n-Hexane Insoluble Fraction of Plantago lanceolata Exerts Anti-Inflammatory Activity in Mice by Inhibiting Cyclooxygenase-2 and Reducing Chemokines Levels
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Extraction and Fractionation
2.3. Animal Experiments
2.3.1. Animal
2.3.2. Carrageenan-Induced Paw Edema in Mice
2.3.3. Immunohistochemistry
2.3.4. Thioglycollate-Induced Leukocytes Migration
2.3.5. Analysis of Chemokines Level
2.4. Cyclooxygenase Enzymatic Assays
2.5. Statistical Analysis
3. Results
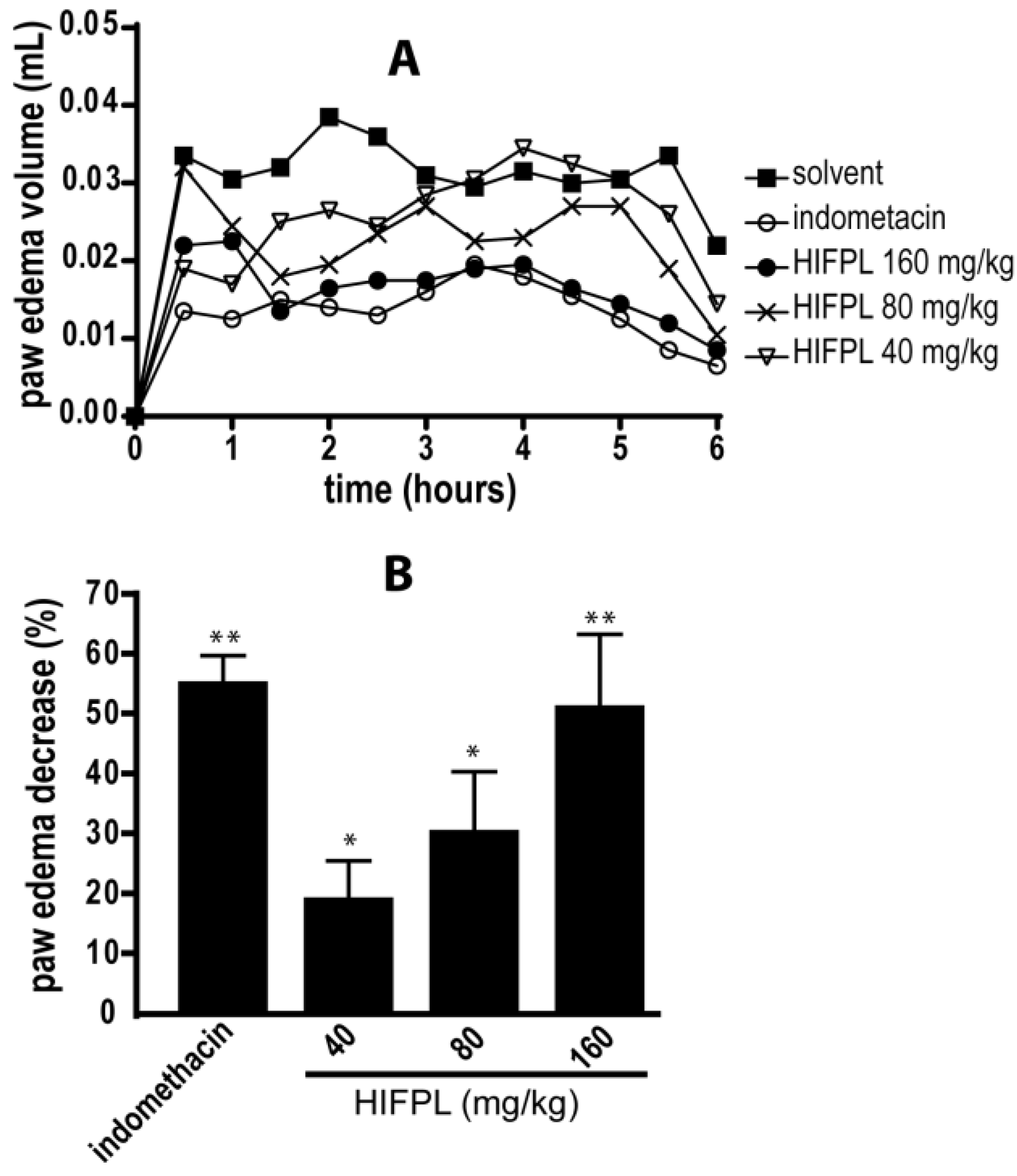
3.1. Anti-Inflammatory Activity in Carrageenan-Induced Paw Edema
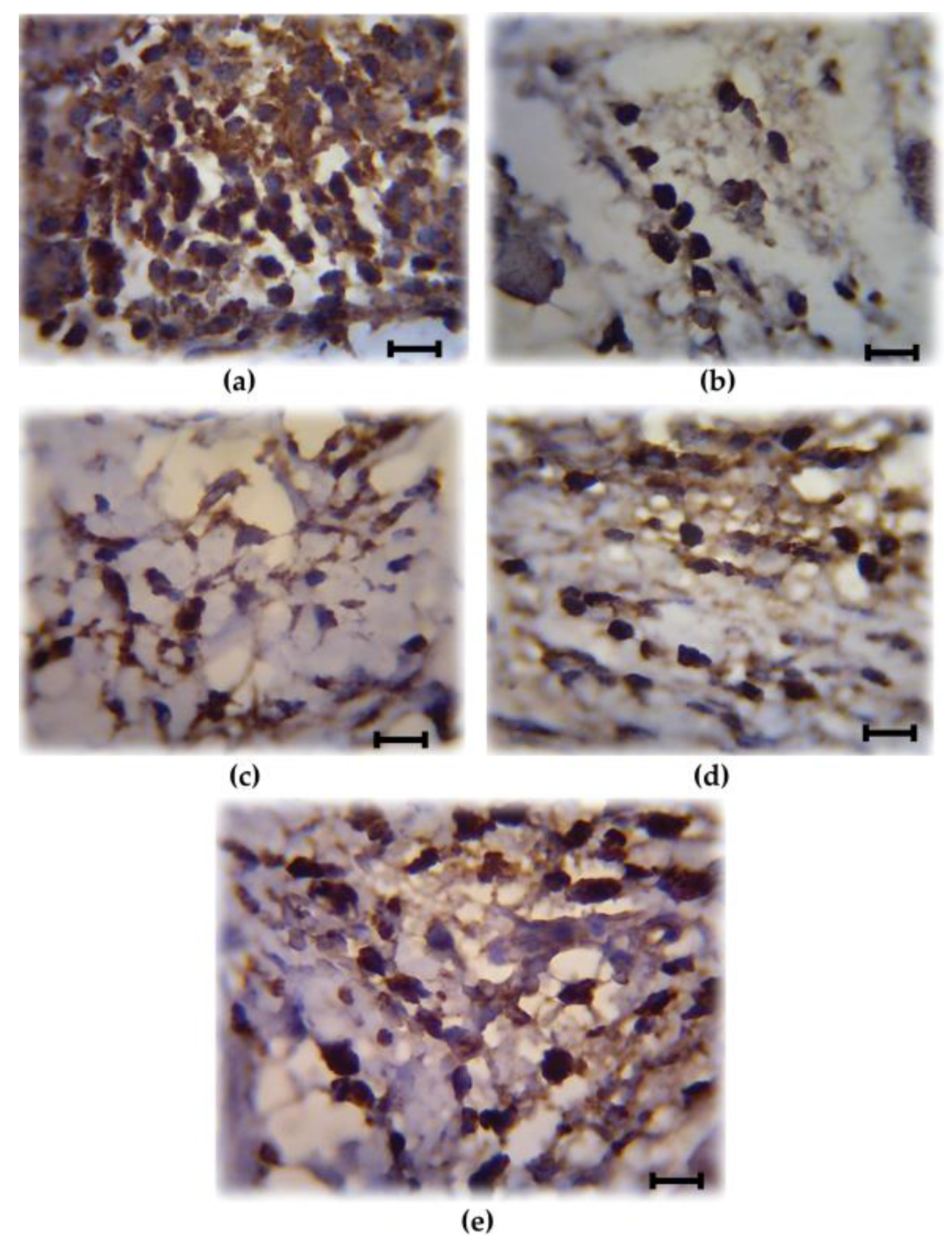
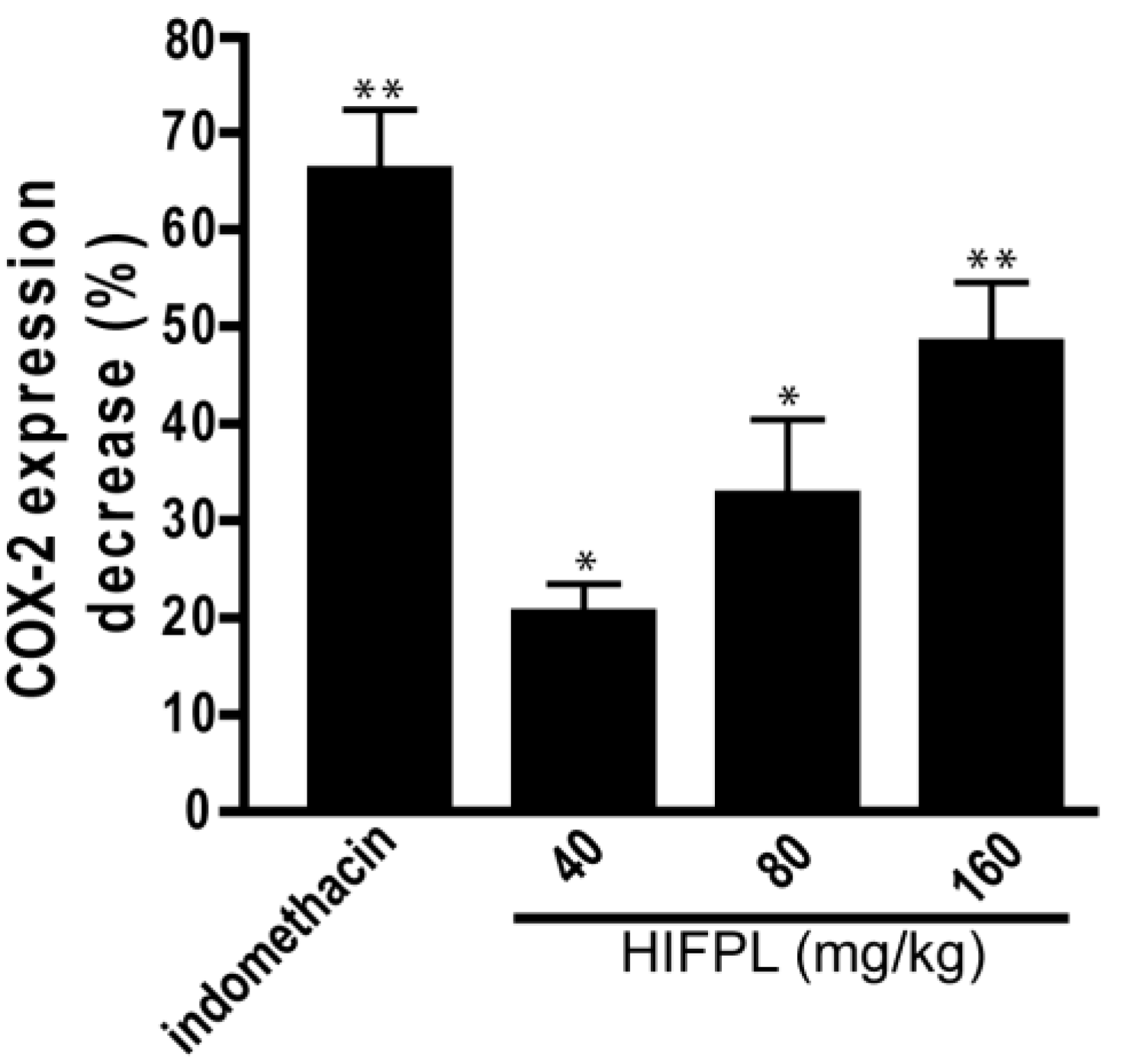
3.2. Analysis of COX-2 Expression
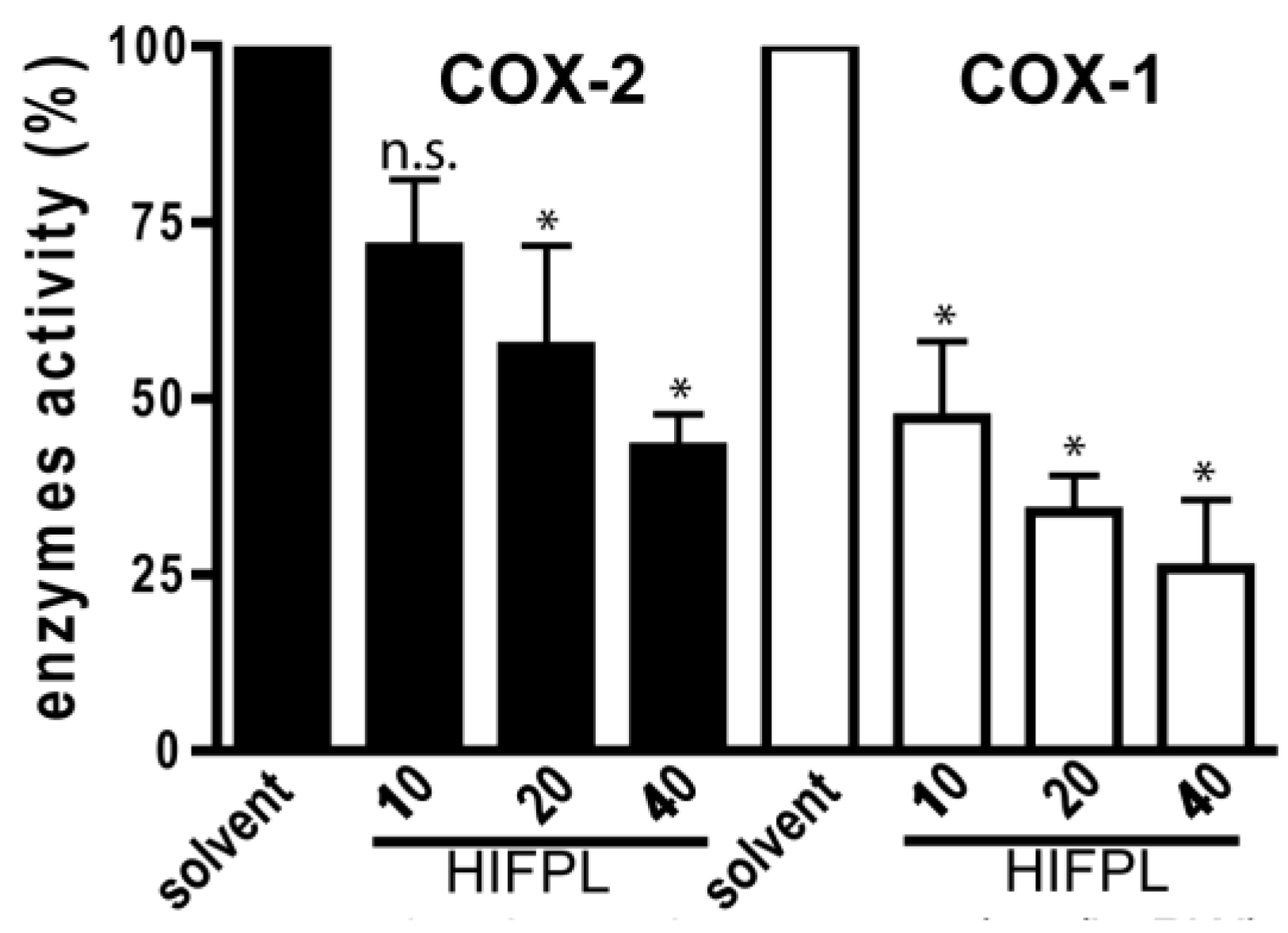
3.3. Cyclooxygenases Inhibition Enzymatic Assays
3.4. Leukocytes Migration Assay
3.5. Analysis of Chemokines Level
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ashley, N.T.; Weil, Z.M.; Nelson, R.J. Inflammation: Mechanisms, Costs, and Natural Variation. Annu. Rev. Ecol. Evolut. Syst. 2012, 43, 385–406. [Google Scholar] [CrossRef]
- Weiss, U. Inflammation. Nature 2008, 454, 427. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S. Inflammation and metabolic disorders. Nature 2006, 444, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Dequeker, J. NSAIDs/corticosteroids—Primum non nocere. Adv. Exp. Med. Biol. 1999, 455, 319–325. [Google Scholar] [PubMed]
- Dhingra, A.K.; Chopra, B.; Dass, R.; Mittal, S.K. An update on Anti-inflammatory Compounds: A Review. Anti-Inflamm. Anti-Allergy Agents Med. Chem. 2015, 14, 81–97. [Google Scholar] [CrossRef]
- Bacchi, S.; Palumbo, P.; Sponta, A.; Coppolino, M.F. Clinical pharmacology of non-steroidal anti-inflammatory drugs: A review. Anti-Inflamm. Anti-Allergy Agents Med. Chem. 2012, 11, 52–64. [Google Scholar] [CrossRef]
- Ricciotti, E.; Fitzgerald, G.A. Prostaglandins and Inflammation. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 986–1000. [Google Scholar] [CrossRef] [PubMed]
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Dubois, R.N.; Abramson, S.B.; Crofford, L.; Gupta, R.A.; Simon, L.S.; van de Putte, L.B.; Lipsky, P.E. Cyclooxygenase in biology and disease. FASEB J. 1998, 12, 1063–1073. [Google Scholar] [PubMed]
- FitzGerald, G.A. COX-2 and beyond: Approaches to prostaglandin inhibition in human disease. Nat. Rev. Drug Discov. 2003, 2, 879–890. [Google Scholar] [CrossRef] [PubMed]
- Flower, R.J. The development of COX2 inhibitors. Nat. Rev. Drug Discov. 2003, 2, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Zarghi, A.; Arfaei, S. Selective COX-2 Inhibitors: A Review of Their Structure-Activity Relationships. Iran. J. Pharm. Res. IJPR 2011, 10, 655–683. [Google Scholar] [PubMed]
- Bresalier, R.S.; Sandler, R.S.; Quan, H.; Bolognese, J.A.; Oxenius, B.; Horgan, K.; Lines, C.; Riddell, R.; Morton, D.; Lanas, A.; et al. Cardiovascular Events Associated with Rofecoxib in a Colorectal Adenoma Chemoprevention Trial. N. Engl. J. Med. 2005, 352, 1092–1102. [Google Scholar] [CrossRef] [PubMed]
- Kearney, P.M.; Baigent, C.; Godwin, J.; Halls, H.; Emberson, J.R.; Patrono, C. Do selective cyclo-oxygenase-2 inhibitors and traditional non-steroidal anti-inflammatory drugs increase the risk of atherothrombosis? Meta-analysis of randomised trials. BMJ (Clin. Res. Ed.) 2006, 332, 1302–1308. [Google Scholar] [CrossRef] [PubMed]
- Dawid-Pać, R. Medicinal plants used in treatment of inflammatory skin diseases. Postepy Dermatol Alergol. 2013, 30, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Vogl, S.; Picker, P.; Mihaly-Bison, J.; Fakhrudin, N.; Atanasov, A.G.; Heiss, E.H.; Wawrosch, C.; Reznicek, G.; Dirsch, V.M.; Saukel, J.; et al. Ethnopharmacological in vitro studies on Austria’s folk medicine—An unexplored lore in vitro anti-inflammatory activities of 71 Austrian traditional herbal drugs. J. Ethnopharmacol. 2013, 149, 750–771. [Google Scholar] [CrossRef] [PubMed]
- Fakhrudin, N.; Hastuti, S.; Andriani, A.; Widyarini, S.; Nurrochmad, A. Study on the Antiinflammatory Activity of Artocarpus altilis Leaves Extract in Mice. Int. J. Pharmacogn. Phytochem. Res. 2015, 7, 1080–1085. [Google Scholar]
- Rowland, M.; Tozer, T.N. Assessment of AUC; Balado, D., Ed.; Williams and Wilkins, Media: Ambler, PA, USA, 1995. [Google Scholar]
- Lucetti, D.; Lucetti, E.; Bandeira, M.; Veras, H.; Silva, A.; Leal, L.; Lopes, A.A.; Alves, V.C.; Silva, G.S.; Brito, G.A.; et al. Anti-inflammatory effects and possible mechanism of action of lupeol acetate isolated from Himatanthus drasticus (Mart.) Plumel. J. Inflamm. 2010, 7, 60. [Google Scholar] [CrossRef] [PubMed]
- Hussein, S.Z.; Mohd Yusoff, K.; Makpol, S.; Mohd Yusof, Y.A. Gelam honey attenuates carrageenan-induced rat paw inflammation via NF-kappaB pathway. PLoS ONE 2013, 8, e72365. [Google Scholar] [CrossRef] [PubMed]
- Fakhrudin, N.; Ladurner, A.; Atanasov, A.G.; Heiss, E.H.; Baumgartner, L.; Markt, P.; Schuster, D.; Ellmerer, E.P.; Wolber, G.; Rollinger, J.M.; et al. Computer-Aided Discovery, Validation, and Mechanistic Characterization of Novel Neolignan Activators of Peroxisome Proliferator-Activated Receptor γ. Mol. Pharmacol. 2010, 77, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Fakhrudin, N.; Putri, P.S.; Sutomo, S.; Wahyuono, S. Antiinflamatory activity of methanolic extract of Mangifera casturi in thioglycollate-induced leukocyte migration on mice. Trad. Med. J. 2013, 18, 151–156. [Google Scholar]
- Badieyan, Z.S.; Moallem, S.A.; Mehri, S.; Shahsavand, S.; Hadizadeh, F. Virtual Screening for Finding Novel COX-2 Inhibitors as Antitumor Agents. Open Med. Chem. J. 2012, 6, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Wiig, H. Pathophysiology of tissue fluid accumulation in inflammation. J. Physiol. 2011, 589, 2945–2953. [Google Scholar] [CrossRef] [PubMed]
- Hoozemans, J.J.; Rozemuller, J.M.; van Haastert, E.S.; Veerhuis, R.; Eikelenboom, P. Cyclooxygenase-1 and -2 in the different stages of Alzheimer’s disease pathology. Curr. Pharm. Des. 2008, 14, 1419–1427. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.-H.; Aid, S.; Bosetti, F. The distinct roles of cyclooxygenase-1 and -2 in neuroinflammation: Implications for translational research. Trends Pharmacol. Sci. 2009, 30, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Beara, I.N.; Orcic, D.Z.; Lesjak, M.M.; Mimica-Dukic, N.M.; Pekovic, B.A.; Popovic, M.R. Liquid chromatography/tandem mass spectrometry study of anti-inflammatory activity of Plantain (Plantago L.) species. J. Pharm. Biomed. Anal. 2010, 52, 701–706. [Google Scholar] [CrossRef] [PubMed]
- Muller, W.A. Getting Leukocytes to the Site of Inflammation. Vet. Pathol. 2013, 50, 7–22. [Google Scholar] [CrossRef] [PubMed]
- Ley, K.; Laudanna, C.; Cybulsky, M.I.; Nourshargh, S. Getting to the site of inflammation: The leukocyte adhesion cascade updated. Nat. Rev. Immunol. 2007, 7, 678–689. [Google Scholar] [CrossRef] [PubMed]
- Geng, J.G. Directional migration of leukocytes: Their pathological roles in inflammation and strategies for development of anti-inflammatory therapies. Cell Res. 2001, 11, 85–88. [Google Scholar] [CrossRef] [PubMed]
- Wright, H.L.; Moots, R.J.; Bucknall, R.C.; Edwards, S.W. Neutrophil function in inflammation and inflammatory diseases. Rheumatology 2010, 49, 1618–1631. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulou, C.; Corrigall, V.; Taylor, P.R.; Poston, R.N. The role of the chemokines MCP-1, GRO-alpha, IL-8 and their receptors in the adhesion of monocytic cells to human atherosclerotic plaques. Cytokine 2008, 43, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Zagotta, I.; Dimova, E.Y.; Debatin, K.M.; Wabitsch, M.; Kietzmann, T.; Fischer-Posovszky, P. Obesity and inflammation: Reduced cytokine expression due to resveratrol in a human in vitro model of inflamed adipose tissue. Front. Pharmacol. 2015, 6, 79. [Google Scholar] [CrossRef] [PubMed]
- Charo, I.F.; Taubman, M.B. Chemokines in the pathogenesis of vascular disease. Circ. Res. 2004, 95, 858–866. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Kakkar, V.; Lu, X. Impact of MCP-1 in atherosclerosis. Curr. Pharm. Des. 2014, 20, 4580–4588. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, C.M.; Dorf, M.E.; Proudfoot, A.; Salant, D.J.; Gutierrez-Ramos, J.C. Role of MCP-1 and RANTES in inflammation and progression to fibrosis during murine crescentic nephritis. J. Leukoc. Biol. 1997, 62, 676–680. [Google Scholar] [PubMed]
- Gupta, M.; Chaturvedi, R.; Jain, A. Role of monocyte chemoattractant protein-1 (MCP-1) as an immune-diagnostic biomarker in the pathogenesis of chronic periodontal disease. Cytokine 2013, 61, 892–897. [Google Scholar] [CrossRef] [PubMed]
- Pavkova Goldbergova, M.; Lipkova, J.; Pavek, N.; Gatterova, J.; Vasku, A.; Soucek, M.; Nemec, P. RANTES, MCP-1 chemokines and factors describing rheumatoid arthritis. Mol. Immunol. 2012, 52, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Sosa, S.; Faudale, M.; Zacchigna, M.; Cateni, F.; Del Favero, G.; Tubaro, A.; Della Loggia, D. Topical anti-inflammatory activity of Plantago lanceolata L. leaves: The relevance of triterpenic acids. Planta Med. 2011, 77, PM132. [Google Scholar] [CrossRef]
- Vigo, E.; Cepeda, A.; Gualillo, O.; Perez-Fernandez, R. In-vitro anti-inflammatory activity of Pinus sylvestris and Plantago lanceolata extracts: Effect on inducible NOS, COX-1, COX-2 and their products in J774A.1 murine macrophages. J. Pharm. Pharmacol. 2005, 57, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Marchesan, M.; Paper, D.H.; Hose, S.; Franz, G. Investigation of the antiinflammatory activity of liquid extracts of Plantago lanceolata L. Phytother. Res. 1998, 12 (Suppl. 1), S33–S34. [Google Scholar] [CrossRef]
- Checker, R.; Sandur, S.K.; Sharma, D.; Patwardhan, R.S.; Jayakumar, S.; Kohli, V.; Sethi, G.; Aggarwal, B.B.; Sainis, K.B. Potent Anti-Inflammatory Activity of Ursolic Acid, a Triterpenoid Antioxidant, Is Mediated through Suppression of NF-κB, AP-1 and NF-AT. PLoS ONE 2012, 7, e31318. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.Q.; Ding, J.; Xiao, Z.H.; Liu, C.M. Ursolic acid ameliorates carbon tetrachloride-induced oxidative DNA damage and inflammation in mouse kidney by inhibiting the STAT3 and NF-κB activities. Int. Immunopharmacol. 2014, 21, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.Y.; Yoon, S.Y.; Roh, D.H.; Jeon, M.J.; Seo, H.S.; Uh, D.K.; Kwon, Y.B.; Kim, H.W.; Han, H.J.; Lee, H.J.; et al. The anti-arthritic effect of ursolic acid on zymosan-induced acute inflammation and adjuvant-induced chronic arthritis models. J. Pharm. Pharmacol. 2008, 60, 1347–1354. [Google Scholar] [CrossRef] [PubMed]
- Ringbom, T.; Segura, L.; Noreen, Y.; Perera, P.; Bohlin, L. Ursolic Acid from Plantago major, a Selective Inhibitor of Cyclooxygenase-2 Catalyzed Prostaglandin Biosynthesis. J. Nat. Prod. 1998, 61, 1212–1215. [Google Scholar] [CrossRef] [PubMed]
- Murai, M.; Tamayama, Y.; Nishibe, S. Phenylethanoids in the Herb of Plantago lanceolata and Inhibitory Effect on Arachidonic Acid-Induced Mouse Ear Edema. Planta Med. 1995, 61, 479–480. [Google Scholar] [CrossRef] [PubMed]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fakhrudin, N.; Dwi Astuti, E.; Sulistyawati, R.; Santosa, D.; Susandarini, R.; Nurrochmad, A.; Wahyuono, S. n-Hexane Insoluble Fraction of Plantago lanceolata Exerts Anti-Inflammatory Activity in Mice by Inhibiting Cyclooxygenase-2 and Reducing Chemokines Levels. Sci. Pharm. 2017, 85, 12. https://doi.org/10.3390/scipharm85010012
Fakhrudin N, Dwi Astuti E, Sulistyawati R, Santosa D, Susandarini R, Nurrochmad A, Wahyuono S. n-Hexane Insoluble Fraction of Plantago lanceolata Exerts Anti-Inflammatory Activity in Mice by Inhibiting Cyclooxygenase-2 and Reducing Chemokines Levels. Scientia Pharmaceutica. 2017; 85(1):12. https://doi.org/10.3390/scipharm85010012
Chicago/Turabian StyleFakhrudin, Nanang, Eny Dwi Astuti, Rini Sulistyawati, Djoko Santosa, Ratna Susandarini, Arief Nurrochmad, and Subagus Wahyuono. 2017. "n-Hexane Insoluble Fraction of Plantago lanceolata Exerts Anti-Inflammatory Activity in Mice by Inhibiting Cyclooxygenase-2 and Reducing Chemokines Levels" Scientia Pharmaceutica 85, no. 1: 12. https://doi.org/10.3390/scipharm85010012
APA StyleFakhrudin, N., Dwi Astuti, E., Sulistyawati, R., Santosa, D., Susandarini, R., Nurrochmad, A., & Wahyuono, S. (2017). n-Hexane Insoluble Fraction of Plantago lanceolata Exerts Anti-Inflammatory Activity in Mice by Inhibiting Cyclooxygenase-2 and Reducing Chemokines Levels. Scientia Pharmaceutica, 85(1), 12. https://doi.org/10.3390/scipharm85010012






