.; Mo, H.; Soelaiman, I.-N. A review of the possible mechanisms of action of tocotrienol—A potential antiosteoporotic agent. Curr. Drug Targets 2013, 14, 1533–1541. [Google Scholar] [CrossRef] [PubMed]
- Pang, S.; Guo, M.; Zhang, X.; Yu, L.; Zhang, Z.; Huang, L.; Gao, J.; Li, X. Myclobutanil developmental toxicity, bioconcentration and sex specific response in cholesterol in zebrafish (Denio rerio). Chemosphere 2020, 242, 125209. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Sun, Q.; Lam, S.M.; Chen, R.; Zhu, J.; Gu, W.; Zhang, L.; Tian, H.; Zhang, K.; Chen, L.-C.; et al. Sex-dependent effects of ambient PM2.5 pollution on insulin sensitivity and hepatic lipid metabolism in mice. Part. Fibre Toxicol. 2020, 17, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Godfrey, K.M.; Barker, D.J. Maternal nutrition in relation to fetal and placental growth. Eur. J. Obstet. Gynecol. Reprod. Biol. 1995, 61, 15–22. [Google Scholar] [CrossRef]
- Valvi, D.; Oulhote, Y.; Weihe, P.; Dalgård, C.; Bjerve, K.S.; Steuerwald, U.; Grandjean, P. Gestational diabetes and offspring birth size at elevated environmental pollutant exposures. Environ. Int. 2017, 107, 205–215. [Google Scholar] [CrossRef]
- Chen, M.; Liang, S.; Qin, X.; Zhang, L.; Qiu, L.; Chen, S.; Hu, Z.; Xu, Y.; Wang, W.; Zhang, Y.; et al. Prenatal exposure to diesel exhaust PM2.5 causes offspring β cell dysfunction in adulthood. Am. J. Physiol. Metab. 2018, 315, E72–E80. [Google Scholar] [CrossRef]
- Nuñez, P.; Fernandez, T.; García-Arévalo, M.; Alonso-Magdalena, P.; Nadal, A.; Perillan, C.; Arguelles, J. Effects of bisphenol A treatment during pregnancy on kidney development in mice: A stereological and histopathological study. J. Dev. Orig. Health Dis. 2017, 9, 208–214. [Google Scholar] [CrossRef]
- Choe, S.-A.; Eliot, M.N.; Savitz, D.A.; Wellenius, G.A. Ambient air pollution during pregnancy and risk of gestational diabetes in New York City. Environ. Res. 2019, 175, 414–420. [Google Scholar] [CrossRef]
- Meng, Z.; Tian, S.; Yan, J.; Jia, M.; Yan, S.; Li, R.; Zhang, R.; Zhu, W.; Zhou, Z. Effects of perinatal exposure to BPA, BPF and BPAF on liver function in male mouse offspring involving in oxidative damage and metabolic disorder. Environ. Pollut. 2019, 247, 935–943. [Google Scholar] [CrossRef]
- Golomb, B.A.; Evans, M.A. Statin adverse effects: A review of the literature and evidence for a mitochondrial mechanism. Am. J. Cardiovasc. Drugs 2008, 8, 373–418. [Google Scholar] [CrossRef] [PubMed]
- Maltese, W.A.; Sheridan, K.M. Differentiation of neuroblastoma cells induced by an inhibitor of mevalonate synthesis: Relation of neurite outgrowth and acetylcholinesterase activity to changes in cell proliferation and blocked isoprenoid synthesis. J. Cell. Physiol. 1985, 125, 540–558. [Google Scholar] [CrossRef] [PubMed]
- Cartocci, V.; Segatto, M.; Di Tunno, I.; Leone, S.; Pfrieger, F.; Pallottini, V. Modulation of the Isoprenoid/Cholesterol Biosynthetic Pathway During Neuronal Differentiation In Vitro. J. Cell. Biochem. 2016, 117, 2036–2044. [Google Scholar] [CrossRef] [PubMed]
- Can, Ö.D.; Ulupinar, E.; Özkay, Ü.D.; Yegin, B.; Ozturk, Y. The effect of simvastatin treatment on behavioral parameters, cognitive performance, and hippocampal morphology in rats fed a standard or a high-fat diet. Behav. Pharmacol. 2012, 23, 582–592. [Google Scholar] [CrossRef]
- Genaro-Mattos, T.C.; Allen, L.B.; Anderson, A.; Tallman, K.A.; Porter, N.A.; Korade, Z.; Mirnics, K. Maternal aripiprazole exposure interacts with 7-dehydrocholesterol reductase mutations and alters embryonic neurodevelopment. Mol. Psychiatry 2019, 24, 491–500. [Google Scholar] [CrossRef]
- Cartocci, V.; Catallo, M.; Tempestilli, M.; Segatto, M.; Pfrieger, F.W.; Bronzuoli, M.R.; Scuderi, C.; Servadio, M.; Trezza, V.; Pallottini, V. Altered Brain Cholesterol/Isoprenoid Metabolism in a Rat Model of Autism Spectrum Disorders. Neuroscience 2018, 372, 27–37. [Google Scholar] [CrossRef]
- Cartocci, V.; Tonini, C.; Di Pippo, T.; Vuono, F.; Schiavi, S.; Marino, M.; Trezza, V.; Pallottini, V. Prenatal exposure to valproate induces sex-, age-, and tissue-dependent alterations of cholesterol metabolism: Potential implications on autism. J. Cell. Physiol. 2018, 234, 4362–4374. [Google Scholar] [CrossRef]
- Schiavi, S.; Iezzi, D.; Manduca, A.; Leone, S.; Melancia, F.; Carbone, C.; Petrella, M.; Mannaioni, G.; Masi, A.; Trezza, V. Reward-Related Behavioral, Neurochemical and Electrophysiological Changes in a Rat Model of Autism Based on Prenatal Exposure to Valproic Acid. Front. Cell. Neurosci. 2019, 13, 479. [Google Scholar] [CrossRef]
- Soscia, S.J.; Tong, M.; Xu, J.; Cohen, A.C.; Chu, J.; Wands, J.R.; De La Monte, S.M. Chronic gestational exposure to ethanol causes insulin and IGF resistance and impairs acetylcholine homeostasis in the brain. Cell. Mol. Life Sci. 2006, 63, 2039–2056. [Google Scholar] [CrossRef]
- Zhou, C.; Chen, J.; Zhang, X.; Costa, L.G.; Guizzetti, M. Prenatal Ethanol Exposure Up-Regulates the Cholesterol Transporters ATP-Binding Cassette A1 and G1 and Reduces Cholesterol Levels in the Developing Rat Brain. Alcohol Alcohol. 2014, 49, 626–634. [Google Scholar] [CrossRef]
- Barceló-Coblijn, G.; Wold, L.E.; Ren, J.; Murphy, E. Prenatal Ethanol Exposure Increases Brain Cholesterol Content in Adult Rats. Lipids 2013, 48, 1059–1068. [Google Scholar] [CrossRef] [PubMed]
- Holahan, M.R.; Smith, C.A. Phthalates and neurotoxic effects on hippocampal network plasticity. Neurotoxicology 2015, 48, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhu, H.; Kannan, K. A Review of Biomonitoring of Phthalate Exposures. Toxics 2019, 7, 21. [Google Scholar] [CrossRef]
- Xu, Y.; Agrawal, S.; Cook, T.; Knipp, G.T. Di-(2-ethylhexyl)-phthalate affects lipid profiling in fetal rat brain upon maternal exposure. Arch. Toxicol. 2006, 81, 57–62. [Google Scholar] [CrossRef]
- Acconcia, F.; Pallottini, V.; Marino, M. Molecular Mechanisms of Action of BPA. Dose Response 2015, 13. [Google Scholar] [CrossRef] [PubMed]
- Miyawaki, J.; Sakayama, K.; Kato, H.; Yamamoto, H.; Masuno, H. Perinatal and Postnatal Exposure to Bisphenol A Increases Adipose Tissue Mass and Serum Cholesterol Level in Mice. J. Atheroscler. Thromb. 2007, 14, 245–252. [Google Scholar] [CrossRef]
- Silva, B.; Bertasso, I.; Pietrobon, C.; Lopes, B.; Santos, T.; Peixoto-Silva, N.; Carvalho, J.; Claudio-Neto, S.; Manhães, A.C.; Cabral, S.; et al. Effects of maternal bisphenol A on behavior, sex steroid and thyroid hormones levels in the adult rat offspring. Life Sci. 2019, 218, 253–264. [Google Scholar] [CrossRef]
- Negri-Cesi, P. Bisphenol A Interaction With Brain Development and Functions. Dose Response 2015, 13, 1559325815590394. [Google Scholar] [CrossRef]
- Shikimi, H.; Sakamoto, H.; Mezaki, Y.; Ukena, K.; Tsutsui, K. Dendritic growth in response to environmental estrogens in the developing Purkinje cell in rats. Neurosci. Lett. 2004, 364, 114–118. [Google Scholar] [CrossRef]
- Kawato, S. Endocrine disrupters as disrupters of brain function: A neurosteroid viewpoint. Environ. Sci. Int. J. Environ. Physiol. Toxicol. 2004, 11, 1–14. [Google Scholar]
- Nakamura, D.; Yanagiba, Y.; Duan, Z.; Ito, Y.; Okamura, A.; Asaeda, N.; Tagawa, Y.; Li, C.; Taya, K.; Zhang, S.-Y. Bisphenol A may cause testosterone reduction by adversely affecting both testis and pituitary systems similar to estradiol. Toxicol. Lett. 2010, 194, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Frye, C.; Bo, E.; Calamandrei, G.; Calza, L.; Dessi-Fulgheri, F.; Fernández, M.; Fusani, L.; Kah, O.; Kajta, M.; Le Page, Y.; et al. Endocrine Disrupters: A Review of Some Sources, Effects, and Mechanisms of Actions on Behaviour and Neuroendocrine Systems. J. Neuroendocr. 2011, 24, 144–159. [Google Scholar] [CrossRef] [PubMed]
- Samova, S.; Patel, C.; Doctor, H.; Pandya, H.A.; Verma, R. The effect of bisphenol A on testicular steroidogenesis and its amelioration by quercetin: An in vivo and in silico approach. Toxicol. Res. 2018, 7, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Baker, M.E.; Lathe, R. The promiscuous estrogen receptor: Evolution of physiological estrogens and response to phytochemicals and endocrine disruptors. J. Steroid Biochem. Mol. Biol. 2018, 184, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Sobolewski, M.; Conrad, K.; Allen, J.L.; Weston, H.; Martin, K.; Lawrence, B.P.; Cory-Slechta, D.A. Sex-specific enhanced behavioral toxicity induced by maternal exposure to a mixture of low dose endocrine-disrupting chemicals. NeuroToxicology 2014, 45, 121–130. [Google Scholar] [CrossRef]
- Ghassabian, A.; Bell, E.M.; Ma, W.-L.; Sundaram, R.; Kannan, K.; Louis, G.M.B.; Yeung, E.H. Concentrations of perfluoroalkyl substances and bisphenol A in newborn dried blood spots and the association with child behavior. Environ. Pollut. 2018, 243, 1629–1636. [Google Scholar] [CrossRef]
- Shu, L.; Meng, Q.; Diamante, G.; Tsai, B.; Chen, Y.-W.; Mikhail, A.; Luk, H.; Ritz, B.; Allard, P.; Yang, X. Prenatal Bisphenol A Exposure in Mice Induces Multitissue Multiomics Disruptions Linking to Cardiometabolic Disorders. Endocrinology 2018, 160, 409–429. [Google Scholar] [CrossRef]
- Tonini, C.; Segatto, M.; Gagliardi, S.; Bertoli, S.; Leone, A.; Barberio, L.; Mandalà, M.; Pallottini, V. Maternal Dietary Exposure to Low-Dose Bisphenol A Affects Metabolic and Signaling Pathways in the Brain of Rat Fetuses. Nutrients 2020, 12, 1448. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tonini, C.; Segatto, M.; Pallottini, V. Impact of Sex and Age on the Mevalonate Pathway in the Brain: A Focus on Effects Induced by Maternal Exposure to Exogenous Compounds. Metabolites 2020, 10, 304. https://doi.org/10.3390/metabo10080304
Tonini C, Segatto M, Pallottini V. Impact of Sex and Age on the Mevalonate Pathway in the Brain: A Focus on Effects Induced by Maternal Exposure to Exogenous Compounds. Metabolites. 2020; 10(8):304. https://doi.org/10.3390/metabo10080304
Chicago/Turabian StyleTonini, Claudia, Marco Segatto, and Valentina Pallottini. 2020. "Impact of Sex and Age on the Mevalonate Pathway in the Brain: A Focus on Effects Induced by Maternal Exposure to Exogenous Compounds" Metabolites 10, no. 8: 304. https://doi.org/10.3390/metabo10080304
APA StyleTonini, C., Segatto, M., & Pallottini, V. (2020). Impact of Sex and Age on the Mevalonate Pathway in the Brain: A Focus on Effects Induced by Maternal Exposure to Exogenous Compounds. Metabolites, 10(8), 304. https://doi.org/10.3390/metabo10080304





