Recent Approaches for Solid Dose Vaccine Delivery
Abstract
1. Introduction
1.1. Rationale for Solid Dosage Form of Vaccine
1.2. Dose-Sparing and Cost-Effectiveness
1.3. Cold-Chain
1.4. Safety
1.5. Compliance
1.6. The Training of Vaccinators
1.7. Rapid Distribution
2. Types of Solid Dose Vaccine Delivery System
2.1. Mucosal Routes
2.1.1. The Oral Route
2.1.2. Pulmonary Route
2.1.3. Intranasal Route
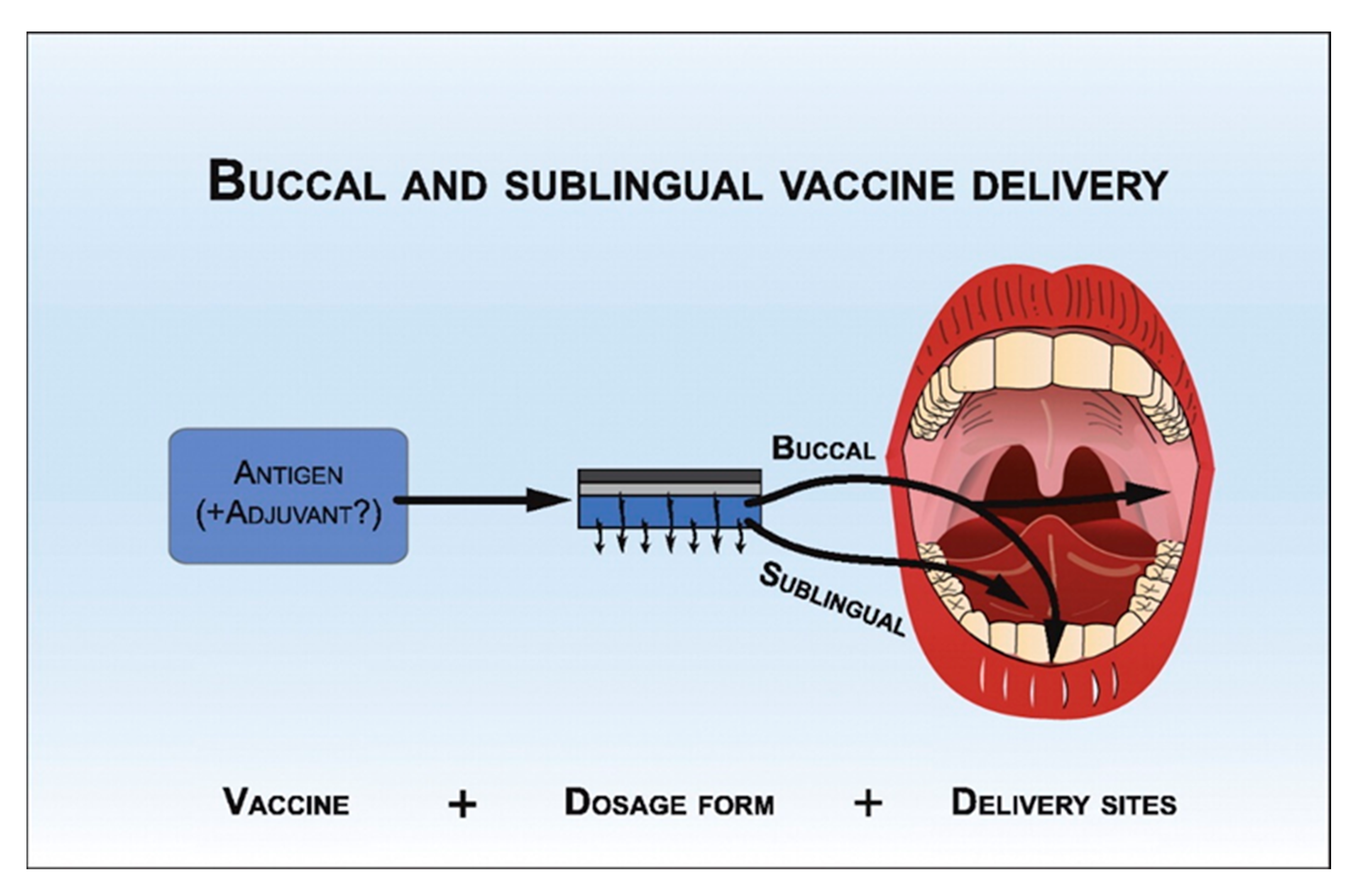
2.1.4. The Buccal and Sublingual Routes
2.2. Transdermal Route
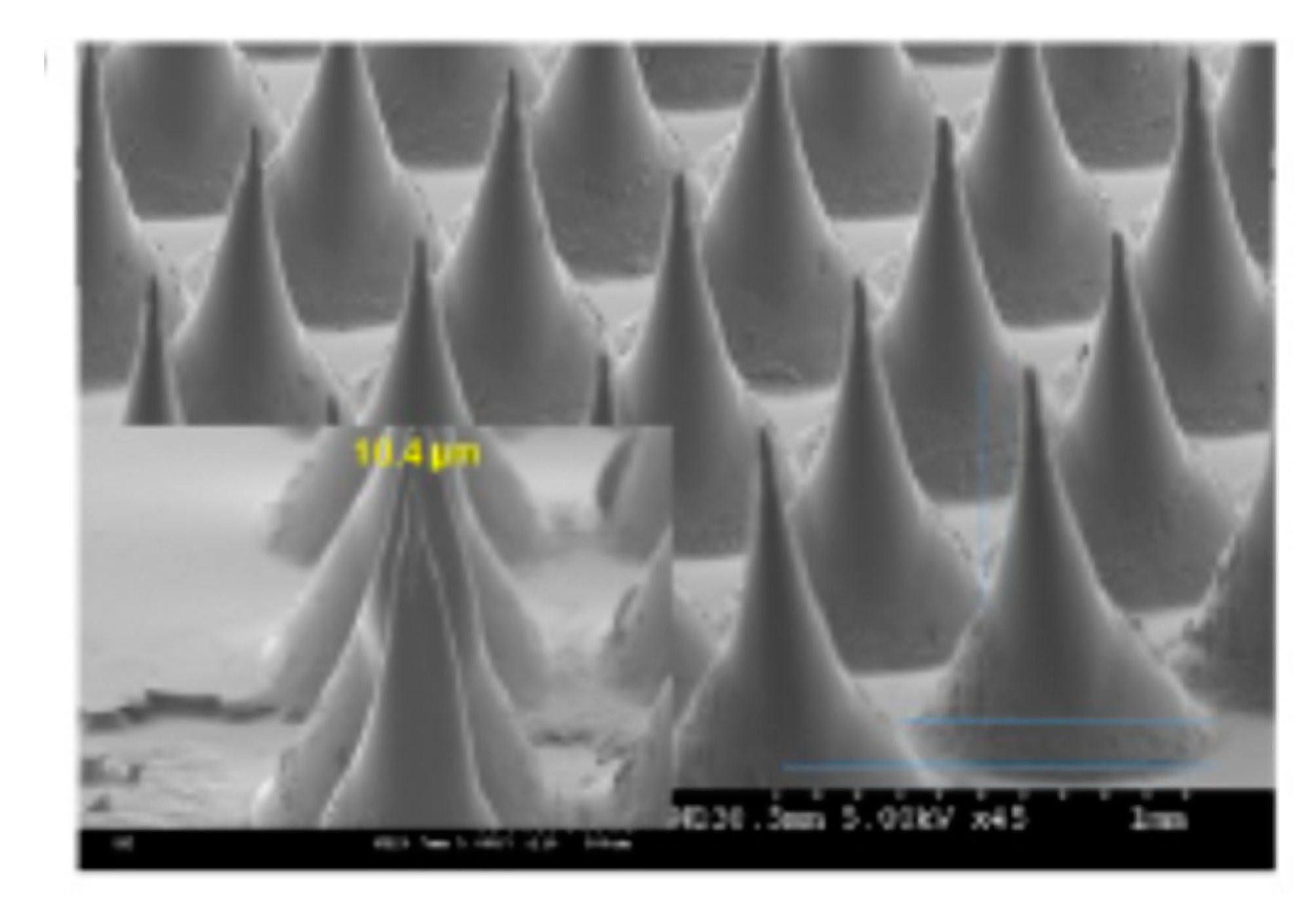
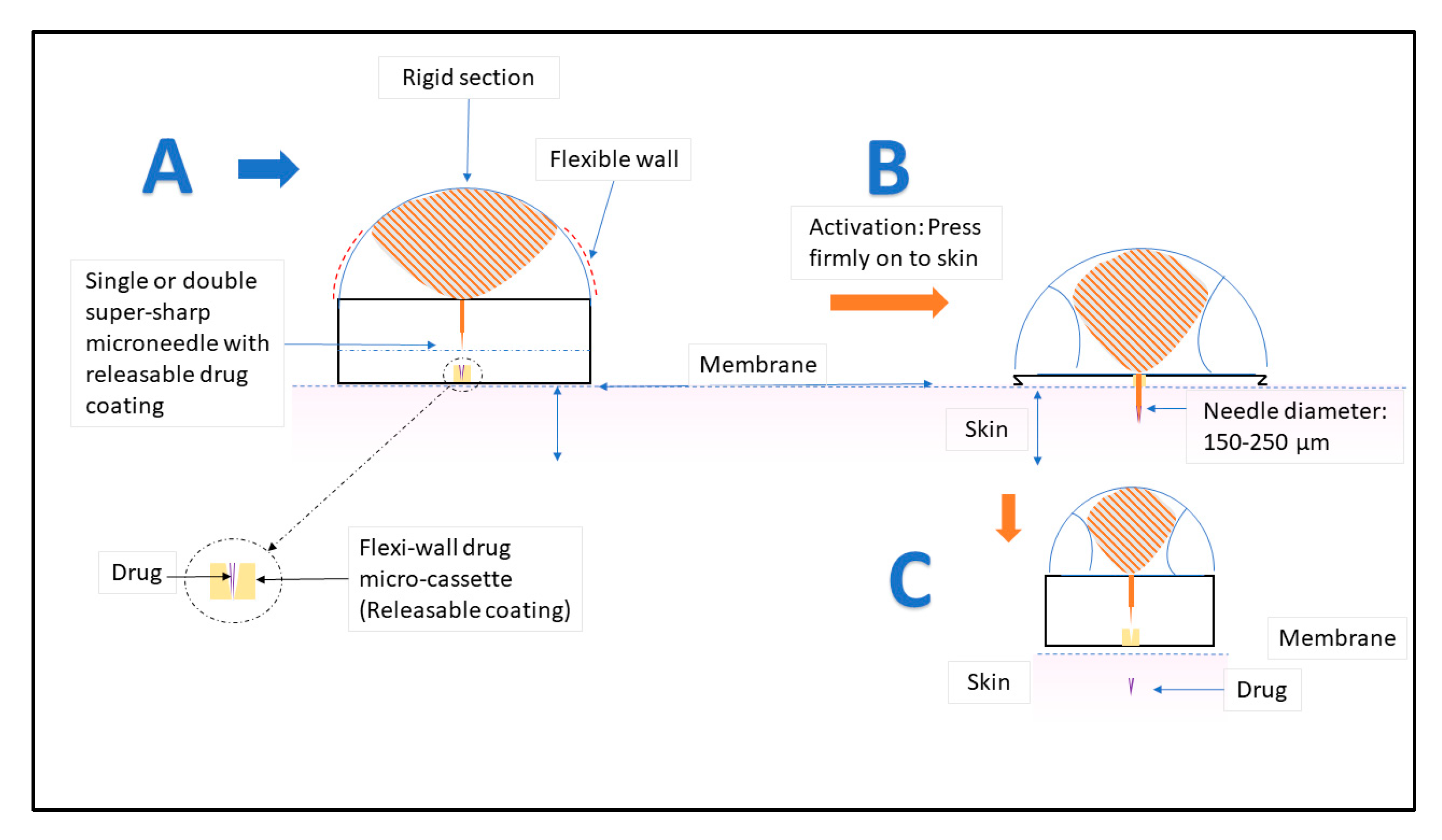
2.2.1. Microneedle Delivery System
2.2.2. Jet Injectors and Solid Dose Injectors
2.2.3. Epidermal Powder Immunisation (EPI)
3. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Riedel, S. Edward Jenner and the history of smallpox and vaccination. Proceeding 2005, 18, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Borde, A.; Ekman, A.; Holmgren, J.; Larsson, A. Effect of protein release rates from tablet formulations on the immune response after sublingual immunization. Eur. J. Pharm. Sci. 2012, 47, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Broadhead, J.; Cheung, K. Vaccine Delivery Using the Nemaura Solid Dose Injector. ONdrugDelivery Mag. 2016, 65, 18–22. [Google Scholar]
- Liao, J.-F.; Lee, J.-C.; Lin, C.-K.; Wei, K.-C.; Chen, P.-Y.; Yang, H.-W. Self-Assembly DNA Polyplex Vaccine inside Dissolving Microneedles for High-Potency Intradermal Vaccination. Theranostics 2017, 7, 2593–2605. [Google Scholar] [CrossRef] [PubMed]
- Kolluru, C.; Gomaa, Y.; Prausnitz, M.R. Development of a thermostable microneedle patch for polio vaccination. Drug Deliv. Transl. Res. 2019, 9, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Vaccines. Available online: http://www.who.int/topics/vaccines/en/ (accessed on 26 July 2016).
- Wang, J.; Thorson, L.; Stokes, R.W.; Santosuosso, M.; Huygen, K.; Zganiacz, A.; Hitt, M.; Xing, Z. Single Mucosal, but Not Parenteral, Immunization with Recombinant Adenoviral-Based Vaccine Provides Potent Protection from Pulmonary Tuberculosis. J. Immunol. 2004, 173, 6357–6365. [Google Scholar] [CrossRef] [PubMed]
- Lycke, N. Recent progress in mucosal vaccine development: Potential and limitations. Nat. Rev. Immunol. 2012, 12, 592–605. [Google Scholar] [CrossRef]
- Allen, L.V.; Popovich, N.G.; Ansel, H.C. Ansel’s Pharmaceutical Dosage Forms and Drug Delivery Systems, 9th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2011; pp. 184–185. [Google Scholar]
- Borde, A. Design of Solid Dosage Forms for Mucosal Vaccination Investigations on the Influence of Excipients on Product Performance. Ph.D. Thesis, Chalmers University of Technology, Gothenburg, Sweden, 2012. [Google Scholar]
- Vivotif. Available online: http://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm094070.htm (accessed on 9 August 2016).
- FluMist® Quadrivalent (Influenza Vaccine Live, Intranasal). Available online: https://www.flumistquadrivalent.com/ (accessed on 6 September 2019).
- Rotarix (Rotavirus Vaccine, Live, Oral Suspension): Side Effects, Interactions, Warning, Dosage &Amp; Uses. Available online: https://www.rxlist.com/rotarix-drug.htm (accessed on 6 September 2019).
- Potter, C.; Nabahi, S. Solid Pharmaceutical and Vaccine Dose 2013. U.S. Patent 14/095,101, 3 December 2013. [Google Scholar]
- Saluja, V.; Amorij, J.-P.; Kapteyn, J.C.; de Boer, A.H.; Frijlink, H.W.; Hinrichs, W.L.J. A comparison between spray drying and spray freeze drying to produce an influenza subunit vaccine powder for inhalation. J. Control. Release 2010, 144, 127–133. [Google Scholar] [CrossRef]
- Amorij, J.-P.; Saluja, V.; Petersen, A.H.; Hinrichs, W.L.J.; Huckriede, A.; Frijlink, H.W. Pulmonary delivery of an inulin-stabilized influenza subunit vaccine prepared by spray-freeze drying induces systemic, mucosal humoral as well as cell-mediated immune responses in BALB/c mice. Vaccine 2007, 25, 8707–8717. [Google Scholar] [CrossRef]
- Kenney, R.T.; Frech, S.A.; Muenz, L.R.; Villar, C.P.; Glenn, G.M. Dose Sparing with Intradermal Injection of Influenza Vaccine. N. Engl. J. Med. 2004, 351, 2295–2301. [Google Scholar] [CrossRef]
- Kremer, M.; Snyder, C.M. Preventives Versus Treatments. Q. J. Econ. 2015, 130, 1167–1239. [Google Scholar] [CrossRef]
- Giudice, E.L.; Campbell, J.D. Needle-free vaccine delivery. Adv. Drug Deliv. Rev. 2006, 58, 68–89. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Epidemiology and Prevention of Vaccine-Preventable Diseases; Hamborsky, J., Kroger, A., Wolfe, S., Eds.; Public Health Foundation: Washington, DC, USA, 2015; pp. 63–78.
- Liebowitz, D.; Lindbloom, J.D.; Brandl, J.R.; Garg, S.J.; Tucker, S.N. High titre neutralising antibodies to influenza after oral tablet immunisation: A phase 1, randomised, placebo-controlled trial. Lancet Infect. Dis. 2015, 15, 1041–1048. [Google Scholar] [CrossRef]
- Bal, S.M.; Ding, Z.; Van Riet, E.; Jiskoot, W.; Bouwstra, J.A. Advances in transcutaneous vaccine delivery: Do all ways lead to Rome? J. Control. Release 2010, 148, 266–282. [Google Scholar] [CrossRef]
- Kraan, H.; Vrieling, H.; Czerkinsky, C.; Jiskoot, W.; Kersten, G.; Amorij, J.P. Buccal and sublingual vaccine delivery. J. Control. Release 2014, 190, 580–592. [Google Scholar] [CrossRef]
- Sheikh, Z.; Jahan, N.; Karim, R. Mucosal delivery of vaccines: A review. Int. J. Pharm. Technol. 2017, 9, 6498–6520. [Google Scholar]
- Huang, J.; Garmise, R.J.; Crowder, T.M.; Mar, K.; Hwang, C.R.; Hickey, A.J.; Mikszta, J.A.; Sullivan, V.J. A novel dry powder influenza vaccine and intranasal delivery technology: Induction of systemic and mucosal immune responses in rats. Vaccine 2004, 23, 794–801. [Google Scholar] [CrossRef]
- Wang, S.; Liu, H.; Zhang, X.; Qian, F. Intranasal and oral vaccination with protein-based antigens: Advantages, challenges and formulation strategies. Protein Cell 2015, 6, 480–503. [Google Scholar] [CrossRef]
- Lal, M.; Priddy, S.; Bourgeois, L.; Walker, R.; Pebley, W.; Brown, J.; Desai, J.; Darsley, M.J.; Kristensen, D.; Chen, D. Development of a fast-dissolving tablet formulation of a live attenuated enterotoxigenic E. coli vaccine candidate. Vaccine 2013, 31, 4759–4764. [Google Scholar] [CrossRef]
- Talavera, A.; Año, G.; Pino, Y.; Castaño, J.; Uribarri, E.; Riverón, L.; Gil, S.; Fernández, S.; Cedré, B.; Valmaseda, T.; et al. Formulation in tablets of a cholera whole cells inactivated vaccine candidate. Vaccine 2006, 24, 3381–3387. [Google Scholar] [CrossRef]
- Fernández, S.; Año, G.; Castaño, J.; Pino, Y.; Uribarri, E.; Riverón, L.A.; Cedré, B.; Valmaseda, T.; Falero, G.; Pérez, J.L.; et al. Evaluation of enteric-coated tablets as a whole cell inactivated vaccine candidate against Vibrio cholerae. Travel Med. Infect. Dis. 2013, 11, 103–109. [Google Scholar] [CrossRef] [PubMed]
- López, Y.; Infante, J.F.; Sifontes, S.; Díaz, D.; Pérez, V.; No, G.; Hernández, T.; Fernández, S.; Castã No, J.L.; Cedré, B.; et al. Pharmacology and toxicology of an oral tablet whole cells inactivated cholera vaccine in Sprague Dawley rats. Vaccine 2011, 29, 3596–3599. [Google Scholar] [CrossRef] [PubMed]
- Ciabattini, A.; Olivieri, R.; Lazzeri, E.; Medaglini, D. Role of the Microbiota in the Modulation of Vaccine Immune Responses. Front. Microbiol. 2019, 10, 1305. [Google Scholar] [CrossRef] [PubMed]
- Lynn, M.A.; Tumes, D.J.; Choo, J.M.; Sribnaia, A.; Blake, S.J.; Leong, L.E.X.; Young, G.P.; Marshall, H.S.; Wesselingh, S.L.; Rogers, G.B.; et al. Early-Life Antibiotic-Driven Dysbiosis Leads to Dysregulated Vaccine Immune Responses in Mice. Cell Host Microbe 2018, 23, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, D.R.; De Calisto, J.; Simmons, N.L.; Cruz, A.N.; Villablanca, E.J.; Mora, J.R.; Barouch, D.H. Vitamin A Deficiency Impairs Vaccine-Elicited Gastrointestinal Immunity. J. Immunol. 2011, 187, 1877–1883. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, W.; Treuer, T.; Karagianis, J.; Ascher-Svanum, H.; Harrison, G. Orally disintegrating olanzapine review: Effectiveness, patient preference, adherence, and other properties. Patient Prefer. Adherence 2012, 6, 109–125. [Google Scholar] [CrossRef] [PubMed]
- Surman, S.L.; Penkert, R.R.; Jones, B.G.; Sealy, R.E.; Hurwitz, J.L. Vitamin Supplementation at the Time of Immunization with a Cold-Adapted Influenza Virus Vaccine Corrects Poor Mucosal Antibody Responses in Mice Deficient for Vitamins A and D. Clin. Vaccine Immunol. 2016, 23, 219–227. [Google Scholar] [CrossRef]
- Tonnis, W.F.; Kersten, G.F.; Frijlink, H.W.; Hinrichs, W.L.J.; de Boer, A.H.; Amorij, J.-P. Pulmonary vaccine delivery: A realistic approach? J. Aerosol Med. Pulm. Drug Deliv. 2012, 25, 249–260. [Google Scholar] [CrossRef]
- de Swart, R.L.; LiCalsi, C.; Quirk, A.V.; van Amerongen, G.; Nodelman, V.; Alcock, R.; Yüksel, S.; Ward, G.H.; Hardy, J.G.; Vos, H.; et al. Measles vaccination of macaques by dry powder inhalation. Vaccine 2007, 25, 1183–1190. [Google Scholar] [CrossRef]
- Kisich, K.O.; Higgins, M.P.; Park, I.; Cape, S.P.; Lindsay, L.; Bennett, D.J.; Winston, S.; Searles, J.; Sievers, R.E. Dry powder measles vaccine: Particle deposition, virus replication, and immune response in cotton rats following inhalation. Vaccine 2011, 29, 905–912. [Google Scholar] [CrossRef]
- Licalsi, C.; Christensen, T.; Bennett, J.V.; Phillips, E.; Witham, C. Dry powder inhalation as a potential delivery method for vaccines. Vaccine 1999, 17, 1796–1803. [Google Scholar] [CrossRef]
- Licalsi, C.; Maniaci, M.J.; Christensen, T.; Phillips, E.; Ward, G.H.; Witham, C. A powder formulation of measles vaccine for aerosol delivery. Vaccine 2001, 19, 2629–2636. [Google Scholar] [CrossRef]
- Zaman, M.; Chandrudu, S.; Toth, I. Strategies for intranasal delivery of vaccines. Drug Deliv. Transl. Res. 2013, 3, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Muszkat, M.; Friedman, G.; Schein, M.H.; Naveh, P.; Greenbaum, E.; Schlesinger, M.; Zakay-Rones, Z.; Yehuda, A.B. Local SIgA response following administration of a novel intranasal inactivated influenza virus vaccine in community residing elderly. Vaccine 2000, 18, 1696–1699. [Google Scholar] [CrossRef]
- Hashigucci, K.; Ogawa, H.; Ishidate, T.; Yamashita, R.; Kamiya, H.; Watanabe, K.; Hattori, N.; Sato, T.; Suzuki, Y.; Nagamine, T.; et al. Antibody responses in volunteers induced by nasal influenza vaccine combined with Escherichia coli heat-labile enterotoxin B subunit containing a trace amount of the holotoxin. Vaccine 1996, 14, 113–119. [Google Scholar] [CrossRef]
- Moldoveanu, Z.; Clements, M.L.; Prince, S.J.; Murphy, B.R.; Mestecky, J. Human immune responses to influenza virus vaccines administered by systemic or mucosal routes. Vaccine 1995, 13, 1006–1012. [Google Scholar] [CrossRef]
- Smith, D.J.; Bot, S.; Dellamary, L.; Bot, A. Evaluation of novel aerosol formulations designed for mucosal vaccination against influenza virus. Vaccine 2003, 21, 2805–2812. [Google Scholar] [CrossRef]
- Birkhoff, M.; Leitz, M.; Marx, D. Advantages of Intranasal Vaccination and Considerations on Device Selection. Indian J. Pharm. Sci. 2009, 71, 729. [Google Scholar]
- Bahamondez-Canas, T.F.; Cui, Z. Intranasal immunization with dry powder vaccines. Eur. J. Pharm. Biopharm. 2018, 122, 167–175. [Google Scholar] [CrossRef]
- Zuercher, A.W.; Coffin, S.E.; Thurnheer, M.C.; Fundova, P.; Cebra, J.J. Nasal-associated lymphoid tissue is a mucosal inductive site for virus-specific humoral and cellular immune responses. J. Immunol. 2002, 168, 1796–1803. [Google Scholar] [CrossRef]
- Hiroi, T.; Iwatani, K.; Iijima, H.; Kodama, S.; Yanagita, M.; Kiyono, H. Nasal immune system: distinctive Th0 and Th1/Th2 type environments in murine nasal-associated lymphoid tissues and nasal passage, respectively. Eur. J. Immunol. 1998, 28, 3346–3353. [Google Scholar] [CrossRef]
- Wu, H.Y.; Nguyen, H.H.; Russell, M.W. Nasal lymphoid tissue (NALT) as a mucosal immune inductive site. Scand. J. Immunol. 1997, 46, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Cesta, M.F. Normal Structure, Function, and Histology of Mucosa-Associated Lymphoid Tissue. Toxicol. Pathol. 2006, 34, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Whaley, K.J.; Zeitlin, L. Preventing transmission: Plant-derived microbicides and mucosal vaccines for reproductive health. Vaccine 2005, 23, 1819–1822. [Google Scholar] [CrossRef] [PubMed]
- Corr, S.C.; Gahan, C.C.G.M.; Hill, C. M-cells: Origin, morphology and role in mucosal immunity and microbial pathogenesis. FEMS Immunol. Med. Microbiol. 2008, 52, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Belshe, R.B.; Mendelman, P.M.; Treanor, J.; King, J.; Gruber, W.C.; Piedra, P.; Bernstein, D.I.; Hayden, F.G.; Kotloff, K.; Zangwill, K.; et al. The Efficacy of Live Attenuated, Cold-Adapted, Trivalent, Intranasal Influenzavirus Vaccine in Children. N. Engl. J. Med. 1998, 338, 1405–1412. [Google Scholar] [CrossRef] [PubMed]
- Izurieta, H.S.; Haber, P.; Wise, R.P.; Iskander, J.; Pratt, D.; Mink, C.; Chang, S.; Braun, M.M.; Ball, R. Adverse Events Reported Following Live, Cold-Adapted, Intranasal Influenza Vaccine. JAMA 2005, 294, 2720. [Google Scholar] [CrossRef]
- Garg, N.K.; Mangal, S.; Khambete, H.; Sharma, P.K.; Tyagi, R.K. Mucosal delivery of vaccines: role of mucoadhesive/biodegradable polymers. Recent Pat. Drug Deliv. Formul. 2010, 4, 114–128. [Google Scholar] [CrossRef]
- Yang, J.; Zhao, J.; Fang, Y. Calorimetric studies of the interaction between sodium alginate and sodium dodecyl sulfate in dilute solutions at different pH values. Carbohydr. Res. 2008, 343, 719–725. [Google Scholar] [CrossRef]
- Sahoo, S.; Chakraborti, C.K.; Mishra, S.C. Qualitative analysis of controlled release ciprofloxacin/carbopol 934 mucoadhesive suspension. J. Adv. Pharm. Technol. Res. 2011, 2, 195–204. [Google Scholar] [CrossRef]
- Singh, M.; O’Hagan, D. The preparation and characterization of polymeric antigen delivery systems for oral administration. Adv. Drug Deliv. Rev. 1998, 34, 285–304. [Google Scholar] [CrossRef]
- Makhlof, A.; Fujimoto, S.; Tozuka, Y.; Takeuchi, H. In vitro and in vivo evaluation of WGA–carbopol modified liposomes as carriers for oral peptide delivery. Eur. J. Pharm. Biopharm. 2011, 77, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Witschi, C.; Mrsny, R.J. In vitro evaluation of microparticles and polymer gels for use as nasal platforms for protein delivery. Pharm. Res. 1999, 16, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.-S.; Xu, Y.-L.; Zou, X.-T.; Xu, Z.-R. Chitosan Nanoparticles Act as an Adjuvant to Promote both Th1 and Th2 Immune Responses Induced by Ovalbumin in Mice. Mar. Drugs 2011, 9, 1038–1055. [Google Scholar] [CrossRef] [PubMed]
- Rauw, F.; Gardin, Y.; Palya, V.; Anbari, S.; Gonze, M.; Lemaire, S.; van den Berg, T.; Lambrecht, B. The positive adjuvant effect of chitosan on antigen-specific cell-mediated immunity after chickens vaccination with live Newcastle disease vaccine. Vet. Immunol. Immunopathol. 2010, 134, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Verheul, R.J.; Hagenaars, N.; van Es, T.; van Gaal, E.V.B.; de Jong, P.H.J.L.F.; Bruijns, S.; Mastrobattista, E.; Slütter, B.; Que, I.; Heldens, J.G.M.; et al. A step-by-step approach to study the influence of N-acetylation on the adjuvanticity of N,N,N-trimethyl chitosan (TMC) in an intranasal nanoparticulate influenza virus vaccine. Eur. J. Pharm. Sci. 2012, 45, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Shen, Y.; Jiang, Z.; Wang, Y.; Chu, Y.; Xiong, S. Intranasal delivery of chitosan–DNA vaccine generates mucosal SIgA and anti-CVB3 protection. Vaccine 2004, 22, 3603–3612. [Google Scholar] [CrossRef]
- Aspden, T.J.; Mason, J.D.T.; Jones, N.S.; Lowe, J.; Skaugrud, O.; Illum, L. Chitosan as a Nasal Delivery System: The Effect of Chitosan Solutions on in Vitro and in Vivo Mucociliary Transport Rates in Human Turbinates and Volunteers. J. Pharm. Sci. 1997, 86, 509–513. [Google Scholar] [CrossRef]
- Almeida, A.; Souto, E. Solid lipid nanoparticles as a drug delivery system for peptides and proteins. Adv. Drug Deliv. Rev. 2007, 59, 478–490. [Google Scholar] [CrossRef]
- Jabbal-Gill, I. Nasal vaccine innovation. J. Drug Target. 2010, 18, 771–786. [Google Scholar] [CrossRef]
- Sharma, S.; Mukkur, T.K.S.; Benson, H.A.E.; Chen, Y. Pharmaceutical Aspects of Intranasal Delivery of Vaccines Using Particulate Systems. J. Pharm. Sci. 2009, 98, 812–843. [Google Scholar] [CrossRef] [PubMed]
- Henriksen-Lacey, M.; Korsholm, K.S.; Andersen, P.; Perrie, Y.; Christensen, D. Liposomal vaccine delivery systems. Expert Opin. Drug Deliv. 2011, 8, 505–519. [Google Scholar] [CrossRef] [PubMed]
- Cusi, M.G.; Terrosi, C.; Savellini, G.G.; Di Genova, G.; Zurbriggen, R.; Correale, P. Efficient delivery of DNA to dendritic cells mediated by influenza virosomes. Vaccine 2004, 22, 735–739. [Google Scholar] [CrossRef] [PubMed]
- Glück, U.; Gebbers, J.O.; Glück, R. Phase 1 evaluation of intranasal virosomal influenza vaccine with and without Escherichia coli heat-labile toxin in adult volunteers. J. Virol. 1999, 73, 7780–7786. [Google Scholar]
- Glück, R.; Mischler, R.; Durrer, P.; Fürer, E.; Lang, A.B.; Herzog, C.; Cryz, S.J. Safety and Immunogenicity of Intranasally Administered Inactivated Trivalent Virosome-Formulated Influenza Vaccine Containing Escherichia coli Heat-Labile Toxin as a Mucosal Adjuvant. J. Infect. Dis. 2000, 181, 1129–1132. [Google Scholar] [CrossRef]
- Bomsel, M.; Tudor, D.; Drillet, A.-S.; Alfsen, A.; Ganor, Y.; Roger, M.-G.; Mouz, N.; Amacker, M.; Chalifour, A.; Diomede, L.; et al. Immunization with HIV-1 gp41 Subunit Virosomes Induces Mucosal Antibodies Protecting Nonhuman Primates against Vaginal SHIV Challenges. Immunity 2011, 34, 269–280. [Google Scholar] [CrossRef]
- Pearse, M.J.; Drane, D. ISCOMATRIX® adjuvant for antigen delivery. Adv. Drug Deliv. Rev. 2005, 57, 465–474. [Google Scholar] [CrossRef]
- Bessler, W.G.; Baier, W.; vd Esche, U.; Hoffmann, P.; Heinevetter, L.; Wiesmüller, K.H.; Jung, G. Bacterial lipopeptides constitute efficient novel immunogens and adjuvants in parenteral and oral immunization. Behring Inst. Mitt. 1997, 390–399. [Google Scholar]
- Gonzalez-Miro, M.; Chen, S.; Gonzaga, Z.J.; Evert, B.; Wibowo, D.; Rehm, B.H.A. Polyester as Antigen Carrier toward Particulate Vaccines. Biomacromolecules 2019, 20, 3213–3232. [Google Scholar] [CrossRef]
- Zeng, L. Mucosal adjuvants: Opportunities and challenges. Hum. Vaccin. Immunother. 2016, 12, 2456–2458. [Google Scholar] [CrossRef]
- Price, R.; Young, P.M.; Edge, S.; Staniforth, J.N. The influence of relative humidity on particulate interactions in carrier-based dry powder inhaler formulations. Int. J. Pharm. 2002, 246, 47–59. [Google Scholar] [CrossRef]
- Coelho, M.C.; Harnby, N. The effect of humidity on the form of water retention in a powder. Powder Technol. 1978, 20, 197–200. [Google Scholar] [CrossRef]
- USP 32 General Notices and Requirements: Applying to Standards, Tests, Assays, and Other Specifications of the United States Pharmacopeia. Available online: https://www.uspnf.com/sites/default/files/usp_pdf/EN/USPNF/generalNoticesandRequirementsFinal.pdf (accessed on 13 July 2019).
- Tiozzo Fasiolo, L.; Manniello, M.D.; Tratta, E.; Buttini, F.; Rossi, A.; Sonvico, F.; Bortolotti, F.; Russo, P.; Colombo, G. Opportunity and challenges of nasal powders: Drug formulation and delivery. Eur. J. Pharm. Sci. 2018, 113, 2–17. [Google Scholar] [CrossRef]
- Çuburu, N.; Kweon, M.-N.; Song, J.-H.; Hervouet, C.; Luci, C.; Sun, J.-B.; Hofman, P.; Holmgren, J.; Anjuère, F.; Czerkinsky, C. Sublingual immunization induces broad-based systemic and mucosal immune responses in mice. Vaccine 2007, 25, 8598–8610. [Google Scholar] [CrossRef] [PubMed]
- Tajarobi, F.; Abrahmsén-Alami, S.; Hansen, M.; Larsson, A. The Impact of Dose and Solubility of Additives on the Release from HPMC Matrix Tablets—Identifying Critical Conditions. Pharm. Res. 2009, 26, 1496–1503. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.J. Formulation of HPV Dry Powder Wafers for Sublingual Vaccination, 2012. Bachelor’s Thesis, University of Colorado Boulder, Boulder, CO, USA, 2012. Available online: https://scholar.colorado.edu/cgi/viewcontent.cgi?referer=https://www.google.com/&httpsredir=1&article=1473&context=honr_theses (accessed on 13 July 2019).
- Indermun, S.; Luttge, R.; Choonara, Y.E.; Kumar, P.; Du Toit, L.C.; Modi, G.; Pillay, V. Current advances in the fabrication of microneedles for transdermal delivery. J. Control. Release 2014, 185, 130–138. [Google Scholar] [CrossRef]
- Kim, Y.-C.; Park, J.-H.; Prausnitz, M.R. Microneedles for drug and vaccine delivery. Adv. Drug Deliv. Rev. 2012, 64, 1547–1568. [Google Scholar] [CrossRef]
- Gill, H.S.; Prausnitz, M.R. Coated microneedles for transdermal delivery. J. Control. Release 2007, 117, 227–237. [Google Scholar] [CrossRef]
- Vrdoljak, A.; McGrath, M.G.; Carey, J.B.; Draper, S.J.; Hill, A.V.S.; O’Mahony, C.; Crean, A.M.; Moore, A.C. Coated microneedle arrays for transcutaneous delivery of live virus vaccines. J. Control. Release 2012, 159, 34–42. [Google Scholar] [CrossRef]
- Hiraishi, Y.; Nandakumar, S.; Choi, S.-O.; Lee, J.W.; Kim, Y.-C.; Posey, J.E.; Sable, S.B.; Prausnitz, M.R. Bacillus Calmette-Guérin vaccination using a microneedle patch. Vaccine 2011, 29, 2626–2636. [Google Scholar] [CrossRef]
- Prow, T.W.; Chen, X.; Prow, N.A.; Fernando, G.J.P.; Tan, C.S.E.; Raphael, A.P.; Chang, D.; Ruutu, M.P.; Jenkins, D.W.K.; Pyke, A.; et al. Nanopatch-Targeted Skin Vaccination against West Nile Virus and Chikungunya Virus in Mice. Small 2010, 6, 1776–1784. [Google Scholar] [CrossRef] [PubMed]
- Perez Cuevas, M.B.; Kodani, M.; Choi, Y.; Joyce, J.; O’Connor, S.M.; Kamili, S.; Prausnitz, M.R. Hepatitis B vaccination using a dissolvable microneedle patch is immunogenic in mice and rhesus macaques. Bioeng. Transl. Med. 2018, 3, 186–196. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-C.; Lee, S.-H.; Choi, W.-H.; Choi, H.-J.; Goo, T.-W.; Lee, J.-H.; Quan, F.-S. Microneedle delivery of trivalent influenza vaccine to the skin induces long-term cross-protection. J. Drug Target. 2016, 24, 943–951. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.-H.; Noh, J.-Y.; Kim, K.-H.; Park, J.-K.; Lee, J.-H.; Jeong, S.D.; Jung, D.-Y.; Song, C.-S.; Kim, Y.-C. Effect of zymosan and poly (I:C) adjuvants on responses to microneedle immunization coated with whole inactivated influenza vaccine. J. Control. Release 2017, 265, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Edens, C.; Collins, M.L.; Goodson, J.L.; Rota, P.A.; Prausnitz, M.R. A microneedle patch containing measles vaccine is immunogenic in non-human primates. Vaccine 2015, 33, 4712–4718. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Vlasova, A.; Velasquez, D.E.; Saif, L.J.; Kandasamy, S.; Kochba, E.; Levin, Y.; Jiang, B. Skin Vaccination against Rotavirus Using Microneedles: Proof of Concept in Gnotobiotic Piglets. PLoS ONE 2016, 11, e0166038. [Google Scholar] [CrossRef]
- Yang, H.-W.; Ye, L.; Guo, X.D.; Yang, C.; Compans, R.W.; Prausnitz, M.R. Ebola Vaccination Using a DNA Vaccine Coated on PLGA-PLL/γPGA Nanoparticles Administered Using a Microneedle Patch. Adv. Healthc. Mater. 2017, 6, 1600750. [Google Scholar] [CrossRef]
- Schipper, P.; van der Maaden, K.; Groeneveld, V.; Ruigrok, M.; Romeijn, S.; Uleman, S.; Oomens, C.; Kersten, G.; Jiskoot, W.; Bouwstra, J. Diphtheria toxoid and N-trimethyl chitosan layer-by-layer coated pH-sensitive microneedles induce potent immune responses upon dermal vaccination in mice. J. Control. Release 2017, 262, 28–36. [Google Scholar] [CrossRef]
- Moreno, E.; Schwartz, J.; Calvo, A.; Blanco, L.; Larrea, E.; Irache, J.M.; Sanmartín, C.; Coulman, S.A.; Soto, M.; Birchall, J.C.; et al. Skin vaccination using microneedles coated with a plasmid DNA cocktail encoding nucleosomal histones of Leishmania spp. Int. J. Pharm. 2017, 533, 236–244. [Google Scholar] [CrossRef]
- Duong, H.T.T.; Kim, N.W.; Thambi, T.; Giang Phan, V.H.; Lee, M.S.; Yin, Y.; Jeong, J.H.; Lee, D.S. Microneedle arrays coated with charge reversal pH-sensitive copolymers improve antigen presenting cells-homing DNA vaccine delivery and immune responses. J. Control. Release 2018, 269, 225–234. [Google Scholar] [CrossRef]
- Seok, H.; Noh, J.Y.; Lee, D.Y.; Kim, S.J.; Song, C.S.; Kim, Y.C. Effective humoral immune response from a H1N1 DNA vaccine delivered to the skin by microneedles coated with PLGA-based cationic nanoparticles. J. Control. Release 2017, 265, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Tu, J.; Du, G.; Reza Nejadnik, M.; Mönkäre, J.; van der Maaden, K.; Bomans, P.H.H.; Sommerdijk, N.A.J.M.; Slütter, B.; Jiskoot, W.; Bouwstra, J.A.; et al. Mesoporous Silica Nanoparticle-Coated Microneedle Arrays for Intradermal Antigen Delivery. Pharm. Res. 2017, 34, 1693–1706. [Google Scholar] [CrossRef] [PubMed]
- Turvey, M.E.; Uppu, D.S.S.M.; Mohamed Sharif, A.R.; Bidet, K.; Alonso, S.; Ooi, E.E.; Hammond, P.T. Microneedle-based intradermal delivery of stabilized dengue virus. Bioeng. Transl. Med. 2019, 4, e10127. [Google Scholar] [CrossRef] [PubMed]
- Bachy, V.; Hervouet, C.; Becker, P.D.; Chorro, L.; Carlin, L.M.; Herath, S.; Papagatsias, T.; Barbaroux, J.-B.; Oh, S.-J.; Benlahrech, A.; et al. Langerin negative dendritic cells promote potent CD8+ T-cell priming by skin delivery of live adenovirus vaccine microneedle arrays. Proc. Natl. Acad. Sci. USA 2013, 110, 3041–3046. [Google Scholar] [CrossRef]
- Matsuo, K.; Hirobe, S.; Yokota, Y.; Ayabe, Y.; Seto, M.; Quan, Y.-S.; Kamiyama, F.; Tougan, T.; Horii, T.; Mukai, Y.; et al. Corrigendum to “Transcutaneous immunization using a dissolving microneedle array protects against tetanus, diphtheria, malaria, and influenza” [J. Control. Release 160 (2012) 495–501]. J. Control. Release 2014, 184, 18–19. [Google Scholar] [CrossRef]
- Zaric, M.; Becker, P.D.; Hervouet, C.; Kalcheva, P.; Ibarzo Yus, B.; Cocita, C.; O’Neill, L.A.; Kwon, S.-Y.; Klavinskis, L.S. Long-lived tissue resident HIV-1 specific memory CD8+ T cells are generated by skin immunization with live virus vectored microneedle arrays. J. Control. Release 2017, 268, 166–175. [Google Scholar] [CrossRef]
- Esser, E.S.; Romanyuk, A.; Vassilieva, E.V.; Jacob, J.; Prausnitz, M.R.; Compans, R.W.; Skountzou, I. Tetanus vaccination with a dissolving microneedle patch confers protective immune responses in pregnancy. J. Control. Release 2016, 236, 47–56. [Google Scholar] [CrossRef]
- Chu, L.Y.; Ye, L.; Dong, K.; Compans, R.W.; Yang, C.; Prausnitz, M.R. Enhanced Stability of Inactivated Influenza Vaccine Encapsulated in Dissolving Microneedle Patches. Pharm. Res. 2016, 33, 868–878. [Google Scholar] [CrossRef]
- Arya, J.M.; Dewitt, K.; Scott-Garrard, M.; Chiang, Y.-W.; Prausnitz, M.R. Rabies vaccination in dogs using a dissolving microneedle patch. J. Control. Release 2016, 239, 19–26. [Google Scholar] [CrossRef]
- Mistilis, M.J.; Joyce, J.C.; Esser, E.S.; Skountzou, I.; Compans, R.W.; Bommarius, A.S.; Prausnitz, M.R. Long-term stability of influenza vaccine in a dissolving microneedle patch. Drug Deliv. Transl. Res. 2017, 7, 195–205. [Google Scholar] [CrossRef]
- Chen, F.; Yan, Q.; Yu, Y.; Wu, M.X. BCG vaccine powder-laden and dissolvable microneedle arrays for lesion-free vaccination. J. Control. Release 2017, 255, 36–44. [Google Scholar] [CrossRef]
- Transdermal Patches, Microneedles & Needle-Free Injection: Manufacturing Lines Roll as Concepts Become Products. ONdrugDelivery Mag. 2013, 40, 4–7.
- Schiffelers, R. Drug Delivery—Select Biosciences Inaugural Summit. 2–4 September 2009, London, UK. IDrugs 2009, 12, 679–682. [Google Scholar] [PubMed]
- Chen, D.; Maa, Y.-F.; Haynes, J.R. Needle-free epidermal powder immunization. Expert Rev. Vaccines 2002, 1, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Burger, M.; Chu, Q.; Endres, R.; Zuleger, C.; Dean, H.; Payne, L.G. Epidermal powder immunization: Cellular and molecular mechanisms for enhancing vaccine immunogenicity. Virus Res. 2004, 103, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Osorio, J.E.; Zuleger, C.L.; Burger, M.; Chu, Q.; Payne, L.G.; Chen, D. Immune responses to hepatitis B surface antigen following epidermal powder immunization. Immunol. Cell Biol. 2003, 81, 52–58. [Google Scholar] [CrossRef]
- Johnston, S.A.; Tang, D.C. Gene gun transfection of animal cells and genetic immunization. Methods Cell Biol. 1994, 43, 353–365. [Google Scholar]
- Barry, M.A.; Johnston, S.A. Biological features of genetic immunization. Vaccine 1997, 15, 788–791. [Google Scholar] [CrossRef]
- Chen, D.; Endres, R.L.; Erickson, C.A.; Weis, K.F.; McGregor, M.W.; Kawaoka, Y.; Payne, L.G. Epidermal immunization by a needle-free powder delivery technology: Immunogenicity of influenza vaccine and protection in mice. Nat. Med. 2000, 6, 1187–1190. [Google Scholar] [CrossRef]
- Burkoth, T.; Bellhouse, B.; Sarphie, D. Transdermal and Transmucosal Powdered Drug Delivery—PubMed Labs. Crit. Rev. Ther. Drug Carr. Syst. 1999, 16, 331–384. [Google Scholar] [CrossRef]
- Amorij, J.-P.; Hinrichs, W.L.; Frijlink, H.W.; Wilschut, J.C.; Huckriede, A. Needle-free influenza vaccination. Lancet. Infect. Dis. 2010, 10, 699–711. [Google Scholar] [CrossRef]
- Kim, Y.-C. Skin Vaccination Methods: Gene Gun, Jet Injector, Tattoo Vaccine, and Microneedle. In Percutaneous Penetration Enhancers Physical Methods in Penetration Enhancement; Springer: Berlin, Germany, 2017; pp. 485–499. [Google Scholar]
- Cheung, K.; Das, D.B. Microneedles for drug delivery: Trends and Progress. Drug Deliv. 2014, 23, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Doherty, M.; Buchy, P.; Standaert, B.; Giaquinto, C.; Prado- Cohrs, D. Vaccine impact: Benefits for human health. Vaccine 2016, 34, 6707–6714. [Google Scholar] [CrossRef] [PubMed]
- WHO The Top 10 Causes of Death. Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 5 September 2019).
- Irvine, D.J.; Swartz, M.A.; Szeto, G.L. Engineering synthetic vaccines using cues from natural immunity. Nat. Mater. 2013, 12, 978–990. [Google Scholar] [CrossRef] [PubMed]
- Saroja, C.; Lakshmi, P.; Bhaskaran, S. Recent trends in vaccine delivery systems: A review. Int. J. Pharm. Investig. 2011, 1, 64–74. [Google Scholar]
- Neutra, M.R.; Kozlowski, P.A. Mucosal vaccines: The promise and the challenge. Nat. Rev. Immunol. 2006, 6, 148–158. [Google Scholar] [CrossRef]
- Ryan, E.J.; Daly, L.M.; Mills, K.H. Immunomodulators and delivery systems for vaccination by mucosal routes. Trends Biotechnol. 2001, 19, 293–304. [Google Scholar] [CrossRef]
- Arakawa, T.; Prestrelski, S.J.; Kenney, W.C.; Carpenter, J.F. Factors affecting short-term and long-term stabilities of proteins. Adv. Drug Deliv. Rev. 2001, 46, 307–326. [Google Scholar] [CrossRef]
- Ohtake, S.; Martin, R.A.; Yee, L.; Chen, D.; Kristensen, D.D.; Lechuga-Ballesteros, D.; Truong-Le, V. Heat-stable measles vaccine produced by spray drying. Vaccine 2010, 28, 1275–1284. [Google Scholar] [CrossRef]
- Rathore, N.; Rajan, R.S. Current Perspectives on Stability of Protein Drug Products during Formulation, Fill and Finish Operations. Biotechnol. Prog. 2008, 24, 504–514. [Google Scholar] [CrossRef]
- Amorij, J.-P.; Huckriede, A.; Wilschut, J.; Frijlink, H.W.; Hinrichs, W.L.J. Development of Stable Influenza Vaccine Powder Formulations: Challenges and Possibilities. Pharm. Res. 2008, 25, 1256–1273. [Google Scholar] [CrossRef] [PubMed]
| Name of the Polymer | Property | Advantages |
|---|---|---|
| Sodium Alginate |
|
|
| Carbopol |
|
|
| Chitosan |
| Disease Name | Type of Vaccine | Experimental Species | Advantages | Refs. |
|---|---|---|---|---|
| Adenovirus Infection | Live adenovirus virus | Mice |
| [89] |
| Tuberculosis | Bacillus Calmette-Guérin (BCG) | Guinea pigs |
| [90] |
| Chikungunya | Inactivated whole chikungunya virus | Mice |
| [91] |
| Hepatitis B | Adjuvant-free hepatitis B vaccine antigen | Mice |
| [92] |
| Hepatitis C | Hepatitis C virus NS3/4A protein | Mice |
| [92] |
| Influenza | Trivalent influenza vaccine containing inactivated A/PR/8/34 (H1N1), A/Hong Kong/68 (H3N2), and B/Lee/40 | Mice |
| [93] |
| Zymosan and poly (I:C) adjuvants coated with whole inactivated influenza vaccine |
| [94] | ||
| Measles | Measles vaccine | Rhesus macaques |
| [95] |
| Rota virus | Inactivated rotavirus vaccine | Piglets |
| [96] |
| West Nile virus | DNA-delivered attenuated West Nile virus vaccine | Mice |
| [91] |
| Ebola | DNA coated on PLGA-PLL/γPGA nanoparticles Polylactic-co-glycolic acid—poly-l-lysine/poly-γ-glutamic acid | Mice |
| [97] |
| Diphtheria | Diphtheria toxoid and N-trimethyl chitosan layer-by-layer | Mice |
| [98] |
| Leishmaniasis | Plasmid DNA cocktail encoding the Leishmania infantum nucleosomal histones H2A, H2B, H3 and H4 | Mice |
| [99] |
| Prophylactic vaccines | DNA polyplex vaccine | Mice |
| [100] |
| H1N1 DNA vaccine | Coated with a polyplex containing poly lactic-co-glycolic acid/polyethyleneimine (PLGA/PEI) nanoparticles | Mice |
| [101] |
| Antigen delivery | Lipid bilayer-coated; Mesoporous silica nanoparticles (LB-MSN-OVA) with antigen ovalbumin | Mice |
| [102] |
| Dengue | Dengue virus- stabilized microneedles arrays using saccharide-based formulations | Mice |
| [103] |
| Disease Name | Type of Vaccine with Polymer Composition | Experimental Species | Advantages | Refs. |
|---|---|---|---|---|
| Adenovirus Infection | Live recombinant human adenovirus type 5 (rAdHu5) with silicone template | Mice |
| [104] |
| Diphtheria | Diptheria toxoid (DT) with Sodium hyaluronate | Mice |
| [105] |
| HIV | Recombinant human adenovirus type 5 vector (AdHu5) encoding HIV-1 gag with sodium carboxymethylcellulose (Na-CMC) | Mice |
| [106] |
| Malaria | SE36 recombinant molecule (malaria vaccine) with Sodium hyaluronate | Mice |
| [105] |
| Poliovirus | Inactivated polio vaccine (IPV) with maltodextrin and D-sorbitol | N/A |
| [5] |
| Tetanus | Unadjuvanted tetanus toxoid with polyvinyl alcohol (PVA), sucrose and CMC | Pregnant mice |
| [107] |
| Influenza | Inactivated Influenza Vaccine loaded with polydimethylsiloxane (PDMS) | Mice |
| [108] |
| DNA polyplex vaccine | Branched polyethylenimine (bPEI) pre-coated with polydimethylsiloxane (PDMS) | Mice |
| [4] |
| Rabies | Rabies DNA vaccine molded with Polydimethylsiloxane (PDMS) | Dogs |
| [109] |
| Influenza | Trivalent subunit influenza vaccine formulated with combinations of trehalose/sucrose, sucrose/arginine, and arginine/heptagluconate | Mice |
| [110] |
| Tuberculosis | Live attenuated Bacille Calmette–Guerin (BCG) bacillusis vaccine powder-laden with biocompatible and dissolvable hyaluronic acid | Mice |
| [111] |
| Hepatitis B | Adjuvant-free HBsAg vaccine with trehalose and carboxy methyl cellulose | Mice and rhesus macaques |
| [92] |
| Route of Administration | Type of Vaccine | Current Status | Challenges | Ref. |
|---|---|---|---|---|
| Oral | Vivotif- enteric coated capsules-vaccine for typhoid | Market available |
| [27] |
| Enteric coated and uncoated oral tablets of inactivated cholera vaccine | Preclinical study | [30] | ||
| Vaxart Inc. an oral recombinant adenovirus (rAd5) based vaccine to influenza A H1N1 | Phase I | [21] | ||
| Pulmonary Route | Influenza vaccine by spray-freeze drying (SFD) using the oligosaccharide inulin as stabilizer | Preclinical study |
| [16] |
| Powder formulation of measles vaccine by Dura Pharmaceuticals | Preclinical study | [40] | ||
| Intranasal Route | FluMist, manufactured by Astra-Zeneca | Market product |
| [54,55,56] |
| Buccal and Sublingual Route | Ovalbumin (OVA) in combination with the adjuvant cholera toxin (CT) | Preclinical |
| [41] |
| Two layered ER tablet containing ovalbumin, comprising of a mucoadhesive layer and a controlled release layer | Preclinical study | [42] | ||
| Wafer formulation containing HPV vaccine | Preclinical study | [44] | ||
| Epidermal Powder Immunisation (EPI) | Hepatitis B (DNA) and influenza (protein) | Phase I |
| [114] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jahan, N.; Archie, S.R.; Shoyaib, A.A.; Kabir, N.; Cheung, K. Recent Approaches for Solid Dose Vaccine Delivery. Sci. Pharm. 2019, 87, 27. https://doi.org/10.3390/scipharm87040027
Jahan N, Archie SR, Shoyaib AA, Kabir N, Cheung K. Recent Approaches for Solid Dose Vaccine Delivery. Scientia Pharmaceutica. 2019; 87(4):27. https://doi.org/10.3390/scipharm87040027
Chicago/Turabian StyleJahan, Nishat, Sabrina Rahman Archie, Abdullah Al Shoyaib, Nadia Kabir, and Karmen Cheung. 2019. "Recent Approaches for Solid Dose Vaccine Delivery" Scientia Pharmaceutica 87, no. 4: 27. https://doi.org/10.3390/scipharm87040027
APA StyleJahan, N., Archie, S. R., Shoyaib, A. A., Kabir, N., & Cheung, K. (2019). Recent Approaches for Solid Dose Vaccine Delivery. Scientia Pharmaceutica, 87(4), 27. https://doi.org/10.3390/scipharm87040027





