Cyclodextrins in Drug Delivery Systems and Their Effects on Biological Barriers
Abstract
1. Introduction
2. Cyclodextrin Complex Formation
3. Cyclodextrin-Based Nanostructures
3.1. Cyclodextrin Associates, Polypseudorotaxanes and Polyrotaxanes
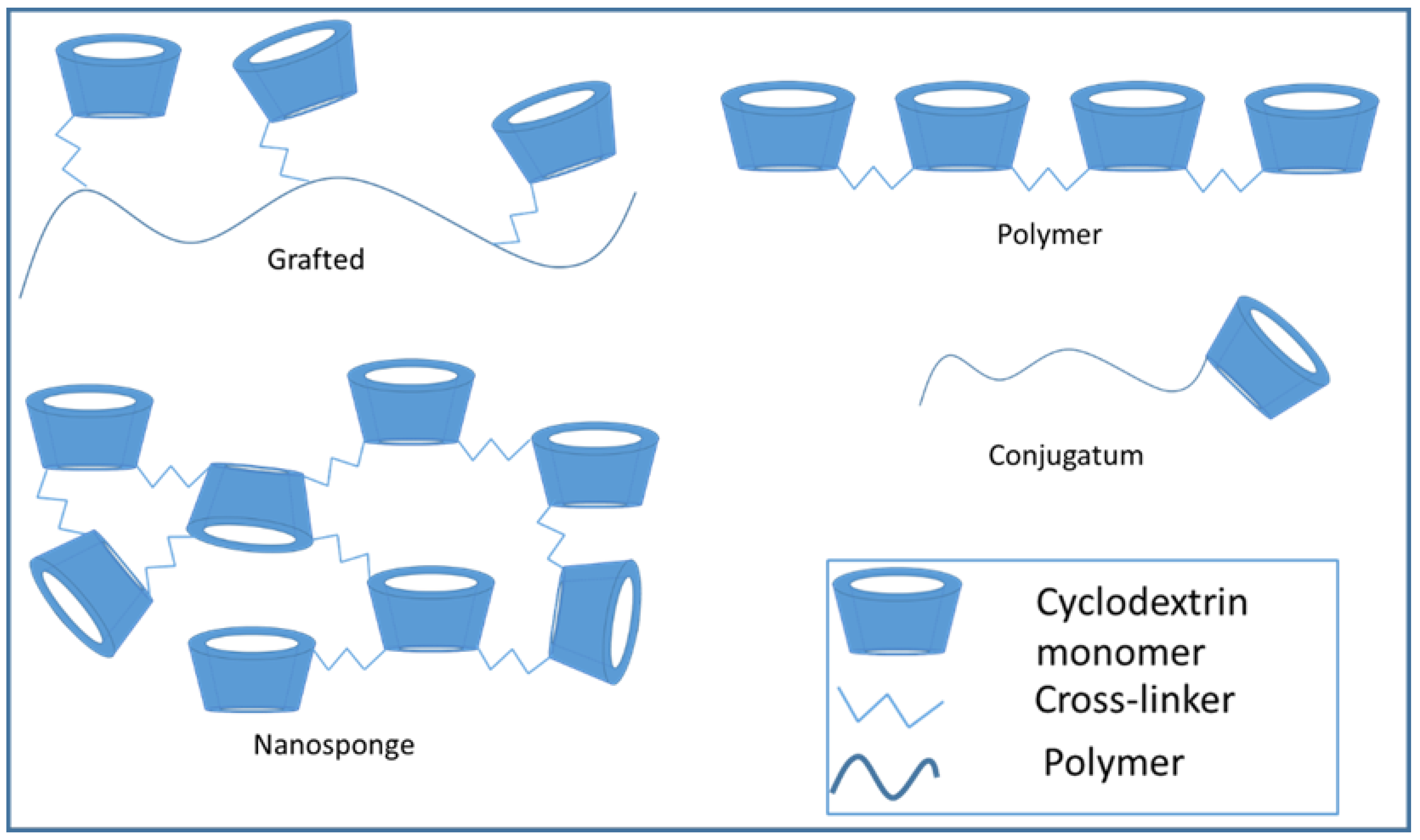
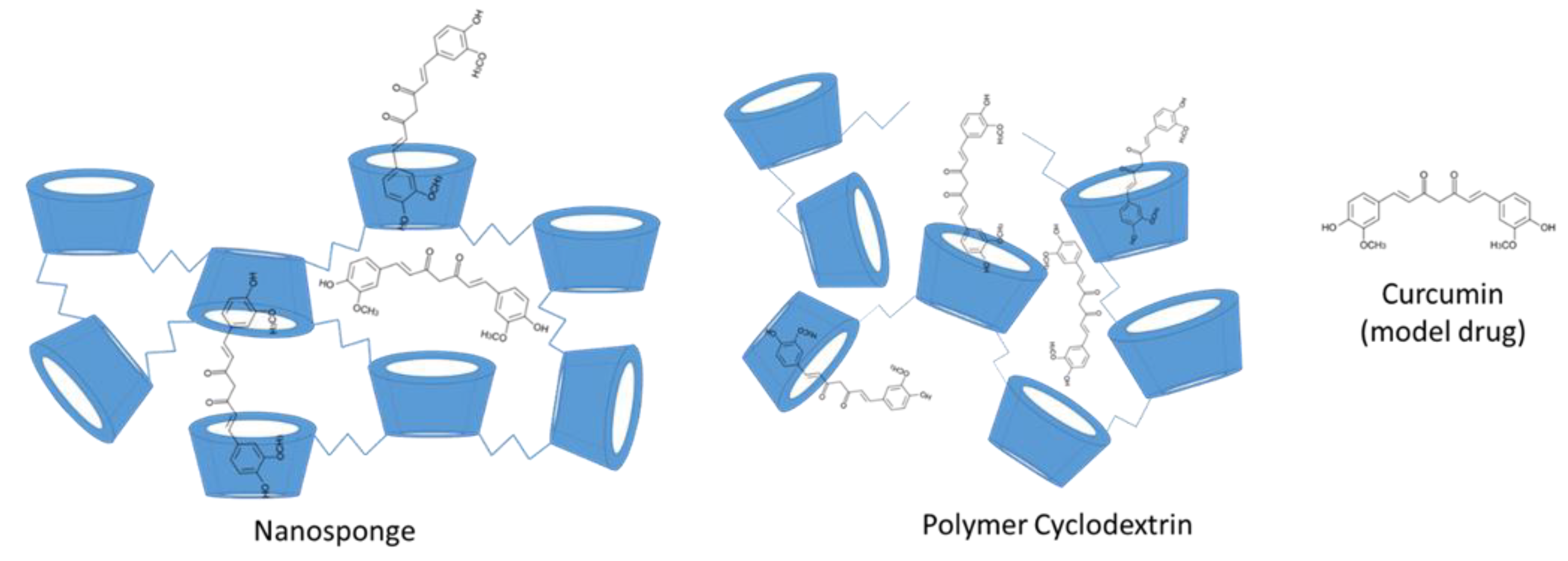
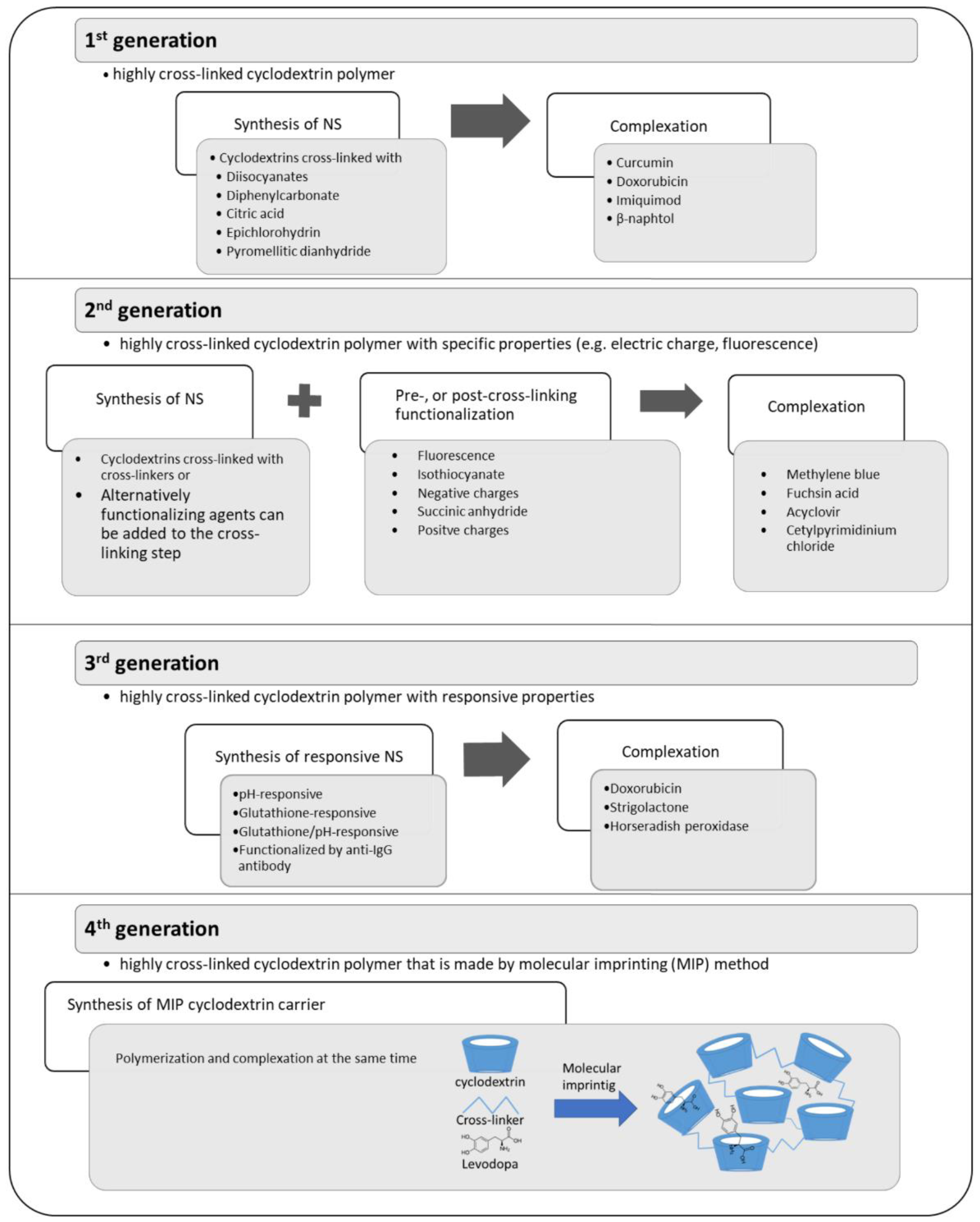
3.2. Formation of Cyclodextrin Conjugates, Polymers, and Nanosponges
3.3. Mucoadhesive Drug Carriers
3.4. Responsive Cyclodextrins
3.5. Cyclodextrin Polymers
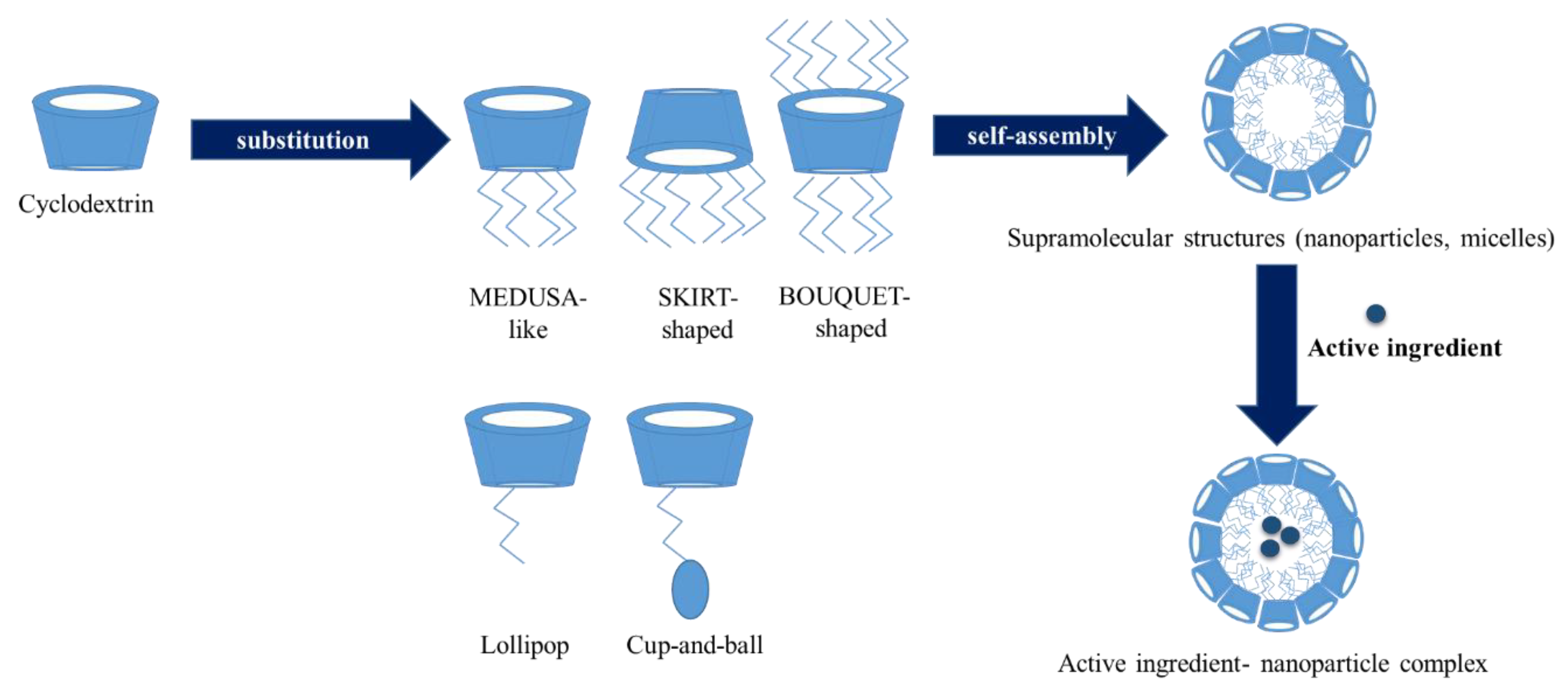
3.6. Amphiphilic Cyclodextrins
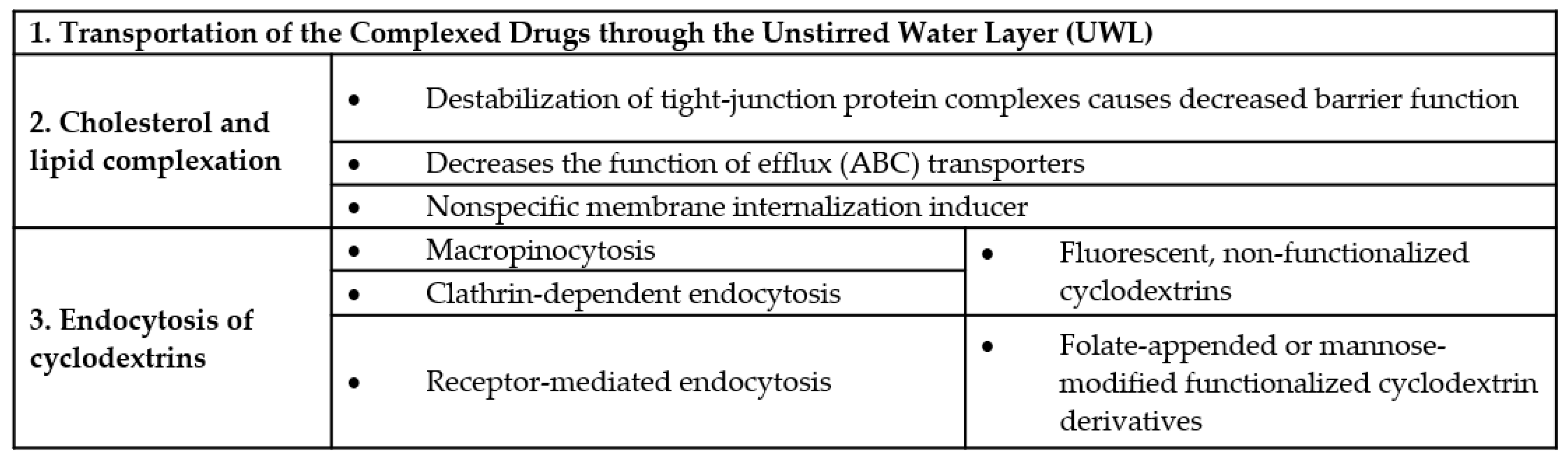
4. Cyclodextrins, Biological Barriers and the Significance of Lipid and Cholesterol Complexation
Endocytosis of Cyclodextrins
5. Conclusions
- The improvement of water solubility of lipophilic drugs;
- increased permeation of lipophilic molecules through the unstirred water layer (UWL);
- permeabilization of cell membrane by removing cholesterol, which leads to further consequences such as:
- Changes in the function of tight junctions by destabilizing the tight junction proteins localized in lipid rafts, causing increased paracellular permeability and
- inhibition the function of efflux pumps;
- endocytosis of free cyclodextrins; and
- endocytosis of cyclodextrin-drug complexes.
Funding
Conflicts of Interest
References
- Szejtli, J. Introduction and General Overview of Cyclodextrin Chemistry. Chem. Rev. 1998, 98, 1743–1754. [Google Scholar] [CrossRef] [PubMed]
- Ikuta, D.; Hirata, Y.; Wakamori, S.; Shimada, H.; Tomabechi, Y.; Kawasaki, Y.; Ikeuchi, K.; Hagimori, T.; Matsumoto, S.; Yamada, H. Conformationally supple glucose monomers enable synthesis of the smallest cyclodextrins. Science 2019, 364, 674–677. [Google Scholar] [CrossRef] [PubMed]
- Assaf, K.I.; Gabel, D.; Zimmermann, W.; Nau, W.M. High-affinity host–guest chemistry of large-ring cyclodextrins. Org. Biomol. Chem. 2016, 14, 7702–7706. [Google Scholar] [CrossRef] [PubMed]
- Sonnendecker, C.; Thürmann, S.; Przybylski, C.; Zitzmann, F.D.; Heinke, N.; Krauke, Y.; Monks, K.; Robitzki, A.A.; Belder, D.; Zimmermann, W. Large-Ring Cyclodextrins as Chiral Selectors for Enantiomeric Pharmaceuticals. Angew. Chem. Int. Ed. 2019, 58, 6411–6414. [Google Scholar] [CrossRef] [PubMed]
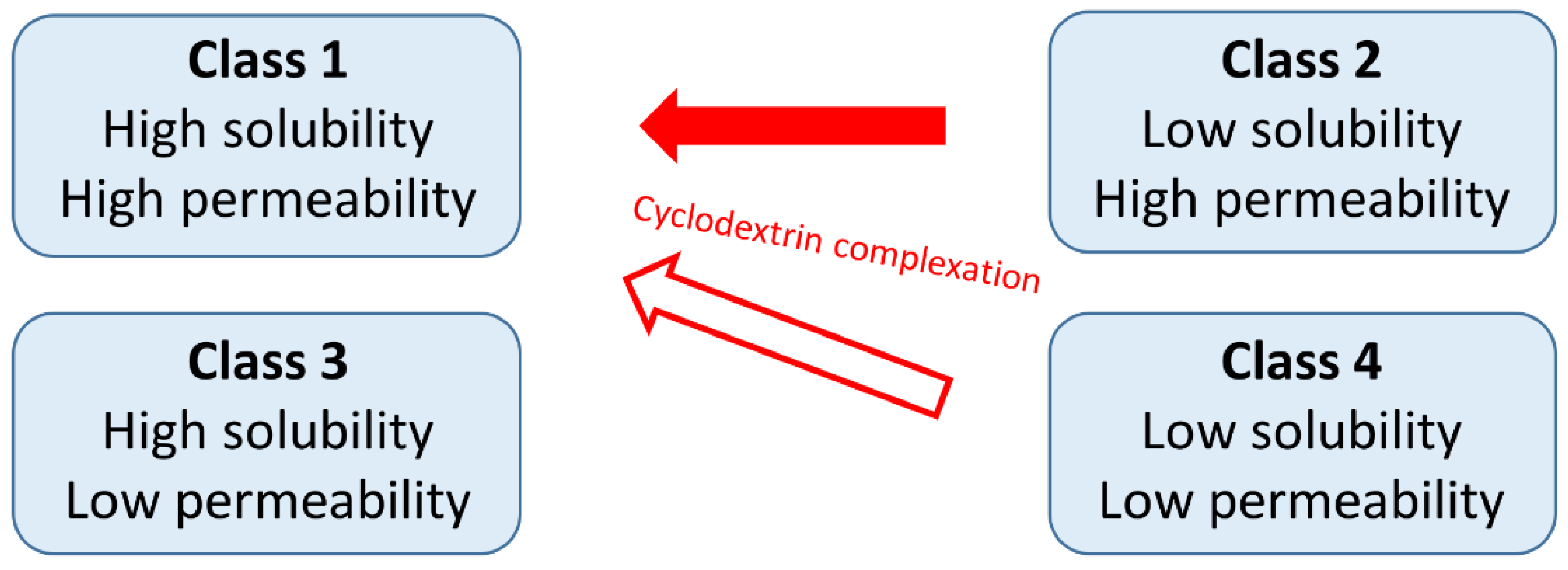
- Loftsson, T. Cyclodextrins and the biopharmaceutics classification system of drugs. J. Incl. Phenom. Macrocycl. Chem. 2002, 44, 63–67. [Google Scholar] [CrossRef]
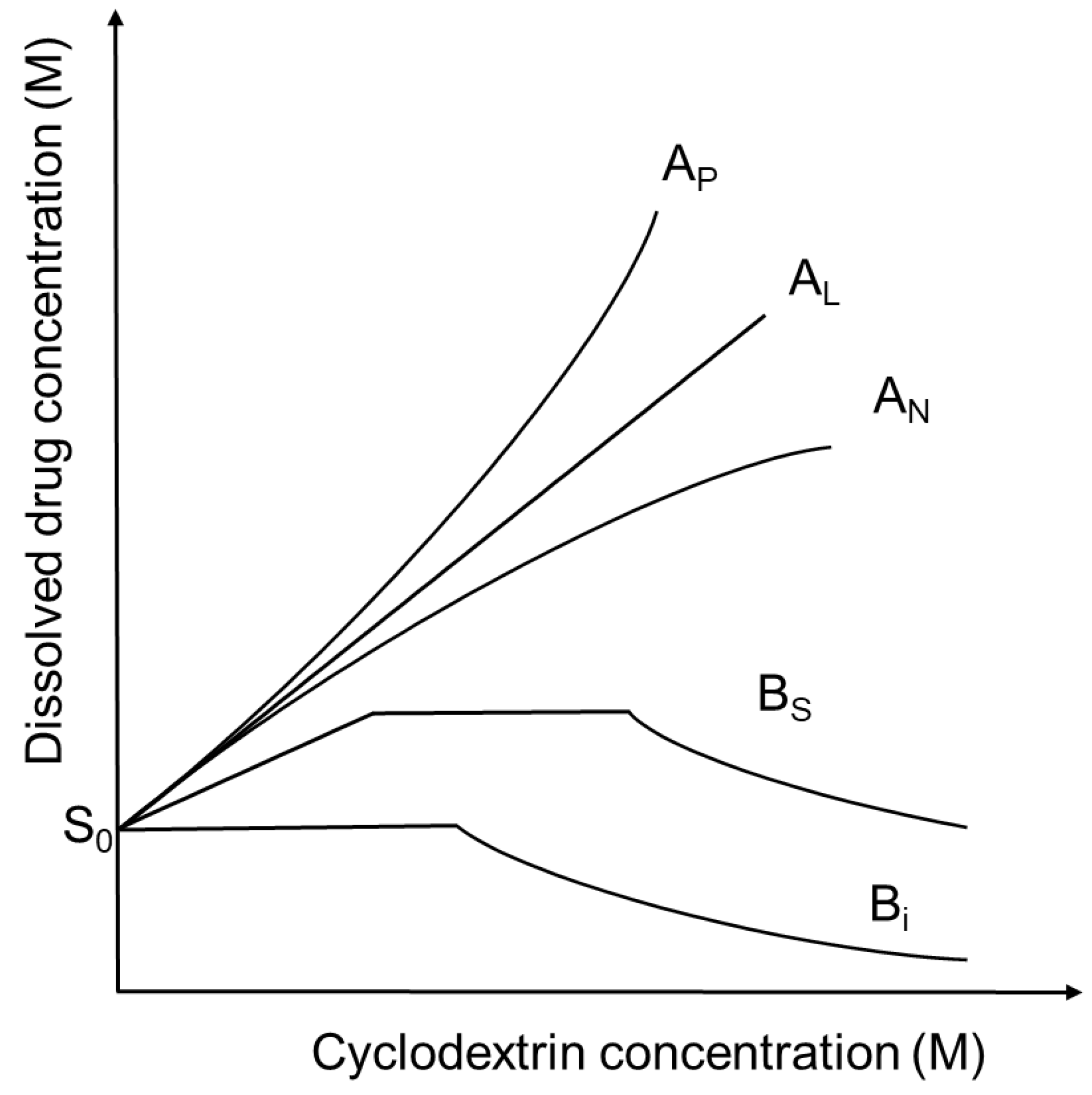
- Higuchi, T.; Connors, K.A. Phase solubility techniques. Adv. Anal. Chem. Instrum. 1965, 4, 117–212. [Google Scholar]
- Loftsson, T.; Brewster, M.E. Pharmaceutical applications of cyclodextrins: Basic science and product development. J. Pharm. Pharmacol. 2010, 62, 1607–1621. [Google Scholar] [CrossRef]
- Loftsson, T.; Magnúsdóttir, A.; Másson, M.; Sigurjónsdóttir, J.F. Self-Association and Cyclodextrin Solubilization of Drugs. J. Pharm. Sci. 2002, 91, 2307–2316. [Google Scholar] [CrossRef]
- Coleman, A.W.; Nicolis, I.; Keller, N.; Dalbiez, J.P. Aggregation of cyclodextrins: An explanation of the abnormal solubility of?-cyclodextrin. J. Incl. Phenom. Mol. Recognit. Chem. 1992, 13, 139–143. [Google Scholar] [CrossRef]
- Becheri, A.; Lo Nostro, P.; Ninham, B.W.; Baglioni, P. The curious world of polypseudorotaxanes: Cyclodextrins as probes of water structure. J. Phys. Chem. B 2003, 107, 3979–3987. [Google Scholar] [CrossRef]
- Harada, A.; Kamachi, M. Complex formation between poly(ethylene glycol) and α-cyclodextrin. Macromolecules 1990, 23, 2821–2823. [Google Scholar] [CrossRef]
- Harada, A.; Kamachi, M. Complex formation between cyclodextrin and poly(propylene glycol). J. Chem. Soc. Chem. Commun. 1990, 1322–1323. [Google Scholar] [CrossRef]
- Higashi, T.; Hirayama, F.; Misumi, S.; Motoyama, K.; Arima, H.; Uekama, K. Polypseudorotaxane Formation of Randomly-Pegylated Insulin with Cyclodextrins: Slow Release and Resistance to Enzymatic Degradation. Chem. Pharm. Bull. 2009, 57, 541–544. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chang, J.; Li, Y.; Wang, G.; He, B.; Gu, Z. Fabrication of novel coumarin derivative functionalized polypseudorotaxane micelles for drug delivery. Nanoscale 2013, 5, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Harada, A. Cyclodextrin-Based Molecular Machines. Acc. Chem. Res. 2001, 34, 456–464. [Google Scholar] [CrossRef]
- Nepogodiev, S.A.; Stoddart, J.F. Cyclodextrin-Based Catenanes and Rotaxanes. Chem. Rev. 1998, 98, 1959–1976. [Google Scholar] [CrossRef]
- Shibaguchi, K.; Tamura, A.; Terauchi, M.; Matsumura, M.; Miura, H.; Yui, N. Mannosylated Polyrotaxanes for Increasing Cellular Uptake Efficiency in Macrophages through Receptor-Mediated Endocytosis. Molecules 2019, 24, 439. [Google Scholar] [CrossRef]
- Wang, H.; Yin, H.; Yan, F.; Sun, M.; Du, L.; Peng, W.; Li, Q.; Feng, Y.; Zhou, Y. Folate-mediated mitochondrial targeting with doxorubicinpolyrotaxane nanoparticles overcomes multidrug resistance. Oncotarget 2015, 6, 2827. [Google Scholar]
- Yu, G.; Yang, Z.; Fu, X.; Yung, B.C.; Yang, J.; Mao, Z.; Shao, L.; Hua, B.; Liu, Y.; Zhang, F.; et al. Polyrotaxane-based supramolecular theranostics. Nat. Commun. 2018, 9, 766. [Google Scholar] [CrossRef]
- Dandekar, P.; Jain, R.; Keil, M.; Loretz, B.; Muijs, L.; Schneider, M.; Auerbach, D.; Jung, G.; Lehr, C.M.; Wenz, G. Cellular delivery of polynucleotides by cationic cyclodextrin polyrotaxanes. J. Control. Release 2012, 164, 387–393. [Google Scholar] [CrossRef]
- Yang, C.; Wang, X.; Li, H.; Goh, S.H.; Li, J. Synthesis and Characterization of Polyrotaxanes Consisting of Cationic α-Cyclodextrins Threaded on Poly[(ethylene oxide)- ran -(propylene oxide)] as Gene Carriers. Biomacromolecules 2007, 8, 3365–3374. [Google Scholar] [CrossRef] [PubMed]
- Hattori, K.; Ikeda, H. Modification Reactions of Cyclodextrins and the Chemistry of Modified Cyclodextrins. In Cyclodextrins and Their Complexes; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2006; pp. 31–64. ISBN 3527312803. [Google Scholar]
- Krause, R.W.; Mamba, B.B.; Bambo, F.M.; Malefetse, T.J. Cyclodextrin polymers: Synthesis and application in water treatment. In Cyclodextrins: Chemistry Physics; Transworld Research Network: Kerala, India, 2010; pp. 168–194. [Google Scholar]
- Mansuri, S.; Kesharwani, P.; Jain, K.; Tekade, R.K.; Jain, N.K. Mucoadhesion: A promising approach in drug delivery system. React. Funct. Polym. 2016, 100, 151–172. [Google Scholar] [CrossRef]
- Ijaz, M.; Prantl, M.; Lupo, N.; Laffleur, F.; Hussain Asim, M.; Matuszczak, B.; Bernkop-Schnürch, A. Development of pre-activated α-cyclodextrin as a mucoadhesive excipient for intra-vesical drug delivery. Int. J. Pharm. 2017, 534, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Ijaz, M.; Matuszczak, B.; Rahmat, D.; Mahmood, A.; Bonengel, S.; Hussain, S.; Huck, C.W.; Bernkop-Schnürch, A. Synthesis and characterization of thiolated β-cyclodextrin as a novel mucoadhesive excipient for intra-oral drug delivery. Carbohydr. Polym. 2015, 132, 187–195. [Google Scholar] [CrossRef]
- Ijaz, M.; Ahmad, M.; Akhtar, N.; Laffleur, F.; Bernkop-Schnürch, A. Thiolated α-Cyclodextrin: The Invisible Choice to Prolong Ocular Drug Residence Time. J. Pharm. Sci. 2016, 105, 2848–2854. [Google Scholar] [CrossRef]
- Ijaz, M.; Griessinger, J.A.; Mahmood, A.; Laffleur, F.; Bernkop-Schnürch, A. Thiolated Cyclodextrin: Development of a Mucoadhesive Vaginal Delivery System for Acyclovir. J. Pharm. Sci. 2016, 105, 1714–1720. [Google Scholar] [CrossRef]
- Asim, M.H.; Moghadam, A.; Ijaz, M.; Mahmood, A.; Götz, R.X.; Matuszczak, B.; Bernkop-Schnürch, A. S-protected thiolated cyclodextrins as mucoadhesive oligomers for drug delivery. J. Colloid Interface Sci. 2018, 531, 261–268. [Google Scholar] [CrossRef]
- Venter, J.P.; Kotzé, A.F.; Auzély-Velty, R.; Rinaudo, M. Synthesis and evaluation of the mucoadhesivity of a CD-chitosan derivative. Int. J. Pharm. 2006, 313, 36–42. [Google Scholar] [CrossRef]
- Sajomsang, W.; Nuchuchua, O.; Gonil, P.; Saesoo, S.; Sramala, I.; Soottitantawat, A.; Puttipipatkhachorn, S.; Ruktanonchai, U.R. Water-soluble β-cyclodextrin grafted with chitosan and its inclusion complex as a mucoadhesive eugenol carrier. Carbohydr. Polym. 2012, 89, 623–631. [Google Scholar] [CrossRef]
- Sajomsang, W.; Gonil, P.; Ruktanonchai, U.R.; Pimpha, N.; Sramala, I.; Nuchuchua, O.; Saesoo, S.; Chaleawlert-umpon, S.; Puttipipatkhachorn, S. Self-aggregates formation and mucoadhesive property of water-soluble β-cyclodextrin grafted with chitosan. Int. J. Biol. Macromol. 2011, 48, 589–595. [Google Scholar] [CrossRef]
- Wang, J.; Zong, J.Y.; Zhao, D.; Zhuo, R.X.; Cheng, S.X. In situ formation of chitosan-cyclodextrin nanospheres for drug delivery. Colloids Surf. B Biointerfaces 2011, 87, 198–202. [Google Scholar] [CrossRef]
- Gomez, C.G.; Chambat, G.; Heyraud, A.; Villar, M.; Auzély-Velty, R. Synthesis and characterization of a β-CD-alginate conjugate. Polymer 2006, 47, 8509–8516. [Google Scholar] [CrossRef]
- Pluemsab, W.; Sakairi, N.; Furuike, T. Synthesis and inclusion property of α-cyclodextrin-linked alginate. Polymer 2005, 46, 9778–9783. [Google Scholar] [CrossRef]
- Parmar, V.; Patel, G.; Abu-Thabit, N.Y. Responsive Cyclodextrins as Polymeric Carriers for Drug Delivery Applications; Elsevier Ltd.: Amsterdam, The Netherlands, 2018; ISBN 9780081019979. [Google Scholar]
- Schmaljohann, D. Thermo- and pH-responsive polymers in drug delivery. Adv. Drug Deliv. Rev. 2006, 58, 1655–1670. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, N.; Wang, D.; He, Y.; Chen, L.; Zhao, Y. Poly (MAH-β-cyclodextrin-co-NIPAAm) hydrogels with drug hosting and thermo/pH-sensitive for controlled drug release. Polym. Degrad. Stab. 2018, 147, 123–131. [Google Scholar] [CrossRef]
- Zhou, Z.; Li, G.; Wang, N.; Guo, F.; Guo, L.; Liu, X. Synthesis of temperature/pH dual-sensitive supramolecular micelles from β-cyclodextrin-poly(N-isopropylacrylamide) star polymer for drug delivery. Colloids Surf. B Biointerfaces 2018, 172, 136–142. [Google Scholar] [CrossRef]
- Liu, L.; Feng, X.; Pei, Y.; Wang, J.; Ding, J.; Chen, L. α-Cyclodextrin concentration-controlled thermo-sensitive supramolecular hydrogels. Mater. Sci. Eng. C 2018, 82, 25–28. [Google Scholar] [CrossRef]
- Danhier, F.; Feron, O.; Préat, V. To exploit the tumor microenvironment: Passive and active tumor targeting of nanocarriers for anti-cancer drug delivery. J. Control. Release 2010, 148, 135–146. [Google Scholar] [CrossRef]
- Zhang, D.; Lv, P.; Zhou, C.; Zhao, Y.; Liao, X.; Yang, B. Cyclodextrin-based delivery systems for cancer treatment. Mater. Sci. Eng. C 2019, 96, 872–886. [Google Scholar] [CrossRef]
- Peng, L.; Liu, S.; Feng, A.; Yuan, J. Polymeric Nanocarriers Based on Cyclodextrins for Drug Delivery: Host-Guest Interaction as Stimuli Responsive Linker. Mol. Pharm. 2017, 14, 2475–2486. [Google Scholar] [CrossRef]
- Hao, W.; Liu, D.; Wang, Y.; Han, X.; Xu, S.; Liu, H. Dual-stimuli responsive nanoparticles (UCNP-CD@APP) assembled by host-guest interaction for drug delivery. Colloids Surf. A Physicochem. Eng. Asp. 2018, 537, 446–451. [Google Scholar] [CrossRef]
- Chen, X.; Wang, Y.; Rong, R.; Zhao, C.; Li, X.; Wang, K. Synthesis, thermo-responsive behavior of cyclodextrin modi fi ed Bi-perylene monoimide derivative. Dye. Pigment. 2019, 160, 779–786. [Google Scholar] [CrossRef]
- Zhou, H.Y.; Wang, Z.Y.; Duan, X.Y.; Jiang, L.J.; Cao, P.P.; Li, J.B.J.X.; Li, J.B.J.X.; Wang, J.J.; Guo, Z.; Xiong, J.; et al. Facile synthesis of chitosan-grafted beta-cyclodextrin for stimuli-responsive drug delivery. Int. J. Biol. Macromol. 2019, 125, 941–947. [Google Scholar]
- Borandeh, S.; Abdolmaleki, A.; Abolmaali, S.S.; Tamaddon, A.M. Synthesis, structural and in-vitro characterization of $β$-cyclodextrin grafted L-phenylalanine functionalized graphene oxide nanocomposite: A versatile nanocarrier for pH-sensitive doxorubicin delivery. Carbohydr. Polym. 2018, 201, 151–161. [Google Scholar] [CrossRef]
- Sawant, V.J.; Bamane, S.R. PEG-beta-cyclodextrin functionalized zinc oxide nanoparticles show cell imaging with high drug payload and sustained pH responsive delivery of curcumin in to MCF-7 cells. J. Drug Deliv. Sci. Technol. 2018, 43, 397–408. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, J.; Jin, Z. Supramolecular hydrogel formation between chitosan and hydroxypropyl $β$-cyclodextrin via Diels-Alder reaction and its drug delivery. Int. J. Biol. Macromol. 2018, 114, 381–391. [Google Scholar] [CrossRef]
- Yang, Z.; Peng, Y.; Qiu, L. pH-Responsive supramolecular micelle based on host-guest interaction of poly(β-amino ester) derivatives and adamantyl-terminated poly(ethylene glycol) for cancer inhibition. Chin. Chem. Lett. 2018, 29, 1839–1844. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, D.; Chen, H.; Lim, W.Q.; Phua, F.S.Z.; An, G.; Yang, P.; Zhao, Y. Reduction-sensitive fluorescence enhanced polymeric prodrug nanoparticles for combinational photothermal-chemotherapy. Biomaterials 2018, 163, 14–24. [Google Scholar] [CrossRef]
- Morin-Crini, N.; Winterton, P.; Fourmentin, S.; Wilson, L.D.; Fenyvesi, É.; Crini, G. Water-insoluble β-cyclodextrin–epichlorohydrin polymers for removal of pollutants from aqueous solutions by sorption processes using batch studies: A review of inclusion mechanisms. Prog. Polym. Sci. 2018, 78, 1–23. [Google Scholar] [CrossRef]
- József, S. Cyclodextrins and Their Inclusion Complexes; Akadémiai Kiadó: Budapest, Hungary, 1982; ISBN 963 05 2850 9. [Google Scholar]
- Rossi, B.; Fontana, A.; Giarola, M.; Mariotto, G.; Mele, A.; Punta, C.; Melone, L.; Toraldo, F.; Trotta, F. Glass-like dynamics of new cross-linked polymeric systems: Behavior of the Boson peak. J. Non. Cryst. Solids 2014, 401, 73–77. [Google Scholar] [CrossRef]
- Gidwani, B.; Vyas, A. Synthesis, characterization and application of Epichlorohydrin-β-cyclodextrin polymer. Colloids Surf. B Biointerfaces 2014, 114, 130–137. [Google Scholar] [CrossRef]
- Garcia-Fernandez, M.J.; Tabary, N.; Chai, F.; Cazaux, F.; Blanchemain, N.; Flament, M.P.; Martel, B. New multifunctional pharmaceutical excipient in tablet formulation based on citric acid-cyclodextrin polymer. Int. J. Pharm. 2016, 511, 913–920. [Google Scholar] [CrossRef]
- Huang, W.; Hu, Y.; Li, Y.; Zhou, Y.; Niu, D.; Lei, Z.; Zhang, Z. Citric acid-crosslinked β-cyclodextrin for simultaneous removal of bisphenol A, methylene blue and copper: The roles of cavity and surface functional groups. J. Taiwan Inst. Chem. Eng. 2018, 82, 189–197. [Google Scholar] [CrossRef]
- Nojavan, S.; Yazdanpanah, M. Micro-solid phase extraction of benzene, toluene, ethylbenzene and xylenes from aqueous solutions using water-insoluble β-cyclodextrin polymer as sorbent. J. Chromatogr. A 2017, 1525, 51–59. [Google Scholar] [CrossRef]
- Morin-Crini, N.; Crini, G. Environmental applications of water-insoluble β-cyclodextrin- epichlorohydrin polymers. Prog. Polym. Sci. 2013, 38, 344–368. [Google Scholar] [CrossRef]
- Moulahcene, L.; Skiba, M.; Senhadji, O.; Milon, N.; Benamor, M.; Lahiani-Skiba, M. Inclusion and removal of pharmaceutical residues from aqueous solution using water-insoluble cyclodextrin polymers. Chem. Eng. Res. Des. 2015, 97, 145–158. [Google Scholar] [CrossRef]
- Tang, P.; Sun, Q.; Zhao, L.; Tang, Y.; Liu, Y.; Pu, H.; Gan, N.; Liu, Y.; Li, H. A simple and green method to construct cyclodextrin polymer for the effective and simultaneous estrogen pollutant and metal removal. Chem. Eng. J. 2019, 366, 598–607. [Google Scholar] [CrossRef]
- Sherje, A.P.; Dravyakar, B.R.; Kadam, D.; Jadhav, M. Cyclodextrin-based nanosponges: A critical review. Carbohydr. Polym. 2017, 173, 37–49. [Google Scholar] [CrossRef]
- Caldera, F.; Tannous, M.; Cavalli, R.; Zanetti, M.; Trotta, F. Evolution of Cyclodextrin Nanosponges. Int. J. Pharm. 2017, 531, 470–479. [Google Scholar] [CrossRef]
- Cavalli, R.; Trotta, F.; Tumiatti, W. Cyclodextrin-based Nanosponges for Drug Delivery. J. Incl. Phenom. Macrocycl. Chem. 2006, 56, 209–213. [Google Scholar] [CrossRef]
- Darandale, S.S.; Vavia, P.R. Cyclodextrin-based nanosponges of curcumin: Formulation and physicochemical characterization. J. Incl. Phenom. Macrocycl. Chem. 2013, 75, 315–322. [Google Scholar] [CrossRef]
- Li, D.; Ma, M. Nanosponges for water purification. Clean Prod. Process. 2000, 2, 112–116. [Google Scholar] [CrossRef]
- Gabr, M.M.; Mortada, S.M.; Sallam, M.A. Carboxylate cross-linked cyclodextrin: A nanoporous scaffold for enhancement of rosuvastatin oral bioavailability. Eur. J. Pharm. Sci. 2018, 111, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Argenziano, M.; Haimhoffer, A.; Bastiancich, C.; Caldera, F.; Trotta, F.; Scutera, S.; Alotto, D.; Fumagalli, M.; Musso, T.; Castagnoli, C.; et al. In Vitro Enhanced Skin Permeation and Retention of Imiquimod Loaded in β -Cyclodextrin Nanosponge Hydrogel. Pharmaceutics 2019, 11, 138. [Google Scholar] [CrossRef]
- Pushpalatha, R.; Selvamuthukumar, S.; Kilimozhi, D. Cyclodextrin-based nanosponges for curcumin delivery—Physicochemical characterization, drug release, stability and cytotoxicity. J. Drug Deliv. Sci. Technol. 2018, 45, 45–53. [Google Scholar] [CrossRef]
- Ansari, K.A.; Vavia, P.R.; Trotta, F.; Cavalli, R. Cyclodextrin-Based Nanosponges for Delivery of Resveratrol: In Vitro Characterisation, Stability, Cytotoxicity and Permeation Study. Aaps Pharmscitech 2011, 12, 279–286. [Google Scholar] [CrossRef]
- Zohrehvand, S.; Evans, C.H. 2-Naphthol-containing β-cyclodextrin-epichlorohydrin copolymers: Synthesis, characterization and fluorescence studies. Polym. Int. 2005, 54, 744–753. [Google Scholar] [CrossRef]
- Lembo, D.; Swaminathan, S.; Donalisio, M.; Civra, A.; Pastero, L.; Aquilano, D.; Vavia, P.; Trotta, F.; Cavalli, R. Encapsulation of Acyclovir in new carboxylated cyclodextrin-based nanosponges improves the agent’s antiviral efficacy. Int. J. Pharm. 2013, 443, 262–272. [Google Scholar] [CrossRef]
- Ncube, P.; Krause, R.W.M.; Mamba, B.B. Detection of chloroform in water using an azo dye-modified β-cyclodextrin—Epichlorohydrin copolymer as a fluorescent probe. Phys. Chem. Earth Parts A/B/C 2014, 67–69, 79–85. [Google Scholar] [CrossRef]
- Wajs, E.; Caldera, F.; Trotta, F.; Fragoso, A. Peroxidase-encapsulated cyclodextrin nanosponge immunoconjugates as a signal enhancement tool in optical and electrochemical assays. Analyst 2014, 139, 375–380. [Google Scholar] [CrossRef]
- Fenyvesi, É.; Ujházy, A.; Szejtli, J.; Potter, S.; Gan, T.G. Controlled release of drugs from CD polymers substituted with ionic groups. J. Incl. Phenom. Mol. Recognit. Chem. 1996, 25, 185–189. [Google Scholar] [CrossRef]
- Daga, M.; Ulllio, C.; Argenziano, M.; Dianzani, C.; Cavalli, R.; Trotta, F.; Ferretti, C.; Zara, G.P.; Gigliotti, C.L.; Ciamporcero, E.S.; et al. GSH-targeted nanosponges increase doxorubicin-induced toxicity “in vitro” and “in vivo” in cancer cells with high antioxidant defenses. Free Radic. Biol. Med. 2016, 97, 24–37. [Google Scholar] [CrossRef]
- Argenziano, M.; Lombardi, C.; Ferrara, B.; Trotta, F.; Caldera, F.; Blangetti, M.; Koltai, H.; Kapulnik, Y.; Yarden, R.; Gigliotti, L.; et al. Glutathione/pH-responsive nanosponges enhance strigolactone delivery to prostate cancer cells. Oncotarget 2018, 9, 35813. [Google Scholar] [CrossRef]
- Gooding, M.; Malhotra, M.; McCarthy, D.J.; Godinho, B.M.D.C.; Cryan, J.F.; Darcy, R.; O’Driscoll, C.M. Synthesis and characterization of rabies virus glycoprotein-tagged amphiphilic cyclodextrins for siRNA delivery in human glioblastoma cells: In vitro analysis. Eur. J. Pharm. Sci. 2015, 71, 80–92. [Google Scholar] [CrossRef]
- Russo, M.; Saladino, M.L.; Chillura Martino, D.; Lo Meo, P.; Noto, R. Polyaminocyclodextrin nanosponges: Synthesis, characterization and pH-responsive sequestration abilities. RSC Adv. 2016, 6, 49941–49953. [Google Scholar] [CrossRef]
- Trotta, F.; Caldera, F.; Cavalli, R.; Soster, M.; Riedo, C.; Biasizzo, M.; Uccello Barretta, G.; Balzano, F.; Brunella, V. Molecularly imprinted cyclodextrin nanosponges for the controlled delivery of L-DOPA: Perspectives for the treatment of Parkinson’s disease. Expert Opin. Drug Deliv. 2016, 13, 1671–1680. [Google Scholar] [CrossRef]
- Deshmukh, K.; Tanwar, Y.S.; Shende, P.; Cavalli, R. Biomimetic estimation of glucose using non-molecular and molecular imprinted polymer nanosponges. Int. J. Pharm. 2015, 494, 244–248. [Google Scholar] [CrossRef]
- Varan, G.; Varan, C.; Erdoğar, N.; Hıncal, A.A.; Bilensoy, E. Amphiphilic cyclodextrin nanoparticles. Int. J. Pharm. 2017, 531, 457–469. [Google Scholar] [CrossRef]
- Roux, M.; Perly, B.; Djedaïni-Pilard, F. Self-assemblies of amphiphilic cyclodextrins. Eur. Biophys. J. 2007, 36, 861–867. [Google Scholar] [CrossRef]
- Donohue, R.; Mazzaglia, A.; Ravoo, B.J.; Darcy, R. Cationic β-cyclodextrin bilayer vesicles. Chem. Commun. 2002, 23, 2864–2865. [Google Scholar] [CrossRef]
- Sallas, F.; Darcy, R. Amphiphilic cyclodextrins-Advances in synthesis and supramolecular chemistry. Eur. J. Org. Chem. 2008, 2008, 957–969. [Google Scholar] [CrossRef]
- Ghera, B.B.; Perret, F.; Chevalier, Y.; Parrot-Lopez, H. Novel nanoparticles made from amphiphilic perfluoroalkyl α-cyclodextrin derivatives: Preparation, characterization and application to the transport of acyclovir. Int. J. Pharm. 2009, 375, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Nacereddine, A.; Bollacke, A.; Róka, E.; Marminon, C.; Bouaziz, Z.; Fenyvesi, F.; Katalin Bácskay, I.; Jose, J.; Perret, F.; Le Borgne, M. Self-assembled supramolecular nanoparticles improve the cytotoxic efficacy of CK2 inhibitor THN7. Pharmaceuticals 2018, 11, 10. [Google Scholar] [CrossRef] [PubMed]
- Godinho, B.M.D.C.; Ogier, J.R.; Quinlan, A.; Darcy, R.; Griffin, B.T.; Cryan, J.F.; O’Driscoll, C.M. PEGylated cyclodextrins as novel siRNA nanosystems: Correlations between polyethylene glycol length and nanoparticle stability. Int. J. Pharm. 2014, 473, 105–112. [Google Scholar] [CrossRef] [PubMed]
- McMahon, A.; O’Neill, M.J.; Gomez, E.; Donohue, R.; Forde, D.; Darcy, R.; O’Driscoll, C.M. Targeted gene delivery to hepatocytes with galactosylated amphiphilic cyclodextrins. J. Pharm. Pharmacol. 2012, 64, 1063–1073. [Google Scholar] [CrossRef] [PubMed]
- Zagami, R.; Rapozzi, V.; Piperno, A.; Scala, A.; Triolo, C.; Trapani, M.; Xodo, L.E.; Monsù Scolaro, L.; Mazzaglia, A. Folate-Decorated Amphiphilic Cyclodextrins as Cell-Targeted Nanophototherapeutics. Biomacromolecules 2019, 20, 2530–2544. [Google Scholar] [CrossRef] [PubMed]
- Lennernaäs, H. Human Intestinal Permeability. J. Pharm. Sci. 1998, 87, 403–410. [Google Scholar] [CrossRef]
- Brewster, M.E.; Noppe, M.; Peeters, J.; Loftsson, T. Effect of the unstirred water layer on permeability enhancement by hydrophilic cyclodextrins. Int. J. Pharm. 2007, 342, 250–253. [Google Scholar] [CrossRef]
- Loftsson, T.; Vogensen, S.B.; Brewster, M.E.; Konráosdóttir, F. Effects of cyclodextrins on drug delivery through biological membranes. J. Pharm. Sci. 2007, 96, 2532–2546. [Google Scholar] [CrossRef]
- Másson, M.; Loftsson, T.; Másson, G.; Stefánsson, E. Cyclodextrins as permeation enhancers: Some theoretical evaluations and in vitro testing. J. Control. Release 1999, 59, 107–118. [Google Scholar] [CrossRef]
- Ren, B.; Gao, H.; Cao, Y.; Jia, L. In Silico understanding of the cyclodextrin–phenanthrene hybrid assemblies in both aqueous medium and bacterial membranes. J. Hazard. Mater. 2015, 285, 148–156. [Google Scholar] [CrossRef]
- Erdoğar, N.; Bilensoy, E. Cyclodextrins in drug delivery. In Nanotechnology and Drug Delivery: Volume 1: Nanoplatforms in Drug Delivery; CRC Press: Boca Raton, FL, USA, 2014; Volume 2, pp. 178–209. ISBN 9781466599482. [Google Scholar]
- Krause, M.R.; Regen, S.L. The Structural Role of Cholesterol in Cell Membranes: From Condensed Bilayers to Lipid Rafts. Acc. Chem. Res. 2014, 47, 3512–3521. [Google Scholar] [CrossRef] [PubMed]
- Fauvelle, F.; Debouzy, J.C.; Crouzy, S.; Göschl, M.; Chapron, Y. Mechanism of α-Cyclodextrin-lnduced Hemolysis. 1. The Two-Step Extraction of Phosphatidylinositol from the Membrane. J. Pharm. Sci. 1997, 86, 935–943. [Google Scholar] [CrossRef] [PubMed]
- Debouzy, J.C.; Fauvelle, F.; Crouzy, S.; Girault, L.; Chapron, Y.; Göschl, M.; Gadelle, A. Mechanism of α-Cyclodextrin Induced Hemolysis. 2. A Study of the Factors Controlling the Association with Serine-, Ethanolamine-, and Choline-Phospholipids. J. Pharm. Sci. 1998, 87, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Kilsdonk, E.P.C.; Yancey, P.G.; Stoudt, G.W.; Bangerter, F.W.; Johnson, W.J.; Phillips, M.C.; Rothblat, G.H. Cellular Cholesterol Efflux Mediated by Cyclodextrins. J. Biol. Chem. 1995, 270, 17250–17256. [Google Scholar] [CrossRef]
- Monnaert, V.; Tilloy, S.; Bricout, H.; Cecchelli, R.; Monflier, E. Behavior of alpha-, beta-, and gamma-cyclodextrins and Their Derivatives on an in Vitro Model of Blood-Brain Barrier. J. Pharmacol. Exp. Ther. 2004, 310, 745–751. [Google Scholar] [CrossRef]
- Ohtani, Y.; Irie, T.; Uekama, K.; Fukunaga, K.; Pitha, J. Differential effects of alpha-, beta- and gamma-cyclodextrins on human erythrocytes. Eur. J. Biochem. 1989, 186, 17–22. [Google Scholar] [CrossRef]
- Kiss, T.; Fenyvesi, F.; Bácskay, I.; Váradi, J.; Fenyvesi, É.; Iványi, R.; Szente, L.; Tósaki, Á.; Vecsernyés, M. Evaluation of the cytotoxicity of β-cyclodextrin derivatives: Evidence for the role of cholesterol extraction. Eur. J. Pharm. Sci. 2010, 40, 376–380. [Google Scholar] [CrossRef]
- Róka, E.; Ujhelyi, Z.; Deli, M.; Bocsik, A.; Fenyvesi, É.; Szente, L.; Fenyvesi, F.; Vecsernyés, M.; Váradi, J.; Fehér, P.; et al. Evaluation of the Cytotoxicity of α-Cyclodextrin Derivatives on the Caco-2 Cell Line and Human Erythrocytes. Molecules 2015, 20, 20269–20285. [Google Scholar] [CrossRef]
- Lambert, D.; O’Neill, C.A.; Padfield, P.J. Depletion of Caco-2 cell cholesterol disrupts barrier function by altering the detergent solubility and distribution of specific tight-junction proteins. Biochem. J. 2005, 387, 553–560. [Google Scholar] [CrossRef]
- Chan, L.M.S.; Lowes, S.; Hirst, B.H. The ABCs of drug transport in intestine and liver: Efflux proteins limiting drug absorption and bioavailability. Eur. J. Pharm. Sci. 2004, 21, 25–51. [Google Scholar] [CrossRef] [PubMed]
- Sharom, F.J. Complex Interplay between the P-Glycoprotein Multidrug Efflux Pump and the Membrane: Its Role in Modulating Protein Function. Front. Oncol. 2014, 4, 41. [Google Scholar] [CrossRef] [PubMed]
- Gutay-Tóth, Z.; Fenyvesi, F.; Bársony, O.; Szente, L.; Goda, K.; Szabó, G.; Bacsó, Z. Cholesterol-dependent conformational changes of P-glycoprotein are detected by the 15D3 monoclonal antibody. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2016, 1861, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Fenyvesi, F.; Fenyvesi, É.; Szente, L.; Goda, K.; Bacsó, Z.; Bácskay, I.; Váradi, J.; Kiss, T.; Molnár, É.; Janáky, T.; et al. P-glycoprotein inhibition by membrane cholesterol modulation. Eur. J. Pharm. Sci. 2008, 34, 236–242. [Google Scholar] [CrossRef]
- Arima, H.; Yunomae, K.; Hirayama, F.; Uekama, K. Contribution of P-glycoprotein to the enhancing effects of dimethyl-beta-cyclodextrin on oral bioavailability of tacrolimus. J. Pharmacol. Exp. Ther. 2001, 297, 547–555. [Google Scholar] [PubMed]
- Fenyvesi, F.; Kiss, T.; Fenyvesi, É.; Szente, L.; Veszelka, S.; Deli, M.A.; Váradi, J.; Fehér, P.; Ujhelyi, Z.; Tósaki, Á.; et al. Randomly methylated β-cyclodextrin derivatives enhance taxol permeability through human intestinal epithelial Caco-2 cell monolayer. J. Pharm. Sci. 2011, 100, 4734–4744. [Google Scholar] [CrossRef]
- Réti-Nagy, K.; Malanga, M.; Fenyvesi, É.; Szente, L.; Vámosi, G.; Váradi, J.; Bácskay, I.; Fehér, P.; Ujhelyi, Z.; Róka, E.; et al. Endocytosis of fluorescent cyclodextrins by intestinal Caco-2 cells and its role in paclitaxel drug delivery. Int. J. Pharm. 2015, 496, 509–517. [Google Scholar] [CrossRef]
- Vanier, M.; Millat, G. Niemann-Pick disease type C. Clin. Genet. 2003, 64, 269–281. [Google Scholar] [CrossRef]
- Abi-Mosleh, L.; Infante, R.E.; Radhakrishnan, A.; Goldstein, J.L.; Brown, M.S. Cyclodextrin overcomes deficient lysosome-to-endoplasmic reticulum transport of cholesterol in Niemann-Pick type C cells. Proc. Natl. Acad. Sci. USA 2009, 106, 19316–19321. [Google Scholar] [CrossRef]
- Rosenbaum, A.I.; Zhang, G.; Warren, J.D.; Maxfield, F.R. Endocytosis of beta-cyclodextrins is responsible for cholesterol reduction in Niemann-Pick type C mutant cells. Proc. Natl. Acad. Sci. USA 2010, 107, 5477–5482. [Google Scholar] [CrossRef]
- Davidson, C.D.; Ali, N.F.; Micsenyi, M.C.; Stephney, G.; Renault, S.; Dobrenis, K.; Ory, D.S.; Vanier, M.T.; Walkley, S.U. Chronic Cyclodextrin Treatment of Murine Niemann-Pick C Disease Ameliorates Neuronal Cholesterol and Glycosphingolipid Storage and Disease Progression. PLoS ONE 2009, 4, e6951. [Google Scholar] [CrossRef] [PubMed]
- Hajdu, I.; Angyal, J.; Szikra, D.; Kertész, I.; Malanga, M.; Fenyvesi, É.; Szente, L.; Vecsernyés, M.; Bácskay, I.; Váradi, J.; et al. Radiochemical synthesis and preclinical evaluation of 68Ga-labeled NODAGA-hydroxypropyl-beta-cyclodextrin (68Ga-NODAGA-HPBCD). Eur. J. Pharm. Sci. 2019, 128, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Pontikis, C.C.; Davidson, C.D.; Walkley, S.U.; Platt, F.M.; Begley, D.J. Cyclodextrin alleviates neuronal storage of cholesterol in Niemann-Pick C disease without evidence of detectable blood–brain barrier permeability. J. Inherit. Metab. Dis. 2013, 36, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Gould, S.; Scott, R.C. 2-Hydroxypropyl-b-cyclodextrin (HP-b-CD): A toxicology review. Food Chem. Toxicol. 2005, 43, 1451–1459. [Google Scholar] [CrossRef] [PubMed]
- Stella, V.J.; He, Q. Cyclodextrins. Toxicol. Pathol. 2008, 36, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Ory, D.S.; Ottinger, E.A.; Farhat, N.Y.; King, K.A.; Jiang, X.; Weissfeld, L.; Berry-Kravis, E.; Davidson, C.D.; Bianconi, S.; Keener, L.A.; et al. Intrathecal 2-hydroxypropyl-β-cyclodextrin decreases neurological disease progression in Niemann-Pick disease, type C1: A non-randomised, open-label, phase 1–2 trial. Lancet 2017, 390, 1758–1768. [Google Scholar] [CrossRef]
- Yasmin, N.; Ishitsuka, Y.; Fukaura, M.; Yamada, Y.; Nakahara, S.; Ishii, A.; Kondo, Y.; Takeo, T.; Nakagata, N.; Motoyama, K.; et al. In Vitro and In Vivo Evaluation of 6-O-α-Maltosyl-β-Cyclodextrin as a Potential Therapeutic Agent Against Niemann-Pick Disease Type C. Int. J. Mol. Sci. 2019, 20, 1152. [Google Scholar] [CrossRef]
- Tamura, A.; Yui, N. Polyrotaxane-based systemic delivery of β-cyclodextrins for potentiating therapeutic efficacy in a mouse model of Niemann-Pick type C disease. J. Control. Release 2018, 269, 148–158. [Google Scholar] [CrossRef]
- Soga, M.; Ishitsuka, Y.; Hamasaki, M.; Yoneda, K.; Furuya, H.; Matsuo, M.; Ihn, H.; Fusaki, N.; Nakamura, K.; Nakagata, N.; et al. HPGCD Outperforms HPBCD as a Potential Treatment for Niemann-Pick Disease Type C During Disease Modeling with iPS Cells. Stem Cells 2015, 33, 1075–1088. [Google Scholar] [CrossRef]
- Szente, L.; Singhal, A.; Domokos, A.; Song, B. Cyclodextrins: Assessing the impact of cavity size, occupancy, and substitutions on cytotoxicity and cholesterol homeostasis. Molecules 2018, 23, 1228. [Google Scholar] [CrossRef]
- Doherty, G.J.; McMahon, H.T. Mechanisms of Endocytosis. Annu. Rev. Biochem. 2009, 78, 857–902. [Google Scholar] [CrossRef]
- Swanson, J.A.; Yoshida, S. Macropinocytosis. In Encyclopedia of Cell Biology; Academic Press: Cambridge, MA, USA, 2015; ISBN 9780123944474. [Google Scholar]
- Kou, L.; Sun, J.; Zhai, Y.; He, Z. The endocytosis and intracellular fate of nanomedicines: Implication for rational design. Asian J. Pharm. Sci. 2013, 8, 1–10. [Google Scholar] [CrossRef]
- Sahay, G.; Alakhova, D.Y.; Kabanov, A.V. Endocytosis of nanomedicines. J. Control. Release 2010, 145, 182–195. [Google Scholar] [CrossRef] [PubMed]
- Fenyvesi, F.; Réti-Nagy, K.; Bacsó, Z.; Gutay-Tóth, Z.; Malanga, M.; Fenyvesi, É.; Szente, L.; Váradi, J.; Ujhelyi, Z.; Fehér, P.; et al. Fluorescently labeled methyl-beta-cyclodextrin enters intestinal epithelial Caco-2 cells by fluid-phase endocytosis. PLoS ONE 2014, 9, e84856. [Google Scholar] [CrossRef] [PubMed]
- Plazzo, A.P.; Höfer, C.T.; Jicsinszky, L.; Fenyvesi, É.; Szente, L.; Schiller, J.; Herrmann, A.; Müller, P. Uptake of a fluorescent methyl-β-cyclodextrin via clathrin-dependent endocytosis. Chem. Phys. Lipids 2012, 165, 505–511. [Google Scholar] [CrossRef]
- Wei, H.; Zheng, W.; Diakur, J.; Wiebe, L.I. Confocal laser scanning microscopy (CLSM) based evidence for cell permeation by mono-4-(N-6-deoxy-6-amino-β-cyclodextrin)-7-nitrobenzofuran (NBD-β-CyD). Int. J. Pharm. 2011, 403, 15–22. [Google Scholar] [CrossRef]
- Onodera, R.; Motoyama, K.; Tanaka, N.; Ohyama, A.; Okamatsu, A.; Higashi, T.; Kariya, R.; Okada, S.; Arima, H. Involvement of Autophagy in Antitumor Activity of Folate-appended Methyl-β-cyclodextrin. Sci. Rep. 2015, 4, 4417. [Google Scholar] [CrossRef]
- Hirama, T.; Fairn, G.D. Induction of spontaneous curvature and endocytosis: Unwanted consequences of cholesterol extraction using methyl-β-Cyclodextrin. Commun. Integr. Biol. 2018, 11, 1–4. [Google Scholar] [CrossRef]
- Zuhorn, I.S.; Kalicharan, R.; Hoekstra, D. Lipoplex-mediated Transfection of Mammalian Cells Occurs through the Cholesterol-dependent Clathrin-mediated Pathway of Endocytosis. J. Biol. Chem. 2002, 277, 18021–18028. [Google Scholar] [CrossRef]
- O’ Neill, M.J.; Guo, J.; Byrne, C.; Darcy, R.; O’Driscoll, C.M. Mechanistic studies on the uptake and intracellular trafficking of novel cyclodextrin transfection complexes by intestinal epithelial cells. Int. J. Pharm. 2011, 413, 174–183. [Google Scholar] [CrossRef]
- Chen, F.W.; Li, C.; Ioannou, Y.A. Cyclodextrin Induces Calcium-Dependent Lysosomal Exocytosis. PLoS ONE 2010, 5, e15054. [Google Scholar] [CrossRef] [PubMed]
| Stimulus | Conjugates/Guests | Cyclodextrin | Drug | Effect | Application | Ref. |
|---|---|---|---|---|---|---|
| Temperature | poly(ethylene glycol) (PEG) | α-CD | No active ingredient was used | Convert from gel to sol state. | Biomedical using as local chemotherapy of cancers, excellent cytocompatibility, controlled drug release. | [12] |
| Temperature | bi-perylene monoimide | permethyl-β-CD | Tetraphenylporphine (TPPS) | LCST shows from 32 ˚C to 48.2 ˚C. | Controlled drug release that is depending on the temperature | [45] |
| Temperature and pH | N-isopropylacrylamide | β-CD | Naproxen sodium | Swollen ratio decreased with the increase of temperature and response to pH is depending on % of the component | Hydrogels show biodegradability and controlled drug release in stomach condition; in intestinal condition the release is faster because of the higher pH. | [38] |
| Temperature and pH | chitosan | β-CD | Etoposide (VP16) | thermo-sensitive hydrogen bonds were between API and the β-CD cavity that is damaged by increasing temperature. Release of the drug reached 90% at pH 4.5 | The pH response is important to treat cancer, because the tumors’ microenvironment is acidic, contrast with the blood pH. | [46] |
| Temperature and pH | N-isopropylacrylamide | β-CD | Doxorubicin (DOX) | The release of DOX was enhanced by the increase of temperature and decrease of pH | It is a supramolecular micelle for anticancer therapy. Therapeutic index is higher than free DOX. | [39] |
| pH | L-phenylalanine functionalized graphene oxide | β-CD | Doxorubicin (DOX) | Changing the pH from 7.2 to 5.4 resulted in triplicated drug release of DOX. | Nanocarrier has excellent biocompatibility, and is a pH-responsive drug delivery system for cancer therapy | [47] |
| pH | zinc oxide nanoparticles with functionalized PEG surface | β-CD | Curcumin | pH stimulated release showed zero order release of curcumin at tumor pH | ZnO nanoparticles have higher antibacterial activity on Staphylococcus Aureus than free drug | [48] |
| pH | chitosan | β-CD | Methyl-orange (not active pharmaceutical ingredients (API)) | Swelling behaviours were changed by pH stimuli | It has sustained release properties, which make it suitable for use in medicine | [49] |
| pH and photo | azobenzene-poly-2-(diisopropylamino) ethyl methacrylate–methoxypolyethylene glycols | β-CD | doxorubicin (DOX) | The low pH and NIR lead to disassembling of nanoparticles. | It provides a new perspective on tumor therapy, tumor targeting, and controlled drug release. | [44] |
| pH | adamantyl-terminated poly (ethylene glycol) | β-cyclodextrin-containing poly(β-amino ester) | Curcumin | Micelle could unload the 70% of drug at pH 5.5 and 30% of the drug at 7.4 until 24 hours. | It is a supramolecular micelle drug delivery system for cancer treatment. | [50] |
| reduction, photo | hyaluronic acid | β-CD | adamantane linked camptothecin | Disulphide bond linkage is reduction sensitive, that is led to release the drug by NIR | A reduction-sensitive drug delivery was developed with photothermal-chemotherapy. | [51] |
| Type of Amphiphilic Cylodextrin | Subtype | Substituents |
|---|---|---|
| Polysubstituted | Medusa-like | Sulfo-, thio-alkyl-, amido- or, amino chains on the primary side |
| Skirt shaped | Modified on the secondary hydroxyl groups with alkyl chains via an ester group | |
| Bouquet-like | Hydrocarbon chains on boths side, or poly(oxyethylene) and polymethylene chains | |
| Monosubstituted | Lollipop | One alkyl chain on the primary side |
| Cup and Ball | Contain a bulky Boc-amino protective group at the end of the alkyl chain | |
| Lipid-like | Cholesteryl, phospholipidyl or dilauryl moiety |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haimhoffer, Á.; Rusznyák, Á.; Réti-Nagy, K.; Vasvári, G.; Váradi, J.; Vecsernyés, M.; Bácskay, I.; Fehér, P.; Ujhelyi, Z.; Fenyvesi, F. Cyclodextrins in Drug Delivery Systems and Their Effects on Biological Barriers. Sci. Pharm. 2019, 87, 33. https://doi.org/10.3390/scipharm87040033
Haimhoffer Á, Rusznyák Á, Réti-Nagy K, Vasvári G, Váradi J, Vecsernyés M, Bácskay I, Fehér P, Ujhelyi Z, Fenyvesi F. Cyclodextrins in Drug Delivery Systems and Their Effects on Biological Barriers. Scientia Pharmaceutica. 2019; 87(4):33. https://doi.org/10.3390/scipharm87040033
Chicago/Turabian StyleHaimhoffer, Ádám, Ágnes Rusznyák, Katalin Réti-Nagy, Gábor Vasvári, Judit Váradi, Miklós Vecsernyés, Ildikó Bácskay, Pálma Fehér, Zoltán Ujhelyi, and Ferenc Fenyvesi. 2019. "Cyclodextrins in Drug Delivery Systems and Their Effects on Biological Barriers" Scientia Pharmaceutica 87, no. 4: 33. https://doi.org/10.3390/scipharm87040033
APA StyleHaimhoffer, Á., Rusznyák, Á., Réti-Nagy, K., Vasvári, G., Váradi, J., Vecsernyés, M., Bácskay, I., Fehér, P., Ujhelyi, Z., & Fenyvesi, F. (2019). Cyclodextrins in Drug Delivery Systems and Their Effects on Biological Barriers. Scientia Pharmaceutica, 87(4), 33. https://doi.org/10.3390/scipharm87040033














