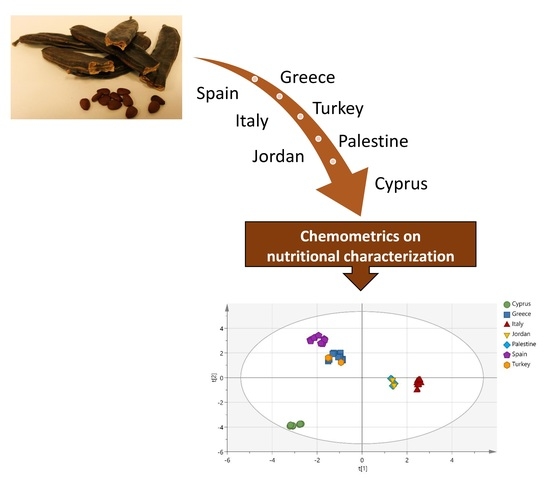
Use of Chemometrics for Correlating Carobs Nutritional Compositional Values with Geographic Origin
Abstract
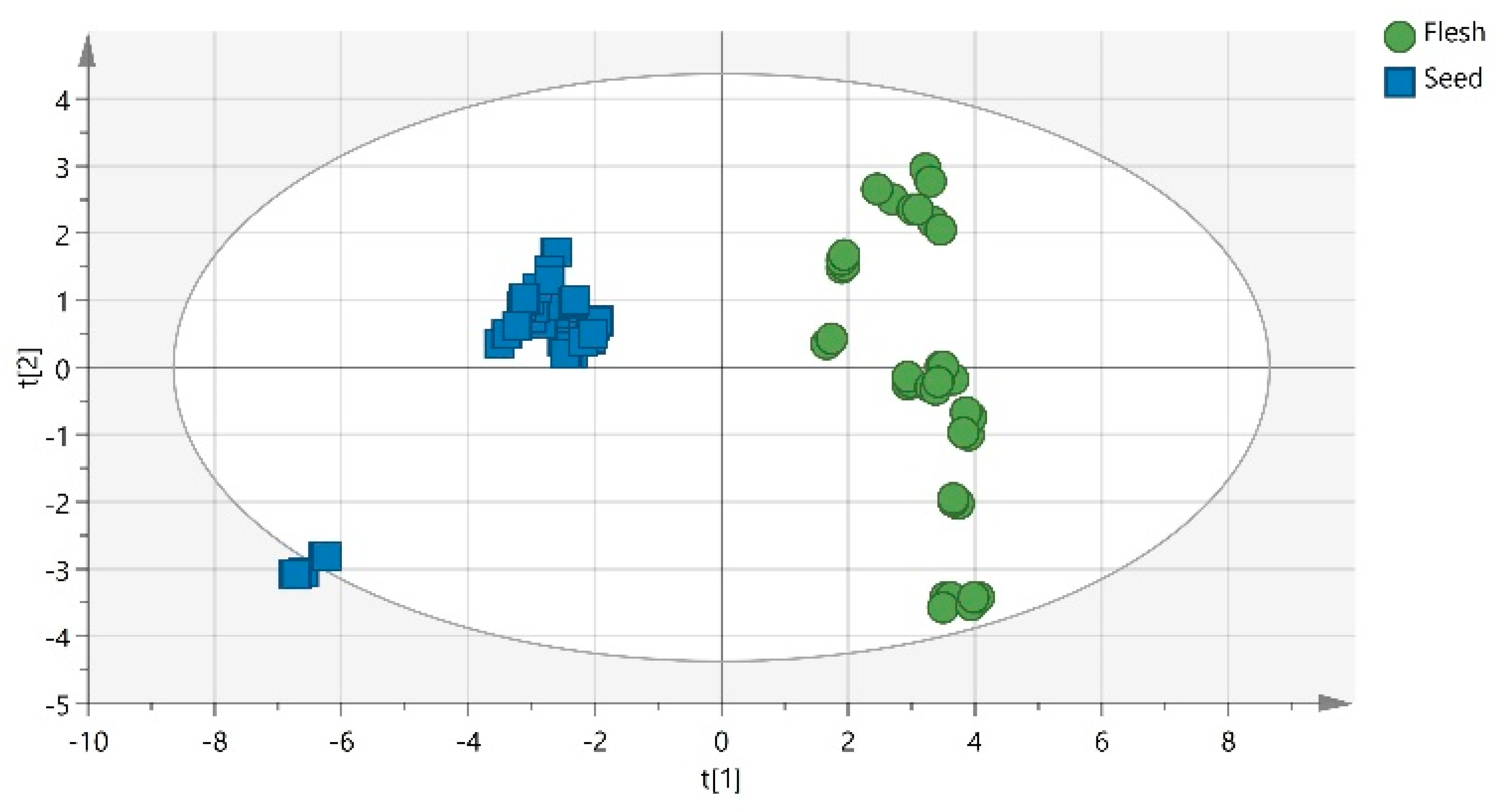
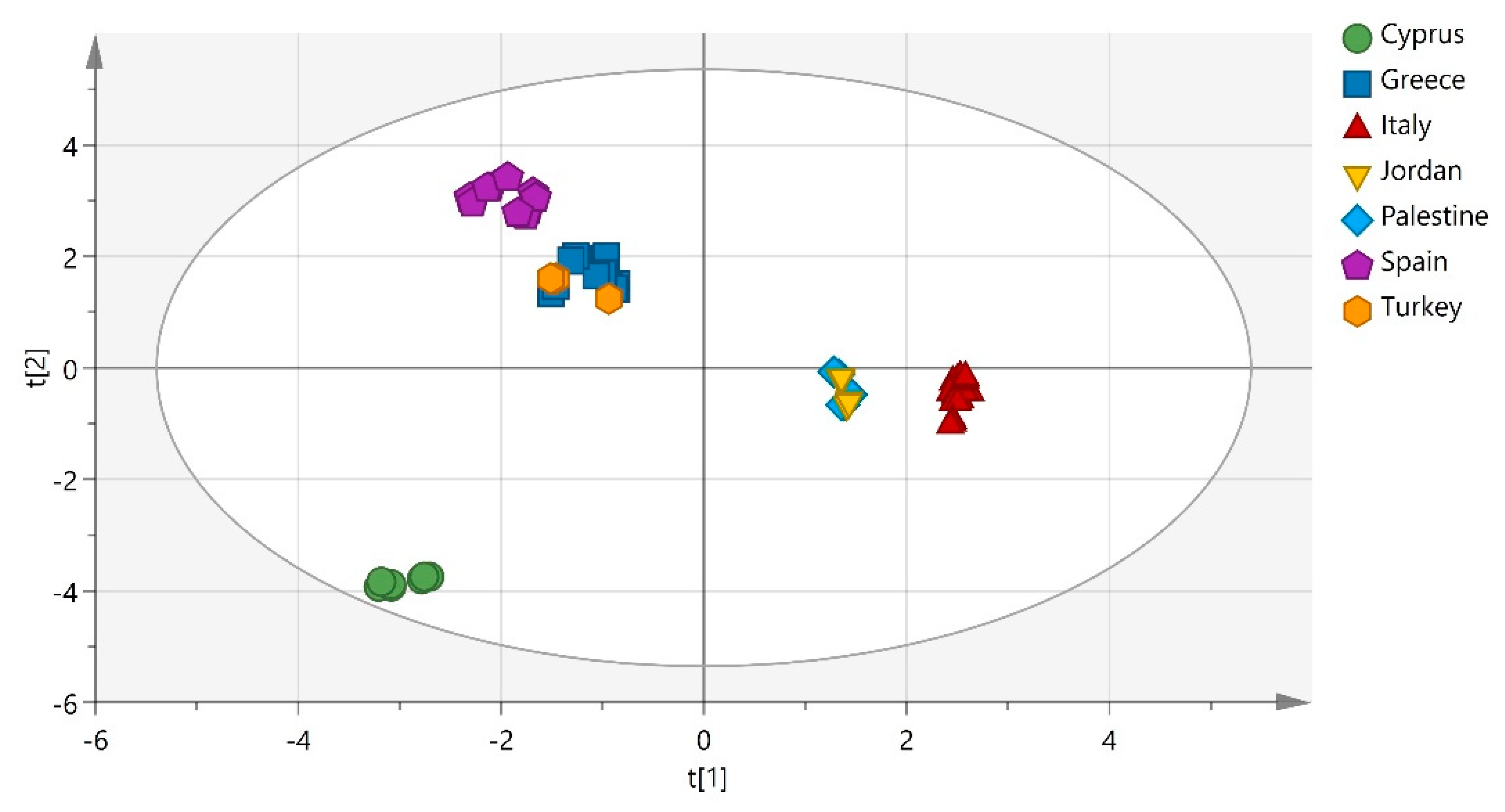
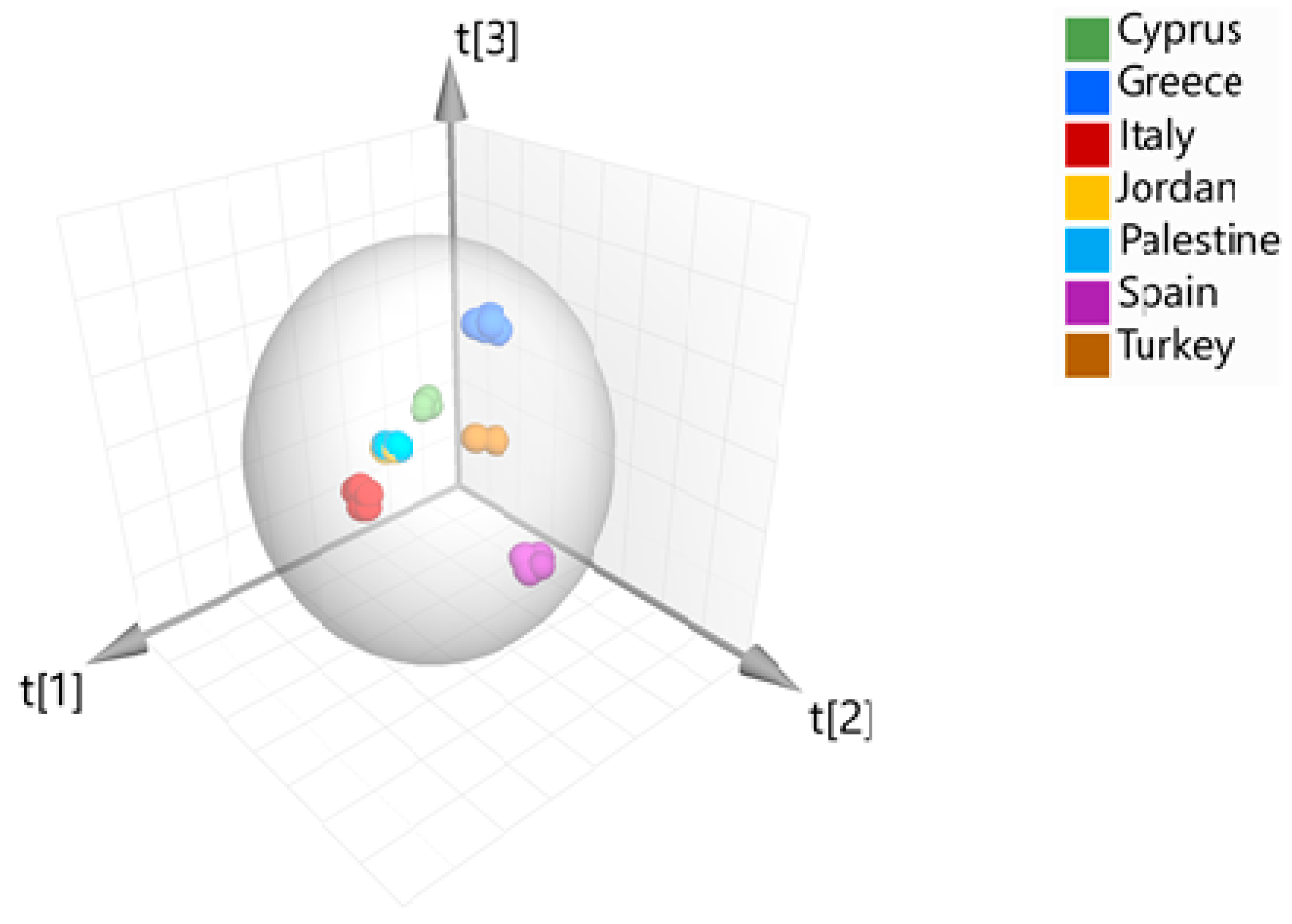
1. Introduction
2. Results and Discussion
3. Conclusions
4. Materials and Methods
4.1. Samples
4.2. Nutritional Composition Analysis
4.3. Multivariate Data Analysis (MDA)
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Luykx, D.M.A.M.; van Ruth, S.M. An overview of analytical methods for determining the geographical origin of food products. Food Chem. 2008, 107, 897–911. [Google Scholar] [CrossRef]
- Ellis, D.I.; Brewster, V.L.; Dunn, W.B.; Allwood, J.W.; Golovanov, A.P.; Goodacre, R. Fingerprinting food: Current technologies for the detection of food adulteration and contamination. Chem. Soc. Rev. 2012, 41, 5706–5727. [Google Scholar] [CrossRef] [PubMed]
- Nasar-Abbas, S.M.; e-Huma, Z.; Vu, T.H.; Khan, M.K.; Esbenshade, H.; Jayasena, V. Carob Kibble: A Bioactive-Rich Food Ingredient. Compr. Rev. Food Sci. Food Saf. 2016, 15, 63–72. [Google Scholar] [CrossRef]
- Mortensen, A.; Aguilar, F.; Crebelli, R.; Di Domenico, A.; Frutos, M.J.; Galtier, P.; Gott, D.; Gundert-Remy, U.; Lambré, C.; Leblanc, J.; et al. Re-evaluation of locust bean gum (E 410) as a food additive. EFSA J. 2017, 15. [Google Scholar] [CrossRef]
- Ayaz, F.A.; Torun, H.; Ayaz, S.; Correia, P.J.; Alaiz, M.; Sanz, C.; Grúz, J.; Strnad, M. Determination of chemical composition of anatolian carob pod (Ceratonia siliqua L.): Sugars, amino and organic acids, minerals and phenolic compounds. J. Food Qual. 2007, 30, 1040–1055. [Google Scholar] [CrossRef]
- Christou, C.; Poulli, E.; Yiannopoulos, S.; Agapiou, A. GC–MS analysis of D-pinitol in carob: Syrup and fruit (flesh and seed). J. Chromatogr. B 2019, 1116, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.J.; Zayed, M.Z.; Zhu, H.X.; Zhao, J.; Li, S.P. Functional polysaccharides of carob fruit: A review. Chinese Med. 2019, 14, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Deans, B.J.; Skierka, B.E.; Karagiannakis, B.W.; Vuong, D.; Lacey, E.; Smith, J.A.; Bissember, A.C. Siliquapyranone: A Tannic Acid Tetrahydropyran-2-one Isolated from the Leaves of Carob (Ceratonia siliqua) by Pressurised Hot Water Extraction. Aust. J. Chem. 2018, 71, 702–707. [Google Scholar] [CrossRef]
- Farag, M.A.; El-Kersh, D.M.; Ehrlich, A.; Choucry, M.A.; El-Seedi, H.; Frolov, A.; Wessjohann, L.A. Variation in Ceratonia siliqua pod metabolome in context of its different geographical origin, ripening stage and roasting process. Food Chem. 2019, 283, 675–687. [Google Scholar] [CrossRef]
- Begou, O.; Deda, O.; Agapiou, A.; Taitzoglou, I.; Gika, H.; Theodoridis, G. Urine and fecal samples targeted metabolomics of carobs treated rats. J. Chromatogr. B 2019, 1114–1115, 76–85. [Google Scholar] [CrossRef]
- Rodríguez-Solana, R.; Coelho, N.; Santos-Rufo, A.; Gonçalves, S.; Pérez-Santín, E.; Romano, A. The Influence of In Vitro Gastrointestinal Digestion on the Chemical Composition and Antioxidant and Enzyme Inhibitory Capacities of Carob Liqueurs Obtained with Different Elaboration Techniques. Antioxidants 2019, 8, 563. [Google Scholar] [CrossRef] [PubMed]
- Krokou, A.; Stylianou, M.; Agapiou, A. Assessing the volatile profile of carob tree (Ceratonia siliqua L.). Environ. Sci. Pollut. Res. 2019, 1–10. [Google Scholar] [CrossRef]
- Loullis, A.; Pinakoulaki, E. Carob as cocoa substitute: A review on composition, health benefits and food applications. Eur. Food Res. Technol. 2018, 244, 959–977. [Google Scholar] [CrossRef]
- Youssef, M.K.E.; El-Manfaloty, M.M.; Ali, H.M. Assessment of proximate chemical composition, nutritional status, fatty acid composition and phenolic compounds of carob (Ceratonia siliqua L.). Food Public Health 2013, 3, 304–308. [Google Scholar] [CrossRef]
- Özcan, M.M.; Arslan, D.; Gökçalik, H. Some compositional properties and mineral contents of carob (Ceratonia siliqua) fruit, flour and syrup. Int. J. Food Sci. Nutr. 2007, 58, 652–658. [Google Scholar] [CrossRef]
- Avallone, R.; Plessi, M.; Baraldi, M.; Monzani, A. Determination of Chemical Composition of Carob (Ceratonia siliqua): Protein, Fat, Carbohydrates, and Tannins. J. Food Compos. Anal. 1997, 10, 166–172. [Google Scholar] [CrossRef]
- El-Shatnawi, M.K.J.; Ereifej, K.I. Chemical composition and livestock ingestion of carob (Ceratonia siliqua L.) seeds. J. Range Manag. 2001, 54, 669–673. [Google Scholar] [CrossRef]
- El Batal, H.; Hasib, A.; Dehbi, F.; Zaki, N.; Ouatmane, A.; Boulli, A. Assessment of nutritional composition of Carob pulp (Ceratonia Siliqua L.) collected from various locations in Morocco. J. Mater. Environ. Sci. 2016, 7, 3278–3285. [Google Scholar]
- Sigge, G.O.; lipumbu, L.; Britz, T.J. Proximate composition of carob cultivars growing in South Africa. S. Afr. J. Plant Soil 2011, 28, 17–22. [Google Scholar] [CrossRef]
- Khlifa, M.; Bahloul, A.; Kitane, S. Determination of chemical composition of carob pod (Ceratonia siliqua L) and its morphological study. J. Mater. Environ. Sci. 2013, 4, 348–353. [Google Scholar]
- Rodríguez-Solana, R.; Carlier, J.D.; Costa, M.C.; Romano, A. Multi-element characterisation of carob, fig and almond liqueurs by MP-AES. J. Inst. Brew. 2018, 124, 300–309. [Google Scholar] [CrossRef]
- Papaefstathiou, E.; Agapiou, A.; Giannopoulos, S.; Kokkinofta, R. Nutritional characterization of carobs and traditional carob products. Food Sci. Nutr. 2018, 6, 2151–2161. [Google Scholar] [CrossRef] [PubMed]
- Medina, S.; Perestrelo, R.; Silva, P.; Pereira, J.A.M.; Câmara, J.S. Current trends and recent advances on food authenticity technologies and chemometric approaches. Trends Food Sci. Technol. 2019, 85, 163–176. [Google Scholar] [CrossRef]
- Christou, C.; Agapiou, A.; Kokkinofta, R. Use of FTIR spectroscopy and chemometrics for the classification of carobs origin. J. Adv. Res. 2018, 10, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.K.; Mo, C.; Lee, D.K.; Long, N.P.; Lim, J.; Kwon, S.W. Non-destructive profiling of volatile organic compounds using HS-SPME/GC–MS and its application for the geographical discrimination of white rice. J. Food Drug Anal. 2018, 26, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Dryahina, K.; Smith, D.; Španěl, P. Quantification of volatile compounds released by roasted coffee by selected ion flow tube mass spectrometry. Rapid Commun. Mass Spectrom. 2018, 32, 739–750. [Google Scholar] [CrossRef]
- Medina, S.; Pereira, J.A.; Silva, P.; Perestrelo, R.; Câmara, J.S. Food fingerprints—A valuable tool to monitor food authenticity and safety. Food Chem. 2019, 278, 144–162. [Google Scholar] [CrossRef]
- Kokkinofta, R.; Petrakis, P.V.; Mavromoustakos, T.; Theocharis, C.R. Authenticity of the traditional Cypriot spirit “zivania” on the basis of metal content using a combination of coupled plasma spectroscopy and statistical analysis. J. Agric. Food Chem. 2003, 51, 6233–6239. [Google Scholar] [CrossRef]
- Baker, I. Copper. In Fifty Materials That Make the World; Springer International Publishing: Cham, Switzerland, 2018; pp. 43–47. ISBN 978-3-319-78766-4. [Google Scholar]
- Granato, D.; Putnik, P.; Kovačević, D.B.; Santos, J.S.; Calado, V.; Rocha, R.S.; Cruz, A.G.; Da Jarvis, B.; Rodionova, O.Y.; Pomerantsev, A. Trends in Chemometrics: Food Authentication, Microbiology, and Effects of Processing. Compr. Rev. Food Sci. Food Saf. 2018, 17, 663–677. [Google Scholar] [CrossRef]
- AOAC. 925.10 Association of Official Analytical Chemists, Solids (total) and loss on drying (moisture) in flour. In Official Methods of Analysis of AOAC International; AOAC International: Rockville, MD, USA, 2010. [Google Scholar]
- AACC. 44-15A Moisture—Air oven method. In Approved Methods of the American Association of Cereal Chemists; AACC: Washington, DC, USA, 1975. [Google Scholar]
- Pearsons Chemical Analysis of Foods, 8th ed.; Longman Scientific & Technical: England, UK, 1981.
- AOAC. 991.20 Nitrogen (total) in milk, Kjeldahl methods. In Association of Official Analytical Chemists, Official Methods of Analysis of AOAC International; AOAC International: Rockville, MD, USA, 2011. [Google Scholar]
- AOAC. 920.87 AOAC Official Method 920.87, Protein (total) in flour. In Association of Official Analytical Chemists, Official Methods of Analysis of AOAC International; AOAC International: Rockville, MD, USA, 2010. [Google Scholar]
- Meat and Meat Products—Determination of Nitrogen Content, Reference Method; ISO-937-1978; ISO: Geneva, Switzerland, 1978.
- AOAC. 977.20 Separation of sugars chromatographic method, JAOAC, 60, 838. In Association of Official Analytical Chemists, Official Methods of Analysis of AOAC International; AOAC International: Rockville, MD, USA, 1977. [Google Scholar]
- AOAC. 991.36 Fat (crude) in meat and meat products, Soxhlet extraction method. In Association of Official Analytical Chemists, Official Methods of Analysis of AOAC International; AOAC International: Rockville, MD, USA, 2010. [Google Scholar]
- AOAC. 963.15 Fat in cacao products, Soxhlet extraction method. In Association of Official Analytical Chemists, Official Methods of Analysis of AOAC International; AOAC International: Rockville, MD, USA, 2010. [Google Scholar]
- AOAC. 985.29 Total dietary fiber in foods (Enzymatic—Gravimetric method). In Association of Official Analytical Chemists, Official Methods of Analysis of AOAC International; AOAC International: Rockville, MD, USA, 1990. [Google Scholar]
- AACC. 32-05.01 Megazyme Kit. In Approved Methods of the American Association of Cereal Chemists; AACC: Washington, DC, USA.
- AOAC. 985.01-1988 Metals and other elements in plants and pet foods, Inductively coupled plasma spectroscopic method. In Association of Official Analytical Chemists, Official Methods of Analysis of AOAC International; AOAC International: Rockville, MD, USA, 1996. [Google Scholar]
- AOAC. 984.27 Determination of nine nutritional elements in food products by Inductively coupled plasma-atomic emission spectroscopy after microwave digestion. In Association of Official Analytical Chemists, Official Methods of Analysis of AOAC International; AOAC International: Rockville, MD, USA.
- Umetrics SIMCA® Version15.02. Multivariate Data Analysis Solution (User’s Manual-Online). Umeå, Sweden. 2017. [Google Scholar]
- Kokkinofta, R.I.; Theocharis, C.R. Chemometric characterization of the cypriot spirit “Zivania”. J. Agric. Food Chem. 2005, 53, 5067–5073. [Google Scholar] [CrossRef]
- Ashurst, P.R.; Dennis, M.J. Food Authentication; Ashurst, P.R., Dennis, M.J., Eds.; Blackie Academic & Professional, Chapman and Hall: London, UK, 1997. [Google Scholar]
- Kowalski, B.R.; Bender, C.F. Pattern recognition. Powerful approach to interpreting chemical data. J. Am. Chem. Soc. 1972, 94, 5632–5639. [Google Scholar] [CrossRef]
- Cocchi, M.; Li Vigni, M.; Durante, C. Food Authentication: Management, Analysis and Regulation Chemometrics—Bioinformatics; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2017. [Google Scholar]
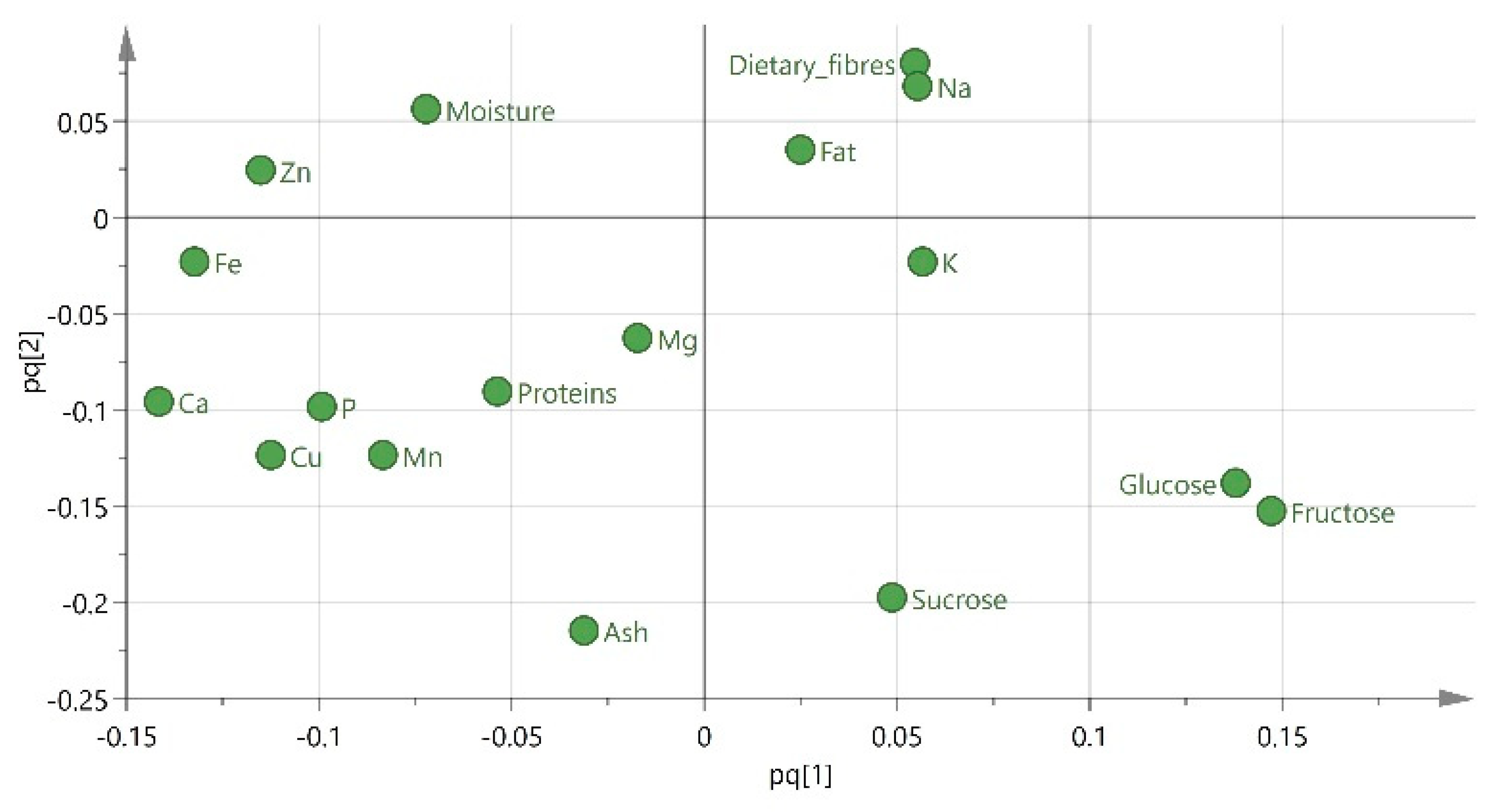
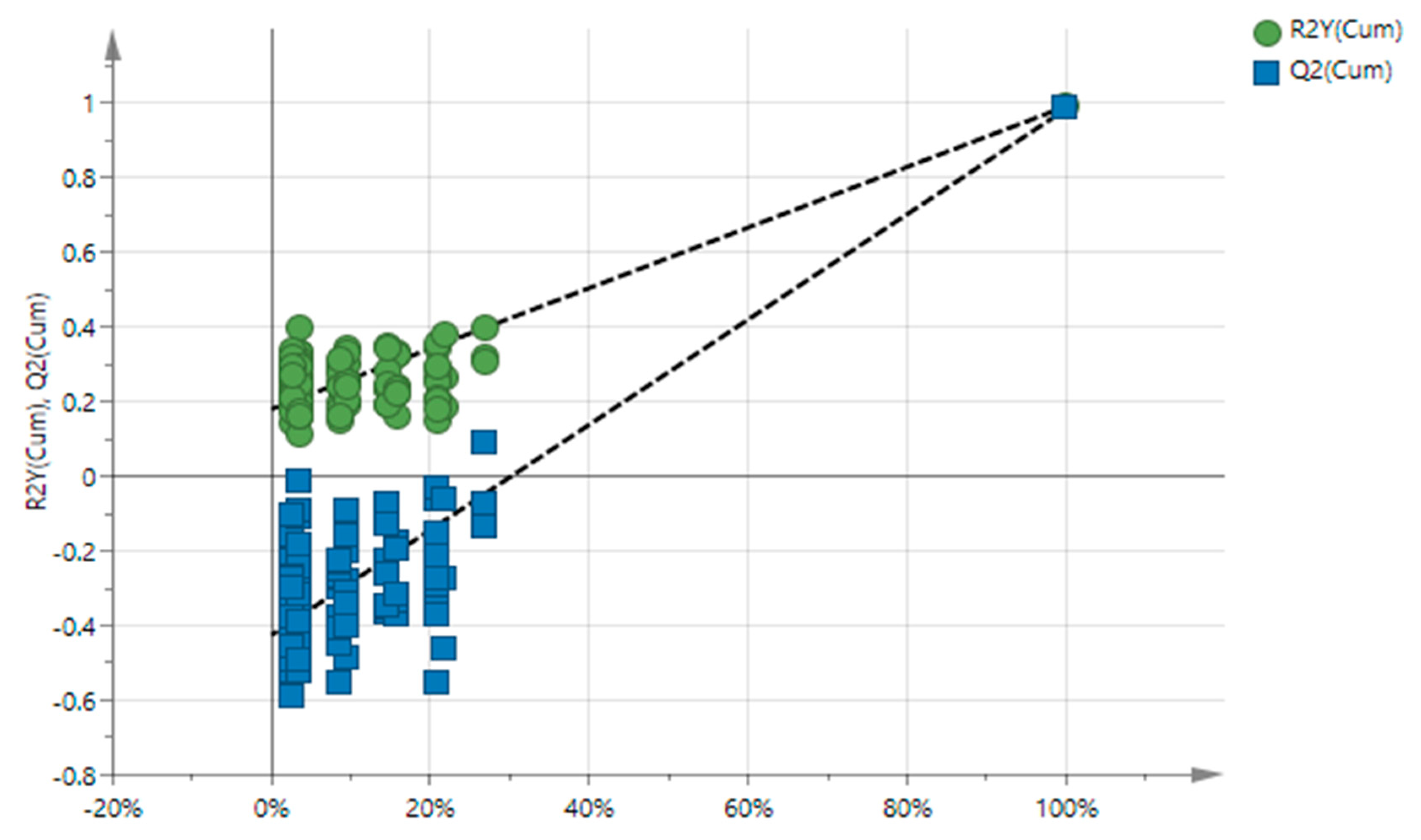
| Chemical Composition (% w/w) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Sucrose | Fructose | Glucose | Dietary Fiber | Ash | Protein | Fat | Moisture | Source |
| 35–45 | 6–7 | 2–4 | Up to 40 | 2–3 | 2–7 | 0.5–1 | - | [3] |
| 40.7–54.7 (sugars) 1 | 7.6–38.0 | 2.0–3.4 | 2.0–7.6 | 0.4–1.3 | 6–11 | [13] | ||
| 75.9 (carbohydrates) 2 | 7.3 | 3.2 | 6.3 | 2.0 | 5.3 | [14] | ||
| 48.3 (total sugars) 3 | 9.7 | 3.3 | 4.7 | 0.2 | 6.0 | [15] | ||
| 35 | 7 | 5 | - | 2 | 2 | 0.4 | 6 | [16] |
| 79.8 (carbohydrates) 2 | 9.1 | 3 | 6.8 | 1.2 | - | [17] | ||
| 31.5–50.1 (total sugars) 3 | - | 2.4–3.9 | 3.1–4.5 | 0.5–0.8 | 12.2–13.5 | [18] | ||
| 33.7–45.1 | 1.8–5.2 | 1.8–4.9 | 29.9–36.1 | 2.1–2.4 | 3.1–4.4 | 0.4–0.9 | 8.2–9.6 | [19] |
| 32.6–45.4 | 6.9–7.4 | 2.0–2.3 | 6.8–7.1 | 2.7–3.2 | 2.6–2.8 | 1–1.3 | 9.7–10.7 | [20] |
| Component | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| Cumulative | 45.5 | 57.1 | 65.6 | 72.8 | 78.1 | 82.4 | 86.6 | 90.7 | 92.8 | 95.0 |
| Class | No of Samples | Correct (%) | 1 | 2 | 3 | 4 | 5 | 6 | 7 | No Class (YPred ≤ 0) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 (Cyprus) | 12 | 100 | 12 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2 (Greece) | 12 | 100 | 0 | 12 | 0 | 0 | 0 | 0 | 0 | 0 |
| 3 (Italy) | 24 | 100 | 0 | 0 | 24 | 0 | 0 | 0 | 0 | 0 |
| 4 (Spain) | 12 | 100 | 0 | 0 | 0 | 12 | 0 | 0 | 0 | 0 |
| 5 (Palestine) | 6 | 0 | 0 | 0 | 6 | 0 | 0 | 0 | 0 | 0 |
| 6 (Jordan) | 6 | 0 | 0 | 0 | 6 | 0 | 0 | 0 | 0 | 0 |
| 7 (Turkey) | 4 | 0 | 0 | 2 | 0 | 2 | 0 | 0 | 0 | 0 |
| No class | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Total | 76 | 78.95 | 12 | 14 | 36 | 14 | 0 | 0 | 0 | 0 |
| Fisher’s probability 3.7 × 10−7 | ||||||||||
| Country | Cultivars | Sample Type |
|---|---|---|
| Cyprus | Tylliria, Koumpota, Kountourka | Flesh and seed |
| Greece | Imera, Imera, Unknown | Flesh and seed |
| Italy | Raexmosa, Giubiliana, Saccarata, Unknown | Flesh and seed |
| Spain | Negra, Rojal, Metalafera | Flesh and seed |
| Turkey | Fleshy | Flesh and seed |
| Jordan | Unknown | Flesh and seed |
| Palestine | Unknown | Flesh and seed |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kokkinofta, R.; Yiannopoulos, S.; Stylianou, M.A.; Agapiou, A. Use of Chemometrics for Correlating Carobs Nutritional Compositional Values with Geographic Origin. Metabolites 2020, 10, 62. https://doi.org/10.3390/metabo10020062
Kokkinofta R, Yiannopoulos S, Stylianou MA, Agapiou A. Use of Chemometrics for Correlating Carobs Nutritional Compositional Values with Geographic Origin. Metabolites. 2020; 10(2):62. https://doi.org/10.3390/metabo10020062
Chicago/Turabian StyleKokkinofta, Rebecca, Stelios Yiannopoulos, Marinos A. Stylianou, and Agapios Agapiou. 2020. "Use of Chemometrics for Correlating Carobs Nutritional Compositional Values with Geographic Origin" Metabolites 10, no. 2: 62. https://doi.org/10.3390/metabo10020062
APA StyleKokkinofta, R., Yiannopoulos, S., Stylianou, M. A., & Agapiou, A. (2020). Use of Chemometrics for Correlating Carobs Nutritional Compositional Values with Geographic Origin. Metabolites, 10(2), 62. https://doi.org/10.3390/metabo10020062