Abstract
Plants are an untapped natural resource; their secondary metabolites take part in a variety of pharmacological activities, making them an essential ingredient in the synthesis of novel medications and the source of reserve resources in this process. Hepatitis and liver cancer are two conditions that can result from non-alcoholic fatty liver disease (NAFLD). NAFLD is a condition that now affects a significant section of the global population. There is a need for preventative action on predisposing factors. Due to their effectiveness and few side effects, herbal medications are frequently utilized for the prevention and treatment of NAFLD. This review discusses the pathogenetic processes of NAFLD and the evidence brought to support the potential of botanical species and their derivatives in limiting the causes that predispose to the onset of NAFLD.
1. Introduction
The liver is the most important multifunctional organ in the body. It has a particular role in the triglyceride cycle and in the synthesis of lipoproteins, which are the form of elimination of triglycerides from the liver; it also carries out biotransformation and detoxification activities and is closely involved in glucose homeostasis. Any interference with these functions can lead to the development of liver disease [1].
Among the most common liver diseases is fatty liver disease (FLD), also known as hepatic steatosis, which currently affects a large part of the world population. Its prevalence has been estimated at 20–30% in the general population (non-alcoholic fatty liver disease (NAFLD) 16–24%, nonalcoholic steatohepatitis (NASH) 2.1–6.3%), to be higher in obese people (mean prevalence 34.2%) [2].
The term steatosis generally indicates pathological processes during which there is an accumulation of lipids, generally triglycerides, in cells where it is not commonly possible to highlight them with common histochemical techniques. The primary feature of fatty liver disease is the unusual presence of TG in hepatocytes, which is produced by the esterification of free fatty acids and glycerol inside hepatocytes, which results in a variety of histological pictures: (1) simple fatty liver disease, defined by an amount of TG > 5–10% of the liver’s weight; (2) non-alcoholic fatty liver disease (NAFLD), which is triggered when fat accumulation begins to exert harmful toxic effects and the percentage of hepatocytes that contain lipid droplets is greater than 5% [3]; and (3) non-alcoholic inflammatory steatohepatitis (NASH), which can be associated with fibrosis and cirrhosis, leading to the eventual development of hepatocellular carcinoma [4].
Previous epidemiological research has shown that approximately 20% of patients with NAFLD progress to the NASH stage, which can lead to cirrhosis, portal hypertension, and hepatocellular carcinoma [5]. Once developed, 30–40% of patients with cirrhosis die from liver complications within 10 years [6].
NAFLD should be managed with a combination of exercise, calorie restriction, and progressive weight reduction through lifestyle changes [7].
To treat NAFLD, several pharmacological approaches have been tried. To reverse the comorbidities of the metabolic syndrome, existing drug therapies are mainly based on the combination of various substances. These treatments include the use of antioxidants (vitamins E and C; betaine), insulin-sensitizing drugs (thiazolidinediones and metformin), lipid-lowering drugs (statins; orlistat; probucol), cytoprotective drugs (ursodeoxycholic acid), and anti-inflammatory or antifibrotic drugs (pentoxifylline; angiotensin receptor blockers). Unfortunately, none of the suggested drugs have yet been successful [8]. Consequently, it is clear that new therapeutic options need to be investigated. Numerous natural substances have been shown to have positive effects on cellular processes involved in the onset and development of various diseases [9,10].
In recent years, research on natural products and their derivatives has had a certain ferment. The use of plants as therapeutic agents has very ancient origins and still holds considerable interest today [11].
Some valid alternatives to the treatment of NAFLD and its progression into NASH can be exemplified by using specific chemical compounds naturally produced by some plants. It is known that different plant substances can treat different conditions that coexist in this disease, including hyperglycemia, hyperlipidemia, inflammation, and oxidative stress [12,13]. There are also studies on the direct use of natural remedies for the treatment of NAFLD [14].
This review provides a summary of the knowledge gained over the years regarding the use of natural products for the treatment of NAFLD. In the first section, we provide a summary of the signaling pathways and damage processes that underlie the onset of NAFLD. We then evaluate the plants and derivates that have been thoroughly investigated for the treatment of NAFLD, as well as their mechanisms.
2. Pathophysiology of NAFLD
Fatty liver disease includes two distinct categories: alcoholic fatty liver disease (AFLD) and non-alcoholic fatty liver disease (NAFLD) [15]. The first is triggered by excessive alcohol consumption; the underlying causes of the second condition have not yet been fully clarified, but a multifactorial etiology is clearly emerging. It recognizes various elements, including obesity, associated insulin resistance (IR), type 2 diabetes mellitus, dyslipidemia, atherosclerosis, hypertension, and fatty liver disease itself [16,17].
Interestingly, these are the same risk factors associated with cardiovascular disease (CVD). In patients with NAFLD, liver enzymes have been shown to predict the incidence of CVD, independent of traditional risk factors, including C-reactive protein and metabolic syndrome (MS). Indeed, the extent of liver damage has been correlated with early carotid atherosclerosis, suggesting that both vessel and liver damage share similar inflammatory mediators [18].
For these reasons, NAFLD has recently been proposed as an early marker of atherosclerosis and endothelial dysfunction and, consequently, as a cardiovascular risk factor. Considering the wide spectrum of effects often related to NAFLD, several scientists have recently provided a more accurate terminology for this disease. The term NAFLD has been changed to “MAFLD,” meaning metabolic dysfunction associated with fatty liver disease. Therefore, NAFLD can be regarded as the hepatic manifestation of a metabolic syndrome [19,20,21].
In this MS, also the intestinal microbiota plays a key role. Recent studies have shown that, in addition to genetic predisposition and diet, the intestinal microbiota influences the hepatic metabolism of carbohydrates and lipids [22]. It also appears to affect the balance between pro-inflammatory and anti-inflammatory effectors in the liver, thereby affecting the progression of MAFLD [23]. Changes in the composition of the intestinal microbiota, in fact, increase intestinal permeability and, consequently, the translocation of bacteria and their products; this induces endotoxemia, which contributes to liver inflammation in patients with NASH [24].
Species belonging to the Gram-negative class grow during MAFLD, while those belonging to the Gram-positive class decrease [25,26]. Therefore, the gut microbiota may be a useful tool as a predictor for the progression and severity of MAFLD [27,28].
Among the first hypotheses about etiopathogenesis, we have that of Day et al., 1998 [29]. The authors proposed the so-called “two-hit” pathogenetic model to explain the appearance of NAFLD and its progression to NASH. The first “hit” is the accumulation of triglycerides in the liver (steatosis) due to several conditions. The liver may become overloaded by excessive dietary intake, increased TG synthesis, excessive influx of fatty acids into the liver as a result of lipolysis of adipose tissue, decreased export of lipids from the liver in the form of VLDL, or decreased oxidation of fatty acids. In this model, the first hit must be followed by a subsequent insult (second hit), capable of activating a consistent lipid peroxidation that generates reactive oxygen species (ROS) and cytotoxic aldehydes that trigger processes such as the transcription of NF-kB (regulator of inflammation genes), leading to necro-inflammation and fibrosis [29].
It has recently emerged that free fatty acids have a direct ability to promote liver injury and the activation of inflammation, even in the absence of a second hit [30].
The initial two-hit theory became obsolete and was reformulated in the form of a “multi-hit” hypothesis. The “hits” could be represented by several injuries, such as obesity, insulin resistance, oxidative stress, and pro-inflammatory processes [31,32].
Tilg and Moschen, 2010 [33] revised this pathogenetic reconstruction, considering NASH as distinct from simple fatty liver disease and not an evolution of the latter. The authors have proposed this new model in which multiple causal factors, acting simultaneously, are able to determine the onset of liver disease (the “parallel multiple stroke model”) [33].
According to this interpretation, steatohepatitis and steatosis represent two distinct nosological entities. Inflammation may also precede and lead to the onset of steatosis in NASH. In fact, there is evidence of patients with NASH who have almost no steatosis and of regression of steatosis in mice models with the obese phenotype ob/ob after anti-inflammatory therapy with anti-TNF-α monoclonal antibodies or metformin (also capable of reducing the expression of TNF-α).
The hypothesis of Tilg and Moshen, 2010 [33] brings to light the prominent role of factors of extrahepatic derivation in determining inflammation and secondary liver fibrosis [33].
Genetic factors, such as polymorphisms of some phospholipases, modifications of the intestinal microflora (dysbiosis)—secondary to a diet rich in fats/carbohydrates—insulin resistance, an increase in circulation of bacterial endotoxins (in relation to an altered liver sequestration function), reduced synthesis of short-chain fatty acids (capable of performing an anti-inflammatory action), and an altered expression in the intestinal epithelium of toll-like receptors (TLRs) (regulators of inflammation and involved in innate immunity), may represent “molecular mediators” of liver damage, inducing lipid accumulation in the liver, lipotoxicity, fibrosis and, finally, the onset of neoplasms.
In the next section, the causes that contribute to the onset of liver disease are analyzed.
Accumulation of Lipids and Steatosis
The control of lipid metabolism involves many critical organs, including the liver. The buildup of TG in hepatocytes is a hallmark of NAFLD. It is believed that there is an imbalance between the amount of FA input (uptake and synthesis with subsequent esterification to TGs) and outflow (oxidation and secretion), which results in hepatic steatosis. Hepatic fatty acids, used for TG synthesis, derive from diet, lipolysis of adipose tissue, or de novo lipogenesis in the presence of excess glucose [1].
According to research by Donnelly et al., 2005 [34], approximately 59% of liver-free fatty acids (FFAs) in NAFLD patients come from circulation, 26% from de novo lipogenesis, and 15% from food [34].
From the small intestine, dietary fatty acids are absorbed, organized into lipoprotein-rich particles (chylomicrons), and released into the blood and lymph. The liver receives the remaining chylomicrons, with the majority going to adipose tissue. The liver cells will not be able to get rid of the extra lipid if the fat meal is repeated often; if the exposure is just once in a while, the triglyceride elimination is accomplished quickly. Increased lipid mobilization from adipose deposits has the same result as intracellular triglyceride buildup. [35]. Typical steatosis of this kind occurs in diabetics, in which lipolysis is activated, and lipemia remains high due to insulin deficiency [36].
There are instances when lipid overflow develops inside the cell owing to increased fatty acid synthesis; this circumstance is known as de novo lipogenesis. The transcription factors SREBP-1c (sterol regulatory element binding protein-1c) and ChREBP (carbohydrate-responsive element-binding protein), which regulate the key lipogenesis genes, ensure that acetyl-CoA is converted to fatty acids in the liver. Insulin is primarily responsible for this conversion [37].
In patients with NAFLD, it is common to find an overexpression of the genes involved in the de novo synthesis of fatty acids, such as acetyl-CoA carboxylase 1 and 2 (ACC1, ACC2) and fatty acid synthase (FAS) [35].
Therefore, insulin plays a role in NAFLD appearance. Specific receptors found on the plasma membrane of the cells allow insulin to carry out its activity. When blood glucose levels rise after a meal, pancreatic beta-cells naturally generate insulin to lower them. Several target proteins are activated, and a cascade of cascading processes is set off when insulin binds to the cell receptor. Insulin is also responsible for de novo lipogenesis in the liver, as previously mentioned. Another function attributable to insulin is the inhibition of lipolysis in adipose tissue. NAFLD often develops in those who have insulin resistance. Increased consumption of free fatty acids (FFAs), hyperglycemia, which promotes the formation of free radicals (glycotoxicity), and inflammatory cytokines released by adipocytes are the key contributors to insulin resistance [38].
In the case of IR, the adipose tissue’s resistance to insulin triggers lipolysis, which increases the release of free fatty acids from the adipose tissue. These free fatty acids are then transported to the liver via the hepatic artery and portal vein, increasing the influence of hepatic fatty acids [39].
High blood glucose levels (in the context of IR) lead to hyperinsulinemia, which activates SREBP-1c and encourages de novo hepatic lipogenesis (through the malonyl- and acetyl-CoA pathways). The increased lipogenesis is also indirect due to the increased production of malonyl-CoA, which inhibits carnitine-palmitoyl transferase-1 and the entry of fatty acids into the mitochondria by reducing ꞵ-oxidation, thus increasing the accumulation of fatty acids and triglycerides [40] (Figure 1).
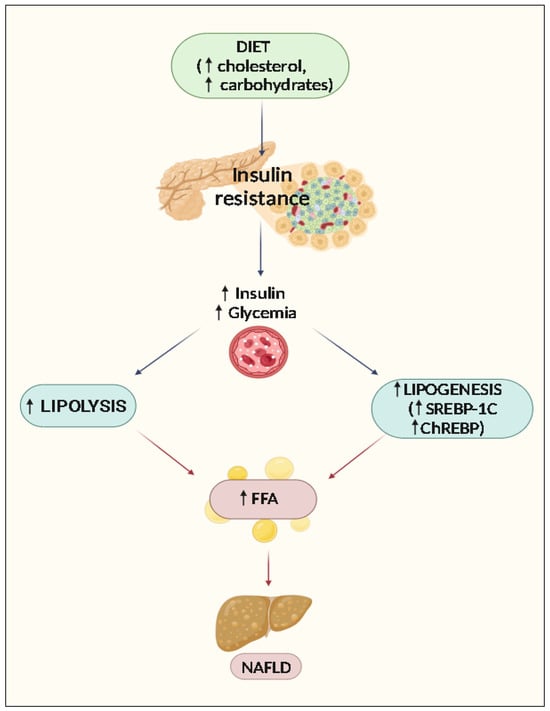
Figure 1.
The role of insulin resistance in NAFLD. ↑: Increase of expression, concentrations, or activity.
3. Progression of Liver Damage: The Role of Oxidative Stress, Mitochondrial Dysfunction, and Endoplasmic Reticulum Stress
It has been shown that individuals with NASH have biochemical and ultrastructural lesions of the mitochondria; these lesions are not completely understood at this time, but it is thought that TNF, non-esterified fatty acids and lipoperoxidation products may be to blame individually or in combination. Non-esterified fatty acids uncouple oxidative phosphorylations in this way. This condition, which is typically accompanied by an increase in oxygen consumption, causes an increase in the production of ROS, which in turn lowers the concentration of antioxidants, causes lipoperoxidation, and mediates the release of TNF from Kupffer cells (KC), which are inflammatory cytokines that are also released by steatotic hepatocytes and increase their impact by activating Kupffer cells. ROS has a growing role in the activation of Ito cells (hepatic stellate cells (HSC)), which leads to hepatic fibrogenesis, a late effect of NASH. Liver steatosis is mostly linked to oxidative stresses, mitochondrial dysfunction, and endoplasmic reticulum (ER) stress [41] (Figure 2).
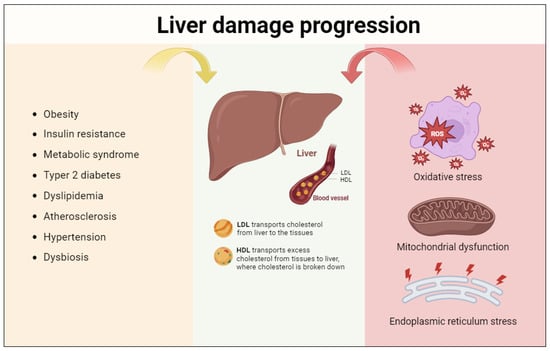
Figure 2.
Liver damage progression. Several risk factors contribute to NAFLD onset; these are highly connected and multi-compartmentalized. In addition to the involvement of the cardiovascular and lymphatic systems, cellular alterations are fundamental for the pathogenesis of this disease and concern the cellular organelles’ involvement, such as mitochondria, lysosomes, peroxisomes, and RE.
3.1. Oxidative Stress
As it was feasible to infer, a major mechanism underlying NASH is oxidative stress (OS), which is defined as an imbalance between oxidant and antioxidant chemical species. ROS are hazardous chemicals that the body’s antioxidant system must eliminate through interacting with proteins, lipids, and nucleic acids [42]. Oxidative stress is brought on by either increased ROS production in liver cells or weakened antioxidant defenses. The body’s fight against free radicals is made up of both enzymatic and nonenzymatic processes. While glutathione (GSH), ascorbic acid (vitamin C), retinol (vitamin A), and tocopherol (vitamin E) are nonenzymatic systems that can protect biomolecules and cellular structures from ROS damage by acting as electron receptors, superoxide dismutase (SOD), catalase (CAT), and GPx are examples of enzyme systems. In the context of the liver, hepatocytes counteract the harmful effects of ROS to avoid cellular damage by turning on antioxidant enzymes such as heme oxygenase 1 (HO1), NAD(P)H:Quinone Oxidoreductase 1 (NQO1), and SOD. It is significant because many of the genes responsible for encoding these antioxidant enzymes are controlled by the transcription factor nuclear factor-erythroid 2-related factor 2 (Nrf2) [43]. Nrf2 protects against NAFLD in addition to activating genes that encourage the removal of ROS by negatively regulating genes that encourage lipid synthesis [44].
Damage to cell membranes and modifications to lipid, protein, carbohydrate, and DNA biomolecules are brought on by ROS [45]. OS is generated in mitochondria, peroxisomes, and ER, organelles in which oxygen consumption is high [46]. Lipid peroxidation, which causes the histological characteristics seen with NASH, is the primary modification mediated by reactive oxygen species [47]. By uncoupling the oxidative cycle, FFA promotes the expression of the microsomal monooxygenases CYP4A and CYP2E1, which are in charge of producing ROS [45]. CYP2E1 is mainly expressed in the liver, with the highest expression in hepatocytes. The ER is where CYP2E1 is mostly found, although the mitochondria also express it. A wide range of compounds, including numerous drugs, polyunsaturated fatty acids, ethanol, paracetamol, and the majority of organic solvents, are metabolized by CYP2E1. The expression of CYP2E1 mRNA and protein is regulated by a number of substances, including insulin, acetone, leptin, adiponectin, and cytokines. Because insulin inhibits the cytochrome CYP2E1, peripheral insulin resistance results in increased levels of its expression [48]. According to Chen et al., 2020, [49], OS is linked to the release of pro-inflammatory cytokines, lipid peroxidation, and disruption of phospholipid membranes, all of which cause hepatocellular damage and subsequently affect the course of NAFLD [32]. HSC, the primary cells responsible for the extracellular fibrous matrix deposition that is intimately related to liver fibrosis, are likewise activated by ROS [50].
3.2. Mitochondrial Dysfunction
Fat acid oxidation process imbalances may contribute to an excessive buildup of lipids in the liver. This process is normally proportional to the concentrations of fatty acids released by the adipose tissue following the stimulus provided by glucagon. Oxidation takes place in the three main subcellular organelles of the organism: the mitochondrion and the peroxisomes, which carry out β-oxidation, and the ER, which carries out the ω-oxidation, catalyzed by cytochrome CYP4A. Several cytochrome P450 enzymes that may hydroxylate the omega terminal carbon and, to a lesser degree, the omega 1 position of saturated and unsaturated fatty acids, as well as enzymes involved in the omega hydroxylation of different prostaglandins, are encoded by the CYP4A subfamily [51,52]. Peroxisome proliferator-activated receptor-α (PPARα), a nuclear hormone receptor that controls the oxidation and transport of fatty acids, controls the activity of some of the main enzymes in these three oxidation systems [34]. As soon as it is activated, it forms a heterodimer with the retinoid X receptor (RXR) and binds to peroxisome response elements in the genes responsible for fatty acid oxidation. According to Suhaili et al., 2017 [53], lipotoxicity and increased inward flux of FFA cause lysosomal membrane permeabilization and mitochondrial dysfunction, which in turn trigger hepatocyte apoptosis. Hepatocyte apoptosis causes an immune response that generates a release of proinflammatory cytokines; this leads to further aggravation of liver inflammation [53].
Mitochondrial β-oxidation is implicated primarily in the oxidation of short (<C8), medium (C8–C12), and long (C12–C20) chain fatty acids. As a result of this process, fatty acids gradually shorten into minor units of acetyl-CoA, which can either be condensed to create ketone bodies (oxidizable substrates that serve as an alternative source of energy for extrahepatic tissues) or enter the tricarboxylic acid cycle to further oxidize into water (H2O) and carbon dioxide (CO2). Carnitine palmitoyl-transferase 1a (CPT1a), which is in charge of moving fatty acids from the cytosol to the mitochondrion, the level of carnitine, and the substrate malonyl-CoA, which inhibits CPT1a, are the primary regulators of β-oxidation [54].
Four different dehydrogenases, each particular for the length of the fatty acid chains, catalyze the initial stage of mitochondrial β-oxidation. A single trifunctional mitochondrial protein (MTP), which catalyzes three distinct enzymatic processes (2-enoyl-CoA hydratase, 3-hydroxyacyl-CoA dehydrogenase, and 3-ketoacyl-CoA thiolase), is responsible for the second, third, and fourth stages. Long-chain fatty acids (>C20) undergo a shortening process in peroxisomes before being transported to the mitochondrion, where they carry out the β-oxidation process. The long-chain dicarboxylic acids (highly toxic to the mitochondrion), generated by microsomal ω-oxidation, are metabolized by the peroxisomal β-oxidation system. Although ω-oxidation is the least important metabolization pathway, in conditions of fatty acid overload, such as obesity or diabetes, large amounts of dicarboxylic acids are formed, inducing greater activation of this metabolic pathway [55].
CPT1a expression is 50% lower in NAFLD patients, although long-chain acyl-CoA dehydrogenase (LCAD) and long-chain L-3-hydroxyacyl-CoA dehydrogenase-α (HADHα) are more expressed. Uncoupling protein 2 (UCP2), which regulates the loss of protons along the inner membrane and uncouples the oxidation energy from ATP synthesis, is a significant enzyme involved in the mitochondrial regulation of ROS. NAFLD patients have increased levels of this enzyme [55,56]. Mitochondrial damage and the increase of aberrant mitochondria in the liver generate excessive oxidative stress responsible for advancing the NAFLD course [57].
3.3. Endoplasmic Reticulum Stress
The ER is the organelle in which secretory and membrane proteins achieve correct folding, thanks to the presence of chaperones. The pathogenesis of NASH is influenced by variables including stress (short of nutrition and viral infections), increased production of misfolded proteins, and ER stress, which promotes the activation of a number of transcription factors and kinases [58]. Through the SREBP pathway, the activated transcription factors cause an increase in lipid production, as well as the transcription of proapoptotic genes. The activation of ER kinases, such as inositol-requiring enzyme 1α (IRE1α) and PKR-like ER kinase (PERK), in turn, involves the induction of stress kinases, such as c-Jun N-terminal kinase 1 (JNK1), which may contribute to the induction of apoptotic phenomena [59].
4. Natural Compounds Showing Activity towards NAFLD
Numerous studies have demonstrated the potential of antioxidants from natural sources, including foods and plant species, to prevent or treat NAFLD. Over the years, research has been able to identify alkaloids and flavonoids in addition to polyphenols. The biological effects of the flavonoid, phenolic, alkaloid, and terpene constituents are shown in Figure 3.
Figure 3.
Natural compounds and targets in the progression of the NAFLD. ↑: Increase; ↓: decrease of expression, concentrations, or activity.
4.1. Flavonoids Components and NAFLD
The fundamental structure of flavonoids is composed of 15 carbon atoms (C6-C3-C6). Due to the presence of flavonoids and their derivatives, almost all fruits and vegetables contain antioxidant, anti-inflammatory, and lipid-regulating properties. Flavonoids, flavanols, flavanones, isoflavones, and anthocyanins have shown a protective relationship with NAFLD [60]. According to in vitro and animal studies, these compounds protect against hepatosteatosis by lowering de novo lipogenesis, raising fatty acid oxidation, enhancing insulin resistance, and decreasing the production of inflammatory cytokines [61].
4.1.1. Quercetin
Quercetin (QE) is a frequently consumed dietary flavonoid found in conifer cherry, acacia flowers, mulberry leaves, and other plants. Zhu et al., 2018 [62], have shown that this compound regulates the IRE1a/XBP1 pathway and lipid metabolism in the PI3K/AKT pathway, which, in turn, regulates the formation of very low-density lipoprotein (VLDL) and lipid phagocytosis [62]. Mice fed with an HFD showed higher VLDL levels after administration of 1.5-fold quercetin [42,43]. Furthermore, QE reverses fatty liver disease by lowering ALT, AST, and NAFLD activity scores [63].
Researchers Ying et al. have examined the hepatoprotective effects of QE in NASH gerbils induced by an HFD. The gerbils received HFD for 28 days in order to cause NASH. From day 15 to day 28, each animal received the appropriate drugs each day. The results of the research showed that giving QE orally to hyperlipidemic rats for 14 days at dosages of 30 to 60 mg/kg was extremely successful in reducing blood levels of total cholesterol (TC), TG, low-density lipoprotein cholesterol (LDL-C), ALT, and AST [64]. This action is also accompanied by the lowering of elevated FFA levels, hepatic accumulation of TG and FFA, as well as inflammatory factor levels (reduction of TNF-α and IL-6 via modulation of SIRT1, NF-κB, and iNOS). According to several pieces of research, QE significantly reduces oxidative stress, lipid peroxidation, and ER stress [65,66].
Similar characteristics are shared by isoquercetin (IQ), a quercetin-derived glycoside. In a rat model of NAFLD, isoquercetin demonstrated effective downregulation of lipid accumulation, inflammation, and oxidative stress, as well as SOD and lipid levels [67]. Porras et al., 2017 [68], demonstrated that quercetin may correct the imbalance in the gut flora and prevent endotoxemia-mediated toll-like receptor TLR4 signal transduction, decreasing the inflammatory response.
4.1.2. Anthocyanins
Anthocyanins (ACNs) are a class of flavonoids found in dark red, purple, or blue plants, such as black goji berries, mulberries, and grapes. ACNs have been found to reduce body weight and liver fat content and have beneficial antioxidants and anti-inflammatory properties [69,70,71]. Reduced hepatic fat content was assumed to be caused by enhanced PPARα activation, causing lipolysis and decreased lipogenesis [72,73,74].
One research validated enhanced AMPK pathway activity in vivo [75], while several studies using experimental NAFLD models demonstrated improved hepatic antioxidant activity (decrease in ROS levels and increased activity of SOD) following exposure to ACNs [76,77,78].
In the study by Zhang et al., 2015 [70], patients in the anthocyanin group had significantly lower ALT levels than those in the placebo group. They also had significantly lower scores for liver fibrosis and a 30.2% reduction in plasma myeloperoxidase (MPO), a tissue-damaging enzyme [70].
Treatment of HFD-induced hepatocytes with palmitic acid (PA), a polymeric derivative of anthocyanin, resulted in a reduction in white adipose tissue (WAT) weight and hepatic adipocyte size.
According to research by Habanova et al., 2016 [79], frequent consumption of bilberries (Vaccinium myrtillus), which are anthocyanin-rich fruits, decreases LDL-C/TG while raising HDL-C levels, which may help lower the risk of cardiovascular diseases [79].
Furthermore, the mRNA levels of TNF-α, IL-1, IL-6, IL-10, and MCP-1 were markedly reduced, exercising an anti-inflammatory impact in NAFLD [80,81]. Instead, the serum levels of IL-10 and IL-22 were increased [82]. Inhibition of TLR4 protein production and activation of the MAPK signaling pathway contributes to the anti-inflammatory effect [83,84].
4.1.3. Kaempferol
Kaempferol is a flavanol found in plants, such as tomatoes, sea buckthorn, ginkgo biloba, and grapefruit, and has shown promise in preventing NAFLD through a variety of hypolipidemic pathways.
In carbon tetrachloride (CCl4)-induced NAFLD rats, pretreatment with kaempferol restored the activity of hepatic enzymes and also decreased liver damage in rats given acetaminophen through increasing silent mating type information regulation 2 homolog 1 (SIRT1) activity [85]. In particular, mice treatment with kaempferol had normalized serum ALT, AST, hepatic GSH, SOD, CAT, and GPx activity [85].
Wei et al., 2014 [86], demonstrated how kaempferol interacts with specificity protein 1 (Sp1) to increase levels of CPT-1a, which promotes fatty acid oxidation and generates lipid-lowering effects.
SREBP-1c, FAS, SCD-1 levels, and the production of adipogenesis-related proteins (PPAR and C/EBP) in a model of oleic acid-induced steatosis were reduced following kaempferol therapy.
4.1.4. Citrus spp. Flavonoids
Citrus fruits, like oranges, lemons, grapefruits, and bergamots, contain phenolic compounds, terpenes, and flavonoids and their derivatives [87]. There are two types of citrus flavanones: glycosides and aglycones. A flavanone glycoside is a hesperidin. According to Wang et al., 2011 [88], the flavonoid hesperidin has been shown to be protective against NAFLD by reducing hepatic steatosis and lipid droplet accumulation, decreasing adipose tissue and liver weights, and lowering serum total cholesterol. In fact, it reduced the expression of proteins involved in lipid metabolism, such as retinol-binding protein-4 (RBP4), heart fatty acid-binding protein (H-FABP), and cutaneous fatty acid-binding protein (C-FABP), in the liver and adipose tissue, as one of its protective mechanisms [88]. However, changes in low-density lipoprotein cholesterol LDL-C and triglycerides TG have not been statistically significant [64].
Hesperidin has anti-inflammatory and antioxidant effects both in vivo and in vitro, and it also has a specific therapeutic impact on NAFLD. In contrast to the HFD group, the hesperidin-treated group had lower levels of IL-6, TNF-α, and NF-kB, which may be attributed to the antioxidant function of hesperidin, according to Kumar et al., 2023 [89]. Hesperidin can enhance the antioxidant response through ERK/Nrf2-mediated HO-1 production and minimize hydrogen peroxide-induced damage by direct radical scavenging [90]. It is also capable of reducing ROS levels and hepatotoxicity brought on by OA via activating the PI3K/AKT-Nrf2 pathway in HepG2 cells [91]. Additionally, hesperidin can reduce fibrosis, inflammation, and steatosis in the liver, potentially through controlling the NF-kB pathway [92]. Further control of inflammation is also mediated by its ability to activate the SIRT1–AMPK signaling pathway [93].
The two most significant flavanones among the aglycone forms are naringenin and hesperetin. Hesperetin was shown to mitigate oleic acid-induced hepatotoxicity and oxidative stress in vitro, as well as plasma lipid profile, comprising total cholesterol (TC), LDL-C, and TG, in a rat model of HFD-induced non-alcoholic fatty liver disease [91]. Hesperetin has shown its ability to lessen oxidative damage and the overproduction of ROS in the liver caused by fatty acids. Additionally, by lowering ROS overproduction, hesperetin demonstrated prevention of fatty acid-induced NF-κB activation and consequent inflammation [91]. In order to treat non-alcoholic fatty liver disease (NAFLD), anti-macrophage scavenger receptor-1 (anti-MSR1) antibodies, or MSR1 inhibitors, may be a useful therapeutic strategy that needs more clinical research.
Another flavanone that is mostly present in grapefruit is called naringenin. Naringenin inhibits non-alcoholic fatty liver disease (NAFLD) by increasing hepatic fatty acid oxidation, inhibiting lipogenesis in the liver and muscle, lowering hepatic cholesterol and cholesterol ester production, and enhancing insulin sensitivity and glucose tolerance [94]. Naringenin also reduced visceral and subcutaneous obesity, insulin resistance, fasting hyperglycemia, and plasma total cholesterol in ovariectomized mice with metabolic disorders [95]. Reduced caloric intake led to lower adiposity, while induction of hepatic genes involved in fatty acid oxidation, such as peroxisome proliferator-activated receptor gamma coactivator-1 and CPT1a, and decreased expression of the lipogenesis gene, Srebf1c, inhibited the accumulation of triglycerides in the liver [96]. Naringenin has the potential to exercise its hepatoprotective impact by inhibiting oxidative stress, which in turn may lead to the production of pro-inflammatory genes, such as TNF-α, interleukin-6, interleukin-1β, and inducible nitric oxide synthase, as well as NF-κB [97]. One of the conditions for liver fibrosis is macrophage infiltration, which is avoided by inhibiting inflammation. Additionally, naringenin inhibits the production of matrix metalloproteinases that are triggered by the fibrosis process [97]. The processes behind naringin’s lipid-lowering and insulin-sensitizing properties have been identified for naringin, naringenin’s glycoside form [98]. Glycosylated flavonoids are more readily absorbed as compared to their aglycon counterparts [99]. Although the majority of research on flavonoids have focused on the aglycon forms, less doses of the compounds may be required to achieve the same effects if glycosylated forms of flavonoids are used due to their higher absorption rates.
4.1.5. Baicalein
The flavone 5,6,7-trihydroxyflavone, or baicalein, was first identified as a form of flavonoid from the roots of Scutellaria lateriflora and Scutellaria baicalensis. It has also been seen in Thyme and Oroxylum indicum [100]. Rats given a high-fat diet (HFD) for 16 weeks had a substantial reduction in body weight compared to the control group after receiving baicalin. By increasing the activation of AMPK and ACC (acetyl CoA-carboxylase) through their phosphorylation and inhibiting relevant genes, SREBP-1c (sterol response element binding protein-1c) and FAS (fatty acid synthase), important enzymes for cholesterol and fatty acid synthesis, respectively, baicalin significantly reduced the elevated levels of serum cholesterol, FFA, and TNF-α. Baicalin administration for 24 h in cultured HepG2 cells with high glucose concentration decreased the fat accumulation by AMPK activation in an in vitro study; this effect was similar to that of the positive control, AICAR, an AMP analog and well-known AMPK activator. Baicalin may, thus, be helpful in treating diseases linked to obesity [101]. Xi and colleagues found that baicalin (100, 200, and 400 mg/kg/day, orally) increased the phosphorylation of AMPKα, ACC, and calcium/calmodulin (CaM)-dependent protein kinase β (CaMKKβ) in the liver while decreasing the body and liver weight, serum levels of TG, total cholesterol (TC), LDL, ALT, AST, and liver steatosis in mice fed a high-fat diet [102]. In a related investigation, Chen et al. discovered that S. baicalensis root extract (125, 250, and 500 mg/kg/day, orally) reduced liver weight, serum TG, TC, FFAs, and glucose levels, as well as the expression levels of SREBP-1c, FAS, ACC, and SCD. Additionally, it caused AMPK to be phosphorylated in the liver tissue of KK-Ay spontaneous type 2 diabetic mice. Orotic acid (OA) has been shown to promote NALFD in mice by activating the SREBP-1c pathways and blocking AMPK activation. Additionally, they assessed the impact of oral baicalin (25 and 50 mg/kg/day) on OA-induced NALFD in KK-Ay mice. In OA-induced NALFD KK-Ay mice, baicalin significantly reduced the levels of TG, TC, and FFAs in the blood, as well as the hepatic lipid content, hepatic injuries, and hepatic mRNA levels of SREBP-1c, FAS, ACC, and SCD. However, it also increased the phosphorylation of AMPK in the liver. Furthermore, in HepG2 cells exposed to sodium oleate-induced lipid accumulation, baicalin and baicalein (10 and 100 μM) significantly increased AMPK phosphorylation while inhibiting the expression of the SREBP-1c gene [103].
4.1.6. Troxerutin
A semi-synthetic bioflavonoid produced from rutin called troxerutin (TRX) has been shown to have a number of pharmacological actions, including anti-inflammatory, antihyperlipidemic, and antioxidant properties. Malinska et al., 2019 [104], observed that TRX (150 mg/kg) prevented hyperinsulinemia, liver cholesterol accumulation, and non-fasting blood glucose in an animal model of metabolic syndrome that was genetic hypertriglyceridemic (HHTg). This effect was attributed to the effects of TRX on genes involved in lipid oxidation and cholesterol synthesis. The researchers also discovered that in the hepatic tissue of HHTg rats, TRX raised the expression of PPARα, a crucial regulator of lipid and glucose metabolism, and decreased the expression of 3-hydroxy-3-methylglutaryl-CoA reductase (HMGCR), implicated in cholesterol production. In addition, TRX markedly decreased lipogenic enzymes, including stearoyl-CoA desaturase (SCD1), which may be connected to the liver’s increased CYP1A1 (cytochrome P450, family 1, subfamily A, polypeptide 1) activity [104]. Geetha et al., 2014 [105], used mice given HFFD for 45 days to examine the antioxidant properties of TRX. By decreasing the amount of lipid present in heart tissue and raising the activity of both enzymatic and nonenzymatic antioxidants, treatment of TRX (150 mg/kg) has been shown to considerably improve oxidative stress and whole-body insulin sensitivity. Furthermore, in the cardiac tissue, TRX reduced the expression of genes involved in fatty acid transport (FATP-1) and fatty acid synthesis (SREBP-1c) and increased the expression of genes engaged in fatty acid oxidation (PPAR-α, PGC-1 α, and CPT-1β) [105]. The anti-obesity effects of oral TRX (150 mg/kg) in HFD mice were investigated by Zhang et al., 2016, over 20 weeks. The amount of epididymal adipose tissue was found to be decreased, blood adiponectin levels were raised, and obesity was shown to be enhanced by TRX. Additionally, the researchers demonstrated that TRX decreased oxidative stress by lowering the levels of 4-hydroxynonenal (4-HNE), reducing ROS production, and raising GSH levels in the liver tissue; this was achieved by regulating the nuclear translocation of NF-κB p65 [106].
4.2. Phenolic Components and NAFLD
4.2.1. Resveratrol
Among foods that contain the polyphenol resveratrol (RSV) are thuja, grapes, and peanuts [107]. In a randomized controlled trial, substantial improvement in steatosis, attenuation of inflammatory markers, and improvement in hepatocyte apoptosis were reported after administering 500 mg orally daily of RSV for 12 weeks to 50 NAFLD patients [108]. Silva et al., 2022 [109], using a mouse model of NAFLD, found that an HFD combined with RSV therapy significantly reduced the amount of hepatic lipid droplets [109]; by altering epigenetic processes, RSV also prevented fatty liver disease. In both in vitro and in vivo models of NAFLD, resveratrol led to the upregulation of Nrf2 at the mRNA and protein levels [44]. It reduced Nrf2 promoter methylation in high glucose (HG)-treated HepG2 cells and HFD-induced animals in vivo; this, in turn, led to the reduction of expression of adipogenesis-related genes FAS and SREBP-1c. In addition, a decrease in TG levels was observed. Furthermore, HO-1, NQO1, CAT, and SOD were among the Nrf2 targets whose expression was greatly upregulated by resveratrol treatment [44]. The antioxidant enzymes T-SOD and GPx were more active after RSV feeding, which dramatically reduced the ROS levels in the body of HFD mice [110]. Studies have shown that RSV reduces the liver’s production of proinflammatory mediators, such as IL-1, IL-6, and TNFα [111]. Additionally, stimulation of TNF-β production by RSV led to anti-inflammatory effects on a human lung epithelial cell line [112]. It is also important to note that RSV reduces NF-kB inflammation by stimulating the AMPK-SIRT1 pathway [113].
4.2.2. Curcumin
Curcumin is a naturally occurring phenolic pigment that is extracted from Curcuma longa rhizomes and has been shown to be successful in the treatment of NAFLD. According to a recent study, curcumin greatly improved metabolic function by altering the Nrf2/FXR/LXR pathway, reversing the expression of CYP3A and CYP7A in the fatty liver [114]. In a rat model of obesity and glucose intolerance caused by an HFD, curcumin also has a preventative effect. It restored histological abnormalities, including fibrosis and the accumulation of CD4+ cells in the liver, while delaying the onset of NAFLD [115]. Additionally, it has been shown that curcumin increased blood levels of high-density lipoprotein cholesterol (HDL-C) and lipocalin while lowering serum leptin levels in a randomized, double-blind trial including 65 patients with NAFLD [116]. Curcumin treatment significantly reduced lipid accumulation in the cell model, inhibited TG levels, and was mainly linked to the downregulation of adipogenic genes, such as ACC, FAS, SREBP-1c, CCAAT/enhancer binding protein (C/EBP), PPAR, and stearoyl CoA desaturase (SCD). Curcumin also markedly reduced increased ROS levels in model cells and, by activating the AMPK/mTOR signaling pathway, was able to arrest the progression of NAFLD disease [117].
4.3. Alkaloid Components and NAFLD
4.3.1. Berberine
Berberine (BBR) is a quaternary alkaloid that is abundantly present in plants, including Phellodendron and Chelidonium. According to recent research [118], BBR has the potential to cure liver ailments. In an OA-induced HepG2 cell model, berberine may control the expression of genes associated with adipogenesis by upregulating FXR mRNA and downregulating SREBP-1C and FAS mRNA, which have a specific regulatory function in NAFLD’s lipid metabolism [119]. Previous research has shown that BBR can reduce oxidative stress, inflammation, and fatty liver disease [120]. The effects of BBR on the liver’s SOD, GSH, and malondialdehyde (MDA) levels may be connected to the activation of the Nrf2/ARE signaling pathway [121]. Additionally, BBR decreases inflammation, TNF-, IL-6, and IL-1 levels in liver tissues and inhibits NF-kB translocation by downregulating TLR4, MyD88, and NF-kB expression [122]. Furthermore, investigations demonstrated that, after oral administration, BBR preferentially accumulates in the liver and elevates the expression of genes associated with energy metabolism, including MTTP, CPT-1a, and GCK [123]. The levels of TC, TG, ALT, and AST were considerably lower in BBR-treated HFD rats than in HFD rats and polyenylphosphatidylcholine (PPC)-treated rats. Ren et al., 2021, found that BBR intervention effectively decreased liver lipid content and reduced liver damage in MCD rats [120].
4.3.2. Betaine
The quaternary alkaloid betaine, extracted from sugar beets, serves as an osmotic agent to preserve the integrity of cell membranes and is a crucial part of the methionine cycle in the inner mitochondrial matrix. Betaine may reduce fatty liver and inflammation in response to NAFLD [124,125]. Blood levels of ALT, AST, TC, TG, and FFA were significantly lowered in NAFLD rats induced by a high-fat diet treated with betaine. After betaine therapy, histological changes were also visible; scores for steatosis, inflammation, and necrosis were significantly decreased [125]. Betaine supplementation for 8 weeks reduced fatty liver disease by 25%, and liver biopsy results after 12 months of therapy demonstrated that betaine prevented the deterioration of fatty liver disease in 55 NASH patients. These findings were reported in a randomized controlled trial of 191 individuals with NASH [126]. Inhibition of the inflammatory reaction is related to betaine’s ability to reduce the expression of NF-κB and TLR4 [125]. Betaine supplementation also affects markers of oxidative stress by increasing levels of SOD and CAT and decreasing ROS and MDA synthesis [124].
4.4. Terpenes Components and NAFLD
4.4.1. Celastrol
Celastrol is a pentacyclic triterpenoid derived from the Celastraceae family of plants. It has been proven to have anti-lipidogenic properties. These properties are mainly due to celastrol’s capacity to improve lipid metabolism, increase antioxidant defenses by inhibiting NF-kB activity, and increase leptin sensitivity [127]. According to Zhang et al., 2017 [127], celastrol lessens NAFLD by reducing lipid production and enhancing the anti-oxidative and anti-inflammatory state [127]. Additionally, SIRT1 plays a crucial part in celastrol’s ability to reverse HFD-induced liver metabolic damage. Celastrol can decrease the oxidative stress response and liver cell death in type 2 diabetes mellitus mice with NAFLD by modulating the Nrf2/HO-1 pathway, according to a recent study [128]. In accordance with findings from several pieces of research, celastrol improves the biological performance of mitochondria by increasing intracellular ATP levels, mitochondrial membrane potential, and fatty acid oxidation. Additionally, celastrol raises intracellular ATP levels, preserves the potential of the mitochondrial membrane, boosts citrate synthase activity, and lowers the amount of superoxide in the mitochondria, all of which improve mitochondrial function [129,130].
4.4.2. Boswellic Acid
Boswellic acid (BA), an apentacyclic triterpenoid, is the major active ingredient in Boswellia. Boswellic acid administration increases insulin sensitivity, liver enzyme activity, hepatic index, and hepatic iNOS expression in the HFD-fed rat model. It reduces the levels of TNF and IL-6 in blood and the expression of iNOS in CPT1 and UCP1 in liver tissue. Boswellic acid also enhances the expression of WAT carnitine to have antisteatosis effects [131]. Zaitone et al., 2015 [131] assessed the protective effects of BAs in a model of diet-induced NAFLD in rodents. Therefore, rats were fed an HFD for 3 months, and inflammation and steatosis were confirmed by deviation of the key liver biomarkers on measurement. Hepatic steatosis and inflammation (NAFLD) were induced in rats by feeding them a high-fat diet (HFD) for three months, which was confirmed by deviation of the key liver biomarkers on measurement. When compared to the control HFD group, rats treated with BA (125 or 250 mg/kg) or pioglitazone had enhanced insulin sensitivity and had lower levels of blood TNF- and IL-6, liver enzyme activity (ALT and AST), liver index, and hepatic iNOS expression. BA also reduced hypercholesterolemia brought on by the HFD. Furthermore, at the cellular level, BA (250 mg/kg) ameliorated the expression of UCP1 and CPT1 in WATs.
The following Table 1 summarizes the biological effects of the flavonoid, phenolic, alkaloid, and terpene constituents.

Table 1.
Biological effects of flavonoid, phenolic, alkaloid, and terpene components.
4.5. Natural Products in NASH Treatment
4.5.1. Tea (Camellia sinensis)
Tea is made using the Camellia sinensis germ and leaf as the basic material; this is accomplished using straightforward mashing, roasting, and exsiccating procedures. Contrary to what may happen during the fermenting process needed to produce black tea, natural antioxidants are rarely altered in these procedures.
The primary polyphenols found in green tea are catechins (flavan-3-ols). Epicatechin (EC), epicatechin gallate (ECG), epigallocatechin (EGC), and epigallocatechin gallate (EGCG) are the four major catechins found in tea. These components make green tea one of the most important food sources of natural antioxidants, and epigallocatechin gallate (EGCG) contributes significantly to its antioxidant action [136]. Bruno et al., 2008 [137], used the ob/ob mouse model of obesity-triggered NAFLD to offer the first evidence of the preventive function of green tea extract (GTE) against liver injury and steatosis.
GTE efficacy has also been observed in several animal models of NASH, including nitrite injection, rats fed an HFD, choline-deficient diets, and SREBP-1c overexpressing models [138,139]. GTE and its constituent catechins have been shown to protect the liver from damage, steatosis, and the development of NASH. In a NASH model of ob/ob mice, adding green tea extract to the diet for six weeks had inhibitory effects on hepatic steatosis, NASH, and impaired hepatic function that may be related to decreased hepatic NADPH activity, expression of myeloperoxidase (MPO), generation of ROS, and reduced lipid peroxidation [140].
Adding 1–2% of GTE to the diet has been shown to help control body weight without significantly changing food intake. Furthermore, GTE-treated experimental animals demonstrated reduced levels of hepatic lipid accumulation and plasma levels of the injury-related markers AST and ALT. Specifically, the administration of fermented green tea extract (100 to 300 mg/kg body weight daily for six weeks) decreased the levels of AST and ALP and improved fibrosis and liver steatosis in a NASH model where Wistar male rats were given HFD deficient in choline and intraperitoneal nitrite injections. This improvement may have been brought on by the prevention of lipid peroxidation and ROS production [141].
4.5.2. Curcuma (Curcuma longa)
Curcuma longa (CL) is a common colorful spice of which the powdered rhizome is used worldwide. Human diseases, including metabolic syndrome and inflammatory diseases, can be prevented with its help. Preclinical and clinical investigations conducted by different research groups have amply demonstrated the beneficial effects of CL extract and its active component, curcumin, in the control of obesity and type 2 diabetes [132,142,143]. A recent study on the preventive effects of CL rhizome powder on fatty liver disease induced by an HFD found that supplementing the diet with turmeric (5% of the diet) for six weeks significantly lowered the lipids levels (TC, TG, LDL, HDL) and enzymes (ALT, ASP, ALP) indicating liver damage and serum dyslipidemia [144]. Another study found that CL extract improves antioxidant status and reduces hepatic lipid peroxidation. In a mouse model of HFD-induced obesity and glucose intolerance receiving CL supplementation, liver histopathological analysis indicated less steatosis and inflammatory changes. Curcumin corrected histological abnormalities, including fibrosis and the accumulation of CD4+ cells in the liver [115]. Curcumin treatment was reported to drastically lower cytochrome c, caspase 3, and caspase 8 expression in rat models of NASH, hence lowering cell death [145]; this may be connected to curcumin’s downregulation of NF-kB’s P65 subunit [133]. Additionally, curcumin has the ability to activate AMPK, which suppresses the expression of SREBP-1 and fatty acid synthase (FAS), thus inhibiting the formation of fat. Clinical studies have demonstrated that taking supplements containing nano-curcumin reduces lipid levels, inflammation, liver enzymes, and the degree of fatty liver in people with NAFLD, which may be connected to curcumin’s role in lipid metabolism [134].
4.5.3. Loquat (Eriobotrya japonica)
A fruit tree known as Eriobotrya japonica has been employed in traditional medicine for its therapeutic effects [146]. Using animals fed a high-fat diet, EJ extracts have been shown to control adipogenesis and body weight gain [147], reduce hyperglycemia in type II diabetic rats and mice [148], and improve hyperlipidemia and insulin resistance. Furthermore, the ameliorative ability of EJ seed extract (70% ethanol) against experimentally produced NASH was examined. Compared with rats fed the MCD diet alone, the plasma levels of AST and ALT were considerably lower in rats fed the seed extract of MCD+EJ. Liver antioxidant enzymes in the group receiving EJ supplements showed substantial improvement. Furthermore, in rats receiving EJ supplements, there was less formation of lipid droplets in the liver and subsequent pathological changes. Compared to rats fed the MCD diet, EJ-supplemented animals showed substantially lower expression of oxidative stress markers (8-hydroxy-2-deoxyguanosine and 4-hydroxy-2-nonenal(4-HNE)) and fibrosis markers (TGF-α and collagen).
4.5.4. Ginkgo (Ginkgo biloba)
G. biloba, often known as the maidenhair tree or ginkgo, is a kind of gymnosperm tree that is native to China. High-fat diet-induced dyslipidemia and insulin resistance were both improved by its extract [149,150]. Through in vitro and in vivo experimental evaluations, Wang et al., 2012 [151] recently reported its benefits in regulating NASH.
A dosage of 0.25%, w/w in rats with experimentally produced NASH, could dramatically lower triglyceride and liver fatty acid levels. Notably, after treatment, the expression and overall activity level of rate-limiting fatty acid oxidation enzyme and carnitine palmitoyltransferase-1a (CPT-1a) were reduced. The combination of Ginko biloba and its active components, quercetin, kaempferol, and isorhamnetin, dramatically reduced the accumulation of cellular triglyceride content in HepG2 cells and increased the expression and activity of CPT-1a overall [151]. Therefore, the regulation of CPT-1a caused by the extract may serve as a potential underlying mechanism for NASH prevention.
4.5.5. Olive Tree (Olea europaea)
The fruit tree known as O. europaea is reputed to have originated in Asia Minor and Siria since it grows there naturally and has done so since antiquity.
A diet rich in olive oil has been shown to have a positive impact on diseases, including metabolic syndrome (MetS), obesity, and diabetes mellitus [152,153]. Dietary supplementation of 3% olive leaf extract (OLE) for eight weeks counteracted the negative alterations of rats’ cardiovascular, hepatic, and metabolic systems caused by a diet rich in fats and carbohydrates [154].
Notably, lipid accumulation, inflammatory cell infiltration, and fibrosis were lower in OLE-fed animals. Although there have been reports of the protective effects of OLE against NASH, its underlying mechanisms have not been thoroughly investigated [155]. Omagari et al., 2010) [156], reported the beneficial effects of OLE (1000 or 2000 mg/kg) on the liver of NASH mice, improving liver histological features and downregulating thioredoxin-1 (TRX-1) and 4-HNE expressions [156].
FAS, malic enzyme, CPT-1, and phosphatidic acid phosphohydrolase activity levels in the liver were not substantially changed. Therefore, the positive effects of OLE have been related to its strong antioxidant capacity. In fact, it is able to reduce ROS levels by activating Nrf2 but also the expression of oxidative stress markers, such as SOD, CAT, GPx, and inflammatory markers, such as IL-8, IL-8, TNFα [156].
4.5.6. Pomegranate (Punica granatum)
The pomegranate (Punica granatum) is a fruit-bearing deciduous shrub in the family Punicaceae. It grows in the Mediterranean area, the Himalayas, Southeast Asia, California, and Arizona. Punica granatum trees are cultivated for their use in various medical systems [157]. Strong antioxidants exist in pomegranates. The antiatherogenic, antihypertensive, and anti-inflammatory characteristics of this fruit are due to its abundance in flavonoids, anthocyanins, punicic acid, ellagitannins, alkaloids, fructose, sucrose, glucose, and other components [158]. Numerous bioactive substances, including punicalagin, ellagic acid, and gallic acid, may be found in pomegranates [159,160]. PGF has a well-established positive effect on the regulation of experimental hyperlipidemia, insulin resistance, and diabetes [161,162]. It is an excellent candidate for the treatment of insulin resistance-induced NASH due to its strong PPAR/activating activity [163]. Reductions in liver weight and liver lipid content were observed in ZDF rats after receiving PGF treatment (500 mg/kg for 6 weeks). Hepatic gene expression of PPAR, CPT-1, acyl-CoA oxidase (ACO), and decreased SCD-1 were all increased concomitantly with these effects. By promoting hepatic expression of genes involved in fatty acid oxidation, the authors concluded that PGF improves diabetes and obesity-related fatty liver, at least in part [164].
4.5.7. Milk Thistle (Silybum marianum)
Milk thistle (Silybum marianum) is a biennial or annual tree that originated in the Mediterranean area and is now found all over the world. It is a member of the Compositae family. In addition to raising CAT and GSH levels, silymarin is a potent antioxidant that may scavenge free lipid peroxyl radicals in hepatocytes [165,166]. In addition to improving poly-(ADP-ribose)-polymerase (PARP) function and protecting the cell from oxidative damage seen in NAFLD, silymarin’s antioxidative properties may restore NAD+ homeostasis, SIRT1 activity, and the AMPK pathway [167]. The PPARγ, acetyl-CoA carboxylase (ACC), and fatty acid synthase (FAS) are all downregulated by this plant, which in turn lowers hepatic de novo lipogenesis [168]. Additionally, silymarin may stimulate the IRS-1/PI3K/Akt pathway, which helps lessen steatosis and insulin resistance associated with NAFLD [165]. Through activation of the farnesyl X receptor (FXR), which is correlated with the reduction of NF-kB transactivity, silymarin is also capable of reducing hepatic inflammation [169]. The main active component of silymarin is silibinin, sometimes referred to as silybin. In a recent study, Haddad et al., 2011 [170], investigated the therapeutic effects of silibinin in a rat model of experimental NASH. Lipid peroxidation, plasma insulin, and TNF were all reduced with silibinin therapy, which also relieved fatty liver and inflammation. In addition to restoring liver relative weight and GSH levels, silibinin also reduced the production of free radicals. The efficacy of silybin–phospholipid complex (SILIPHOS) was evaluated in research by Serviddio et al., 2010 [171], on liver redox balance and mitochondrial activity in a dietary model of NASH. Treatment with SILIPHOS reduced GSH depletion and mitochondrial hydrogen peroxide formation, preserved mitochondrial bioenergetics, and stopped mitochondrial proton leakage and ATP reduction. Furthermore, it prevented the liver from producing protein adducts consisting of 4-HNE and MDA. It has been argued that SILIPHOS may act by mediating changes in the fatty acid content of the mitochondrial membrane.
4.5.8. Coffea spp.
Numerous studies have been conducted on coffee’s bioactive ingredients. The in vitro study demonstrated that caffeic acid might reduce ER stress and improve hepatic steatosis by boosting autophagy in AML12 cells [172]. In HepG2 cells treated with oleate, coffee might also influence hepatocyte steatosis via activating SIRT3 and AMPK [173]. Coffee intake has been linked to a reduction in the risk of developing NAFLD, according to an in vivo investigation [174]. By increasing the expression of PPAR, the coffee pulp aqueous extract reduced hepatic steatosis, insulin resistance, and oxidative stress in Wistar rats; however, simvastatin treatment in combination with the extract might also inhibit the expression of PPAR-α and SREBP-1c [175]. Additionally, by enhancing liver fat oxidation, intestinal cholesterol efflux, energy metabolism, and gut permeability, coffee administration might reduce hepatic fat accumulation and metabolic disturbance in NAFLD mice [176]. Additionally, coffee’s trigonelline, which increases the expression of Bcl-2 protein and decreases the expression of Bax protein in the liver, can reduce NAFLD in SD rats [177]. By stimulating the SIRT3–AMPK–ACC and STAT3 signal transducer and activator of transcription 3 (STAT3) pathways, caffeine, another significant bioactive component in coffee, might inhibit hepatic steatosis [173,178]. By boosting the expression of tight junction proteins and reducing the expression of TLR4, decaffeinated coffee may also restore normal function of the intestinal barrier and gut permeability [179]. Coffee’s hepatoprotective benefits may be more closely tied to its polyphenols than its caffeine. Simply put, there is evidence that polyphenols have preventative qualities that can appear through a number of channels, such as antioxidant, anti-inflammatory, and antifibrotic pathways, change in energy metabolism, decreased insulin resistance, and milder forms of diabetes [180]. According to Vitaglione et al., 2010 [181], coffee polyphenol intake in rats given a high-fat diet had the same protective effects on liver damage as whole coffee intake; specifically, coffee polyphenol intake mirrored the effects of whole coffee in reducing oxidative stress, insulin resistance, and fibrosis [181]. A further investigation into the effects of coffee polyphenols on diet-induced obesity in rats showed that the polyphenolic fraction upregulates the expression of cell chaperones, such as DJ-1, which controls autophagy, and GRP-78, which is important in proper protein folding [182,183]. Pietrocola et al., 2014, intriguingly expanded on these results, showing that coffee polyphenols increase autophagic flow in the heart, liver, and muscle [183]. Recent research has shown that the primary coffee polyphenol, chlorogenic acid, inhibits the activation of hepatic stellate cells in vitro [184], offering a strong molecular justification for the use of these substances as antifibrogenic medications in clinical settings.
4.5.9. Red Rice (Oryza sativa)
Red yeast rice derives from the fermentation of Oryza sativa by the yeast (Monascus purpureus). It has been a part of Chinese folk medicine and diet since 800 BC, when it was largely employed as a preservative, coloring agent, and flavoring in food preparations, such as cheese, pork, fish, rice, wine, and vegetables. The pigments that are created during fermentation give rice its distinctive red color and give it the ability to function as a coloring agent. It was shown that rice bran had antioxidant and hypolipidemic properties in an in vivo investigation on male Wistar rats given an HFD and treated with an aqueous extract of the grain. In addition, compared to the group following the hyperlipidic diet alone, the antioxidant parameters (catalase and carbonylated proteins) were considerably enhanced in the group treated with the extract. The group treated with the extract had, compared to HFD rats, lower TC and TG levels, the activity of HMG-CoA reductase was decreased, and the amount of cholesterol and lipids in the feces increased [185,186]. The hypoglycemic effects of O. sativa have, on the other hand, also been mentioned in other publications [187,188].
The primary cause of the hypocholesterolemic effects of fermented red rice is the presence of monacolin K, a synthetic chemical compound with chemical properties similar to lovastatin that works by blocking the major enzyme involved in the metabolism of cholesterol (HMG-CoA reductase). But in addition to monoacolin K, red rice contains other compounds, like sterols, isoflavones, and monounsaturated fatty acids, that not only control cholesterol levels but are also thought to have a preventative effect on conditions like diabetes, cancer, osteoporosis, and Alzheimer’s. Given that a serving of red rice contains 1.02–7.2 mg of monacolin K, which is substantially less than 20–40 mg of lovastatin needed to provide the same pharmacological effect, it is clear that the aforementioned components work together synergistically [189]. Nine distinct varieties of fermented red rice were examined in a chemical–analytical analysis, and it was shown that the levels of monacolin K are significantly varied. Seven of the analyzed samples furthermore included citrinin, a mycotoxin that is produced by a broad range of fungi and which has been shown to be nephrotoxic in all studied animal species and to be cytotoxic at high doses in cultured human cells. As a result, citrinin concentrations in supplements must be kept to a minimum. Red yeast rice was beneficial and well tolerated by a variety of individuals, according to several controlled and double-blind clinical investigations. However, some individuals who had received kidney transplants and were on cyclosporine have also been documented with incidences of muscular myopathy and rhabdomyolysis. Due to these findings, the FDA has deemed red yeast rice dangerous for a particular group of patients [190,191]. The Panel on Dietetic Products, Nutrition, and Allergies (NDA) determined that the recommended daily dose of monacolin K for a man weighing approximately 70 kg corresponds to 10 mg. This determination was made after establishing a clear cause-and-effect relationship between the consumption of monacolin K from red yeast rice preparations and the maintenance of normal blood LDL cholesterol levels [192].
4.5.10. Artichoke (Cynara scolymus)
Artichoke (Cynara scolymus) is a perennial herbaceous plant that belongs to the Asteraceae family, cultivated in Italy and other countries for alimentary and curative use. Artichokes mostly consist of carbohydrates, with inulin and fibers standing out among them, in addition to water content. Sodium, potassium, phosphorus, and calcium are the primary minerals. B1, B3, and trace levels of vitamin C are the most prevalent vitamins. Artichoke displays a diverse array of secondary metabolites, which can be readily identified. These include flavonoids (particularly rutin), sesquiterpene lactones (including cynaropicrin, dehydro-cynaropicrin, grosseimin, and cinaratriol), caffeic acid derivatives (including acid chlorogenic acid, neochlorogenic acid, cryptochlorogenic acid, and cynarine), flavonoids (particularly acid chlorogenic acid), and flavonoids. This plant’s phenols’ most well-known pharmacological effects are liver protection, anti-cancer, antioxidant, antibacterial, choleretic, and diuretic.
Artichoke leaf extracts’ antioxidant effects on rat hepatocytes were shown in vitro tests [193]. The bioavailability of polyphenols in vivo, however, has been shown to be limited (does not surpass blood values of 10 M), which may be due to the glucuronidation or sulphation of the hydroxyl groups in the chemical structures of these compounds. However, dietary polyphenols are still available in high quantities in the gastrointestinal lumen despite their decreased bioavailability. Here, they may play a significant role in preventing oxidative damage and postponing the development of colon, intestine, or rectal cancer [194]. This antioxidant effect has been observed at a general level by Magielse J. and collaborators, 2014 [195], through in vivo experiments. The latter examined the effects of an aqueous extract of artichoke leaves on various biomarkers of oxidative stress and on antioxidant defenses, including MDA levels in the blood, 8-deoxyguanosine levels in the urine, and erythrocyte levels of coenzyme Q9 (CoQ9) and reduced GSH, which are both indicators of antioxidant defense systems. In this experiment, two different doses of artichoke extract—0.2 g/kg and 1 g/kg of body weight—were examined and given orally to rats exposed to STZ-induced oxidative stress (diabetic rats) for three weeks. Only at a dose of 0.2 g/kg did the artichoke leaf extract elicit a decrease in the levels of MDA, 8-deoxyguanosine, CoQ9, or GSH, indicating the plant’s antioxidant effects even in vivo. But the administration of the greater dosage (1g/kg) led to a potentially dangerous level of MDA [195,196]. In fact, current research has shown that the administration of large doses of antioxidants does not always have a positive impact, which could result in genotoxic effects [197,198].
4.5.11. Soy (Glycine max)
Soy (Glycine max) is an annual herbaceous plant belonging to the Leguminous family, native to eastern Asia and cultivated for food purposes. Isoflavones are one of several bioactive soy components that may have a positive impact on human health. The biological actions of the other soy components, such as saponins, soy cystatin, lecithin, raffinose, stachyose, and 2-pentyl-pyridine, vary from those of isoflavones and include hormonal, immunological, bacterial, and digestive effects [199]. Isoflavones are found in soybeans in the physiologically inactive form of glucosides, the most prevalent of which are genistein and daizein. These are also found in soybeans in the biologically inactive form of -D-glucosides, genistin, and daizine. Intestinal glucosidase hydrolyzes these glucosides into the equivalent active form of aglycones. Isoflavones, which are heterocyclic phenols that may scavenge free radicals by donating a hydrogen atom to them, have an inhibitory impact on the buildup of body fat and have significant antioxidant characteristics. They facilitate the breakdown of hydrogen peroxide by reducing the generation of radical species and ROS. Studies on mice that had suffered severe oxidative stress revealed that the antioxidant defenses SOD and CAT may be restored after being treated with isoflavones derived from soy. These defenses had been dramatically reduced in response to the high quantities of ROS [200]. According to certain experimental data, probiotic-assisted in vitro fermentation enhances the bioavailability of isoflavones through glycosidic portion removal and aglycones (functional structures) formation. Studies on rats fed 1% cholesterol diets and then given fermented soy demonstrated improved lipid metabolism overall and decreased intestinal absorption of cholesterol [201,202].
4.5.12. Alfalfa (Medicago sativa)
Alfalfa (Medicago sativa) is a perennial herbaceous plant belonging to the Leguminosae species. The word “Medicago” comes from Persian ancestry, namely from the area of Media; “Medicago” means “herb of Media” and is not a result of the plant’s therapeutic qualities or advantages. However, it is also widely utilized in human nutrition and has been known since ancient times for its beneficial effects. Numerous nutrients are abundant in it, including more protein than eggs; vitamins A, E, C, D, K, B1, and B2; mineral salts including calcium, selenium, potassium, phosphorus, zinc, and copper; fiber; antioxidants; beta-carotene; saponins; and chlorophyll. As much as 30% of a plant’s dry weight may be made up of saponins (or saponosides), terpenic glycosides of vegetable origin that receive their name from the Saponaria officinalis plant that was traditionally used for washing wool. According to many studies, eating this plant lowers the amount of cholesterol absorbed from the intestines, enhances the excretion of endogenous and exogenous steroids in the feces, and prevents atherosclerotic plaques from forming. Along with controlling lipid metabolism, this plant’s aqueous extracts also have an intriguing effect on glucose metabolism. They promote the transport of 2-deoxy-glucose, the synthesis of glycogen in mouse abdominal muscles, and the production of insulin in pancreatic beta cells [203]. The saponin extract plays a vital function in lowering blood cholesterol, according to research that looked at the extract’s hypocholesterolemic effects on rats fed a hyperlipidic diet [204]. The induction of CYP7A1, which is involved in the conversion of cholesterol into bile acids; the induction of LDLr, which is in charge of removing LDL cholesterol from the bloodstream; and finally, the down-regulation of the HMGCoA reductase and ACAT2 genes, the former of which is in charge of cholesterol biosynthesis and the latter of which is involved in the pathway for triglyceride synthesis, are the genes that are responsible for this effect. In the end, the regulation of these genes and the enhanced excretion of cholesterol from the body might both be responsible for the hypocholesterolemic effects of saponin extract [205].
4.5.13. Bergamot (Citrus bergamia)
Bergamot (Citrus bergamia) is a citrus fruit in the Rutaceae family. Bergamot has been revealed over time to be a promising ally against various ailments, demonstrating antimicrobial, hypoglycemic, hypolipidemic, and neuroprotective properties [206,207,208,209,210,211,212]. The polyphenolic fraction of bergamot (BPF) is characterized by the presence of many polyphenol compounds, such as melitidin, brutieridin, neoeriocitrin, naringin, and neohesperidin [208]. When compared to other juices like apple and apricot juice, bergamot juice (BJ) has a remarkable antioxidant activity that is significantly superior [213]. HDL levels increased in vivo, while blood cholesterol, triglyceride, and LDL levels significantly decreased following long-term administration of BJ (1 mL per day) [209]. BPF, which was extracted by BJ, has also been linked to cardioprotective properties in addition to antioxidant activities. Recent studies found that BPF inhibited the cardiotoxicity brought on by the well-known anticancer medication doxorubicin by reducing the overproduction of reactive oxygen species (ROS), restoring protective autophagy, and reducing cardiomyocyte death [214,215]. Bergamot has recently received attention for its anti-inflammatory, hypolipidemic, hypoglycemic [216,217,218], and anticancer properties [219,220]. In individuals with mixed hyperlipidemia, oral treatment of BPF (1000 mg/day for 30 days straight; n = 15) dramatically decreased TC, LDL-C, and TG and increased HDL-C levels. In comparison to the impact of rosuvastatin alone, the hypolipidemic effect of rosuvastatin was significantly improved when BPF (1000 mg/day) and the lowest dose of rosuvastatin (10 mg) were taken together for 30 consecutive days [221]. Interestingly, the effect of bergamot juice on NAFLD-associated biomarkers (e.g., SOD, MDA, GPx, and TNF) is also enhanced by association with the compound Cynara cardunculus, as shown in a double-blind study by Musolino et al., 2020 [222]. The BPF has demonstrated great outcomes in clinical practice in a few investigations. It has been demonstrated that the flavonoids it contains, particularly naringin and hesperidin, exhibit action like that of statins, well-known compounds implicated in the metabolism of triglycerides and cholesterol. Therefore, BPF can be used as an adjunctive therapy for the treatment of NAFLD [223,224].
4.5.14. Rosemary (Rosmarinus officinalis)
Rosemary (Rosmarinus officinalis) is a fascinating source of a number of bioactive phytochemicals that fall into two categories: the volatile fraction and the phenolic components. The latter category mostly consists of flavonoids with well-established antioxidant properties, such as rosmarinic acid, carnosic acid (CA), carnosol, rosmanol, and 7-methyl-epirosmanol [225]. Afonso et al., 2013 [226] and Labban et al., 2014 [227] found that rosemary derivatives improved serum hyperglycemia and dyslipidemia when given abruptly to adult men and women or animals fed an HFD. An improvement in blood oxidative stress markers was seen along with this impact. According to research by Harach et al., 2010 [228] and Ibarra et al., 2011 [229], the limiting impact on lipid absorption may contribute to the favorable effects of chronic administration of rosemary extract (RE) on both lipid and glucose metabolism in HFD mice. Studies conducted in the laboratory revealed that RE, carnosol, and CA directly affected glycolysis and FFA oxidation in HepG2 cells, where they also increased the phosphorylation of the AMPK and PPAR signaling pathways [230]. Hepatic steatosis was prevented when CA-enriched RE or CA alone was chronically given to HFD-fed mice or ob/ob mice, and the recovery of hepatic TAG, ALT, AST, and FFA levels was linked to the reduction of HFD-induced oxidative stress and inflammation [231,232]. By blocking the SIRT1/p66shc proapoptotic pathway, CA was also able to shield hepatocytes from NAFLD-induced apoptosis [233]
4.5.15. Peppermint (Mentha piperita)
According to Schuhmacher et al., 2003 [234], the primary bioactive components of this plant include various kinds of flavonoids, such as luteolin, apigenin, and hesperetin, as well as phenolic acids like caffeic and rosmarinic acids. Apigenin and hesperetin chronic therapy in mice fed an HFD decreased hepatic CYP2B9 expression and decreased body weight growth, mesenteric adipose tissue weight, and serum leptin levels [71]. Chronic luteolin supplementation in HFD-fed mice alleviated hepatic steatosis by inhibiting hepatic lipogenesis and lipid absorption and decreased adiposity by upregulating genes for lipolysis and the tricarboxylic acid cycle and enhancing PPAR protein expression. Additionally, by inhibiting SREBP1, which affects insulin receptor substrate 2 expression via negative feedback and gluconeogenesis, it increased hepatic insulin sensitivity [235]. According to Mesbahzadeh et al., 2015 [236], peppermint extract had favorable effects on body weight increase, blood parameters including TAG, TC, LDL, and glucose in rats, and fructose-induced hyperlipidemia. It also exhibited high antioxidant activity.
4.5.16. Sage (Salvia officinalis)
Sage (Salvia officinalis) has antioxidant and anti-inflammatory characteristics, inhibits pancreatic lipase and fat absorption, and exerts PPAR agonistic effects [230,237,238]. Rosmarinic acid, CA, and luteolin-7-glucoside are the two phenolic chemicals that are most prevalent in sage extract. The hypolipidemic effects of sage ingestion have been proven in humans. Drinking sage tea led to an immediate improvement in the cholesterol profile in a non-randomized crossover experiment, reducing plasma LDL and TC and raising HDL levels [239]. Sage may be safe and effective in the treatment of hyperlipidemia and fatty liver, according to research from randomized, double-blind, placebo-controlled clinical trials that showed administration of sage leaf extract improved lipid profiles in both hyperlipidemic and T2DM patients [240].
4.5.17. Hot Pepper (Capsicum annuum)
The hot pepper (Capsicum annuum) is included in the Solanaceae family. The most prevalent ingredient in hot peppers, capsaicin, is what gives them their spicy qualities. The health advantages of capsaicin and the non-pungent capsiate have been shown in recent years.
Transient receptor potential vanilloid 1 (TRPV1), a Ca2+ permeable ion channel, is activated by capsaicin at nanomolar concentrations [241]. According to McCarty, DiNicolantonio, and O’Keefe (2015) [242], TRPV1 is expressed in a wide variety of cells from neuronal and other organs, including the liver. Capsinoids, which are non-pungent analogs of capsaicin, have recently been shown to accelerate catabolism in the liver and adipose tissue, opening the door for novel medications to treat NAFLD [243].
NAFLD in mice has been shown to be improved by capsaicin-rich meals [244]. Capsaicin administration reduced the inflammation caused by fat in adipose tissue, which had an impact on the overall health of the liver and the whole body. In this situation, capsaicin boosted adiponectin expression while decreasing IL-6, TNF-α, MCP-1, and COX-2 production [245]. According to Lee et al., 2013 [246], PPAR activation in adipose tissue appears to be a mediator of these effects. In the liver and adipose tissue, where chronic capsaicin administration dramatically decreased obesity-induced glucose intolerance and hepatic steatosis, dietary capsaicin action was likewise mediated via PPAR activation and upregulation [247]. According to Li et al., 2013 [244], and McCarty et al., 2015 [242], capsaicin-mediated stimulation of TRPV1 also stimulated PPAR activation, which most likely caused downstream induction of UCP2. The efficiency of hepatic FFA oxidation was enhanced by increased uncoupling in mitochondria as a result of UCP2 activation [248]. By increasing the effectiveness of hepatic FFA oxidation, weight-management techniques that aim to maximize appetite control, selective fat oxidation, and thermogenesis may be supplemented by inducing UCP2 in hepatocytes.
The following Table 2 summarizes the biological effects of the natural products summarizes the biological effects of the natural products aforementioned.

Table 2.
Biological effects of natural products in NAFLD treatment.
5. Clinical Studies
Several clinical studies have been conducted that have demonstrated the usefulness of NPs in improving NAFLD. Some examples will be given below.
For 12 weeks, 20 g/day of either sunflower oil or olive oil was given to 66 NAFLD patients who were divided into two groups in a double-blinded randomized controlled study (RCT). Each participant was advised to follow a hypocaloric diet of around 500 kcal per day. The severity of fatty liver, hepatic enzymes, anthropometric measurements, pressure, insulin, glucose, malondialdehyde, serum lipid profile, total antioxidant capacity, and interleukin-6 (IL-6) levels were all assessed before and after administration. The AST in serum was only reduced by olive oil. Following olive oil consumption, serum TG and fat mass were dramatically reduced. Although there were no changes in body fat % reported from the studies, modifications in fatty liver damage grade and skeletal muscle mass were most significant in participants who were in the olive oil group [250].
Two placebo capsules or 300 mg resveratrol capsules were administered twice daily for three months to 60 NAFLD patients in a double-blind, placebo-controlled RCT. GPT, glucose, LDL-C, TC, and HOMA-IR all dropped considerably in the resveratrol group compared to the placebo group. A substantial decrease in the concentrations of TNF-a, cytokeratin 18 fragments, and FGF-21, as well as an increase in the amount of APN, were seen in the resveratrol group [251].
In an RCT, individuals with NAFLD of varying disease severity received 1 g/day of curcumin for eight weeks. In both the curcumin and placebo groups, taking supplements containing curcumin was linked to a substantial decrease in waist circumference and body mass index (BMI). When compared to 4.7% of the control group, ultrasound scanning showed a substantial improvement in 75.0% of the patients treated with curcumin. Only the curcumin group’s serum ALT and AST levels slowed down appreciably. A considerable reduction in TG, LDL-C, fasting blood glucose (FBG), HOMA-IR, body weight, and AST levels was observed after curcumin treatment. Though not significant, curcumin’s observed reduction in TC, HbA1c, ALT, and insulin levels [252].
Intake of a green tea beverage optimized with catechins and an Epigallocatechin-3-gallate-HFD was shown to lower body weight (BW) in 126 obese adult patients in a double-blinded RCT. The patients were split into three groups: low-dose, high-dose, and placebo. Both the low-dose and high-dose groups’ BW considerably dropped [253].
In a recent clinical experiment, 30 overweight individuals with NAFLD were divided into three groups and randomly assigned to receive either a customized Mediterranean diet in groups A and B or no therapy at all in group C (control). The Realsil combination, which combines 94 mg of silybin with 194 mg of phosphatidylcholine and 30 mg of α-tocopherol, was also administered twice daily to the participants in group B. NAFLD was identified with the use of liver ultrasonography. Compared to the control group, group A and group B’s weight, BMI, waist and hip circumferences, serum TG, total cholesterol, and fatty liver index significantly decreased after the 6-month intervention. Additionally, compared to the control group, there were significant improvements in the Realsil complex group’s fasting blood sugar, insulin, and HOMA-IR levels. Additionally, both intervention groups’ Hamaguchi scores—which measure the buildup of hepatic fat—were considerably lower at the study’s conclusion compared to the baseline. None of the research participants mentioned any negative outcomes [254].
In a double-blind, randomized experiment, 74 individuals with NAFLD who had been identified by ultrasonography were enrolled to either take a placebo for 12 weeks or 320 mg/day of pure anthocyanins from bilberries and black currants (four capsules, each containing 80 mg anthocyanins). There were no significant changes in BMI, WC, blood pressure, serum AST, NAFLD fibrosis score, total cholesterol, LDL-C, or insulin at the conclusion of the intervention. However, anthocyanin therapy substantially decreased blood ALT, cytokeratin-18 M30 fragment, and myeloperoxidase concentrations (markers of inflammation and oxidative stress) when compared to placebo. There were no negative consequences from the intervention [70].
In total, 184 NAFLD patients with proton magnetic resonance spectroscopy (1H MRS) confirmation were recruited, split into three intervention groups, and monitored for 16 weeks in a randomized, controlled, open-label clinical study. A lifestyle intervention (dietary changes and exercise program) was given to the first group. A second group also received a lifestyle intervention plus 15 mg of pioglitazone (PGZ) daily, and a third group also received a lifestyle intervention plus 0.5 g of berberine three times daily, taken 30 min before meals. The addition of berberine substantially decreased body weight, BMI, WC, hepatic fat content (by 1H MRS), blood total cholesterol, TG, apolipoprotein B, ALT, and AST when compared to lifestyle intervention alone. Additionally, the lifestyle intervention plus berberine group saw substantially larger weight, BMI, WC, serum total cholesterol, and TG reductions than the lifestyle intervention plus pioglitazone group. Additionally, when berberine was used instead of pioglitazone, hepatic fat content reductions appeared to be more pronounced. Overall, a lifestyle intervention with berberine was just as effective as a lifestyle intervention plus pioglitazone in lowering NAFLD-related biomarkers. The predominant side effects in the berberine group of participants were anorexia, upset stomach, diarrhea, and constipation. The most common side effects in patients using pioglitazone were weariness, muscle discomfort, and heart problems [123].
Hajagha Mohammadi et al., 2008 [255], studied the effectiveness of silymarin in 50 NAFLD patients in a randomized clinical study. The study’s 64 percent male and 36 percent female participants were split into case and control groups. Elevated liver enzyme levels and enhanced hepatic echogenicity were seen in all individuals. One silymarin pill containing 140 mg was given to the case group each day for two months, whereas a placebo was given to the control group. Each patient’s weight, body mass index (BMI), and liver transaminase levels were assessed both before and after the trial. Before and after the trial, neither group’s mean weight nor BMI showed any significant variations, according to the scientists. Aspartate transaminase (AST) and alkaline transaminase (ALT) levels decreased in the case group from 53.7 to 29.1 IU/mL and 103.1 to 41.4 U/L, respectively (p < 0.001 and p < 0.001, respectively). The reductions in mean ALT and AST (7.8 and 2.2 IU/mL, respectively) in the control group were not statistically significant [255].
A double-blind, placebo-controlled clinical trial with 102 individuals with hepatic steatosis was conducted by Ferro et al. in 2020 [212]. For 12 weeks, a nutraceutical comprising 300 mg/day of Cynara cardunculus extract and a Bergamot polyphenol fraction was given to the intervention group. The placebo was given to the control group each day. At the beginning and conclusion of the trial, serum transaminases, lipids, glucose, and liver fat content were assessed by transient elastography. The change in liver fat content, measured as a percentage of the controlled attenuation parameter (CAP) score, was established. The decrease in CAP was statistically significant in individuals with android obesity, overweight/obesity, and women. The authors discovered that participants using the nutraceutical saw a larger reduction in liver fat content than those receiving a placebo (48.2 39 vs. 26.9 43 dB/m, p = 0.02). The percentage CAP score decrease, however, was statistically significant only in those over 50 years old (44 vs. 78% in placebo and nutraceutical, respectively; p = 0.007 after weight change correction) [212].
6. Prevention and Management: The Role of Lifestyle and Physical Activity
The percentage of obese individuals with NAFLD varies from 30% to 37%. There is a clear link between increased waist circumference in NAFLD and abdominal obesity [256]. According to research, 80% of NAFLD participants had a body mass index (BMI) of more than 30 kg/m2, and morbidly obese people (BMI > 40 kg/m2) have more visceral adipose tissue, which increases the morbidity of NAFLD [257]. Proof that NAFLD and obesity are associated suggests that certain macro- and micronutrients are more closely linked to the occurrence of NAFLD. This component involves the tight correlation between high carbohydrate consumption and the risk of non-alcoholic fatty liver disease (NAFLD), with fructose being a prominent factor. It is possible to hypothesize that carbohydrates trigger c-Jun-N-terminal pathway-mediated de novo lipogenesis (DNL) and an inflammatory response that results in hepatocyte apoptosis [256]. With this in mind, changes in diet and lifestyle can make a strong contribution to improving the clinical condition. Current guidelines recognize that “weight loss is the key to improvement in the histopathological features of NASH” [258,259] and “structured programs aimed at lifestyle changes towards healthy diet and habitual physical activity are advisable.” Not every dietary strategy is designed or anticipated to result in weight reduction. Similar to this, physical activity regimens may differ in terms of length, frequency, and intensity, with a concentration on either resistance or aerobic training [260]. In a prospective analysis of 293 people with histologically established NASH, after a year, 88 had lost ≥5% of their weight, with a mean weight loss of 9.3%; 72 (25%) had resolved steatohepatitis, 138 (47%) had reduced NAS, and 56 (19%) had seen regression of fibrosis [261]. A follow-up analysis of these data revealed a direct correlation between the extent of weight reduction and improvements in fibrosis and steatosis; among the 10% of participants who lost more than 10% of their body weight, 90% had NASH resolution, 81% had fibrosis regression, and 100% had steatosis improvement [262]. Several clinical studies have examined the so-called Mediterranean diet (MD) and its expansion to a Mediterranean lifestyle [263] for individuals with NAFLD as a therapeutic intervention. The diet replicates the 1960s “traditional” (albeit hardly followed) eating patterns of Greece, Italy, and Spain. It is typified by a low consumption of meat and dairy products and a high consumption of fruits, vegetables, legumes, nuts, beans, cereals, grains, seafood, and unsaturated fats, like olive oil. The MD also allows for modest use of red wine. From a macronutrient standpoint, the MD is based on nutritional interventions rather than caloric restriction. It is a moderate carb diet (~40% carbs) with moderate to slightly raised total fat (35–40%), mostly omega-3 polyunsaturated fat and monounsaturated fat. Many bioactive polyphenols with suggested anti-inflammatory qualities, such as hydroxytyrosol, tyrosol, oleocanthal, and resveratrol, are found in red wine and extra virgin olive oil [264]. Frequent exercise, which is essential to the MD paradigm, may also significantly (although not dramatically) contribute to any advantages. In comparison to conventional healthy diet and lifestyle recommendations, a meta-analysis of 20 randomized controlled trials including 1073 individuals with NAFLD, MD, and exercise resulted in better aminotransferase levels, hepatic fat levels, and NAS, regardless of weight loss [265]. Higher adherence to the MD was linked to better insulin resistance as assessed by the homeostatic model assessment (HOMA), less severe steatosis at histology, and a lower probability of progression to NASH, according to an observational research examining the impact of the MD on NASH [266].
7. Discussion and Conclusions
In addition to summarizing the natural products that have been recently reported in the literature to have modifying effects on in vitro and in vivo models of NAFLD, this study reviews the present status of research on the pathogenesis of NAFLD. Most often, lipid accumulation, oxidative stress, ER stress, and lipotoxicity are linked to the development of NAFLD. It is noteworthy to note that these active substances treat NAFLD by modulating the amounts of proteins, including AMPK, PPAR, SREBP-1c, FAS, ACC, SIRT1, Nrf2, JNK, and others. These organic ingredients could open new avenues for the investigation and development of NAFLD therapeutic candidates. We encounter several problems at the same moment. The absence of uniform criteria and norms in current natural product research has led to immature assessment systems and ambiguous potential processes. In addition, many natural products still lack fully developed extraction technologies for their active components. Furthermore, the extraction method used for natural substances affects the bioavailability of the active components you are trying to isolate. In the same case, the bioavailability is significantly reduced as a result. Synergisms and antagonisms of products taken in the diet should be investigated in large-scale studies. Warfarin, aspirin, alkylating drugs, and cyclosporine have all been linked to harm in individuals due to herb-drug interactions. Therefore, additional research is required to use natural items to treat NAFLD.
Author Contributions
Conceptualization, S.N., R.M.B. and R.B.; methodology, S.N., R.M.B., R.B., G.S., V.M. and E.P.; writing—original draft preparation, S.N., R.M.B., S.R., R.B., R.C., M.S., C.L., M.M., F.C. (Fabio Castagna), C.L., M.M., F.C. (Filomena Conforti), G.S., V.M. and E.P.; writing—review and editing, S.N., R.M.B., S.R., R.B., R.C., M.S., C.L., M.M., F.C. (Fabio Castagna), C.L., M.M., F.C. (Filomena Conforti), G.S., V.M. and E.P.; supervision, G.S., V.M. and E.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hassan, K.; Bhalla, V.; El Regal, M.E.; A-Kader, H.H. Nonalcoholic fatty liver disease: A comprehensive review of a growing epidemic. World J. Gastroenterol. 2014, 20, 12082. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease—Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Scorletti, E.; Carr, R.M. A new perspective on NAFLD: Focusing on lipid droplets. J. Hepatol. 2022, 76, 934–945. [Google Scholar] [CrossRef]
- Wree, A.; Broderick, L.; Canbay, A.; Hoffman, H.M.; Feldstein, A.E. From NAFLD to NASH to cirrhosis—New insights into disease mechanisms. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 627–636. [Google Scholar] [CrossRef] [PubMed]
- Henao-Mejia, J.; Elinav, E.; Jin, C.; Hao, L.; Mehal, W.Z.; Strowig, T.; Thaiss, C.A.; Kau, A.L.; Eisenbarth, S.C.; Jurczak, M.J. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature 2012, 482, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Propst, A. Prognosis in nonalcoholic steatohepatitis. Gastroenterology 1995, 108, 1607. [Google Scholar] [CrossRef]
- Nseir, W.; Hellou, E.; Assy, N. Role of diet and lifestyle changes in nonalcoholic fatty liver disease. World J. Gastroenterol. 2014, 20, 9338. [Google Scholar]
- Mantovani, A.; Dalbeni, A. Treatments for NAFLD: State of art. Int. J. Mol. Sci. 2021, 22, 2350. [Google Scholar] [CrossRef]
- Talebi, S.; Bagherniya, M.; Atkin, S.L.; Askari, G.; Orafai, H.M.; Sahebkar, A. The beneficial effects of nutraceuticals and natural products on small dense LDL levels, LDL particle number and LDL particle size: A clinical review. Lipids Health Dis. 2020, 19, 1–21. [Google Scholar] [CrossRef]
- Noce, A.; Di Lauro, M.; Di Daniele, F.; Pietroboni Zaitseva, A.; Marrone, G.; Borboni, P.; Di Daniele, N. Natural bioactive compounds useful in clinical management of metabolic syndrome. Nutrients 2021, 13, 630. [Google Scholar] [CrossRef]
- Bora, K.S.; Sharma, A. The genus Artemisia: A comprehensive review. Pharm. Biol. 2011, 49, 101–109. [Google Scholar] [CrossRef]
- Jo, H.K.; Kim, G.W.; Jeong, K.J.; Do, Y.K.; Chung, S.H. Eugenol ameliorates hepatic steatosis and fibrosis by down-regulating SREBP1 gene expression via AMPK-mTOR-p70S6K signaling pathway. Biol. Pharm. Bull. 2014, 37, 1341–1351. [Google Scholar] [CrossRef]
- Andrade, J.M.O.; Paraíso, A.F.; de Oliveira, M.V.M.; Martins, A.M.E.; Neto, J.F.; Guimarães, A.L.S.; de Paula, A.M.; Qureshi, M.; Santos, S.H.S. Resveratrol attenuates hepatic steatosis in high-fat fed mice by decreasing lipogenesis and inflammation. Nutrition 2014, 30, 915–919. [Google Scholar] [CrossRef] [PubMed]
- Perdomo, C.M.; Frühbeck, G.; Escalada, J. Impact of nutritional changes on nonalcoholic fatty liver disease. Nutrients 2019, 11, 677. [Google Scholar] [CrossRef] [PubMed]
- Mills, S.J.; Harrison, S.A. Comparison of the natural history of alcoholic and nonalcoholic fatty liver disease. Curr. Gastroenterol. Rep. 2005, 7, 32–36. [Google Scholar] [CrossRef]
- Moore, J.B. Non-alcoholic fatty liver disease: The hepatic consequence of obesity and the metabolic syndrome. Proc. Nutr. Soc. 2010, 69, 211–220. [Google Scholar] [CrossRef]
- Masarone, M.; Federico, A.; Abenavoli, L.; Loguercio, C.; Persico, M. Non alcoholic fatty liver: Epidemiology and natural history. Rev. Recent Clin. Trials 2014, 9, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Targher, G.; Bertolini, L.; Padovani, R.; Rodella, S.; Zoppini, G.; Zenari, L.; Cigolini, M.; Falezza, G.; Arcaro, G. Relations between carotid artery wall thickness and liver histology in subjects with nonalcoholic fatty liver disease. Diabetes Care 2006, 29, 1325–1330. [Google Scholar] [CrossRef]
- Kasper, P.; Martin, A.; Lang, S.; Kuetting, F.; Goeser, T.; Demir, M.; Steffen, H.-M. NAFLD and cardiovascular diseases: A clinical review. Clin. Res. Cardiol. 2021, 110, 921–937. [Google Scholar] [CrossRef]
- Eslam, M.; Sanyal, A.J.; George, J.; Sanyal, A.; Neuschwander-Tetri, B.; Tiribelli, C.; Kleiner, D.E.; Brunt, E.; Bugianesi, E.; Yki-Järvinen, H. MAFLD: A consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology 2020, 158, 1999–2014. [Google Scholar] [CrossRef]
- Nucera, S.; Ruga, S.; Cardamone, A.; Coppoletta, A.R.; Guarnieri, L.; Zito, M.C.; Bosco, F.; Macrì, R.; Scarano, F.; Scicchitano, M. MAFLD progression contributes to altered thalamus metabolism and brain structure. Sci. Rep. 2022, 12, 1207. [Google Scholar] [CrossRef]
- Kobyliak, N.; Abenavoli, L.; Mykhalchyshyn, G.; Kononenko, L.; Boccuto, L.; Kyriienko, D.; Dynnyk, O. A multi-strain probiotic reduces the fatty liver index, cytokines and aminotransferase levels in NAFLD patients: Evidence from a randomized clinical trial. J. Gastrointest. Liver Dis. 2018, 27, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Kolodziejczyk, A.A.; Zheng, D.; Shibolet, O.; Elinav, E. The role of the microbiome in NAFLD and NASH. EMBO Mol. Med. 2019, 11, e9302. [Google Scholar] [CrossRef] [PubMed]
- Michail, S.; Lin, M.; Frey, M.R.; Fanter, R.; Paliy, O.; Hilbush, B.; Reo, N.V. Altered gut microbial energy and metabolism in children with non-alcoholic fatty liver disease. FEMS Microbiol. Ecol. 2015, 91, 1–9. [Google Scholar] [CrossRef]
- Wang, B.; Jiang, X.; Cao, M.; Ge, J.; Bao, Q.; Tang, L.; Chen, Y.; Li, L. Altered fecal microbiota correlates with liver biochemistry in nonobese patients with non-alcoholic fatty liver disease. Sci. Rep. 2016, 6, 32002. [Google Scholar] [CrossRef]
- Loomba, R.; Seguritan, V.; Li, W.; Long, T.; Klitgord, N.; Bhatt, A.; Dulai, P.S.; Caussy, C.; Bettencourt, R.; Highlander, S.K. Gut microbiome-based metagenomic signature for non-invasive detection of advanced fibrosis in human nonalcoholic fatty liver disease. Cell Metab. 2017, 25, 1054–1062. [Google Scholar] [CrossRef] [PubMed]
- Boursier, J.; Mueller, O.; Barret, M.; Machado, M.; Fizanne, L.; Araujo-Perez, F.; Guy, C.D.; Seed, P.C.; Rawls, J.F.; David, L.A. The severity of nonalcoholic fatty liver disease is associated with gut dysbiosis and shift in the metabolic function of the gut microbiota. Hepatology 2016, 63, 764–775. [Google Scholar] [CrossRef]
- Ren, Z.; Li, A.; Jiang, J.; Zhou, L.; Yu, Z.; Lu, H.; Xie, H.; Chen, X.; Shao, L.; Zhang, R. Gut microbiome analysis as a tool towards targeted non-invasive biomarkers for early hepatocellular carcinoma. Gut 2019, 68, 1014–1023. [Google Scholar] [CrossRef]
- Day, C.P.; James, O.F.W. Steatohepatitis: A tale of two “hits”? Gastroenterology 1998, 114, 842–845. [Google Scholar] [CrossRef]
- Liu, Q.; Bengmark, S.; Qu, S. The role of hepatic fat accumulation in pathogenesis of non-alcoholic fatty liver disease (NAFLD). Lipids Health Dis. 2010, 9, 42. [Google Scholar] [CrossRef]
- Loomba, R.; Friedman, S.L.; Shulman, G.I. Mechanisms and disease consequences of nonalcoholic fatty liver disease. Cell 2021, 184, 2537–2564. [Google Scholar] [CrossRef]
- Takaki, A.; Kawai, D.; Yamamoto, K. Multiple hits, including oxidative stress, as pathogenesis and treatment target in non-alcoholic steatohepatitis (NASH). Int. J. Mol. Sci. 2013, 14, 20704–20728. [Google Scholar] [CrossRef]
- Tilg, H.; Moschen, A.R. Evolution of inflammation in nonalcoholic fatty liver disease: The multiple parallel hits hypothesis. Hepatology 2010, 52, 1836–1846. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, K.L.; Smith, C.I.; Schwarzenberg, S.J.; Jessurun, J.; Boldt, M.D.; Parks, E.J. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J. Clin. Investig. 2005, 115, 1343–1351. [Google Scholar] [CrossRef] [PubMed]
- Kawano, Y.; Cohen, D.E. Mechanisms of hepatic triglyceride accumulation in non-alcoholic fatty liver disease. J. Gastroenterol. 2013, 48, 434–441. [Google Scholar] [CrossRef]
- Goldberg, I.J. Diabetic dyslipidemia: Causes and consequences. J. Clin. Endocrinol. Metab. 2001, 86, 965–971. [Google Scholar] [CrossRef] [PubMed]
- Nassir, F.; Ibdah, J.A. Role of mitochondria in nonalcoholic fatty liver disease. Int. J. Mol. Sci. 2014, 15, 8713–8742. [Google Scholar] [CrossRef]
- Berg, A.H.; Scherer, P.E. Adipose tissue, inflammation, and cardiovascular disease. Circ. Res. 2005, 96, 939–949. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, P.; Stumvoll, M. Fatty acids and insulin resistance in muscle and liver. Best Pract. Res. Clin. Endocrinol. Metab. 2005, 19, 625–635. [Google Scholar] [CrossRef]
- Pan, M.; Lai, C.; Tsai, M.; Ho, C. Chemoprevention of nonalcoholic fatty liver disease by dietary natural compounds. Mol. Nutr. Food Res. 2014, 58, 147–171. [Google Scholar] [CrossRef]
- Yuzefovych, L.V.; Musiyenko, S.I.; Wilson, G.L.; Rachek, L.I. Mitochondrial DNA damage and dysfunction, and oxidative stress are associated with endoplasmic reticulum stress, protein degradation and apoptosis in high fat diet-induced insulin resistance mice. PLoS ONE 2013, 8, e54059. [Google Scholar] [CrossRef]
- Morgan, M.J.; Liu, Z. Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res. 2011, 21, 103–115. [Google Scholar] [CrossRef]
- Lee, J.-M.; Calkins, M.J.; Chan, K.; Kan, Y.W.; Johnson, J.A. Identification of the NF-E2-related factor-2-dependent genes conferring protection against oxidative stress in primary cortical astrocytes using oligonucleotide microarray analysis. J. Biol. Chem. 2003, 278, 12029–12038. [Google Scholar] [CrossRef]
- Hosseini, H.; Teimouri, M.; Shabani, M.; Koushki, M.; Khorzoughi, R.B.; Namvarjah, F.; Izadi, P.; Meshkani, R. Resveratrol alleviates non-alcoholic fatty liver disease through epigenetic modification of the Nrf2 signaling pathway. Int. J. Biochem. Cell Biol. 2020, 119, 105667. [Google Scholar] [CrossRef]
- Robertson, G.; Leclercq, I.; Farrell, G.C., II. Cytochrome P-450 enzymes and oxidative stress. Am. J. Physiol. Liver Physiol. 2001, 281, G1135–G1139. [Google Scholar]
- Phaniendra, A.; Jestadi, D.B.; Periyasamy, L. Free radicals: Properties, sources, targets, and their implication in various diseases. Indian J. Clin. Biochem. 2015, 30, 11–26. [Google Scholar] [CrossRef]
- Mansouri, A.; Demeilliers, C.; Amsellem, S.; Pessayre, D.; Fromenty, B. Acute ethanol administration oxidatively damages and depletes mitochondrial DNA in mouse liver, brain, heart, and skeletal muscles: Protective effects of antioxidants. J. Pharmacol. Exp. Ther. 2001, 298, 737–743. [Google Scholar]
- Weltman, M.D.; Farrell, G.C.; Hall, P.; Ingelman-Sundberg, M.; Liddle, C. Hepatic cytochrome P450 2E1 is increased in patients with nonalcoholic steatohepatitis. Hepatology 1998, 27, 128–133. [Google Scholar] [CrossRef]
- Chen, J.; Deng, X.; Liu, Y.; Tan, Q.; Huang, G.; Che, Q.; Guo, J.; Su, Z. Kupffer cells in non-alcoholic fatty liver disease: Friend or foe? Int. J. Biol. Sci. 2020, 16, 2367. [Google Scholar] [CrossRef]
- Zisser, A.; Ipsen, D.H.; Tveden-Nyborg, P. Hepatic stellate cell activation and inactivation in NASH-fibrosis—Roles as putative treatment targets? Biomedicines 2021, 9, 365. [Google Scholar] [CrossRef]
- Oktia, R.T.; Okita, J.R. Cytochrome P450 4A fatty acid omega hydroxylases. Curr. Drug Metab. 2001, 2, 265–281. [Google Scholar] [CrossRef] [PubMed]
- Hardwick, J.P.; Osei-Hyiaman, D.; Wiland, H.; Abdelmegeed, M.A.; Song, B.-J. PPAR/RXR regulation of fatty acid metabolism and fatty acid ω-hydroxylase (CYP4) isozymes: Implications for prevention of lipotoxicity in fatty liver disease. PPAR Res. 2009, 2009, 952734. [Google Scholar] [CrossRef] [PubMed]
- Suhaili, S.H.; Karimian, H.; Stellato, M.; Lee, T.-H.; Aguilar, M.-I. Mitochondrial outer membrane permeabilization: A focus on the role of mitochondrial membrane structural organization. Biophys. Rev. 2017, 9, 443–457. [Google Scholar] [CrossRef] [PubMed]
- Schreurs, M.; Kuipers, F.; Van Der Leij, F.R. Regulatory enzymes of mitochondrial β-oxidation as targets for treatment of the metabolic syndrome. Obes. Rev. 2010, 11, 380–388. [Google Scholar] [CrossRef]
- Reddy, J.K.; Sambasiva Rao, M. Lipid metabolism and liver inflammation. II. Fatty liver disease and fatty acid oxidation. Am. J. Physiol. Liver Physiol. 2006, 290, G852–G858. [Google Scholar] [CrossRef]
- Kohjima, M.; Enjoji, M.; Higuchi, N.; Kato, M.; Kotoh, K.; Yoshimoto, T.; Fujino, T.; Yada, M.; Yada, R.; Harada, N. Re-evaluation of fatty acid metabolism-related gene expression in nonalcoholic fatty liver disease. Int. J. Mol. Med. 2007, 20, 351–358. [Google Scholar] [CrossRef]
- Durand, M.; Coué, M.; Croyal, M.; Moyon, T.; Tesse, A.; Atger, F.; Ouguerram, K.; Jacobi, D. Changes in key mitochondrial lipids accompany mitochondrial dysfunction and oxidative stress in NAFLD. Oxid. Med. Cell. Longev. 2021, 2021, 9986299. [Google Scholar] [CrossRef]
- Ron, D. Proteotoxicity in the endoplasmic reticulum: Lessons from the Akita diabetic mouse. J. Clin. Investig. 2002, 109, 443–445. [Google Scholar] [CrossRef]
- Demirtas, L.; Guclu, A.; Erdur, F.M.; Akbas, E.M.; Ozcicek, A.; Onk, D.; Turkmen, K. Apoptosis, autophagy & endoplasmic reticulum stress in diabetes mellitus. Indian J. Med. Res. 2016, 144, 515–524. [Google Scholar]
- Zhong, Q.; Wu, Y.; Xiong, F.; Liu, M.; Liu, Y.; Wang, C.; Chen, Y. Higher flavonoid intake is associated with a lower progression risk of non-alcoholic fatty liver disease in adults: A prospective study. Br. J. Nutr. 2021, 125, 460–470. [Google Scholar] [CrossRef]
- Rodriguez-Ramiro, I.; Vauzour, D.; Minihane, A.M. Polyphenols and non-alcoholic fatty liver disease: Impact and mechanisms. Proc. Nutr. Soc. 2016, 75, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Xiong, T.; Liu, P.; Guo, X.; Xiao, L.; Zhou, F.; Tang, Y.; Yao, P. Quercetin ameliorates HFD-induced NAFLD by promoting hepatic VLDL assembly and lipophagy via the IRE1a/XBP1s pathway. Food Chem. Toxicol. 2018, 114, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Yang, T.; Heng, C.; Zhou, Y.; Jiang, Z.; Qian, X.; Du, L.; Mao, S.; Yin, X.; Lu, Q. Quercetin improves nonalcoholic fatty liver by ameliorating inflammation, oxidative stress, and lipid metabolism in db/db mice. Phyther. Res. 2019, 33, 3140–3152. [Google Scholar] [CrossRef] [PubMed]
- Ying, H.-Z.; Liu, Y.-H.; Yu, B.; Wang, Z.-Y.; Zang, J.-N.; Yu, C.-H. Dietary quercetin ameliorates nonalcoholic steatohepatitis induced by a high-fat diet in gerbils. Food Chem. Toxicol. 2013, 52, 53–60. [Google Scholar] [CrossRef]
- Mahesh, T.; Menon, V.P. Quercetin allievates oxidative stress in streptozotocin-induced diabetic rats. Phyther. Res. An Int. J. Devoted to Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2004, 18, 123–127. [Google Scholar] [CrossRef]
- Wagner, C.; Fachinetto, R.; Dalla Corte, C.L.; Brito, V.B.; Severo, D.; Dias, G.d.O.C.; Morel, A.F.; Nogueira, C.W.; Rocha, J.B.T. Quercitrin, a glycoside form of quercetin, prevents lipid peroxidation in vitro. Brain Res. 2006, 1107, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Qin, G.; Ma, J.; Huang, Q.; Yin, H.; Han, J.; Li, M.; Deng, Y.; Wang, B.; Hassan, W.; Shang, J. Isoquercetin improves hepatic lipid accumulation by activating AMPK pathway and suppressing TGF-β signaling on an HFD-induced nonalcoholic fatty liver disease rat model. Int. J. Mol. Sci. 2018, 19, 4126. [Google Scholar] [CrossRef]
- Porras, D.; Nistal, E.; Martínez-Flórez, S.; Pisonero-Vaquero, S.; Olcoz, J.L.; Jover, R.; González-Gallego, J.; García-Mediavilla, M.V.; Sánchez-Campos, S. Protective effect of quercetin on high-fat diet-induced non-alcoholic fatty liver disease in mice is mediated by modulating intestinal microbiota imbalance and related gut-liver axis activation. Free Radic. Biol. Med. 2017, 102, 188–202. [Google Scholar] [CrossRef]
- Mehmood, A.; Zhao, L.; Wang, Y.; Pan, F.; Hao, S.; Zhang, H.; Iftikhar, A.; Usman, M. Dietary anthocyanins as potential natural modulators for the prevention and treatment of non-alcoholic fatty liver disease: A comprehensive review. Food Res. Int. 2021, 142, 110180. [Google Scholar]
- Zhang, P.-W.; Chen, F.-X.; Li, D.; Ling, W.-H.; Guo, H.-H. A CONSORT-compliant, randomized, double-blind, placebo-controlled pilot trial of purified anthocyanin in patients with nonalcoholic fatty liver disease. Medicine 2015, 94, e758. [Google Scholar] [CrossRef]
- Hoek-van den Hil, E.F.; van Schothorst, E.M.; van der Stelt, I.; Swarts, H.J.M.; van Vliet, M.; Amolo, T.; Vervoort, J.J.M.; Venema, D.; Hollman, P.C.H.; Rietjens, I.M.C.M. Direct comparison of metabolic health effects of the flavonoids quercetin, hesperetin, epicatechin, apigenin and anthocyanins in high-fat-diet-fed mice. Genes Nutr. 2015, 10, 23. [Google Scholar] [PubMed]
- Seymour, E.M.; Tanone, I.I.; Urcuyo-Llanes, D.E.; Lewis, S.K.; Kirakosyan, A.; Kondoleon, M.G.; Kaufman, P.B.; Bolling, S.F. Blueberry intake alters skeletal muscle and adipose tissue peroxisome proliferator-activated receptor activity and reduces insulin resistance in obese rats. J. Med. Food 2011, 14, 1511–1518. [Google Scholar] [PubMed]
- Vendrame, S.; Daugherty, A.; Kristo, A.S.; Klimis-Zacas, D. Wild blueberry (Vaccinium angustifolium)-enriched diet improves dyslipidaemia and modulates the expression of genes related to lipid metabolism in obese Zucker rats. Br. J. Nutr. 2014, 111, 194–200. [Google Scholar] [CrossRef]
- Takayama, F.; Nakamoto, K.; Kawasaki, H.; Mankura, M.; Egashira, T.; Ueki, K.; Hasegawa, A.; Okada, S.; Mori, A. Beneficial effects of Vitis coignetiae Pulliat leaves on nonalcoholic steatohepatitis in a rat model. Acta Med. Okayama 2009, 63, 105–111. [Google Scholar]
- Hwang, Y.P.; Choi, J.H.; Han, E.H.; Kim, H.G.; Wee, J.-H.; Jung, K.O.; Jung, K.H.; Kwon, K.; Jeong, T.C.; Chung, Y.C. Purple sweet potato anthocyanins attenuate hepatic lipid accumulation through activating adenosine monophosphate–activated protein kinase in human HepG2 cells and obese mice. Nutr. Res. 2011, 31, 896–906. [Google Scholar]
- Zhu, W.; Jia, Q.; Wang, Y.; Zhang, Y.; Xia, M. The anthocyanin cyanidin-3-O-β-glucoside, a flavonoid, increases hepatic glutathione synthesis and protects hepatocytes against reactive oxygen species during hyperglycemia: Involvement of a cAMP–PKA-dependent signaling pathway. Free Radic. Biol. Med. 2012, 52, 314–327. [Google Scholar]
- Frank, J.; Kamal-Eldin, A.; Lundh, T.; Määttä, K.; Törrönen, R.; Vessby, B. Effects of dietary anthocyanins on tocopherols and lipids in rats. J. Agric. Food Chem. 2002, 50, 7226–7230. [Google Scholar]
- Dubey, P.; Jayasooriya, A.P.; Cheema, S.K. Fish oil induced hyperlipidemia and oxidative stress in BioF1B hamsters is attenuated by elderberry extract. Appl. Physiol. Nutr. Metab. 2012, 37, 472–479. [Google Scholar] [PubMed]
- Habanova, M.; Saraiva, J.A.; Haban, M.; Schwarzova, M.; Chlebo, P.; Predna, L.; Gažo, J.; Wyka, J. Intake of bilberries (Vaccinium myrtillus L.) reduced risk factors for cardiovascular disease by inducing favorable changes in lipoprotein profiles. Nutr. Res. 2016, 36, 1415–1422. [Google Scholar]
- Sangouni, A.A.; Hosseinzadeh, M.; Sangsefidi, Z.S.; Yarhosseini, F.; Akhondi-Meybodi, M.; Ranjbar, A.; Madadizadeh, F.; Mozaffari-Khosravi, H. Effect of Total Anthocyanin-base Standardized Cornus mas L. Fruit Extract on Hepatic Steatosis and Visceral Adiposity Index in Patients With Non-alcoholic Fatty Liver Disease: A Double-blind Randomized Controlled Trial. Res. Square 2021. preprint. [Google Scholar] [CrossRef]
- Reis, J.F.; Monteiro, V.V.S.; de Souza Gomes, R.; do Carmo, M.M.; da Costa, G.V.; Ribera, P.C.; Monteiro, M.C. Action mechanism and cardiovascular effect of anthocyanins: A systematic review of animal and human studies. J. Transl. Med. 2016, 14, 315. [Google Scholar] [PubMed]
- Roth, S.; Spalinger, M.R.; Gottier, C.; Biedermann, L.; Zeitz, J.; Lang, S.; Weber, A.; Rogler, G.; Scharl, M. Bilberry-derived anthocyanins modulate cytokine expression in the intestine of patients with ulcerative colitis. PLoS ONE 2016, 11, e0154817. [Google Scholar]
- Zhang, Y.; Meng, Q.; Yin, J.; Zhang, Z.; Bao, H.; Wang, X. Anthocyanins attenuate neuroinflammation through the suppression of MLK3 activation in a mouse model of perioperative neurocognitive disorders. Brain Res. 2020, 1726, 146504. [Google Scholar] [CrossRef]
- Karunarathne, W.A.H.M.; Lee, K.T.; Choi, Y.H.; Jin, C.-Y.; Kim, G.-Y. Anthocyanins isolated from Hibiscus syriacus L. attenuate lipopolysaccharide-induced inflammation and endotoxic shock by inhibiting the TLR4/MD2-mediated NF-κB signaling pathway. Phytomedicine 2020, 76, 153237. [Google Scholar] [CrossRef]
- Zang, Y.; Zhang, D.; Yu, C.; Jin, C.; Igarashi, K. Antioxidant and hepatoprotective activity of kaempferol 3-O-β-D-(2, 6-di-O-α-L-rhamnopyranosyl) galactopyronoside against carbon tetrachloride-induced liver injury in mice. Food Sci. Biotechnol. 2017, 26, 1071–1076. [Google Scholar] [CrossRef]
- Wei, T.; Xiong, F.; Wang, S.; Wang, K.; Zhang, Y.; Zhang, Q. Flavonoid ingredients of Ginkgo biloba leaf extract regulate lipid metabolism through Sp1-mediated carnitine palmitoyltranferase 1A up-regulation. J. Biomed. Sci. 2014, 21, 87. [Google Scholar] [CrossRef]
- Bava, R.; Castagna, F.; Piras, C.; Palma, E.; Cringoli, G.; Musolino, V.; Lupia, C.; Perri, M.R.; Statti, G.; Britti, D.; et al. In vitro evaluation of acute toxicity of five Citrus spp. Essential oils towards the parasitic mite Varroa destructor. Pathogens 2021, 10, 1182. [Google Scholar] [CrossRef]
- Wang, X.; Hasegawa, J.; Kitamura, Y.; Wang, Z.; Matsuda, A.; Shinoda, W.; Miura, N.; Kimura, K. Effects of hesperidin on the progression of hypercholesterolemia and fatty liver induced by high-cholesterol diet in rats. J. Pharmacol. Sci. 2011, 117, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Khan, M.I.; Ashfaq, F.; Alsayegh, A.A.; Khatoon, F.; Altamimi, T.N.; Rizvi, S.I. Hesperidin Supplementation Improves Altered PON-1, LDL Oxidation, Inflammatory Response and Hepatic Function in an Experimental Rat Model of Hyperlipidemia. Indian J. Clin. Biochem. 2023, 1–7. [Google Scholar] [CrossRef]
- Parhiz, H.; Roohbakhsh, A.; Soltani, F.; Rezaee, R.; Iranshahi, M. Antioxidant and anti-inflammatory properties of the citrus flavonoids hesperidin and hesperetin: An updated review of their molecular mechanisms and experimental models. Phyther. Res. 2015, 29, 323–331. [Google Scholar] [CrossRef]
- Li, J.; Wang, T.; Liu, P.; Yang, F.; Wang, X.; Zheng, W.; Sun, W. Hesperetin ameliorates hepatic oxidative stress and inflammation via the PI3K/AKT-Nrf2-ARE pathway in oleic acid-induced HepG2 cells and a rat model of high-fat diet-induced NAFLD. Food Funct. 2021, 12, 3898–3918. [Google Scholar] [CrossRef]
- Cheraghpour, M.; Imani, H.; Ommi, S.; Alavian, S.M.; Karimi-Shahrbabak, E.; Hedayati, M.; Yari, Z.; Hekmatdoost, A. Hesperidin improves hepatic steatosis, hepatic enzymes, and metabolic and inflammatory parameters in patients with nonalcoholic fatty liver disease: A randomized, placebo-controlled, double-blind clinical trial. Phyther. Res. 2019, 33, 2118–2125. [Google Scholar] [CrossRef]
- Shokri Afra, H.; Zangooei, M.; Meshkani, R.; Ghahremani, M.H.; Ilbeigi, D.; Khedri, A.; Shahmohamadnejad, S.; Khaghani, S.; Nourbakhsh, M. Hesperetin is a potent bioactivator that activates SIRT1-AMPK signaling pathway in HepG2 cells. J. Physiol. Biochem. 2019, 75, 125–133. [Google Scholar] [CrossRef]
- Mulvihill, E.E.; Allister, E.M.; Sutherland, B.G.; Telford, D.E.; Sawyez, C.G.; Edwards, J.Y.; Markle, J.M.; Hegele, R.A.; Huff, M.W. Naringenin prevents dyslipidemia, apolipoprotein B overproduction, and hyperinsulinemia in LDL receptor–null mice with diet-induced insulin resistance. Diabetes 2009, 58, 2198–2210. [Google Scholar] [CrossRef]
- Ke, J.-Y.; Kliewer, K.L.; Hamad, E.M.; Cole, R.M.; Powell, K.A.; Andridge, R.R.; Straka, S.R.; Yee, L.D.; Belury, M.A. The flavonoid, naringenin, decreases adipose tissue mass and attenuates ovariectomy-associated metabolic disturbances in mice. Nutr. Metab. 2015, 12, 1. [Google Scholar] [CrossRef]
- Assini, J.M.; Mulvihill, E.E.; Burke, A.C.; Sutherland, B.G.; Telford, D.E.; Chhoker, S.S.; Sawyez, C.G.; Drangova, M.; Adams, A.C.; Kharitonenkov, A. Naringenin prevents obesity, hepatic steatosis, and glucose intolerance in male mice independent of fibroblast growth factor 21. Endocrinology 2015, 156, 2087–2102. [Google Scholar] [CrossRef]
- Chtourou, Y.; Fetoui, H.; Jemai, R.; Slima, A.B.; Makni, M.; Gdoura, R. Naringenin reduces cholesterol-induced hepatic inflammation in rats by modulating matrix metalloproteinases-2, 9 via inhibition of nuclear factor κB pathway. Eur. J. Pharmacol. 2015, 746, 96–105. [Google Scholar] [CrossRef]
- Sharma, A.K.; Bharti, S.; Ojha, S.; Bhatia, J.; Kumar, N.; Ray, R.; Kumari, S.; Arya, D.S. Up-regulation of PPARγ, heat shock protein-27 and-72 by naringin attenuates insulin resistance, β-cell dysfunction, hepatic steatosis and kidney damage in a rat model of type 2 diabetes. Br. J. Nutr. 2011, 106, 1713–1723. [Google Scholar] [CrossRef]
- Ross, J.A.; Kasum, C.M. Dietary flavonoids: Bioavailability, metabolic effects, and safety. Annu. Rev. Nutr. 2002, 22, 19–34. [Google Scholar] [CrossRef]
- Matsumoto, T. Phytochemistry Research Progress; Nova Publishers: New York, NY, USA, 2008; ISBN 1604562323. [Google Scholar]
- Guo, H.; Liu, D.; Ma, Y.; Liu, J.; Wang, Y.; Du, Z.; Wang, X.; Shen, J.; Peng, H. Long-term baicalin administration ameliorates metabolic disorders and hepatic steatosis in rats given a high-fat diet. Acta Pharmacol. Sin. 2009, 30, 1505–1512. [Google Scholar] [CrossRef]
- Xi, Y.; Wu, M.; Li, H.; Dong, S.; Luo, E.; Gu, M.; Shen, X.; Jiang, Y.; Liu, Y.; Liu, H. Baicalin attenuates high fat diet-induced obesity and liver dysfunction: Dose-response and potential role of CaMKKβ/AMPK/ACC pathway. Cell. Physiol. Biochem. 2015, 35, 2349–2359. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Liu, M.; Yu, H.; Li, J.; Wang, S.; Zhang, Y.; Qiu, F.; Wang, T. Scutellaria baicalensis regulates FFA metabolism to ameliorate NAFLD through the AMPK-mediated SREBP signaling pathway. J. Nat. Med. 2018, 72, 655–666. [Google Scholar] [CrossRef] [PubMed]
- Malinska, H.; Hüttl, M.; Oliyarnyk, O.; Markova, I.; Poruba, M.; Racova, Z.; Kazdova, L.; Vecera, R. Beneficial effects of troxerutin on metabolic disorders in non-obese model of metabolic syndrome. PLoS ONE 2019, 14, e0220377. [Google Scholar] [CrossRef]
- Geetha, R.; Yogalakshmi, B.; Sreeja, S.; Bhavani, K.; Anuradha, C.V. Troxerutin suppresses lipid abnormalities in the heart of high-fat–high-fructose diet-fed mice. Mol. Cell. Biochem. 2014, 387, 123–134. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, X.; Zheng, G.; Shan, Q.; Lu, J.; Fan, S.; Sun, C.; Wu, D.; Zhang, C.; Su, W. Troxerutin attenuates enhancement of hepatic gluconeogenesis by inhibiting NOD activation-mediated inflammation in high-fat diet-treated mice. Int. J. Mol. Sci. 2016, 18, 31. [Google Scholar] [CrossRef]
- Wang, N.; Gao, E.; Cui, C.; Wang, F.; Ren, H.; Xu, C.; Ning, C.; Zheng, Y.; Liu, Q.; Yu, Q. The combined anticancer of peanut skin procyanidins and resveratrol to CACO-2 colorectal cancer cells. Food Sci. Nutr. 2023, 11, 6483–6497. [Google Scholar] [CrossRef]
- Faghihzadeh, F.; Adibi, P.; Rafiei, R.; Hekmatdoost, A. Resveratrol supplementation improves inflammatory biomarkers in patients with nonalcoholic fatty liver disease. Nutr. Res. 2014, 34, 837–843. [Google Scholar] [CrossRef]
- Silva, K.A.S.; Freitas, D.F.; Borém, L.M.A.; Oliveira, L.P.; Oliveira, J.R.; Paraíso, A.F.; Guimarães, A.L.S.; de Paula, A.M.B.; D’Angelis, C.E.M.; Santos, S.H.S. Resveratrol Attenuates Non-alcoholic Fatty Liver Disease in Obese Mice Modulating MAF1. Rev. Bras. Farmacogn. 2022, 32, 786–795. [Google Scholar] [CrossRef]
- Cheng, K.; Song, Z.; Zhang, H.; Li, S.; Wang, C.; Zhang, L.; Wang, T. The therapeutic effects of resveratrol on hepatic steatosis in high-fat diet-induced obese mice by improving oxidative stress, inflammation and lipid-related gene transcriptional expression. Med. Mol. Morphol. 2019, 52, 187–197. [Google Scholar] [CrossRef]
- Ji, G.; Wang, Y.; Deng, Y.; Li, X.; Jiang, Z. Resveratrol ameliorates hepatic steatosis and inflammation in methionine/choline-deficient diet-induced steatohepatitis through regulating autophagy. Lipids Health Dis. 2015, 14, 134. [Google Scholar] [CrossRef]
- Suenaga, F.; Hatsushika, K.; Takano, S.; Ando, T.; Ohnuma, Y.; Ogawa, H.; Nakao, A. A possible link between resveratrol and TGF-β: Resveratrol induction of TGF-β expression and signaling. FEBS Lett. 2008, 582, 586–590. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Ma, J.; Wang, W.; Zhang, L.; Xu, J.; Wang, K.; Li, D. Resveratrol supplement inhibited the NF-κB inflammation pathway through activating AMPKα-SIRT1 pathway in mice with fatty liver. Mol. Cell. Biochem. 2016, 422, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Zhang, Y.; Zhang, X.; Aa, J.; Wang, G.; Xie, Y. Curcumin regulates endogenous and exogenous metabolism via Nrf2-FXR-LXR pathway in NAFLD mice. Biomed. Pharmacother. 2018, 105, 274–281. [Google Scholar] [CrossRef]
- Inzaugarat, M.E.; De Matteo, E.; Baz, P.; Lucero, D.; García, C.C.; Gonzalez Ballerga, E.; Daruich, J.; Sorda, J.A.; Wald, M.R.; Cherñavsky, A.C. New evidence for the therapeutic potential of curcumin to treat nonalcoholic fatty liver disease in humans. PLoS ONE 2017, 12, e0172900. [Google Scholar] [CrossRef] [PubMed]
- Mirhafez, S.R.; Farimani, A.R.; Dehhabe, M.; Bidkhori, M.; Hariri, M.; Ghouchani, B.F.N.M.; Abdollahi, F. Effect of phytosomal curcumin on circulating levels of adiponectin and leptin in patients with non-alcoholic fatty liver disease: A randomized, double-blind, placebo-controlled clinical trial. J. Gastrointest. Liver Dis. 2019, 28, 183–189. [Google Scholar] [CrossRef]
- Shan, D.; Wang, J.; Di, Q.; Jiang, Q.; Xu, Q. Steatosis induced by nonylphenol in HepG2 cells and the intervention effect of curcumin. Food Funct. 2022, 13, 327–343. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Colletti, A.; Bellentani, S. Nutraceutical approach to non-alcoholic fatty liver disease (NAFLD): The available clinical evidence. Nutrients 2018, 10, 1153. [Google Scholar] [CrossRef]
- Liu, Y.N.; Zhang, Z.X. Effect of berberine on a cellular model of non-alcoholic fatty liver disease. Int J Clin Exp Med 2017, 10, 16360–16366. [Google Scholar]
- Ren, S.; Ma, X.; Wang, R.; Liu, H.; Wei, Y.; Wei, S.; Jing, M.; Zhao, Y. Preclinical evidence of berberine on non-alcoholic fatty liver disease: A systematic review and meta-analysis of animal studies. Front. Pharmacol. 2021, 12, 742465. [Google Scholar] [CrossRef]
- Deng, Y.; Tang, K.; Chen, R.; Nie, H.; Liang, S.; Zhang, J.; Zhang, Y.; Yang, Q. Berberine attenuates hepatic oxidative stress in rats with non-alcoholic fatty liver disease via the Nrf2/ARE signalling pathway. Exp. Ther. Med. 2019, 17, 2091–2098. [Google Scholar] [CrossRef]
- Wang, L.; Jia, Z.; Wang, B.; Zhang, B. Berberine inhibits liver damage in rats with non-alcoholic fatty liver disease by regulating TLR4/MyD88/NF-κB pathway. Turkish J. Gastroenterol. 2020, 31, 902. [Google Scholar] [CrossRef]
- Yan, H.-M.; Xia, M.-F.; Wang, Y.; Chang, X.-X.; Yao, X.-Z.; Rao, S.-X.; Zeng, M.-S.; Tu, Y.-F.; Feng, R.; Jia, W.-P. Efficacy of berberine in patients with non-alcoholic fatty liver disease. PLoS ONE 2015, 10, e0134172. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, X.; Xu, M.; Jiang, L.; Zhou, M.; Liu, W.; Chen, Z.; Wang, Y.; Zou, Q.; Wang, L. Betaine prevented high-fat diet-induced NAFLD by regulating the FGF10/AMPK signaling pathway in ApoE−/− mice. Eur. J. Nutr. 2021, 60, 1655–1668. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, L.; Wang, L.; Li, X.; Zhang, H.; Luo, L.-P.; Song, J.-C.; Gong, Z. Betaine protects against high-fat-diet-induced liver injury by inhibition of high-mobility group box 1 and Toll-like receptor 4 expression in rats. Dig. Dis. Sci. 2013, 58, 3198–3206. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Y.; Zhou, R.; Chen, X.; Wang, C.; Tan, X.; Wang, L.; Zheng, R.; Zhang, H.; Ling, W. Associations of gut-flora-dependent metabolite trimethylamine-N-oxide, betaine and choline with non-alcoholic fatty liver disease in adults. Sci. Rep. 2016, 6, 19076. [Google Scholar] [CrossRef]
- Zhang, Y.; Geng, C.; Liu, X.; Li, M.; Gao, M.; Liu, X.; Fang, F.; Chang, Y. Celastrol ameliorates liver metabolic damage caused by a high-fat diet through Sirt1. Mol. Metab. 2017, 6, 138–147. [Google Scholar] [CrossRef]
- Sun, J.; Wang, H.; Yu, J.; Li, T.; Han, Y. Protective effect of celastrol on type 2 diabetes mellitus with nonalcoholic fatty liver disease in mice. Food Sci. Nutr. 2020, 8, 6207–6216. [Google Scholar] [CrossRef]
- Bakar, M.H.A.; Tan, J.S. Improvement of mitochondrial function by celastrol in palmitate-treated C2C12 myotubes via activation of PI3K-Akt signaling pathway. Biomed. Pharmacother. 2017, 93, 903–912. [Google Scholar] [CrossRef]
- Yu, X.; Meng, X.; Xu, M.; Zhang, X.; Zhang, Y.; Ding, G.; Huang, S.; Zhang, A.; Jia, Z. Celastrol ameliorates cisplatin nephrotoxicity by inhibiting NF-κB and improving mitochondrial function. EBioMedicine 2018, 36, 266–280. [Google Scholar] [CrossRef]
- Zaitone, S.A.; Barakat, B.M.; Bilasy, S.E.; Fawzy, M.S.; Abdelaziz, E.Z.; Farag, N.E. Protective effect of boswellic acids versus pioglitazone in a rat model of diet-induced non-alcoholic fatty liver disease: Influence on insulin resistance and energy expenditure. Naunyn. Schmiedebergs. Arch. Pharmacol. 2015, 388, 587–600. [Google Scholar] [CrossRef]
- Alappat, L.; Awad, A.B. Curcumin and obesity: Evidence and mechanisms. Nutr. Rev. 2010, 68, 729–738. [Google Scholar] [CrossRef]
- Kuo, J.-J.; Chang, H.-H.; Tsai, T.-H.; Lee, T.-Y. Curcumin ameliorates mitochondrial dysfunction associated with inhibition of gluconeogenesis in free fatty acid-mediated hepatic lipoapoptosis. Int. J. Mol. Med. 2012, 30, 643–649. [Google Scholar] [CrossRef]
- Jazayeri-Tehrani, S.A.; Rezayat, S.M.; Mansouri, S.; Qorbani, M.; Alavian, S.M.; Daneshi-Maskooni, M.; Hosseinzadeh-Attar, M.-J. Nano-curcumin improves glucose indices, lipids, inflammation, and Nesfatin in overweight and obese patients with non-alcoholic fatty liver disease (NAFLD): A double-blind randomized placebo-controlled clinical trial. Nutr. Metab. 2019, 16, 8. [Google Scholar] [CrossRef]
- Varma, K.; Haponiuk, J.T.; Gopi, S. Antiinflammatory Activity of Boswellia. In Inflammation and Natural Products; Elsevier: Amsterdam, The Netherlands, 2021; pp. 147–159. [Google Scholar]
- Tang, G.; Xu, Y.; Zhang, C.; Wang, N.; Li, H.; Feng, Y. Green tea and epigallocatechin gallate (EGCG) for the management of nonalcoholic fatty liver diseases (NAFLD): Insights into the role of oxidative stress and antioxidant mechanism. Antioxidants 2021, 10, 1076. [Google Scholar] [CrossRef]
- Bruno, R.S.; Dugan, C.E.; Smyth, J.A.; DiNatale, D.A.; Koo, S.I. Green tea extract protects leptin-deficient, spontaneously obese mice from hepatic steatosis and injury. J. Nutr. 2008, 138, 323–331. [Google Scholar] [CrossRef]
- Bose, M.; Lambert, J.D.; Ju, J.; Reuhl, K.R.; Shapses, S.A.; Yang, C.S. The major green tea polyphenol,(-)-epigallocatechin-3-gallate, inhibits obesity, metabolic syndrome, and fatty liver disease in high-fat–fed mice. J. Nutr. 2008, 138, 1677–1683. [Google Scholar] [CrossRef]
- Kuzu, N.; Bahcecioglu, I.H.; Dagli, A.F.; Ozercan, I.H.; Ustündag, B.; Sahin, K. Epigallocatechin gallate attenuates experimental non-alcoholic steatohepatitis induced by high fat diet. J. Gastroenterol. Hepatol. 2008, 23, e465–e470. [Google Scholar] [CrossRef]
- Chung, M.-Y.; Park, H.J.; Manautou, J.E.; Koo, S.I.; Bruno, R.S. Green tea extract protects against nonalcoholic steatohepatitis in ob/ob mice by decreasing oxidative and nitrative stress responses induced by proinflammatory enzymes. J. Nutr. Biochem. 2012, 23, 361–367. [Google Scholar] [CrossRef]
- Nakamoto, K.; Takayama, F.; Mankura, M.; Hidaka, Y.; Egashira, T.; Ogino, T.; Kawasaki, H.; Mori, A. Beneficial effects of fermented green tea extract in a rat model of non-alcoholic steatohepatitis. J. Clin. Biochem. Nutr. 2009, 44, 239–246. [Google Scholar] [CrossRef]
- Pivari, F.; Mingione, A.; Brasacchio, C.; Soldati, L. Curcumin and type 2 diabetes mellitus: Prevention and treatment. Nutrients 2019, 11, 1837. [Google Scholar] [CrossRef]
- Chuengsamarn, S.; Rattanamongkolgul, S.; Luechapudiporn, R.; Phisalaphong, C.; Jirawatnotai, S. Curcumin extract for prevention of type 2 diabetes. Diabetes Care 2012, 35, 2121–2127. [Google Scholar] [CrossRef]
- Elahi, R.K. Preventive effects of turmeric (Curcuma longa Linn.) powder on hepatic steatosis in the rats fed with high fat diet. Life Sci. J. 2012, 9, 5462–5468. [Google Scholar]
- Farzaei, M.H.; Zobeiri, M.; Parvizi, F.; El-Senduny, F.F.; Marmouzi, I.; Coy-Barrera, E.; Naseri, R.; Nabavi, S.M.; Rahimi, R.; Abdollahi, M. Curcumin in liver diseases: A systematic review of the cellular mechanisms of oxidative stress and clinical perspective. Nutrients 2018, 10, 855. [Google Scholar] [CrossRef] [PubMed]
- Baljinder, S.; Seena, G.; Dharmendra, K.; Vikas, G.; Bansal, P. Pharmacological potential of Eriobotrya japonica—An overview. Int. Res. J. Pharm 2010, 1, 95–99. [Google Scholar]
- Oh, J.; Min, O.-J.; Kim, H.-A.; Kim, Y.J.; Baek, H.Y.; Rhyu, D.Y. Effect of Eriobotrya japonica on adipogenesis and body weight. J. Korean Soc. Appl. Biol. Chem. 2011, 54, 382–387. [Google Scholar] [CrossRef]
- Tanaka, K.; Nishizono, S.; Makino, N.; Tamaru, S.; Terai, O.; Ikeda, I. Hypoglycemic activity of Eriobotrya japonica seeds in type 2 diabetic rats and mice. Biosci. Biotechnol. Biochem. 2008, 72, 686–693. [Google Scholar] [CrossRef]
- Cong, W.; Tao, R.; Tian, J.; Zhao, J.; Liu, Q.; Ye, F. EGb761, an extract of Ginkgo biloba leaves, reduces insulin resistance in a high-fat-fed mouse model. Acta Pharm. Sin. B 2011, 1, 14–20. [Google Scholar] [CrossRef]
- Xia, J.; Zhang, X.; Ye, X.; Liu, J.; Wang, L.; Zhong, Y. Intervention study of Ginkgo biloba extract in rat model of lipid-induced insulin resistance. J. Med. Plant Res. 2011, 5, 6284–6290. [Google Scholar]
- Wang, S.D.; Xie, Z.Q.; Chen, J.; Wang, K.; Wei, T.; Zhao, A.H.; Zhang, Q.H. Inhibitory effect of G inkgo biloba extract on fatty liver: Regulation of carnitine palmitoyltransferase 1a and fatty acid metabolism. J. Dig. Dis. 2012, 13, 525–535. [Google Scholar] [CrossRef]
- Perez-Martinez, P.; Garcia-Rios, A.; Delgado-Lista, J.; Perez-Jimenez, F.; Lopez-Miranda, J. Mediterranean diet rich in olive oil and obesity, metabolic syndrome and diabetes mellitus. Curr. Pharm. Des. 2011, 17, 769–777. [Google Scholar] [CrossRef]
- Abenavoli, L.; Milanović, M.; Milić, N.; Luzza, F.; Giuffrè, A.M. Olive oil antioxidants and non-alcoholic fatty liver disease. Expert Rev. Gastroenterol. Hepatol. 2019, 13, 739–749. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.-T.; Pang, J.-H.S.; Yang, R.-C. Anti-cancer effects of Phyllanthus urinaria and relevant mechanisms. Chang Gung Med. J. 2010, 33, 477–487. [Google Scholar] [PubMed]
- Poudyal, H.; Campbell, F.; Brown, L. Olive leaf extract attenuates cardiac, hepatic, and metabolic changes in high carbohydrate–, high fat–fed rats. J. Nutr. 2010, 140, 946–953. [Google Scholar] [CrossRef] [PubMed]
- Omagari, K.; Kato, S.; Tsuneyama, K.; Hatta, H.; Sato, M.; Hamasaki, M.; Sadakane, Y.; Tashiro, T.; Fukuhata, M.; Miyata, Y. Olive leaf extract prevents spontaneous occurrence of non-alcoholic steatohepatitis in SHR/NDmcr-cp rats. Pathology 2010, 42, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Jurenka, J. Therapeutic applications of pomegranate (Punica granatum L.): A review. Altern. Med. Rev. 2008, 13, 128–144. [Google Scholar]
- Zarfeshany, A.; Asgary, S.; Javanmard, S.H. Potent health effects of pomegranate. Adv. Biomed. Res. 2014, 3, 100. [Google Scholar]
- Cheng, J.; Li, J.; Xiong, R.-G.; Wu, S.-X.; Huang, S.-Y.; Zhou, D.-D.; Saimaiti, A.; Shang, A.; Feng, Y.; Gan, R.-Y. Bioactive compounds and health benefits of pomegranate: An updated narrative review. Food Biosci. 2023, 53, 102629. [Google Scholar] [CrossRef]
- Castagna, F.; Britti, D.; Oliverio, M.; Bosco, A.; Bonacci, S.; Iriti, G.; Ragusa, M.; Musolino, V.; Rinaldi, L.; Palma, E. In Vitro Anthelminthic Efficacy of Aqueous Pomegranate (Punica granatum L.) Extracts against Gastrointestinal Nematodes of Sheep. Pathogens 2020, 9, 1063. [Google Scholar] [CrossRef]
- Jafri, M.A.; Aslam, M.; Javed, K.; Singh, S. Effect of Punica granatum Linn.(flowers) on blood glucose level in normal and alloxan-induced diabetic rats. J. Ethnopharmacol. 2000, 70, 309–314. [Google Scholar] [CrossRef]
- Bagri, P.; Ali, M.; Aeri, V.; Bhowmik, M.; Sultana, S. Antidiabetic effect of Punica granatum flowers: Effect on hyperlipidemia, pancreatic cells lipid peroxidation and antioxidant enzymes in experimental diabetes. Food Chem. Toxicol. 2009, 47, 50–54. [Google Scholar] [CrossRef]
- Li, Y.; Wen, S.; Kota, B.P.; Peng, G.; Li, G.Q.; Yamahara, J.; Roufogalis, B.D. Punica granatum flower extract, a potent α-glucosidase inhibitor, improves postprandial hyperglycemia in Zucker diabetic fatty rats. J. Ethnopharmacol. 2005, 99, 239–244. [Google Scholar] [CrossRef]
- Xu, K.Z.-Y.; Zhu, C.; Kim, M.S.; Yamahara, J.; Li, Y. Pomegranate flower ameliorates fatty liver in an animal model of type 2 diabetes and obesity. J. Ethnopharmacol. 2009, 123, 280–287. [Google Scholar] [CrossRef]
- Zhang, Y.; Hai, J.; Cao, M.; Zhang, Y.; Pei, S.; Wang, J.; Zhang, Q. Silibinin ameliorates steatosis and insulin resistance during non-alcoholic fatty liver disease development partly through targeting IRS-1/PI3K/Akt pathway. Int. Immunopharmacol. 2013, 17, 714–720. [Google Scholar] [CrossRef]
- Hajiaghamohammadi, A.A.; Ziaee, A.; Oveisi, S.; Masroor, H. Effects of metformin, pioglitazone, and silymarin treatment on non-alcoholic fatty liver disease: A randomized controlled pilot study. Hepat. Mon. 2012, 12, e6099. [Google Scholar] [CrossRef]
- Salomone, F.; Barbagallo, I.; Godos, J.; Lembo, V.; Currenti, W.; Cinà, D.; Avola, R.; D’Orazio, N.; Morisco, F.; Galvano, F. Silibinin restores NAD+ levels and induces the SIRT1/AMPK pathway in non-alcoholic fatty liver. Nutrients 2017, 9, 1086. [Google Scholar] [CrossRef]
- Cui, C.-X.; Deng, J.-N.; Yan, L.; Liu, Y.-Y.; Fan, J.-Y.; Mu, H.-N.; Sun, H.-Y.; Wang, Y.-H.; Han, J.-Y. Silibinin capsules improves high fat diet-induced nonalcoholic fatty liver disease in hamsters through modifying hepatic de novo lipogenesis and fatty acid oxidation. J. Ethnopharmacol. 2017, 208, 24–35. [Google Scholar] [CrossRef]
- Gu, M.; Zhao, P.; Huang, J.; Zhao, Y.; Wang, Y.; Li, Y.; Li, Y.; Fan, S.; Ma, Y.-M.; Tong, Q. Silymarin ameliorates metabolic dysfunction associated with diet-induced obesity via activation of farnesyl X receptor. Front. Pharmacol. 2016, 7, 345. [Google Scholar] [CrossRef]
- Haddad, Y.; Vallerand, D.; Brault, A.; Haddad, P.S. Antioxidant and hepatoprotective effects of silibinin in a rat model of nonalcoholic steatohepatitis. Evid.-Based Complement. Altern. Med. 2011, 2011, 647903. [Google Scholar] [CrossRef] [PubMed]
- Serviddio, G.; Bellanti, F.; Giudetti, A.M.; Gnoni, G.V.; Petrella, A.; Tamborra, R.; Romano, A.D.; Rollo, T.; Vendemiale, G.; Altomare, E. A silybin-phospholipid complex prevents mitochondrial dysfunction in a rodent model of nonalcoholic steatohepatitis. J. Pharmacol. Exp. Ther. 2010, 332, 922–932. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.M.; Kim, Y.; Lee, E.S.; Huh, J.H.; Chung, C.H. Caffeic acid ameliorates hepatic steatosis and reduces ER stress in high fat diet–induced obese mice by regulating autophagy. Nutrition 2018, 55, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.-J.; Li, Y.-F.; Wang, G.-E.; Tan, R.-R.; Tsoi, B.; Mao, G.-W.; Zhai, Y.-J.; Cao, L.-F.; Chen, M.; Kurihara, H. Caffeine ameliorates high energy diet-induced hepatic steatosis: Sirtuin 3 acts as a bridge in the lipid metabolism pathway. Food Funct. 2015, 6, 2578–2587. [Google Scholar] [CrossRef]
- Watanabe, S.; Takahashi, T.; Ogawa, H.; Uehara, H.; Tsunematsu, T.; Baba, H.; Morimoto, Y.; Tsuneyama, K. Daily coffee intake inhibits pancreatic beta cell damage and nonalcoholic steatohepatitis in a mouse model of spontaneous metabolic syndrome, tsumura-suzuki obese diabetic mice. Metab. Syndr. Relat. Disord. 2017, 15, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Ontawong, A.; Boonphang, O.; Pasachan, T.; Duangjai, A.; Pongchaidecha, A.; Phatsara, M.; Jinakote, M.; Amornlerdpison, D.; Srimaroeng, C. Hepatoprotective effect of coffee pulp aqueous extract combined with simvastatin against hepatic steatosis in high-fat diet-induced obese rats. J. Funct. Foods 2019, 54, 568–577. [Google Scholar] [CrossRef]
- Vitaglione, P.; Mazzone, G.; Lembo, V.; D’Argenio, G.; Rossi, A.; Guido, M.; Savoia, M.; Salomone, F.; Mennella, I.; De Filippis, F. Coffee prevents fatty liver disease induced by a high-fat diet by modulating pathways of the gut–liver axis. J. Nutr. Sci. 2019, 8, e15. [Google Scholar] [CrossRef]
- Zhang, D.-F.; Zhang, F.; Zhang, J.; Zhang, R.-M.; Li, R. Protection effect of trigonelline on liver of rats with non-alcoholic fatty liver diseases. Asian Pac. J. Trop. Med. 2015, 8, 651–654. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Cai, X.; Hayashi, S.; Hao, S.; Sakiyama, H.; Wang, X.; Yang, Q.; Akira, S.; Nishiguchi, S.; Fujiwara, N. Caffeine-stimulated muscle IL-6 mediates alleviation of non-alcoholic fatty liver disease. Biochim. Biophys. Acta (BBA)-Molecular Cell Biol. Lipids 2019, 1864, 271–280. [Google Scholar] [CrossRef]
- Brandt, A.; Nier, A.; Jin, C.J.; Baumann, A.; Jung, F.; Ribas, V.; García-Ruiz, C.; Fernández-Checa, J.C.; Bergheim, I. Consumption of decaffeinated coffee protects against the development of early non-alcoholic steatohepatitis: Role of intestinal barrier function. Redox Biol. 2019, 21, 101092. [Google Scholar] [CrossRef]
- Chen, S.; Teoh, N.C.; Chitturi, S.; Farrell, G.C. Coffee and non-alcoholic fatty liver disease: Brewing evidence for hepatoprotection? J. Gastroenterol. Hepatol. 2014, 29, 435–441. [Google Scholar] [CrossRef]
- Vitaglione, P.; Morisco, F.; Mazzone, G.; Amoruso, D.C.; Ribecco, M.T.; Romano, A.; Fogliano, V.; Caporaso, N.; D’Argenio, G. Coffee reduces liver damage in a rat model of steatohepatitis: The underlying mechanisms and the role of polyphenols and melanoidins. Hepatology 2010, 52, 1652–1661. [Google Scholar] [CrossRef]
- Salomone, F.; Volti, G.L.; Vitaglione, P.; Morisco, F.; Fogliano, V.; Zappalà, A.; Palmigiano, A.; Garozzo, D.; Caporaso, N.; D’Argenio, G. Coffee enhances the expression of chaperones and antioxidant proteins in rats with nonalcoholic fatty liver disease. Transl. Res. 2014, 163, 593–602. [Google Scholar] [CrossRef]
- Pietrocola, F.; Malik, S.A.; Mariño, G.; Vacchelli, E.; Senovilla, L.; Chaba, K.; Niso-Santano, M.; Maiuri, M.C.; Madeo, F.; Kroemer, G. Coffee induces autophagy in vivo. Cell Cycle 2014, 13, 1987–1994. [Google Scholar] [CrossRef]
- Shi, H.; Dong, L.; Dang, X.; Liu, Y.; Jiang, J.; Wang, Y.; Lu, X.; Guo, X. Effect of chlorogenic acid on LPS-induced proinflammatory signaling in hepatic stellate cells. Inflamm. Res. 2013, 62, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.I.; Kim, T.H.; Rico, C.W.; Kang, M.Y. Effect of instant cooked giant embryonic rice on body fat weight and plasma lipid profile in high fat-fed mice. Nutrients 2014, 6, 2266–2278. [Google Scholar] [CrossRef]
- Wang, Y.-X.; Li, Y.; Sun, A.-M.; Wang, F.-J.; Yu, G.-P. Hypolipidemic and antioxidative effects of aqueous enzymatic extract from rice bran in rats fed a high-fat and-cholesterol diet. Nutrients 2014, 6, 3696–3710. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.H.; Um, M.Y.; Ahn, J.; Jung, C.H.; Ha, T.Y. Long-term intake of rice improves insulin sensitivity in mice fed a high-fat diet. Nutrition 2014, 30, 920–927. [Google Scholar] [CrossRef]
- Senadheera, S.P.A.S.; Ekanayake, S.; Wanigatunge, C. Anti-diabetic properties of rice-based herbal porridges in diabetic Wistar rats. Phyther. Res. 2014, 28, 1567–1572. [Google Scholar] [CrossRef]
- Yang, C.W.; Mousa, S.A. The effect of red yeast rice (Monascus purpureus) in dyslipidemia and other disorders. Complement. Ther. Med. 2012, 20, 466–474. [Google Scholar] [CrossRef] [PubMed]
- Klimek, M.; Wang, S.; Ogunkanmi, A. Safety and efficacy of red yeast rice (Monascus purpureus) as an alternative therapy for hyperlipidemia. Pharm. Ther. 2009, 34, 313. [Google Scholar]
- Childress, L.; Gay, A.; Zargar, A.; Ito, M.K. Review of red yeast rice content and current Food and Drug Administration oversight. J. Clin. Lipidol. 2013, 7, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Gerards, M.C.; Terlou, R.J.; Yu, H.; Koks, C.H.W.; Gerdes, V.E.A. Traditional Chinese lipid-lowering agent red yeast rice results in significant LDL reduction but safety is uncertain—A systematic review and meta-analysis. Atherosclerosis 2015, 240, 415–423. [Google Scholar] [CrossRef]
- D’Antuono, I.; Garbetta, A.; Linsalata, V.; Minervini, F.; Cardinali, A. Polyphenols from artichoke heads (Cynara cardunculus (L.) subsp. scolymus Hayek): In vitro bio-accessibility, intestinal uptake and bioavailability. Food Funct. 2015, 6, 1268–1277. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Zhao, K.; Whiteman, M. The gastrointestinal tract: A major site of antioxidant action? Free Radic. Res. 2000, 33, 819–830. [Google Scholar] [CrossRef]
- Magielse, J.; Verlaet, A.; Breynaert, A.; Keenoy, B.M.Y.; Apers, S.; Pieters, L.; Hermans, N. Investigation of the in vivo antioxidative activity of Cynara scolymus (artichoke) leaf extract in the streptozotocin-induced diabetic rat. Mol. Nutr. Food Res. 2014, 58, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Küçükgergin, C.; Aydın, A.F.; Özdemirler-Erata, G.; Mehmetçik, G.; Koçak-Toker, N.; Uysal, M. Effect of artichoke leaf extract on hepatic and cardiac oxidative stress in rats fed on high cholesterol diet. Biol. Trace Elem. Res. 2010, 135, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Jacociunas, L.V.; de Andrade, H.H.R.; Lehmann, M.; de Abreu, B.R.R.; Ferraz, A.d.B.F.; da Silva, J.; Grivicich, I.; Dihl, R.R. Artichoke induces genetic toxicity in the cytokinesis-block micronucleus (CBMN) cytome assay. Food Chem. Toxicol. 2013, 55, 56–59. [Google Scholar] [CrossRef]
- Pereira, C.; Barreira, J.C.M.; Calhelha, R.C.; Queiroz, M.J.R.P.; Barros, L.; Ferreira, I.C.F.R. New insights into the effects of formulation type and compositional mixtures on the antioxidant and cytotoxic activities of dietary supplements based-on hepatoprotective plants. Food Funct. 2014, 5, 2052–2060. [Google Scholar] [CrossRef]
- Imai, S. Soybean and processed soy foods ingredients, and their role in cardiometabolic risk prevention. Recent Pat. Food. Nutr. Agric. 2015, 7, 75–82. [Google Scholar] [CrossRef]
- Yoon, G.-A.; Park, S. Antioxidant action of soy isoflavones on oxidative stress and antioxidant enzyme activities in exercised rats. Nutr. Res. Pract. 2014, 8, 618–624. [Google Scholar] [CrossRef]
- Oh, H.-G.; Kang, Y.-R.; Lee, H.-Y.; Kim, J.-H.; Shin, E.-H.; Lee, B.-G.; Park, S.-H.; Moon, D.-I.; Kim, O.-J.; Lee, I.-A. Ameliorative effects of Monascus pilosus-fermented black soybean (Glycine max L. Merrill) on high-fat diet-induced obesity. J. Med. Food 2014, 17, 972–978. [Google Scholar] [CrossRef]
- Kim, Y.; Yoon, S.; Lee, S.B.; Han, H.W.; Oh, H.; Lee, W.J.; Lee, S.-M. Fermentation of soy milk via Lactobacillus plantarum improves dysregulated lipid metabolism in rats on a high cholesterol diet. PLoS ONE 2014, 9, e88231. [Google Scholar] [CrossRef]
- Gray, A.M.; Flatt, P.R. Pancreatic and extra-pancreatic effects of the traditional anti-diabetic plant, Medicago sativa (lucerne). Br. J. Nutr. 1997, 78, 325–334. [Google Scholar] [CrossRef]
- Raeeszadeh, M.; Beheshtipour, J.; Jamali, R.; Akbari, A. The antioxidant properties of Alfalfa (Medicago sativa L.) and its biochemical, antioxidant, anti-inflammatory, and pathological effects on nicotine-induced oxidative stress in the rat liver. Oxid. Med. Cell. Longev. 2022, 2022, 2691577. [Google Scholar] [CrossRef]
- Shi, Y.; Guo, R.; Wang, X.; Yuan, D.; Zhang, S.; Wang, J.; Yan, X.; Wang, C. The regulation of alfalfa saponin extract on key genes involved in hepatic cholesterol metabolism in hyperlipidemic rats. PLoS ONE 2014, 9, e88282. [Google Scholar] [CrossRef]
- Mollace, V.; Scicchitano, M.; Paone, S.; Casale, F.; Calandruccio, C.; Gliozzi, M.; Musolino, V.; Carresi, C.; Maiuolo, J.; Nucera, S. Hypoglycemic and hypolipemic effects of a new lecithin formulation of bergamot polyphenolic fraction: A double blind, randomized, placebo-controlled study. Endocr. Metab. Immune Disord. Targets (Former. Curr. Drug Targets-Immune Endocr. Metab. Disord.) 2019, 19, 136–143. [Google Scholar] [CrossRef]
- Quirino, A.; Giorgi, V.; Palma, E.; Marascio, N.; Morelli, P.; Maletta, A.; Divenuto, F.; De Angelis, G.; Tancrè, V.; Nucera, S. Citrus bergamia: Kinetics of Antimicrobial Activity on Clinical Isolates. Antibiotics 2022, 11, 361. [Google Scholar] [CrossRef] [PubMed]
- Maiuolo, J.; Bosco, F.; Guarnieri, L.; Nucera, S.; Ruga, S.; Oppedisano, F.; Tucci, L.; Muscoli, C.; Palma, E.; Giuffrè, A.M. Protective Role of an Extract Waste Product from Citrus bergamia in an In Vitro Model of Neurodegeneration. Plants 2023, 12, 2126. [Google Scholar] [CrossRef] [PubMed]
- Miceli, N.; Mondello, M.R.; Monforte, M.T.; Sdrafkakis, V.; Dugo, P.; Crupi, M.L.; Taviano, M.F.; De Pasquale, R.; Trovato, A. Hypolipidemic effects of Citrus bergamia Risso et Poiteau juice in rats fed a hypercholesterolemic diet. J. Agric. Food Chem. 2007, 55, 10671–10677. [Google Scholar] [CrossRef] [PubMed]
- Mare, R.; Mazza, E.; Ferro, Y.; Gliozzi, M.; Nucera, S.; Paone, S.; Aversa, I.; Pujia, R.; Marafioti, G.; Musolino, V. A new breakfast brioche containing bergamot fiber prevents insulin and glucose increase in healthy volunteers: A pilot study. Minerva Endocrinol. 2020, 46, 214–225. [Google Scholar] [CrossRef] [PubMed]
- Mirarchi, A.; Mare, R.; Musolino, V.; Nucera, S.; Mollace, V.; Pujia, A.; Montalcini, T.; Romeo, S.; Maurotti, S. Bergamot polyphenol extract reduces hepatocyte neutral fat by increasing beta-oxidation. Nutrients 2022, 14, 3434. [Google Scholar] [CrossRef]
- Ferro, Y.; Montalcini, T.; Mazza, E.; Foti, D.; Angotti, E.; Gliozzi, M.; Nucera, S.; Paone, S.; Bombardelli, E.; Aversa, I. Randomized clinical trial: Bergamot citrus and wild cardoon reduce liver steatosis and body weight in non-diabetic individuals aged over 50 years. Front. Endocrinol. 2020, 11, 494. [Google Scholar] [CrossRef]
- Pernice, R.; Borriello, G.; Ferracane, R.; Borrelli, R.C.; Cennamo, F.; Ritieni, A. Bergamot: A source of natural antioxidants for functionalized fruit juices. Food Chem. 2009, 112, 545–550. [Google Scholar] [CrossRef]
- Carresi, C.; Musolino, V.; Gliozzi, M.; Maiuolo, J.; Mollace, R.; Nucera, S.; Maretta, A.; Sergi, D.; Muscoli, S.; Gratteri, S. Anti-oxidant effect of bergamot polyphenolic fraction counteracts doxorubicin-induced cardiomyopathy: Role of autophagy and c-kitposCD45negCD31neg cardiac stem cell activation. J. Mol. Cell. Cardiol. 2018, 119, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Maiuolo, J.; Bava, I.; Carresi, C.; Gliozzi, M.; Musolino, V.; Scarano, F.; Nucera, S.; Scicchitano, M.; Bosco, F.; Ruga, S. The Effects of Bergamot Polyphenolic Fraction, Cynara cardunculus, and Olea europea L. Extract on Doxorubicin-Induced Cardiotoxicity. Nutrients 2021, 13, 2158. [Google Scholar] [CrossRef]
- Navarra, M.; Ursino, M.R.; Ferlazzo, N.; Russo, M.; Schumacher, U.; Valentiner, U. Effect of Citrus bergamia juice on human neuroblastoma cells in vitro and in metastatic xenograft models. Fitoterapia 2014, 95, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Visalli, G.; Ferlazzo, N.; Cirmi, S.; Campiglia, P.; Gangemi, S.; Di Pietro, A.; Calapai, G.; Navarra, M. Bergamot juice extract inhibits proliferation by inducing apoptosis in human colon cancer cells. Anti-Cancer Agents Med. Chem. (Former. Curr. Med. Chem. Agents) 2014, 14, 1402–1413. [Google Scholar] [CrossRef] [PubMed]
- Delle Monache, S.; Sanità, P.; Trapasso, E.; Ursino, M.R.; Dugo, P.; Russo, M.; Ferlazzo, N.; Calapai, G.; Angelucci, A.; Navarra, M. Mechanisms underlying the anti-tumoral effects of Citrus bergamia juice. PLoS ONE 2013, 8, e61484. [Google Scholar] [CrossRef] [PubMed]
- Mollace, V.; Sacco, I.; Janda, E.; Malara, C.; Ventrice, D.; Colica, C.; Visalli, V.; Muscoli, S.; Ragusa, S.; Muscoli, C. Hypolipemic and hypoglycaemic activity of bergamot polyphenols: From animal models to human studies. Fitoterapia 2011, 82, 309–316. [Google Scholar] [CrossRef]
- Musolino, V.; Gliozzi, M.; Carresi, C.; Maiuolo, J.; Mollace, R.; Bosco, F.; Scarano, F.; Scicchitano, M.; Maretta, A.; Palma, E. Lipid-lowering effect of bergamot polyphenolic fraction: Role of pancreatic cholesterol ester hydrolase. J. Biol. Regul. Homeost. Agents 2017, 31, 1087–1093. [Google Scholar]
- Gliozzi, M.; Walker, R.; Muscoli, S.; Vitale, C.; Gratteri, S.; Carresi, C.; Musolino, V.; Russo, V.; Janda, E.; Ragusa, S. Bergamot polyphenolic fraction enhances rosuvastatin-induced effect on LDL-cholesterol, LOX-1 expression and protein kinase B phosphorylation in patients with hyperlipidemia. Int. J. Cardiol. 2013, 170, 140–145. [Google Scholar] [CrossRef]
- Musolino, V.; Gliozzi, M.; Bombardelli, E.; Nucera, S.; Carresi, C.; Maiuolo, J.; Mollace, R.; Paone, S.; Bosco, F.; Scarano, F. The synergistic effect of Citrus bergamia and Cynara cardunculus extracts on vascular inflammation and oxidative stress in non-alcoholic fatty liver disease. J. Tradit. Complement. Med. 2020, 10, 268–274. [Google Scholar] [CrossRef]
- Maiuolo, J.; Mollace, R.; Bosco, F.; Scarano, F.; Oppedisano, F.; Nucera, S.; Ruga, S.; Guarnieri, L.; Macri, R.; Bava, I. The Phytochemical Synergistic Properties of Combination of Bergamot Polyphenolic Fraction and Cynara cardunculus Extract in Non-Alcoholic Fatty Liver Disease. Agriculture 2023, 13, 249. [Google Scholar] [CrossRef]
- Musolino, V.; Gliozzi, M.; Scarano, F.; Bosco, F.; Scicchitano, M.; Nucera, S.; Carresi, C.; Ruga, S.; Zito, M.C.; Maiuolo, J. Bergamot polyphenols improve dyslipidemia and pathophysiological features in a mouse model of non-alcoholic fatty liver disease. Sci. Rep. 2020, 10, 2565. [Google Scholar] [CrossRef]
- Herrero, M.; Plaza, M.; Cifuentes, A.; Ibáñez, E. Green processes for the extraction of bioactives from Rosemary: Chemical and functional characterization via ultra-performance liquid chromatography-tandem mass spectrometry and in-vitro assays. J. Chromatogr. A 2010, 1217, 2512–2520. [Google Scholar] [CrossRef] [PubMed]
- Afonso, M.S.; de O Silva, A.M.; Carvalho, E.B.T.; Rivelli, D.P.; Barros, S.B.M.; Rogero, M.M.; Lottenberg, A.M.; Torres, R.P.; Mancini-Filho, J. Phenolic compounds from Rosemary (Rosmarinus officinalis L.) attenuate oxidative stress and reduce blood cholesterol concentrations in diet-induced hypercholesterolemic rats. Nutr. Metab. 2013, 10, 19. [Google Scholar] [CrossRef] [PubMed]
- Labban, L.; Mustafa, U.E.-S.; Ibrahim, Y.M. The effects of rosemary (Rosmarinus officinalis) leaves powder on glucose level, lipid profile and lipid perodoxation. Int. J. Clin. Med. 2014, 5, 297–304. [Google Scholar] [CrossRef]
- Harach, T.; Aprikian, O.; Monnard, I.; Moulin, J.; Membrez, M.; Béolor, J.-C.; Raab, T.; Macé, K.; Darimont, C. Rosemary (Rosmarinus officinalis L.) leaf extract limits weight gain and liver steatosis in mice fed a high-fat diet. Planta Med. 2010, 76, 566–571. [Google Scholar] [CrossRef]
- Ibarra, A.; Cases, J.; Roller, M.; Chiralt-Boix, A.; Coussaert, A.; Ripoll, C. Carnosic acid-rich rosemary (Rosmarinus officinalis L.) leaf extract limits weight gain and improves cholesterol levels and glycaemia in mice on a high-fat diet. Br. J. Nutr. 2011, 106, 1182–1189. [Google Scholar] [CrossRef]
- Rau, O.; Wurglics, M.; Paulke, A.; Zitzkowski, J.; Meindl, N.; Bock, A.; Dingermann, T.; Abdel-Tawab, M.; Schubert-Zsilavecz, M. Carnosic acid and carnosol, phenolic diterpene compounds of the labiate herbs rosemary and sage, are activators of the human peroxisome proliferator-activated receptor gamma. Planta Med. 2006, 72, 881–887. [Google Scholar] [CrossRef]
- Wang, T.; Takikawa, Y.; Satoh, T.; Yoshioka, Y.; Kosaka, K.; Tatemichi, Y.; Suzuki, K. Carnosic acid prevents obesity and hepatic steatosis in ob/ob mice. Hepatol. Res. 2011, 41, 87–92. [Google Scholar] [CrossRef]
- Zhao, Y.; Sedighi, R.; Wang, P.; Chen, H.; Zhu, Y.; Sang, S. Carnosic acid as a major bioactive component in rosemary extract ameliorates high-fat-diet-induced obesity and metabolic syndrome in mice. J. Agric. Food Chem. 2015, 63, 4843–4852. [Google Scholar] [CrossRef]
- Shan, W.; Gao, L.; Zeng, W.; Hu, Y.; Wang, G.; Li, M.; Zhou, J.; Ma, X.; Tian, X.; Yao, J. Activation of the SIRT1/p66shc antiapoptosis pathway via carnosic acid-induced inhibition of miR-34a protects rats against nonalcoholic fatty liver disease. Cell Death Dis. 2015, 6, e1833. [Google Scholar] [CrossRef] [PubMed]
- Schuhmacher, A.; Reichling, J.; Schnitzler, P. Virucidal effect of peppermint oil on the enveloped viruses herpes simplex virus type 1 and type 2 in vitro. Phytomedicine 2003, 10, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Kwon, E.-Y.; Jung, U.J.; Park, T.; Yun, J.W.; Choi, M.-S. Luteolin attenuates hepatic steatosis and insulin resistance through the interplay between the liver and adipose tissue in mice with diet-induced obesity. Diabetes 2015, 64, 1658–1669. [Google Scholar] [CrossRef] [PubMed]
- Mesbahzadeh, B.; Akbari, M.; Zadeh, J.B. The effects of different levels of peppermint alcoholic extract on body-weight gain and blood biochemical parameters of adult male Wistar rats. Electron. Physician 2015, 7, 1376. [Google Scholar] [PubMed]
- Lima, C.F.; Valentao, P.C.R.; Andrade, P.B.; Seabra, R.M.; Fernandes-Ferreira, M.; Pereira-Wilson, C. Water and methanolic extracts of Salvia officinalis protect HepG2 cells from t-BHP induced oxidative damage. Chem. Biol. Interact. 2007, 167, 107–115. [Google Scholar] [CrossRef]
- Ninomiya, K.; Matsuda, H.; Shimoda, H.; Nishida, N.; Kasajima, N.; Yoshino, T.; Morikawa, T.; Yoshikawa, M. Carnosic acid, a new class of lipid absorption inhibitor from sage. Bioorg. Med. Chem. Lett. 2004, 14, 1943–1946. [Google Scholar] [CrossRef]
- Sá, C.M.; Ramos, A.A.; Azevedo, M.F.; Lima, C.F.; Fernandes-Ferreira, M.; Pereira-Wilson, C. Sage tea drinking improves lipid profile and antioxidant defences in humans. Int. J. Mol. Sci. 2009, 10, 3937–3950. [Google Scholar] [CrossRef] [PubMed]
- Behradmanesh, S.; Derees, F.; Rafieian-Kopaei, M. Effect of Salvia officinalis on diabetic patients. J. Ren. Inj. Prev. 2013, 2, 51. [Google Scholar]
- Sharma, S.K.; Vij, A.S.; Sharma, M. Mechanisms and clinical uses of capsaicin. Eur. J. Pharmacol. 2013, 720, 55–62. [Google Scholar] [CrossRef]
- McCarty, M.F.; DiNicolantonio, J.J.; O’Keefe, J.H. Capsaicin may have important potential for promoting vascular and metabolic health. Open Hear. 2015, 2, e000262. [Google Scholar] [CrossRef]
- Hong, Q.; Xia, C.; Xiangying, H.; Quan, Y. Capsinoids suppress fat accumulation via lipid metabolism. Mol. Med. Rep. 2015, 11, 1669–1674. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Li, L.; Wang, F.; Chen, J.; Zhao, Y.; Wang, P.; Nilius, B.; Liu, D.; Zhu, Z. Dietary capsaicin prevents nonalcoholic fatty liver disease through transient receptor potential vanilloid 1-mediated peroxisome proliferator-activated receptor δ activation. Pflügers Arch. J. Physiol. 2013, 465, 1303–1316. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.-H.; Kim, C.-S.; Han, I.-S.; Kawada, T.; Yu, R. Capsaicin, a spicy component of hot peppers, modulates adipokine gene expression and protein release from obese-mouse adipose tissues and isolated adipocytes, and suppresses the inflammatory responses of adipose tissue macrophages. FEBS Lett. 2007, 581, 4389–4396. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.; Shin, M.K.; Yoon, D.; Kim, A.; Yu, R.; Park, N.; Han, I. Topical application of capsaicin reduces visceral adipose fat by affecting adipokine levels in high-fat diet-induced obese mice. Obesity 2013, 21, 115–122. [Google Scholar] [CrossRef]
- Kang, J.; Tsuyoshi, G.; Han, I.; Kawada, T.; Kim, Y.M.; Yu, R. Dietary capsaicin reduces obesity-induced insulin resistance and hepatic steatosis in obese mice fed a high-fat diet. Obesity 2010, 18, 780–787. [Google Scholar] [CrossRef]
- McCarty, M.F. High mitochondrial redox potential may promote induction and activation of UCP2 in hepatocytes during hepatothermic therapy. Med. Hypotheses 2005, 64, 1216–1219. [Google Scholar] [CrossRef]
- Perri, M.R.; Marrelli, M.; Statti, G.; Conforti, F. Olea europaea bud extracts: Inhibitory effects on pancreatic lipase and α-amylase activities of different cultivars from Calabria region (Italy). Plant Biosyst. 2020. [Google Scholar] [CrossRef]
- Rezaei, S.; Akhlaghi, M.; Sasani, M.R.; Boldaji, R.B. Olive oil lessened fatty liver severity independent of cardiometabolic correction in patients with non-alcoholic fatty liver disease: A randomized clinical trial. Nutrition 2019, 57, 154–161. [Google Scholar] [CrossRef]
- Chen, S.; Zhao, X.; Ran, L.; Wan, J.; Wang, X.; Qin, Y.; Shu, F.; Gao, Y.; Yuan, L.; Zhang, Q. Resveratrol improves insulin resistance, glucose and lipid metabolism in patients with non-alcoholic fatty liver disease: A randomized controlled trial. Dig. Liver Dis. 2015, 47, 226–232. [Google Scholar] [CrossRef]
- Panahi, Y.; Kianpour, P.; Mohtashami, R.; Jafari, R.; Simental-Mendía, L.E.; Sahebkar, A. Efficacy and safety of phytosomal curcumin in non-alcoholic fatty liver disease: A randomized controlled trial. Drug Res. 2017, 67, 244–251. [Google Scholar] [CrossRef]
- Kobayashi, M.; Kawano, T.; Ukawa, Y.; Sagesaka, Y.M.; Fukuhara, I. Green tea beverages enriched with catechins with a galloyl moiety reduce body fat in moderately obese adults: A randomized double-blind placebo-controlled trial. Food Funct. 2016, 7, 498–507. [Google Scholar] [CrossRef] [PubMed]
- Abenavoli, L.; Greco, M.; Nazionale, I.; Peta, V.; Milic, N.; Accattato, F.; Foti, D.; Gulletta, E.; Luzza, F. Effects of Mediterranean diet supplemented with silybin–vitamin E–phospholipid complex in overweight patients with non-alcoholic fatty liver disease. Expert Rev. Gastroenterol. Hepatol. 2015, 9, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Hajagha, M.A.A.; Ziaei, A.; Rafiei, R. The efficacy of silymarin in decreasing transaminase activities in non-alcoholic fatty liver disease: A randomized controlled clinical trial. Hepat. Mon. 2008, 8, 191–195. [Google Scholar]
- Perumpail, B.J.; Khan, M.A.; Yoo, E.R.; Cholankeril, G.; Kim, D.; Ahmed, A. Clinical epidemiology and disease burden of nonalcoholic fatty liver disease. World J. Gastroenterol. 2017, 23, 8263. [Google Scholar] [CrossRef] [PubMed]
- Pei, K.; Gui, T.; Kan, D.; Feng, H.; Jin, Y.; Yang, Y.; Zhang, Q.; Du, Z.; Gai, Z.; Wu, J. An overview of lipid metabolism and nonalcoholic fatty liver disease. Biomed. Res. Int. 2020, 2020, 4020249. [Google Scholar] [CrossRef] [PubMed]
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Charlton, M.; Cusi, K.; Rinella, M.; Harrison, S.A.; Brunt, E.M.; Sanyal, A.J. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018, 67, 328–357. [Google Scholar] [CrossRef] [PubMed]
- Glen, J.; Floros, L.; Day, C.; Pryke, R. Non-alcoholic fatty liver disease (NAFLD): Summary of NICE guidance. BMJ 2016, 354, i4428. [Google Scholar] [CrossRef]
- van Baak, M.A.; Pramono, A.; Battista, F.; Beaulieu, K.; Blundell, J.E.; Busetto, L.; Carraça, E.V.; Dicker, D.; Encantado, J.; Ermolao, A. Effect of different types of regular exercise on physical fitness in adults with overweight or obesity: Systematic review and meta-analyses. Obes. Rev. 2021, 22, e13239. [Google Scholar] [CrossRef]
- Vilar-Gomez, E.; Martinez-Perez, Y.; Calzadilla-Bertot, L.; Torres-Gonzalez, A.; Gra-Oramas, B.; Gonzalez-Fabian, L.; Friedman, S.L.; Diago, M.; Romero-Gomez, M. Weight loss through lifestyle modification significantly reduces features of nonalcoholic steatohepatitis. Gastroenterology 2015, 149, 367–378. [Google Scholar] [CrossRef]
- Romero-Gómez, M.; Zelber-Sagi, S.; Trenell, M. Treatment of NAFLD with diet, physical activity and exercise. J. Hepatol. 2017, 67, 829–846. [Google Scholar] [CrossRef]
- Diolintzi, A.; Panagiotakos, D.B.; Sidossis, L.S. From Mediterranean diet to Mediterranean lifestyle: A narrative review. Public Health Nutr. 2019, 22, 2703–2713. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Hoffmann, G. Mediterranean dietary pattern, inflammation and endothelial function: A systematic review and meta-analysis of intervention trials. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 929–939. [Google Scholar] [CrossRef] [PubMed]
- Katsagoni, C.N.; Georgoulis, M.; Papatheodoridis, G.V.; Panagiotakos, D.B.; Kontogianni, M.D. Effects of lifestyle interventions on clinical characteristics of patients with non-alcoholic fatty liver disease: A meta-analysis. Metabolism 2017, 68, 119–132. [Google Scholar] [CrossRef] [PubMed]
- Kontogianni, M.D.; Tileli, N.; Margariti, A.; Georgoulis, M.; Deutsch, M.; Tiniakos, D.; Fragopoulou, E.; Zafiropoulou, R.; Manios, Y.; Papatheodoridis, G. Adherence to the Mediterranean diet is associated with the severity of non-alcoholic fatty liver disease. Clin. Nutr. 2014, 33, 678–683. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).