MoS2/Au Heterojunction Catalyst for SERS Monitoring of a Fenton-like Reaction
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of MoS2 and MoS2/Au Nanomaterials
2.3. Instrument Characterizations and Experimental Tests
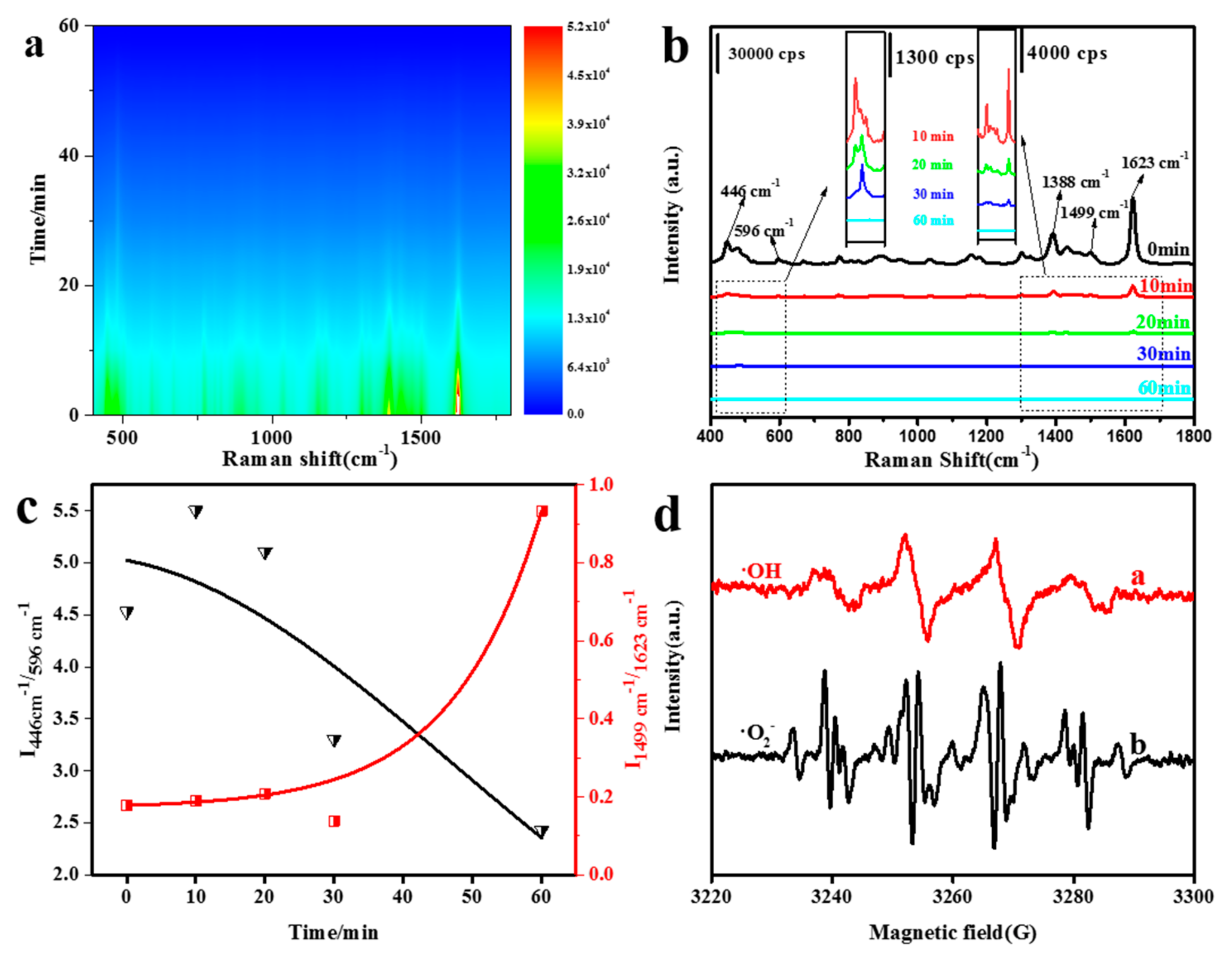
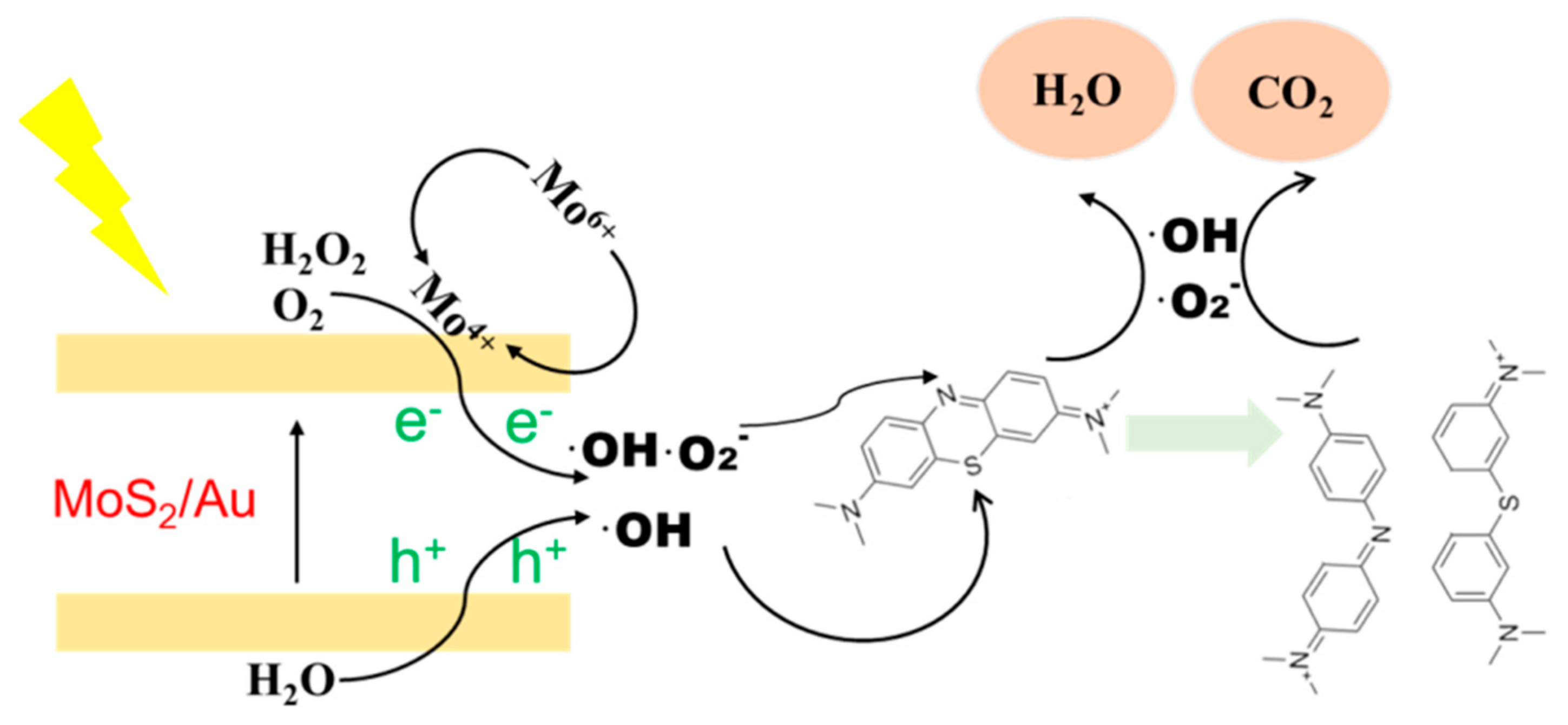
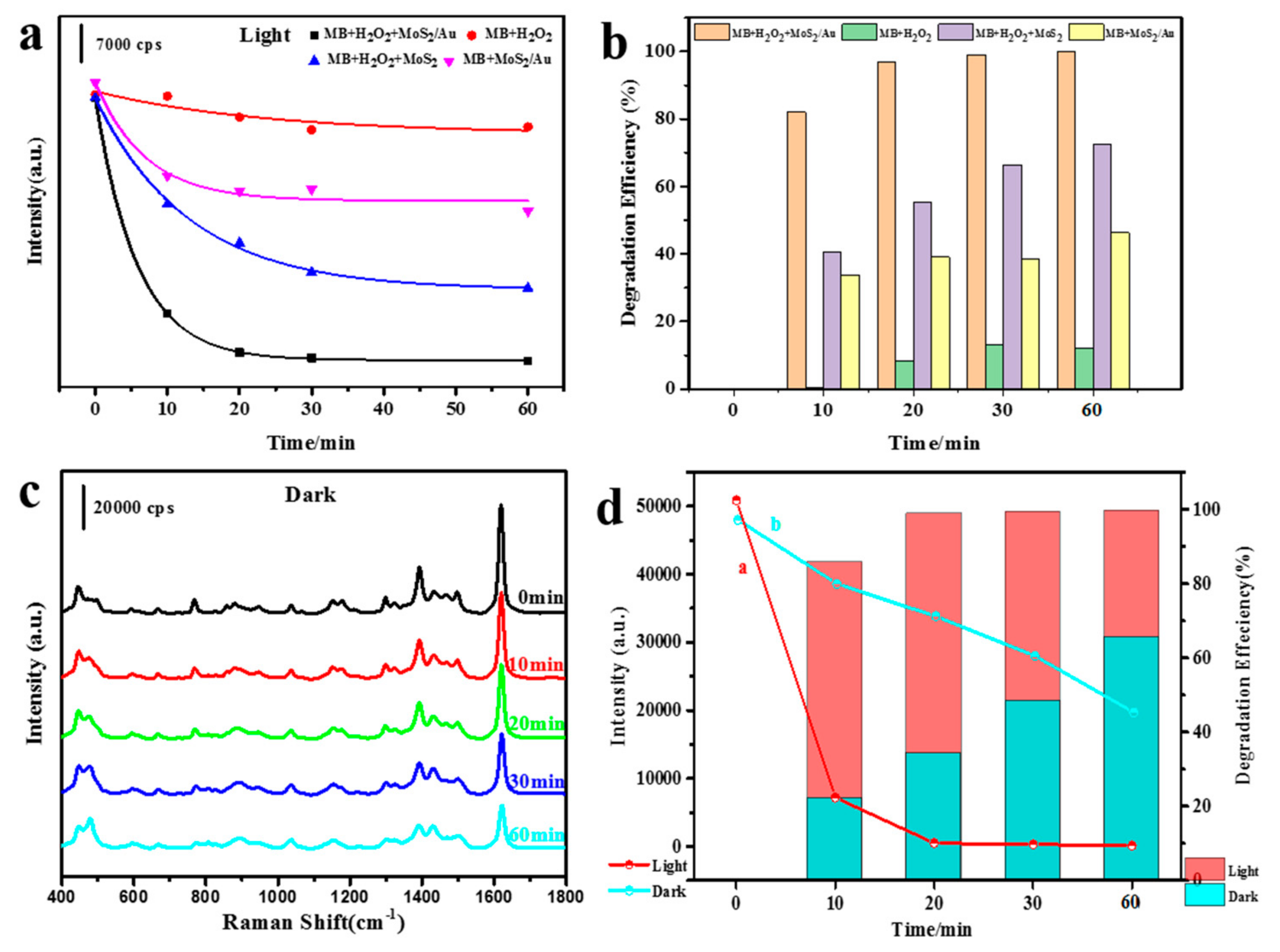
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
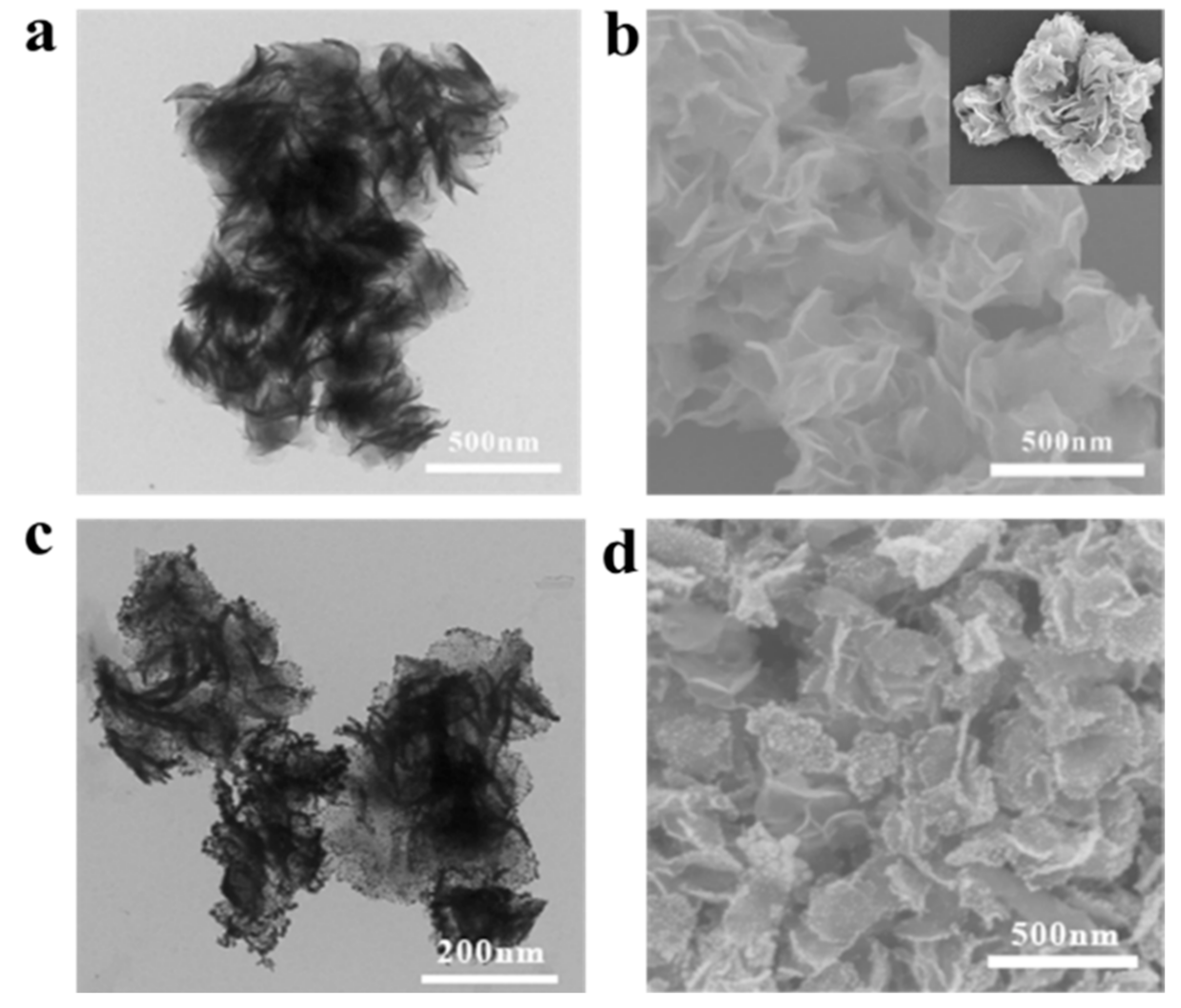
Appendix A.1. Characterizations of MoS2 and MoS2/Au
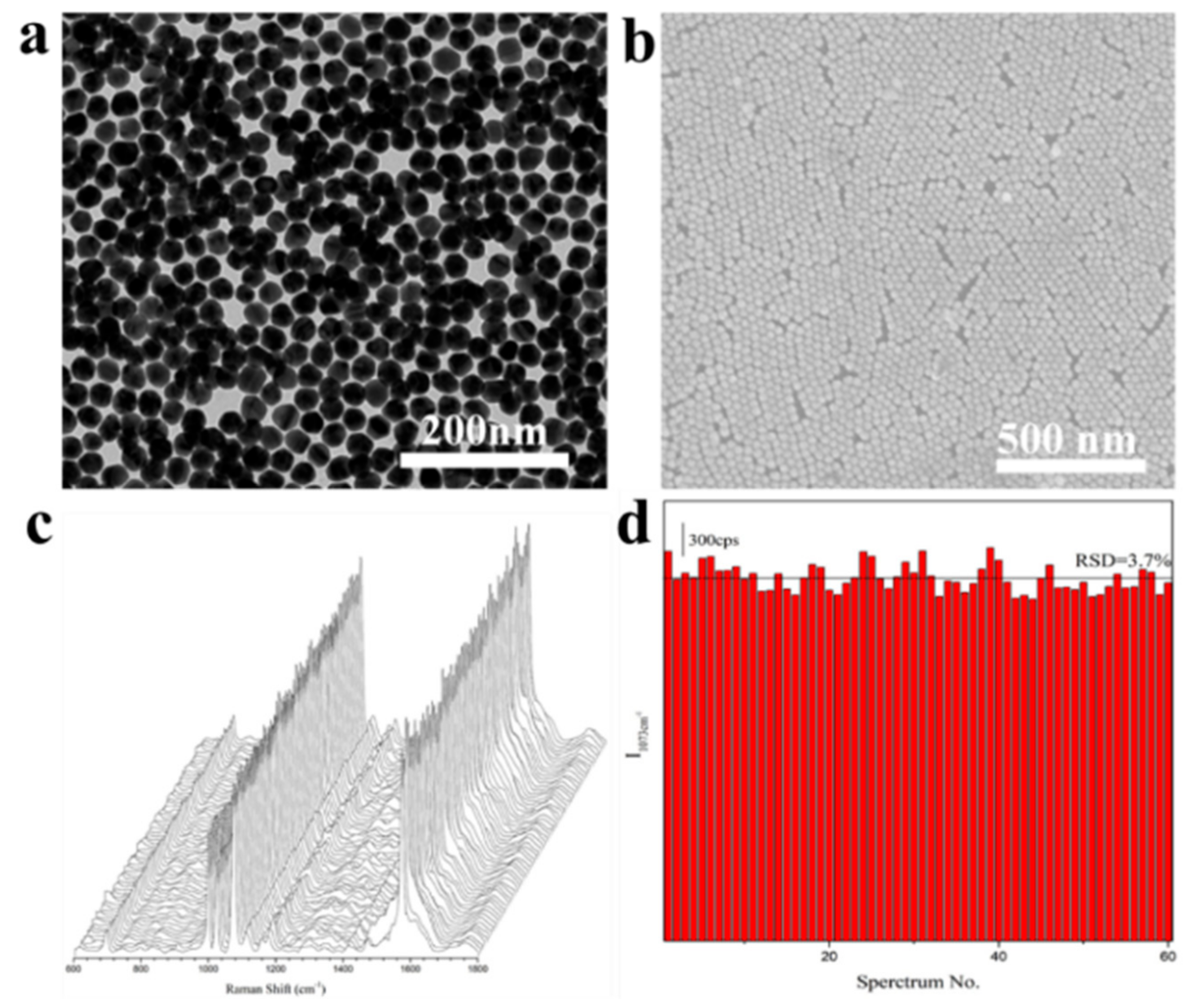
Appendix A.2. Preparation of Au Monolayer Film (MLF)
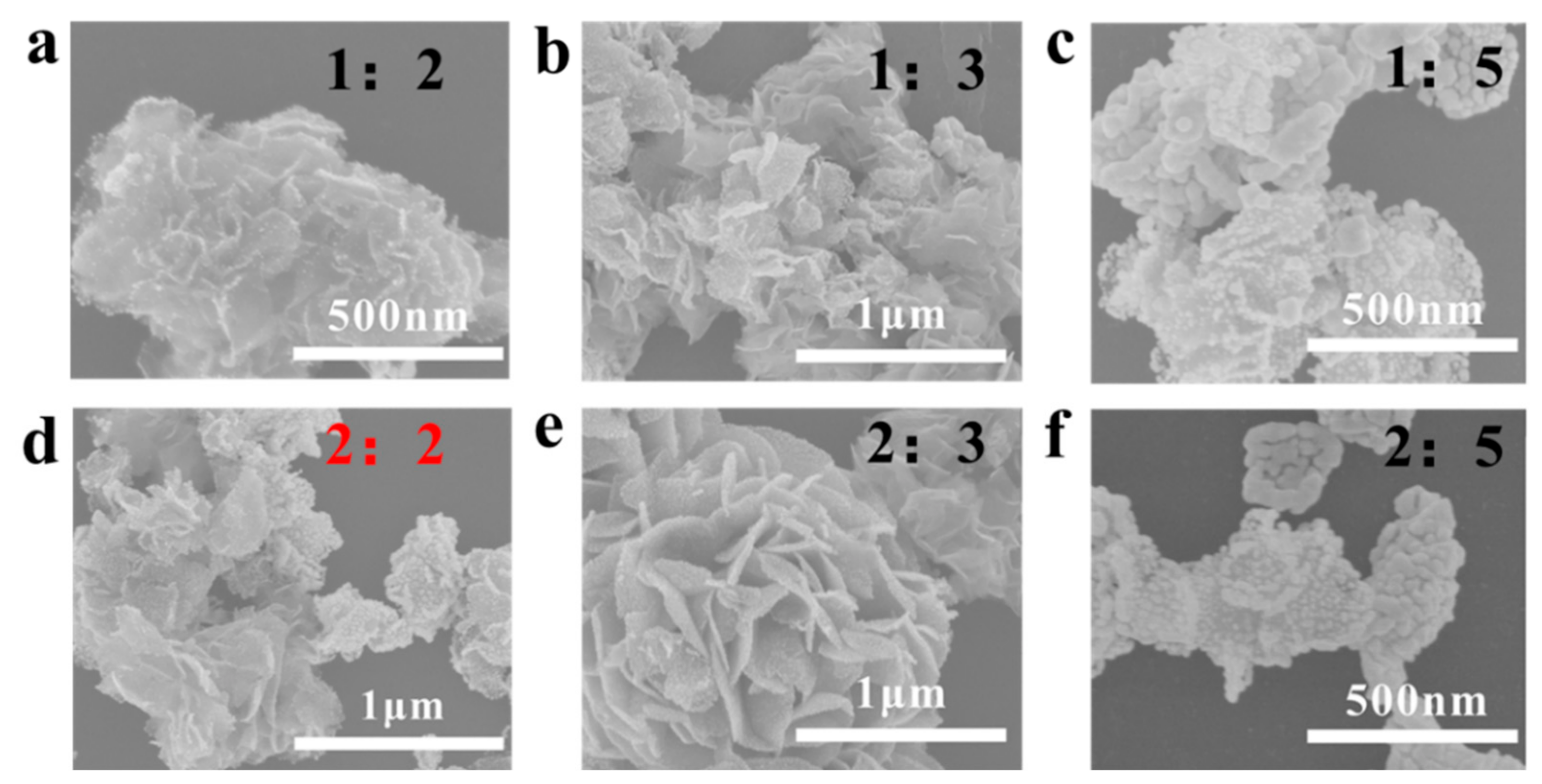
Appendix A.3. Control Au Coverages on the MoS2 Surface
References
- Viktoryova, N.; Szarka, A.; Hrouzkova, S. Recent developments and emerging trends in paint industry wastewater treatment methods. Appl. Sci. 2022, 12, 10678. [Google Scholar] [CrossRef]
- Shrivastava, V.; Ali, I.; Marjub, M.M.; Rene, E.R.; Soto, A.M.F. Wastewater in the food industry: Treatment technologies and reuse potential. Chemosphere 2022, 293, 133553. [Google Scholar] [CrossRef]
- Capodaglio, A.G. Critical perspective on advanced treatment processes for water and wastewater: AOPs, ARPs, and AORPs. Appl. Sci. 2020, 10, 4549. [Google Scholar] [CrossRef]
- Saravanan, A.; Deivayanai, V.C.; Kumar, P.S.; Rangasamy, G.; Hemavathy, R.V.; Harshana, T.; Gayathri, N.; Alagumalai, K. A detailed review on advanced oxidation process in treatment of wastewater: Mechanism, challenges and future outlook. Chemosphere 2022, 308, 136524. [Google Scholar] [CrossRef]
- Tian, K.; Hu, L.M.; Li, L.T.; Zheng, Q.Z.; Xin, Y.J.; Zhang, G.S. Recent advances in persulfate-based advanced oxidation processes for organic wastewater treatment. Chin. Chem. Lett. 2022, 33, 4461–4477. [Google Scholar] [CrossRef]
- Zhu, C.X.; Wang, Y.J.; Qiu, L.Y.; Liu, Y.Q.; Li, H.B.; Yu, Y.S.; Li, J.M.; Yang, W.W. 3D hierarchical Fe-doped Bi4O5I2 microflowers as an efficient Fenton photocatalyst for tetracycline degradation over a wide pH range. Sep. Purif. Technol. 2022, 290, 120878. [Google Scholar] [CrossRef]
- Pinedo-Hernandez, S.; Sanchez-Mendieta, V.; Solache-Rios, M.; Gutierrez-Segura, E. Degradation of brilliant blue by heterogeneous Fenton and UV-Fenton processes. Water Air Soil Pollut. 2022, 233, 264. [Google Scholar] [CrossRef]
- Xia, Q.X.; Zhang, D.J.; Yao, Z.P.; Jiang, Z.H. Investigation of Cu heteroatoms and Cu clusters in Fe-Cu alloy and their special effect mechanisms on the Fenton-like catalytic activity and reusability. Appl. Catal. B Environ. 2021, 299, 120662. [Google Scholar] [CrossRef]
- Hu, H.M.; Yu, H.; Ding, K.Z.; Jiang, Z.Z.; Liao, Y.P.; Ge, X.Q.; Sun, M.; Deng, C.H. Fabrication of hierarchical CuO architectures displaying the (111) facets-enhanced superior fentonlike degradation of organic dyes. J. Mater. Sci. Mater. Electron. 2021, 32, 11989–12000. [Google Scholar] [CrossRef]
- Xing, M.; Xu, W.; Dong, C.; Bai, Y.; Zeng, J.; Zhou, Y.; Zhang, J.; Yin, Y. Metal sulfides as excellent co-catalysts for H2O2 decomposition in advanced oxidation processes. Chem 2018, 4, 1359–1372. [Google Scholar] [CrossRef]
- Brienza, M.; Nir, S.; Plantard, G.; Goetz, V.; Chiron, S. Combining micelle-clay sorption to solar photo-Fenton processes for domestic wastewater treatment. Environ. Sci. Pollut. Res. Int. 2019, 26, 18971–18978. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Huang, X.; Xi, S.; Miao, S.; Ding, J.; Cai, W.; Liu, S.; Yang, X.; Yang, H.; Gao, J.; et al. Single cobalt atoms anchored on porous N-doped graphene with dual reaction sites for efficient Fenton-like catalysis. J. Am. Chem. Soc. 2018, 140, 12469–12475. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Cain, J.D.; Hanson, E.D.; Murthy, A.A.; Hao, S.; Shi, F.; Li, Q.; Wolverton, C.; Chen, X.; Dravid, V.P. Au@MoS2 core-shell heterostructures with strong light-matter interactions. Nano Lett. 2016, 16, 7696–7702. [Google Scholar] [CrossRef] [PubMed]
- Lai, M.T.L.; Lee, K.M.; Yang, T.C.K.; Pan, G.T.; Lai, C.W.; Chen, C.Y.; Johan, M.R.; Juan, J.C. The improved photocatalytic activity of highly expanded MoS2 under visible light emitting diodes. Nanoscale Adv. 2021, 3, 1106. [Google Scholar] [CrossRef]
- Niu, S.; Cai, J.; Wang, G. Two-dimensional MoS2 for hydrogen evolution reaction catalysis: The electronic structure regulation. Nano Res. 2020, 14, 1985–2002. [Google Scholar] [CrossRef]
- Pidamaimaiti, G.; Huang, X.; Pang, K.; Su, Z.; Wang, F. A microenvironment-mediated Cu2O–MoS2 nanoplatform with enhanced Fenton-like reaction activity for tumor chemodynamic/photothermal therapy. New J. Chem. 2021, 45, 10296–10302. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, Q.; Aleisa, R.; Zhao, T.; Ma, S.; Zhang, G.; Yao, T.; Yin, Y. MoS2/FeS nanocomposite catalyst for efficient Fenton reaction. ACS Appl. Mater. Interfaces 2021, 13, 51829–51838. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, M.; Zhang, L.; Bingham, P.A.; Tanaka, M.; Li, W.; Kubuki, S. BiOBr/MoS2 catalyst as heterogenous peroxymonosulfate activator toward organic pollutant removal: Energy band alignment and mechanism insight. J. Colloid Interface Sci. 2021, 594, 635–649. [Google Scholar] [CrossRef]
- Li, W.X.; Ding, C.J.; Kang, S.F.; Gao, W.K.; Yang, Y.; Cui, L.F. A rapid effervescent route to rational activation of Cu/g-C3N4 and bulk MoS2 cocatalyst for highly efficient and robust photo-Fenton process. Adv. Mater. Interfaces 2022, 9, 2201552. [Google Scholar] [CrossRef]
- Li, Z.T.; Gu, Y.F.; Li, F.T. Heterogeneous Fenton system with dual working mechanisms for aqueous pollutants degradation. J. Environ. Chem. Eng. 2022, 10, 107686. [Google Scholar] [CrossRef]
- Hollywood, K.A. Monitoring the succinate dehydrogenase activity isolated from mitochondria by surface enhanced Raman scattering. J. Phys. Chem. 2010, 114, 7308–7313. [Google Scholar] [CrossRef]
- Quan, Y.; Su, R.; Yang, S.; Chen, L.; Wei, M.; Liu, H.; Yang, J.; Gao, M.; Li, B. In-situ surface-enhanced Raman scattering based on MTi20 nanoflowers: Monitoring and degradation of contaminants. J. Hazard. Mater. 2021, 412, 125209. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Zhu, C.; Meng, G.; Wu, N. Review—Surface-enhanced Raman scattering sensors for food safety and environmental monitoring. J. Electrochem. Soc. 2018, 165, 3098–3118. [Google Scholar] [CrossRef]
- Qin, S.; Meng, J.; Tang, X.; Yang, L. Monitoring the inorganic chemical reaction by surface-enhanced Raman spectroscopy: A case of Fe3+ to Fe2+ conversion. Talanta 2016, 146, 452–456. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Zhang, H.; Li, S.; Wang, R.; Sun, X.; Zhou, M.; Zhou, J.; Lou, X.W.; Xie, Y. Defect-rich MoS2 ultrathin nanosheets with additional active edge sites for enhanced electrocatalytic hydrogen evolution. Adv. Mater. 2013, 25, 5807–5813. [Google Scholar] [CrossRef]
- Wang, C.M.; Zhang, Y.; Shutthanandan, V.; Thevuthasan, S.; Duscher, G. Microstructure of precipitated Au nanoclusters in TiO2. J. Appl. Phys. 2004, 95, 8185–8193. [Google Scholar] [CrossRef]
- Addou, R.; Colombo, L.; Wallace, R.M. Surface defects on natural MoS2. ACS Appl. Mater. Interfaces 2015, 7, 11921–11929. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Q.; Yap, C.C.R.; Tay, B.K.; Edwin, T.H.T.; Olivier, A.; Baillargeat, D. From bulk to monolayer MoS2: Evolution of Raman scattering. Adv. Funct. Mater. 2012, 22, 1385–1390. [Google Scholar] [CrossRef]
- Bagnall, A.G.; Liang, W.; Marseglia, E.A. Raman studies of MoS2 at high pressure. Physica B+C. 1980, 99B, 343–346. [Google Scholar] [CrossRef]
- Lee, C.; Yan, H.; Brus, L.; Heinz, F.; Hone, J.; Ryu, S. Anomalous lattice vibrations of single and few-layer MoS2. ACS Nano 2010, 4, 2695–2700. [Google Scholar] [CrossRef]
- Sun, L.; Hu, H.; Zhan, D.; Yan, J.; Liu, L.; Teguh, J.S.; Yeow, E.K.; Lee, P.S.; Shen, Z. Plasma modified MoS2 nanoflakes for surface enhanced raman scattering. Small 2014, 10, 1090–1095. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, M.; Scheuschner, N.; Maultzsch, J.; Duesberg, G.S.; McEvoy, N. Raman spectroscopy of suspended MoS2. Phys. Status Solidi B 2017, 254, 1700218. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, X. Anatase/Bronze TiO2 heterojunction: Enhanced photocatalysis and prospect in photothermal catalysis. Chem. Res. Chin. Univ. 2020, 36, 992–999. [Google Scholar] [CrossRef]
- Christian, E.; Batista, J.R.; Gerrity, D. Use of COD, TOC, and fluorescence spectroscopy to estimate BOD in wastewater. Water Environ. Res. 2017, 89, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.T.; Mateen, A.; Ashraf, F.; Javed, M.R.; Ali, A.; Mahmood, K.; Zohaib, A.; Amin, N.; Ikram, S.; Yusuf, M. A new SERS substrate based on Zn2GeO4 nanostructures for the rapid identification of E.Coli and methylene blue. Ceram. Int. 2021, 47, 27998–28003. [Google Scholar] [CrossRef]
- He, L.; Nam, K.; Li, H.; Hu, Z.; Lin, M. Use of a fractal-like gold nanostructure in surface-enhanced Raman spectroscopy for detection of selected food contaminants. J. Agric. Food Chem. 2018, 56, 9843–9847. [Google Scholar] [CrossRef]
- Guan, Y.; Wang, Z.; Gu, P.; Wang, Y.; Zhang, W.; Zhang, G. An in situ SERS study of plasmonic nanochemistry based on bifunctional “hedgehog-like” arrays. Nanoscale 2019, 11, 9422–9428. [Google Scholar] [CrossRef]
- Utsumi, H.; Han, Y.H.; Ichikawa, K. A kinetic study of 3-chlorophenol enhanced hydroxyl radical generation during ozonation. Water Res. 2003, 37, 4924–4928. [Google Scholar] [CrossRef]
- Pieta, P.; Petr, A.; Kutner, W.; Dunsch, L. In situ ESR spectroscopic evidence of the spin-trapped superoxide radical, O2−, electrochemically generated in DMSO at room temperature. Electrochim. Acta 2008, 53, 3412–3415. [Google Scholar] [CrossRef]
- Diaz-Uribe, C.E.; Daza, M.C.; Martínez, F.; Páez-Mozo, E.A.; Guedes, C.L.B.; Di Mauro, E. Visible light superoxide radical anion generation by tetra(4-carboxyphenyl)porphyrin/TiO2: EPR characterization. J. Photochem. Photobiol. 2010, 215, 172–178. [Google Scholar] [CrossRef]
- Xia, F.; Nan, Z. Excellent Fenton-like catalyst application in alkaline solution. Funct. Mater. Lett. 2019, 13, 2050002. [Google Scholar] [CrossRef]
- Lai, H.; Ma, G.; Shang, W.; Chen, D.; Yun, Y.; Peng, X.; Xu, F. Multifunctional magnetic sphere-MoS2@Au hybrid for surface-enhanced Raman scattering detection and visible light photo-Fenton degradation of aromatic dyes. Chemosphere 2019, 223, 465–473. [Google Scholar] [CrossRef]
- Clarizia, L.; Russo, D.; Di Somma, I.; Marotta, R.; Andreozzi, R. Homogeneous photo-Fenton processes at near neutral pH: A review. Appl. Catal. B 2017, 209, 358–371. [Google Scholar] [CrossRef]
- Zhao, Y.L.; Kang, S.C.; Qin, L.; Wang, W.; Zhang, T.T.; Song, S.X.; Komarneni, S. Self-assembled gels of Fe-chitosan/montmorillonite nanosheets: Dye degradation by the synergistic effect of adsorption and photo-Fenton reaction. Chem. Eng. J. 2020, 379, 122322. [Google Scholar] [CrossRef]
- Dai, H.W.; Miao, X.Z.; Zhu, J.X.; Chen, J.X. Oxalate regulate the redox cycle of iron in heterogeneous UV-Fenton system with Fe3O4 nanoparticles as catalyst: Critical role of homogeneous reaction. Chemosphere 2022, 298, 134240. [Google Scholar] [CrossRef] [PubMed]
- Babuponnusami, A.; Muthukumar, K. Removal of phenol by heterogenous photo electro Fenton-like process using nano-zero valent iron. Sep. Purif. Technol. 2012, 98, 130–135. [Google Scholar] [CrossRef]
- Raheb, I.; Manlla, M.S. Kinetic and thermodynamic studies of the degradation of methylene blue by photo-Fenton reaction. Heliyon 2021, 7, e07427. [Google Scholar] [CrossRef]
- Papegowda, P.K.; Syed, A.A. Isotherm, kinetic and thermodynamic studies on the removal of methylene blue dye from aqueous solution using saw palmetto spent. Int. J. Environ. Res. 2017, 11, 91–98. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, Q.; Lu, B.; Yang, Q.; Shi, C.; Wei, Y.; Xu, M.; Zhang, C.; Yuan, Y. MoS2/Au Heterojunction Catalyst for SERS Monitoring of a Fenton-like Reaction. Materials 2023, 16, 1169. https://doi.org/10.3390/ma16031169
Wei Q, Lu B, Yang Q, Shi C, Wei Y, Xu M, Zhang C, Yuan Y. MoS2/Au Heterojunction Catalyst for SERS Monitoring of a Fenton-like Reaction. Materials. 2023; 16(3):1169. https://doi.org/10.3390/ma16031169
Chicago/Turabian StyleWei, Qian, Beibei Lu, Qing Yang, Can Shi, Yulan Wei, Minmin Xu, Chenjie Zhang, and Yaxian Yuan. 2023. "MoS2/Au Heterojunction Catalyst for SERS Monitoring of a Fenton-like Reaction" Materials 16, no. 3: 1169. https://doi.org/10.3390/ma16031169
APA StyleWei, Q., Lu, B., Yang, Q., Shi, C., Wei, Y., Xu, M., Zhang, C., & Yuan, Y. (2023). MoS2/Au Heterojunction Catalyst for SERS Monitoring of a Fenton-like Reaction. Materials, 16(3), 1169. https://doi.org/10.3390/ma16031169




