Six-Month Chronic Toxicity Study of Tamarind Pulp (Tamarindus indica L.) Water Extract
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material Collection
2.2. Tamarind Pulp Water Extract Preparation
2.3. Experimental Animal
- Group 1: Control group received carboxymethylcellulose sodium (CMC-Na) 0.5% 1 mL/100 g bw per day for six months.
- Group 2: Control satellite group received CMC-Na 0.5% 1 mL/100 g bw per day for six months.
- Group 3: 75 mg/kg bw group received tamarind pulp extract 75 mg/kg bw per day for six months.
- Group 4: 200 mg/kg bw group received tamarind pulp extract 200 mg/kg bw per day for six months.
- Group 5: 1000 mg/kg bw group received tamarind pulp 1000 mg/kg bw per day for six months.
- Group 6: Satellite 1000 mg/kg bw group received tamarind pulp 1000 mg/kg bw per day for six months.
2.4. Six-Month Chronic Toxicity Study
2.5. Relative Organ Weights Determination
2.6. Hematological Analysis
2.7. Biochemistry Profile Analysis
2.7.1. Blood Glucose Determination
2.7.2. Total Cholesterol Determination
2.7.3. Triglyceride Determination
2.7.4. Aspartate Amino Transferase Determination
2.7.5. Alanine Amino Transferase Determination
2.7.6. Blood Creatinine Determination
2.7.7. Urea
2.8. Statistics
3. Results
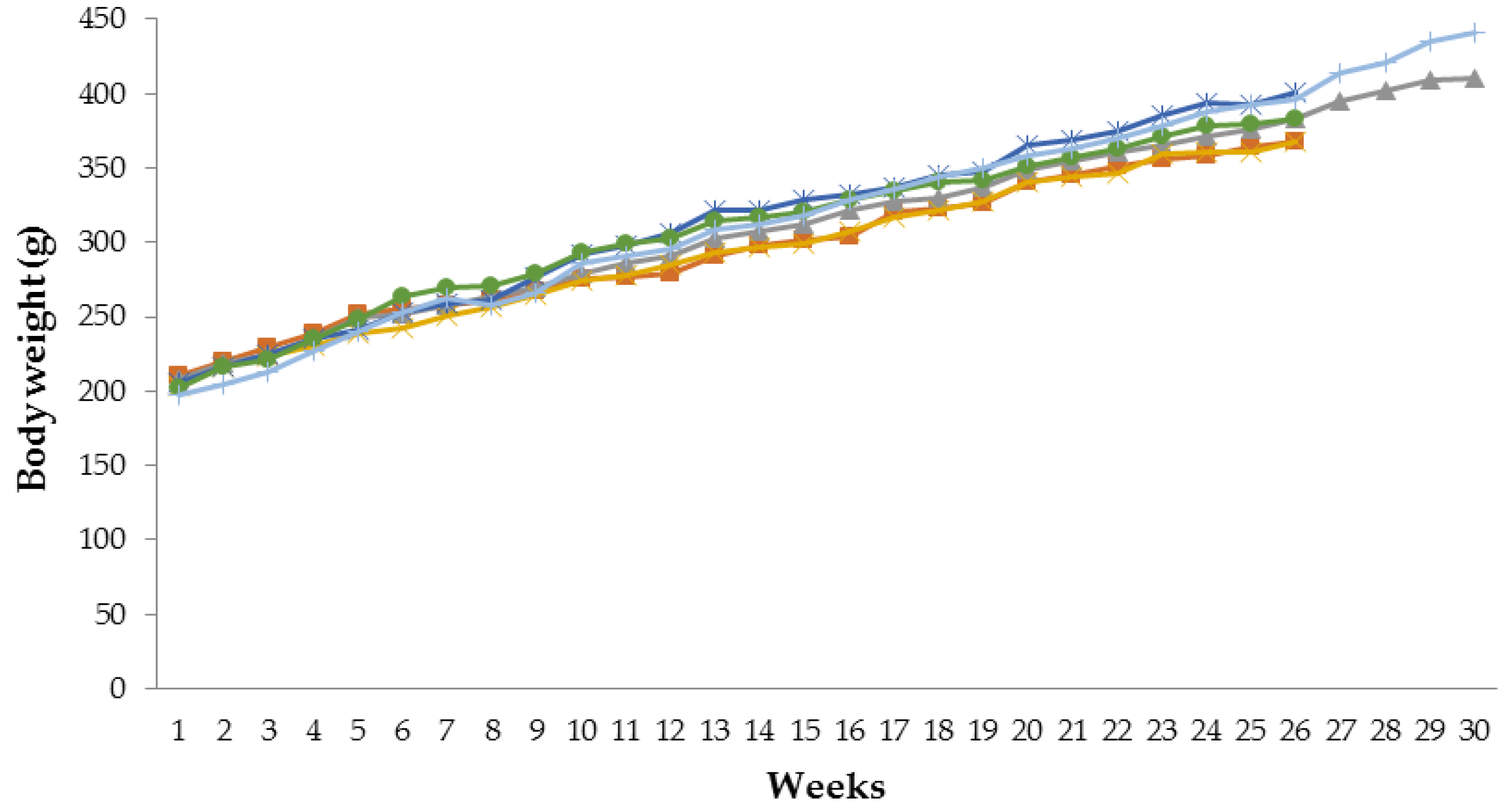
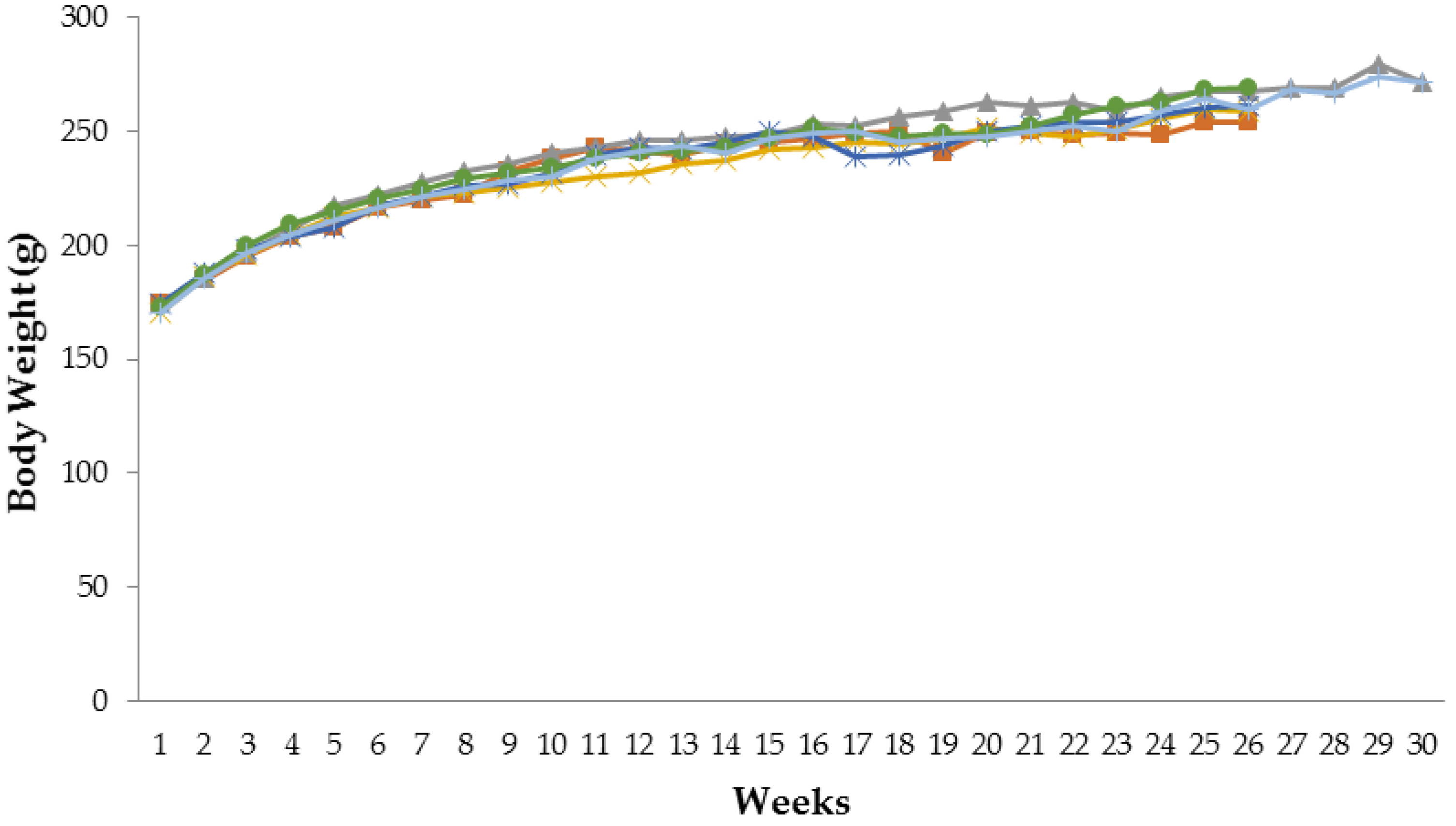
3.1. Effect of Tamarind Pulp Water Extract on Body Weight Profile
3.2. Effect of Tamarind Pulp Water Extract on Hematology Profile
3.3. Effect of Tamarind Pulp Water Extract on Relative Organ Weights
3.4. Effect of Tamarind Pulp Water Extract on Clinical Biochemistry Profile
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kirtikar, K.R.; Basu, B.D. Indian Medicinal Plants, 2nd ed.; International Books Distributor: Dehradun, India, 1987; pp. 887–891. [Google Scholar]
- Departemen Kesehatan R.I. Materia Medika Indonesia, 6th ed.; Departemen Kesehatan R.I.: Jakarta, Indonesia, 1995; pp. 228–291. [Google Scholar]
- Azman, K.F.; Zulkhairi, A.; Arzina, A.; Mohd, E.N.; Mat, A.R.; Zamree, S.; Abdul, K.K.K. Antiobesity effect of Tamarindus indica L. pulp aqueous extract in high fat induced obese rat. J. Nat. Med. 2012, 66, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.Y.; Junit, S.M.; Abdulla, M.A.; Aziz, A.A. In vivo biochemical and gene expression analyses of the antioxidant activities and hypocholesterolaemic properties of Tamarindus indica fruit pulp extract. PLoS ONE 2003, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Arranz, J.C.E.; Roses, R.P.; Amando, J.R.; Quevedo, H.J.M.; Mwasi, L.B.; Sotomayor, O.C.; Garcia, R.M.; Lorez, O.F.; Castillo, A.A.; Zapata, E.P. Antioxidant and toxicological evaluation of a Tamarindus indica L. leaf fluid extract. Nat. Prod. Res. 2016, 30, 456–459. [Google Scholar] [CrossRef] [PubMed]
- Abubakar, M.G.; Yerima, M.B.; Zahriya, A.G.; Ukwuani, A.N. Acute Toxicity and antifungal studies of ethanolic leaves, stem and pulp extract of Tamarindus indica. Res. J. Pharm. Biol. Chem. Sci. 2010, 4, 104–111. [Google Scholar]
- Abukakar, M.G.; Ukwuani, A.N.; Shehu, R.A. An Evaluation of Toxic Effect of Tamarindus indica Pulp Extract in Albino Rats. J. Pharmacol. Toxicol. 2008, 3, 111–118. [Google Scholar] [CrossRef]
- Barham, D.; Trinder, P. An Improved Color Reagent for the Determination of Blood Glucose by the Oxidase System. Analyst 1972, 97, 142–145. [Google Scholar] [CrossRef] [PubMed]
- Allain, C.C.; Poon, L.S.; Chan, C.S.G.; Richmond, W.; Fu, P.C. Enzymatic Determinationof Total Serum Cholesterol. Clin. Chem. 1974, 20, 470–475. [Google Scholar] [PubMed]
- Mc Gowan, M.W.; Artiss, J.D.; Strandbergh, D.R.; Zak, B. A Peroxidase-Coupled Method for the Colorimetric Determination of Serum Triglycerides. Clin. Chem. 1983, 29, 538–542. [Google Scholar] [PubMed]
- Tietz, N.W.; Rinker, A.D.; Shaw, L.M. IFCC Methods for the measurement of catalytic concentration of enzymes, IFCC Method for Alkaline Phosphatase. J. Clin. Chem. Clin. Biochem. 1983, 21, 731–748. [Google Scholar] [PubMed]
- Bergmeyer, H.U.; Horder, M.; Rey, J. Approved recommendation on IFCC methods for the measurement of catalytic concentration of enzymes, IFCC method for Alanine Aminotransferase. J. Clin. Chem. Clin. Biochem. 1986, 24, 481–495. [Google Scholar] [PubMed]
- Bartels, H.; Bohmer, M. Micro-determination of Creatinine. Clin. Chim. Acta 1971, 32, 81–85. [Google Scholar] [PubMed]
- Berthelot, M. Violet d’aniline. Rep. Chem. Appl. 1859, 1, 284–288. [Google Scholar]
- Fawcett, J.K.; Scott, J.E. A rapid and percise method for the determination of urea. J. Clin. Path. 1960, 13, 156–159. [Google Scholar] [CrossRef] [PubMed]
- Setiawan, F.; Sukandar, E.Y.; Sukrasno; Adnyana, I.K. Activity of Tamarindus indica pulp water extract in high carbohydrate diet rats as a treatment for obesity and insulin resistance. J. Chin. Pharm. Sci. 2016, 25, 892–897. [Google Scholar] [CrossRef]
- Piggot, G.H. Animal studies for prediction of chronic toxicity. In Archives of Toxicology Supplement 15 Medical Toxicology, Proceedings of the 1991 EUROTOX Congrees, Maastricht, Netherlands, 1–4 September 1991; Bolt, H.M., de Wolff, F.A., Henderson, P.T., Eds.; Springer: Berlin-Heidelberg, Germany, 1992. [Google Scholar]
- OECD. Test No. 452: Chronic Toxicity Studies; OECD Publishing: Paris, France, 2009; p. 5. [Google Scholar] [CrossRef]
- Wilson, N.H.; Hardisty, J.F.; Hayes, J.R. Short-term, sub-chronic, and chronic toxicity studies. In Principles and Methods of Toxicology, 5th ed.; Hayes, W.A., Ed.; CRC Press: Boca Raton, United States, 2007; p. 1245. [Google Scholar]
- Gamez, R.; Mas, R.; Noa, M.; Menendez, R.; Garcia, H. Six-months toxicity study of oral administration of D-003 in Sprague Dawley Rats. Drugs R&D 2002, 3, 375–386. [Google Scholar] [PubMed]
- Martini, F.H.; Nath, J.L.; Bartholomew, E.F. Fundamentals of Anatomy and Physiology, 9th ed.; Pearson: San Fransisco, CA, USA, 2012; pp. 642–645, 776. [Google Scholar]
- Silva, F.M.V.; Leite, M.F.; Spadaro, A.C.C.; Uyemura, S.A.; Maistro, E.L. Assesment of the potential genotoxic risk of medicinal Tamarindus indica fruit pulp extract using in vivo assays. Genet. Mol. Res. 2009, 8, 1085–1098. [Google Scholar] [CrossRef] [PubMed]
- Frame, S.R.; Mann, P.C. Principles of pathology for toxicology studies. In Principles and Methods of Toxicology, 5th ed.; Hayes, W.A., Ed.; CRC Press: Boca Raton, FL, USA, 2007; p. 595. [Google Scholar]
- Sellers, R.S.; Morton, D.; Michael, B.; Roome, N.; Johnson, J.K.; Yano, B.L.; Perry, R.; Schafer, K. Society of Toxicologic pathology position paper: Organ weight recomendation for toxicology studies. Toxicol. Pathol. 2007, 35, 751–755. [Google Scholar] [CrossRef] [PubMed]
- Hall, R.L.; Everds, N.E. Principles of clinical pathology for toxicity studies. In Principles and Methods of Toxicology, 5th ed.; Hayes, W.A., Ed.; CRC Press: Boca Raton, FL, USA, 2007; pp. 1342–1348. [Google Scholar]
| Groups | White Blood Cell Counts (×103/mm3) | Hemoglobin (g/dL) | MCH (pg/sel) | MCHC (g/dL) | Red Blood Cell Counts (×106/mm3) | MCV (fL) | Hematocrit (%) | Platelet (×103/mL) |
|---|---|---|---|---|---|---|---|---|
| Control | 10.95 ± 5.34 | 14.89 ± 1.58 | 18.35 ± 1.87 | 34.94 ± 3.96 | 8.19 ± 1.17 | 52.59 ± 0.94 | 43.12 ± 6.61 | 317.10 ± 46.76 |
| Control Satellite | 10.02 ± 3.77 | 14.58 ± 1.40 | 17.55 ± 3.22 | 33.45 ± 6.80 | 8.54 ± 1.58 | 52.68 ± 1.47 | 45.15 ± 9.34 | 294.01 ± 62.19 |
| 75 mg/kg bw | 8.60 ± 3.25 | 14.87 ± 1.52 | 17.26 ± 2.17 | 32.89 ± 4.31 | 8.78 ± 1.65 | 52.50 ± 0.81 | 46.13 ± 8.92 | 291.61 ± 37.74 |
| 200 mg/kg bw | 8.18 ± 3.13 | 14.70 ± 1.90 | 18.29 ± 2.37 | 34.95 ± 5.09 | 8.16 ± 1.42 | 52.42 ± 0.97 | 42.88 ± 7.88 | 303.86 ± 51.07 |
| 1000 mg/kg bw | 9.50 ± 4.00 | 14.62 ± 1.85 | 18.41 ± 2.42 | 35.24 ± 4.91 | 8.11 ± 1.69 | 52.31 ± 0.79 | 42.49 ± 9.26 | 301.56 ± 40.15 |
| Satellite 1000 mg/kg bw | 9.24 ± 2.22 | 14.29 ± 1.80 | 17.81 ± 1.55 | 33.94 ± 3.11 | 8.08 ± 1.20 | 52.49 ± 0.82 | 42.42 ± 6.42 | 301.21 ± 51.82 |
| Groups | White Blood Cell Counts (×103/mm3) | Hemoglobin (g/dL) | MCH (pg/sel) | MCHC (g/dL) | Red Blood Cell Counts (×106/mm3) | MCV (fL) | Hematocrit (%) | Platelet (×103/mL) |
|---|---|---|---|---|---|---|---|---|
| Control | 7.55 ± 3.03 | 14.22 ± 2.42 | 16.95 ± 3.73 | 35.01 ± 7.57 | 8.60 ± 1.63 | 48.40 ± 0.90 | 41.68 ± 8.27 | 302.27 ± 65.16 |
| Satellite Control | 7.90 ± 3.52 | 14.78 ± 1.83 | 17.09 ± 2.12 | 35.22 ± 4.77 | 8.75 ± 1.43 | 48.59 ± 0.90 | 42.57 ± 7.30 | 338.32 ± 51.76 |
| 75 mg/kg bw | 7.33 ± 1.80 | 13.10 ± 1.29 | 15.47 ± 1.98 | 32.32 ± 4.06 | 8.48 ± 1.66 | 48.49 ± 0.74 | 41.13 ± 8.18 | 276.20 ± 54.16 |
| 200 mg/kg bw | 9.50 ± 3.05 | 14.04 ± 1.94 | 15.62 ± 2.10 | 31.98 ± 4.54 | 9.16 ± 1.90 | 48.91 ± 0.71 | 44.85 ± 9.49 | 296.27 ± 62.40 |
| 1000 mg/kg bw | 9.00 ± 3.44 | 13.62 ± 1.51 | 15.88 ± 2.74 | 32.59 ± 5.75 | 8.83 ± 1.80 | 48.77 ± 0.70 | 43.10 ± 9.03 | 294.43 ± 54.71 |
| Satellite 1000 mg/kg bw | 7.09 ± 1.98 | 15.03 ± 1.41 | 15.26 ± 1.38 | 33.10 ± 4.40 | 9.51 ± 1.49 | 48.73 ± 0.50 | 46.28 ± 7.53 | 318.14 ± 44.54 |
| Groups | Liver | Lung | Heart | Spleen | Kidneys | Adrenal | Seminal Vesicle | Testicles |
|---|---|---|---|---|---|---|---|---|
| Control | 3.09 ± 0.39 | 0.57 ± 0.07 | 0.36 ± 0.05 | 0.18 ± 0.08 | 0.63 ± 0.05 | 0.02 ± 0.003 | 0.44 ± 0.07 | 0.91 ± 0.10 |
| Satellite Control | 3.23 ± 0.43 | 0.59 ± 0.08 | 0.35 ± 0.07 | 0.21 ± 0.08 | 0.66 ± 0.07 | 0.02 ± 0.003 | 0.40 ± 0.10 | 0.85 ± 0.14 |
| 75 mg/kg bw | 3.10 ± 0.29 | 0.54 ± 0.07 | 0.35 ± 0.05 | 0.21 ± 0.04 | 0.65 ± 0.06 | 0.02 ± 0.004 | 0.49 ± 0.13 | 0.91 ± 0.16 |
| 200 mg/kg bw | 3.06 ± 0.26 | 0.52 ± 0.08 | 0.34 ± 0.03 | 0.19 ± 0.06 | 0.64 ± 0.05 | 0.02 ± 0.003 | 0.48 ± 0.13 | 0.87 ± 0.15 |
| 1000 mg/kg bw | 3.18 ± 0.38 | 0.56 ± 0.09 | 0.36 ± 0.06 | 0.20 ± 0.04 | 0.70 ± 0.07 * | 0.02 ± 0.004 | 0.49 ± 0.10 | 0.93 ± 0.18 |
| Satelit 1000 mg/kg bw | 3.16 ± 0.34 | 0.55 ± 0.05 | 0.34 ± 0.04 | 0.19 ± 0.04 | 0.63 ± 0.05 | 0.02 ± 0.003 | 0.41 ± 0.09 | 0.78 ± 0.07 |
| Groups | Liver | Lung | Heart | Spleen | Kidneys | Adrenal | Ovaries | Uterus | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Control | 3.71 ± 0.67 | 0.71 ± 0.17 | 0.38 ± 0.05 | 0.24 ± 0.06 | 0.66 ± 0.08 | 0.03 ± 0.005 | 0.05 ± 0.02 | 0.29 ± 0.12 | ||
| SatelliteControl | 3.66 ± 0.72 | 0.72 ± 0.13 | 0.35 ± 0.03 | 0.24 ± 0.05 | 0.70 ± 0.08 | 0.03 ± 0.006 | 0.05 ± 0.01 | 0.33 ± 0.10 | ||
| 75 mg/kg bw | 3.54 ± 0.57 | 0.69 ± 0.11 | 0.36 ± 0.03 | 0.22 ± 0.04 | 0.64 ± 0.06 | 0.03 ± 0.004 | 0.05 ± 0.01 | 0.23 ± 0.06 | ||
| 200 mg/kg bw | 3.40 ± 0.40 | 0.67 ± 0.11 | 0.36 ± 0.02 | 0.21 ± 0.03 * | 0.64 ± 0.07 | 0.03 ± 0.006 | 0.05 ± 0.02 | 0.25 ± 0.07 | ||
| 1000 mg/kg bw | 3.68 ± 0.56 | 0.65 ± 0.12 | 0.37 ± 0.03 | 0.22 ± 0.03 | 0.66 ± 0.08 | 0.03 ± 0.005 | 0.06 ± 0.01 | 0.26 ± 0.07 | ||
| Satellite 1000mg/kg bw | 3.67 ± 0.46 | 0.65 ± 0.09 | 0.35 ± 0.03 | 0.23 ± 0.02 | 0.66 ± 0.06 | 0.03 ± 0.004 | 0.05 ± 0.03 | 0.27 ± 0.09 |
| Groups | Glucose (mg/dL) | Cholesterol (mg/dL) | Triglyceride (mg/dL) | AST (U/L) | ALT (U/L) | Urea (mg/dL) | Creatinine (mg/dL) |
|---|---|---|---|---|---|---|---|
| Control | 110.10 ± 20.18 | 43.90 ± 10.04 | 90.36 ± 21.65 | 145.88 ± 34.98 | 56.07 ± 11.57 | 43.14 ± 6.34 | 0.65 ± 0.16 |
| Satellite Control | 97.79 ± 20.10 | 43.85 ± 9.62 | 102.60 ± 37.96 | 139.11 ± 20.90 | 58.10 ± 8.77 | 40.41 ± 7.12 | 0.69 ± 0.15 |
| 75 mg/kg bw | 121.55 ± 29.42 | 42.80 ± 9.01 | 101.85 ± 30.09 | 138.48 ± 27.89 | 52.85 ± 10.62 | 41.13 ± 11.36 | 0.68 ± 0.14 |
| 200 mg/kg bw | 112.69 ± 17.51 | 41.68 ± 12.46 | 106.58 ± 34.18 | 132.84 ± 22.04 | 51.65 ± 10.52 | 41.12 ± 6.23 | 0.62 ± 0.08 |
| 1000 mg/kg bw | 116.66 ± 24.87 | 47.35 ± 29.17 | 109.75 ± 55.16 | 135.99 ± 22.85 | 49.14 ± 13.11 | 44.54 ± 7.53 | 0.69 ± 0.14 |
| Satellite 1000 mg/kg bw | 99.37 ± 18.25 | 41.35 ± 11.13 | 110.75 ± 35.67 | 142.68 ± 23.60 | 59.42 ± 11.10 | 41.97 ± 6.87 | 0.63 ± 0.11 |
| Groups | Glucose (mg/dL) | Cholesterol (mg/dL) | Triglyceride (mg/dL) | AST (U/L) | ALT (U/L) | Urea (mg/dL) | Creatinine (mg/dL) |
|---|---|---|---|---|---|---|---|
| Control | 130.31 ± 43.46 | 50.62 ± 12.58 | 134.94 ± 45.21 | 119.26 ± 39.44 | 48.48 ± 13.32 | 43.18 ± 7.91 | 0.68 ± 0.13 |
| Satellite Control | 122.80 ± 54.49 | 55.90 ± 11.74 | 134.40 ± 34.51 | 125.74 ± 33.66 | 53.18 ± 14.41 | 42.84 ± 9.05 | 0.72 ± 0.08 |
| 75 mg/kg bw | 138.95 ± 46.94 | 52.60 ± 14.69 | 122.20 ± 36.07 | 133.87 ± 42.65 | 53.46 ± 12.75 | 44.60 ± 5.94 | 0.64 ± 0.10 |
| 200 mg/kg bw | 125.30 ± 30.41 | 52.15 ± 13.43 | 116.70 ± 37.32 | 144.93 ± 60.60 | 53.71 ± 15.26 | 45.86 ± 6.70 | 0.64 ± 0.11 |
| 1000 mg/kg bw | 116.79 ± 33.95 | 49.42 ± 12.69 | 126.58 ± 34.85 | 125.57 ± 40.65 | 48.57 ± 13.41 | 43.75 ± 5.93 | 0.63 ± 0.16 |
| Satellite 1000 mg/kg bw | 113.92 ± 50.75 | 55.70 ± 9.76 | 129.80 ± 32.68 | 119.83 ± 22.89 | 51.90 ± 12.10 | 42.70 ± 8.91 | 0.67 ± 0.06 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iskandar, I.; Setiawan, F.; Sasongko, L.D.N.; Adnyana, I.K. Six-Month Chronic Toxicity Study of Tamarind Pulp (Tamarindus indica L.) Water Extract. Sci. Pharm. 2017, 85, 10. https://doi.org/10.3390/scipharm85010010
Iskandar I, Setiawan F, Sasongko LDN, Adnyana IK. Six-Month Chronic Toxicity Study of Tamarind Pulp (Tamarindus indica L.) Water Extract. Scientia Pharmaceutica. 2017; 85(1):10. https://doi.org/10.3390/scipharm85010010
Chicago/Turabian StyleIskandar, Irene, Finna Setiawan, Lucy D N Sasongko, and I Ketut Adnyana. 2017. "Six-Month Chronic Toxicity Study of Tamarind Pulp (Tamarindus indica L.) Water Extract" Scientia Pharmaceutica 85, no. 1: 10. https://doi.org/10.3390/scipharm85010010
APA StyleIskandar, I., Setiawan, F., Sasongko, L. D. N., & Adnyana, I. K. (2017). Six-Month Chronic Toxicity Study of Tamarind Pulp (Tamarindus indica L.) Water Extract. Scientia Pharmaceutica, 85(1), 10. https://doi.org/10.3390/scipharm85010010





