Photoresponsive Activity of the Zn0.94Er0.02Cr0.04O Compound with Hemisphere-like Structure Obtained by Co-Precipitation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material Preparation
2.2. Characterization
2.3. Photocatalytic Test
2.4. Ecotoxicity
3. Results and Discussion
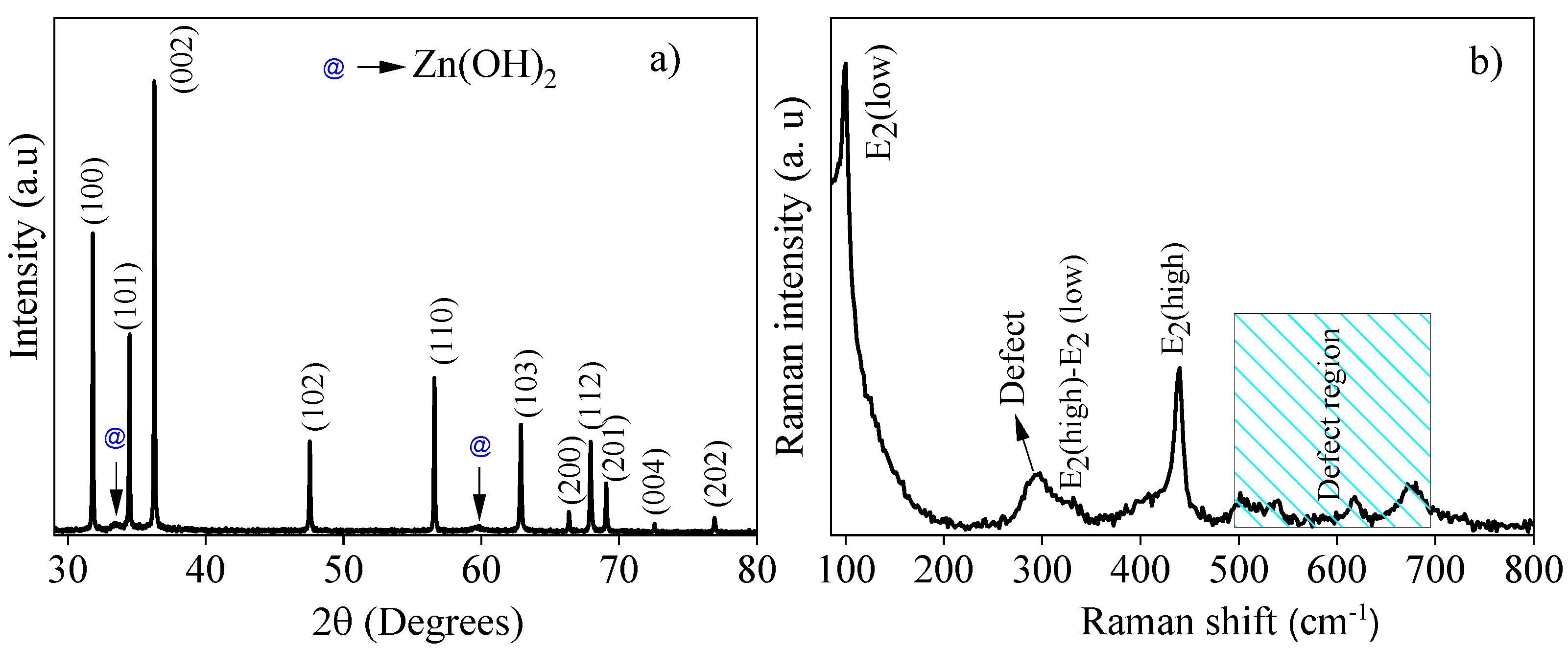
3.1. Structural, Optical and Morphological Characterization
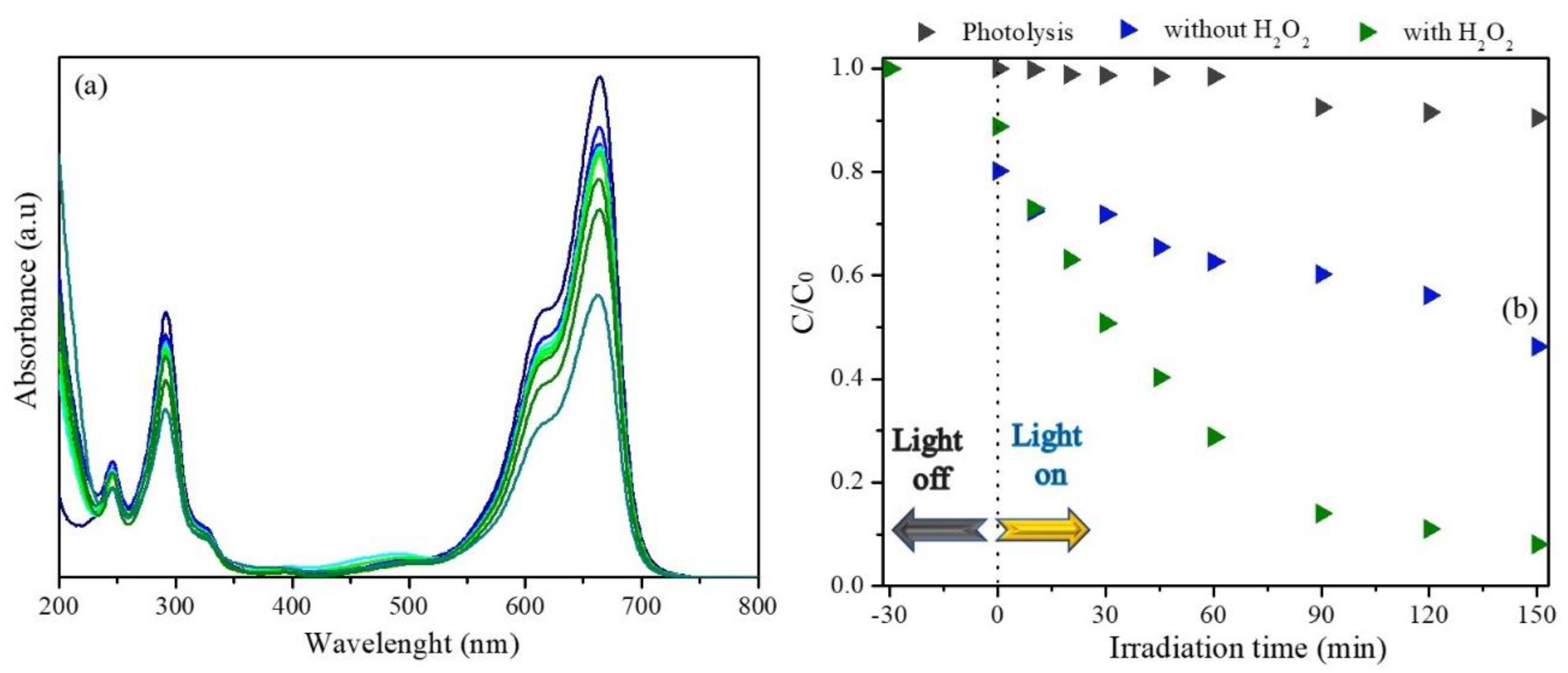
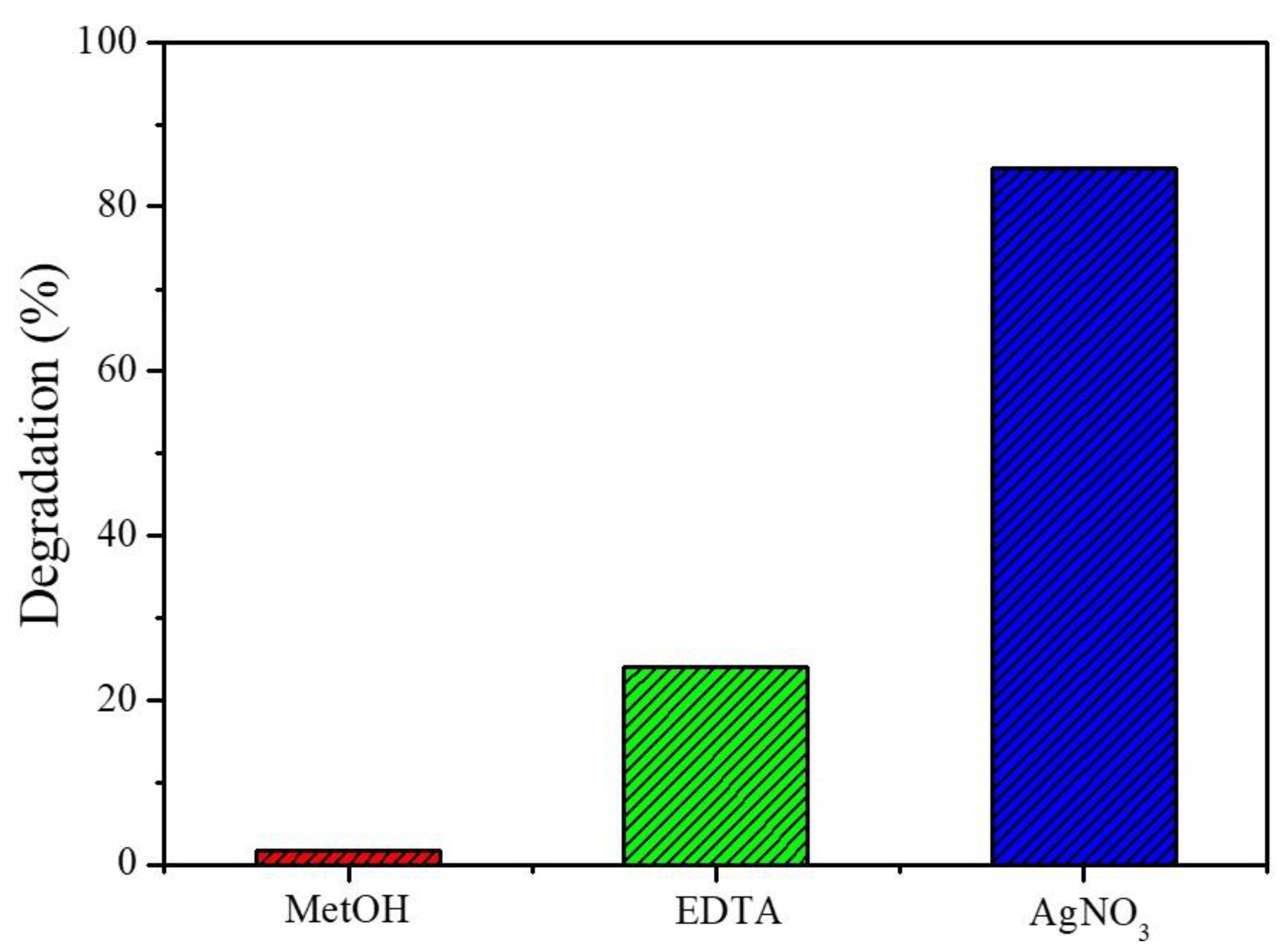
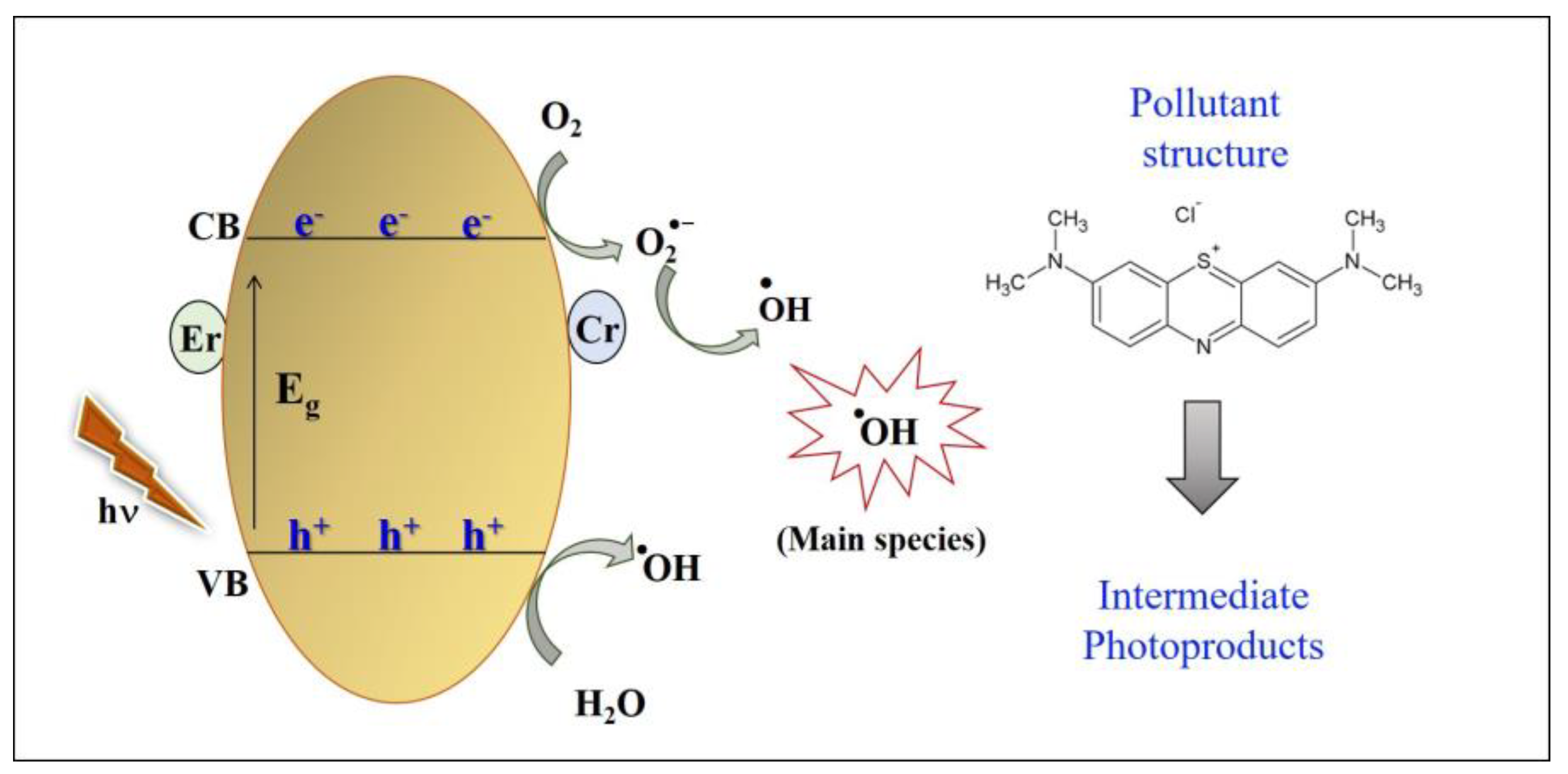
3.2. Photocatalytic Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hamadani, H.; Rashid, S.M.; Rehman, M.U.; Ali, R.; Rashid, M.; Rahman, M.; Hussain, I.; Gul, G.; ul Haq, Z. Global scenario of remediation techniques to combat environmental pollution. In Bioremediation and Biotechnology; Springer: Cham, Switzerland, 2020; ISBN 9783030356910. [Google Scholar]
- Ayub, A.; Irfan, A.; Raza, Z.A.; Abbas, M.; Muhammad, A.; Ahmad, K.; Munwar, A. Development of Poly(1-Vinylimidazole)-Chitosan Composite Sorbent under Microwave Irradiation for Enhanced Uptake of Cd(II) Ions from Aqueous Media. Polym. Bull. 2022, 79, 807–827. [Google Scholar] [CrossRef]
- Sanakousar, F.M.; Vidyasagar, C.; Jiménez-Pérez, V.M.; Prakash, K. Recent Progress on Visible-Light-Driven Metal and Non-Metal Doped ZnO Nanostructures for Photocatalytic Degradation of Organic Pollutants. Mater. Sci. Semicond. Process. 2022, 140, 106390. [Google Scholar] [CrossRef]
- Honorio, L.M.C.; Trigueiro, P.A.; Viana, B.C.; Ribeiro, A.B.; Osajima, J.A. Nanostructured Materials for the Photocatalytic Degradation of Organic Pollutants in Water; Springer: Cham, Switzerland, 2019; ISBN 9783030337445. [Google Scholar]
- Kabra, K.; Chaudhary, R.; Sawhney, R.L. Treatment of Hazardous Organic and Inorganic Compounds through Aqueous-Phase Photocatalysis: A Review. Ind. Eng. Chem. Res. 2004, 43, 7683–7696. [Google Scholar] [CrossRef]
- Wang, J.L.; Xu, L.J. Advanced Oxidation Processes for Wastewater Treatment: Formation of Hydroxyl Radical and Application. Crit. Rev. Environ. Sci. Technol. 2012, 42, 251–325. [Google Scholar] [CrossRef]
- De Araújo, K.S.; Antonelli, R.; Gaydeczka, B.; Granato, A.C.; Malpass, G.R.P. Advanced Oxidation Processes: A Review Regarding the Fundamentals and Applications in Wastewater Treatment and Industrial Wastewater. Ambiente Agua-Interdiscip. J. Appl. Sci. 2016, 11, 387–401. [Google Scholar] [CrossRef]
- Chen, D.; Cheng, Y.; Zhou, N.; Chen, P.; Wang, Y.; Li, K.; Huo, S.; Cheng, P.; Peng, P.; Zhang, R.; et al. Photocatalytic Degradation of Organic Pollutants Using TiO2-Based Photocatalysts: A Review. J. Clean. Prod. 2020, 268, 121725. [Google Scholar] [CrossRef]
- Byrne, C.; Subramanian, G.; Pillai, S.C. Recent Advances in Photocatalysis for Environmental Applications. J. Environ. Chem. Eng. 2017, 6, 3531–3555. [Google Scholar] [CrossRef]
- Morais, A.I.S.; Oliveira, W.V.; Oliveira, V.V.; Araujo, F.P.; Bezerra, R.D.S.; Fechine, P.B.A.; Viana, B.C.; Furtini, M.B.; Silva-filho, E.C.; Osajima, J.A. Semiconductor Supported by Palygorskite and Layered Double Hydroxides Clays to Dye Discoloration in Solution by a Photocatalytic Process. J. Environ. Chem. Eng. 2019, 7, 103431. [Google Scholar] [CrossRef]
- Serpone, N.; Emeline, A.V. Semiconductor Photocatalysis—Past, Present, and Future Outlook. J. Phys. Chem. Lett. 2012, 3, 673–677. [Google Scholar] [CrossRef]
- Maynez-Navarro, O.D.; Sánchez-Salas, J.L. Focus on Zinc Oxide as a Photocatalytic Material for Water Treatment. Int. J. Bioremediat. Biodegrad. 2018, 106. [Google Scholar] [CrossRef]
- Moosavi, F.; Bahrololoom, M.E.; Kamjou, R. Influence of Pb and Co-Doping on Photocatalytic Degradation Performance of ZnO Thin Films. J. Ultrafine Grained Nanostruct. Mater. 2021, 54, 173–179. [Google Scholar] [CrossRef]
- Lemos, S.C.S.; de Lima Rezende, T.K.; Assis, M.; da Costa Romeiro, F.; Peixoto, D.A.; de Oliveira Gomes, E.; Jacobsen, G.M.; Teodoro, M.D.; Gracia, L.; Ferrari, J.L.; et al. Efficient Ni and Fe Doping Process in ZnO with Enhanced Photocatalytic Activity: A Theoretical and Experimental Investigation. Mater. Res. Bull. 2022, 152, 111849. [Google Scholar] [CrossRef]
- Araujo, F.P.; Trigueiro, P.; Honório, L.M.C.; Furtini, M.B.; Oliveirab, D.M.; Almeidac, L.C.; Garcia, R.R.P.; Viana, B.C.; Silva-Filho, E.C.; Osajima, J.A. Novel Green Approach Based on ZnO Nanoparticles and Polysaccharides for Photocatalytic Performance. Dalton Trans. 2020, 49, 16394–16403. [Google Scholar] [CrossRef] [PubMed]
- Ong, C.B.; Ng, L.Y.; Mohammad, A.W. A Review of ZnO Nanoparticles as Solar Photocatalysts: Synthesis, Mechanisms and Applications. Renew. Sustain. Energy Rev. 2018, 81, 536–551. [Google Scholar] [CrossRef]
- Truong, T.K.; Van Doan, T.; Tran, H.H.; Van Le, H.; Lam, V.Q.; Tran, H.N.; Cao, T.M.; Van Pham, V. Effect of Cr Doping on Visible-Light-Driven Photocatalytic Activity of ZnO Nanoparticles. J. Electron. Mater. 2019, 48, 7378–7388. [Google Scholar] [CrossRef]
- Ahmad, I.; Shoaib Akhtar, M.; Ahmed, E.; Ahmad, M.; Keller, V.; Qamar Khan, W.; Khalid, N.R. Rare Earth Co-Doped ZnO Photocatalysts: Solution Combustion Synthesis and Environmental Applications. Sep. Purif. Technol. 2020, 237, 116328. [Google Scholar] [CrossRef]
- Devi, K.N.; Devi, S.A.; Singh, W.J.; Singh, K.J. Nickel Doped Zinc Oxide with Improved Photocatalytic Activity for Malachite Green Dye Degradation and Parameters Affecting the Degradation. J. Mater. Sci. Mater. Electron. 2021, 32, 8733–8745. [Google Scholar] [CrossRef]
- Kannadasan, N.; Shanmugam, N.; Cholan, S.; Sathishkumar, K.; Viruthagiri, G.; Poonguzhali, R. The Effect of Ce4+ Incorporation on Structural, Morphological and Photocatalytic Characters of ZnO Nanoparticles. Mater. Charact. 2014, 97, 37–46. [Google Scholar] [CrossRef]
- Tang, L.; Jia, Y.; Zhu, Z.; Hua, Y.; Wu, J.; Zou, Z.; Zhou, Y. Effects of Co Doping on the Growth and Photocatalytic Properties of ZnO Particles. Molecules 2022, 27, 833. [Google Scholar] [CrossRef]
- Abdel Messih, M.F.; Ahmed, M.A.; Soltan, A.; Anis, S.S. Synthesis and Characterization of Novel Ag/ZnO Nanoparticles for Photocatalytic Degradation of Methylene Blue under UV and Solar Irradiation. J. Phys. Chem. Solids 2019, 135, 109086. [Google Scholar] [CrossRef]
- Kaneva, N.V.; Dimitrov, D.T.; Dushkin, C.D. Effect of Nickel Doping on the Photocatalytic Activity of ZnO Thin Films under UV and Visible Light. Appl. Surf. Sci. 2011, 257, 8113–8120. [Google Scholar] [CrossRef]
- Mittal, M.; Sharma, M.; Pandey, O.P. UV-Visible Light Induced Photocatalytic Studies of Cu Doped ZnO Nanoparticles Prepared by Co-Precipitation Method. Sol. Energy 2014, 110, 386–397. [Google Scholar] [CrossRef]
- Biswas, B.D.; Purkayastha, M.D.; Tiwari, E.; Denrah, S.; Sarkar, M.; Darbha, G.K.; Majumder, T.P. Study of the Photocatalytic Activity of Mn-Doped ZnO Nanocomposites Depending on Their Morphology and Structure with the Variation of Manganese Concentration. Surf. Interfaces 2021, 23, 100902. [Google Scholar] [CrossRef]
- Alkallas, F.H.; Trabelsi, A.B.G.; Nasser, R.; Fernandez, S.; Song, J.M.; Elhouichet, H. Promising Cr-Doped ZnO Nanorods for Photocatalytic Degradation Facing Pollution. Appl. Sci. 2022, 12, 34. [Google Scholar] [CrossRef]
- Akhtar, J.; Tahir, M.B.; Sagir, M.; Bamufleh, H.S. Improved Photocatalytic Performance of Gd and Nd Co-Doped ZnO Nanorods for the Degradation of Methylene Blue. Ceram. Int. 2020, 46, 11955–11961. [Google Scholar] [CrossRef]
- Kumar, R.; Dosanjh, H.S. A Mini-Review on Rare Earth Metal Doped ZnO Nanomaterials for Photocatalytic Remediation of Waste Water. J. Phys. Conf. Ser. 2022, 2267, 012139. [Google Scholar] [CrossRef]
- Alam, U.; Khan, A.; Ali, D.; Bahnemann, D.; Muneer, M. Comparative Photocatalytic Activity of Sol-Gel Derived Rare Earth Metal (La, Nd, Sm and Dy)-Doped ZnO Photocatalysts for Degradation of Dyes. RSC Adv. 2018, 8, 17582–17594. [Google Scholar] [CrossRef]
- Sowik, J.; Miodyńska, M.; Bajorowicz, B.; Mikolajczyk, A.; Lisowski, W.; Klimczuk, T.; Kaczor, D.; Zaleska Medynska, A.; Malankowska, A. Optical and Photocatalytic Properties of Rare Earth Metal-Modified ZnO Quantum Dots. Appl. Surf. Sci. 2019, 464, 651–663. [Google Scholar] [CrossRef]
- Pascariu, P.; Cojocaru, C.; Olaru, N.; Samoila, P.; Airinei, A.; Ignat, M.; Sacarescu, L.; Timpu, D. Novel Rare Earth (RE-La, Er, Sm) Metal Doped ZnO Photocatalysts for Degradation of Congo-Red Dye: Synthesis, Characterization and Kinetic Studies. J. Environ. Manag. 2019, 239, 225–234. [Google Scholar] [CrossRef]
- Costa-Silva, M.; Araujo, F.P.; Guerra, Y.; Viana, B.C.; Silva-Filho, E.C.; Osajima, J.A.; Almeida, L.C.; Skovroinski, E.; Peña-Garcia, R. Photocatalytic, Structural and Optical Properties of Ce–Ni Co-Doped ZnO Nanodisks-like Self-Assembled Structures. Mater. Chem. Phys. 2022, 292, 126814. [Google Scholar] [CrossRef]
- Ghomri, R.; Shaikh, M.N.; Ahmed, M.I.; Bououdina, M.; Ghers, M. (Al, Er) Co-Doped ZnO Nanoparticles for Photodegradation of Rhodamine Blue. Appl. Phys. A 2016, 122, 1–9. [Google Scholar] [CrossRef]
- Irtiqa, S.; Rahman, A. Photocatalytic Studies of La,Ce Co-Doped ZnO Nanoparticles. Russ. J. Appl. Chem. 2020, 93, 1906–1919. [Google Scholar] [CrossRef]
- Asgari, E.; Sheikhmohammadi, A.; Manshouri, M.; Hashemzadeh, B. The Investigation of Removal Performances of UV/ZnO, UV/ZnO/H2O2 and UV/ZnO/O3 Processes in the Degradation of Butoben and Phenylmethyl Ester from Aqueous Solution. Optik 2021, 228, 166208. [Google Scholar] [CrossRef]
- Castro-Lopes, S.; Guerra, Y.; Silva-Sousa, A.; Oliveira, D.M.; Gonçalves, L.A.P.; Franco, A.; Padrón-Hernández, E.; Peña-Garcia, R. Influence of PH on the Structural and Magnetic Properties of Fe-Doped ZnO Nanoparticles Synthesized by Sol Gel Method. Solid State Sci. 2020, 109, 106438. [Google Scholar] [CrossRef]
- Rocha, M.; Araujo, F.P.; Castro-Lopes, S.; de Lima, I.S.; Silva-Filho, E.C.; Osajima, J.A.; Oliveira, C.S.; Viana, B.C.; Almeida, L.C.; Guerra, Y.; et al. Synthesis of Fe–Pr Co-Doped ZnO Nanoparticles: Structural, Optical and Antibacterial Properties. Ceram. Int. 2023, 49, 2282–2295. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised Effective Ionic Radii and Systematic Studies of Interatomic Distances in Halides and Chalcogenides. Acta Crystallogr. 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Peña-Garcia, R.; Guerra, Y.; Milani, R.; Oliveira, D.M.; Rodrigues, A.R.; Padrón-Hernández, E. The Role of Y on the Structural, Magnetic and Optical Properties of Fe-Doped ZnO Nanoparticles Synthesized by Sol Gel Method. J. Magn. Magn. Mater. 2020, 498, 166085. [Google Scholar] [CrossRef]
- Peña-Garcia, R.; Guerra, Y.; Milani, R.; Oliveira, D.M.; de Souza, F.R.; Padrón-Hernández, E. Influence of Ni and Sr on the Structural, Morphological and Optical Properties of ZnO Synthesized by Sol Gel. Opt. Mater. 2019, 98, 109427. [Google Scholar] [CrossRef]
- Fifere, N.; Airinei, A.; Timpu, D.; Rotaru, A.; Sacarescu, L.; Ursu, L. New Insights into Structural and Magnetic Properties of Ce Doped ZnO Nanoparticles. J. Alloys Compd. 2018, 757, 60–69. [Google Scholar] [CrossRef]
- Chen, K.J.; Fang, T.H.; Hung, F.Y.; Ji, L.W.; Chang, S.J.; Young, S.J.; Hsiao, Y.J. The Crystallization and Physical Properties of Al-Doped ZnO Nanoparticles. Appl. Surf. Sci. 2008, 254, 5791–5795. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, S.; Zhang, C.; Yang, Y.; Lv, K. Raman Spectra and Microstructure of Zinc Oxide Irradiated with Swift Heavy Ion. Crystals 2019, 9, 395. [Google Scholar] [CrossRef]
- da Trindade, L.G.; Minervino, G.B.; Trench, A.B.; Carvalho, M.H.; Assis, M.; Li, M.S.; de Oliveira, A.J.A.; Pereira, E.C.; Mazzo, T.M.; Longo, E. Influence of Ionic Liquid on the Photoelectrochemical Properties of ZnO Particles. Ceram. Int. 2018, 44, 10393–10401. [Google Scholar] [CrossRef]
- Musa, I.; Qamhieh, N.; Mahmoud, S.T. Synthesis and Length Dependent Photoluminescence Property of Zinc Oxide Nanorods. Results Phys. 2017, 7, 3552–3556. [Google Scholar] [CrossRef]
- Punia, K.; Lal, G.; Alvi, P.A.; Dolia, S.N.; Dalela, S.; Modi, K.B.; Kumar, S. A Comparative Study on the Influence of Monovalent, Divalent and Trivalent Doping on the Structural, Optical and Photoluminescence Properties of Zn0.96T0.04O (T: Li+, Ca2+& Gd3+) Nanoparticles. Ceram. Int. 2019, 45, 13472–13483. [Google Scholar]
- Poornaprakash, B.; Chalapathi, U.; Reddy, B.P.; Poojitha, P.T.; Park, S. Enhanced Ferromagnetism in ZnGdO Nanoparticles Induced by Al Co-Doping. J. Alloys Compd. 2017, 705, 51–57. [Google Scholar] [CrossRef]
- Punia, K.; Lal, G.; Dalela, S.; Dolia, S.N.; Alvi, P.A.; Barbar, S.K.; Modi, K.B.; Kumar, S. A Comprehensive Study on the Impact of Gd Substitution on Structural, Optical and Magnetic Properties of ZnO Nanocrystals. J. Alloys Compd. 2021, 868, 159142. [Google Scholar] [CrossRef]
- Punia, K.; Lal, G.; Barbar, S.K.; Dolia, S.N.; Alvi, P.A.; Dalela, S.; Kumar, S. Oxygen Vacancies Mediated Cooperative Magnetism in ZnO Nanocrystals: A D0 Ferromagnetic Case Study. Vaccum 2021, 184, 109921. [Google Scholar] [CrossRef]
- Kumar, J.; Sharma, A.; Won, S.O.; Kumar, R.; Chae, K.H.; Kumar, S.; Vij, A. Probing Defects and Electronic Structure of Eu Doped T-Mg2B2O5 Nanocrystals Using X-ray Absorption near Edge Spectroscopy and Luminescence Techniques. Vacuum 2020, 180, 7–12. [Google Scholar] [CrossRef]
- Ma, X.; Wang, Z. The Optical Properties of Rare Earth Gd Doped ZnO Nanocrystals. Mater. Sci. Semicond. Process. 2012, 15, 227–231. [Google Scholar] [CrossRef]
- Mhlongo, G.H.; Shingange, K.; Tshabalala, Z.P.; Dhonge, B.P.; Mahmoud, F.A.; Mwakikunga, B.W.; Motaung, D.E. Room Temperature Ferromagnetism and Gas Sensing in ZnO Nanostructures: Influence of Intrinsic Defects and Mn, Co, Cu Doping. Appl. Surf. Sci. 2016, 390, 804–815. [Google Scholar] [CrossRef]
- Galdámez-Martinez, A.; Santana, G.; Güell, F.; Martínez-Alanis, P.R.; Dutt, A. Photoluminescence of Zno Nanowires: A Review. Nanomaterials 2020, 10, 857. [Google Scholar] [CrossRef]
- Jayachandraiah, C.; Krishnaiah, G. Erbium Induced Raman Studies and Dielectric Properties of Er-Doped ZnO Nanoparticles. Adv. Mater. Lett. 2015, 6, 743–748. [Google Scholar] [CrossRef]
- Soares, A.S.S.; Castro-Lopes, S.; Cabrera-Baez, M.; Milani, R.; Padrón-Hernández, E.; Farias, B.V.V.; Soares, J.M.; Gusmão, S.S.; Viana, B.C.; Guerra, Y.; et al. The Role of PH on the Vibrational, Optical and Electronic Properties of the Zn FeO Compound Synthesized via Sol Gel Method. Solid State Sci. 2022, 128, 106880. [Google Scholar] [CrossRef]
- Mondal, S.; Bhattacharyya, S.R.; Mitra, P. Effect of Al Doping on Microstructure and Optical Band Gap of ZnO Thin Film Synthesized by Successive Ion Layer Adsorption and Reaction. Pramana-J. Phys. 2013, 80, 315–326. [Google Scholar] [CrossRef]
- Araujo, F.P.; Trigueiro, P.; Honório, L.M.C.; Oliveira, D.M.; Almeida, L.C.; Garcia, R.P.; Lobo, A.O.; Cantanhêde, W.; Silva-Filho, E.C.; Osajima, J.A. Eco-Friendly Synthesis and Photocatalytic Application of Flowers-like ZnO Structures Using Arabic and Karaya Gums. Int. J. Biol. Macromol. 2020, 165, 2813–2822. [Google Scholar] [CrossRef]
- Akhir, R.M.; Harrum, W.M.W.; Buniyamin, I.; Rusop, M.; Khusaimi, Z. Enhanced Structural and Morphological Properties of ZnO Nanorods Using Plant Extract-Assisted Solution Immersion Method. Mater. Today Proc. 2021, 48, 1855–1860. [Google Scholar] [CrossRef]
- Trung, D.Q.; Tran, M.T.; Tu, N.; Thu, L.T.H.; Huyen, N.T.; Hung, N.D.; Viet, D.X.; Kien, N.D.T.; Huy, P.T. Synthesis, Structural and Optical Properties of ZnS/ZnO Heterostructure-Alloy Hexagonal Micropyramids. Opt. Mater. 2022, 125, 112077. [Google Scholar] [CrossRef]
- Fe, S. Visible Photocatalytic Degradation of Methylene Blue on Magnetic. J. Mater. Sci. Mater. Electron. 2018, 123, 157–161. [Google Scholar] [CrossRef]
- Matsunami, D.; Yamanaka, K.; Mizoguchi, T.; Kojima, K. Comparison of Photodegradation of Methylene Blue Using Various TiO2 Films and WO3 Powders under Ultraviolet and Visible-Light Irradiation. J. Photochem. Photobiol. A Chem. 2019, 369, 106–114. [Google Scholar] [CrossRef]
- Navarro, S.; Fenoll, J.; Vela, N.; Ruiz, E.; Navarro, G. Photocatalytic Degradation of Eight Pesticides in Leaching Water by Use of ZnO under Natural Sunlight. J. Hazard. Mater. 2009, 172, 1303–1310. [Google Scholar] [CrossRef] [PubMed]
- Sá, A.S.; Feitosa, R.P.; Honório, L.; Peña-Garcia, R.; Almeida, L.C.; Dias, J.S.; Brazuna, L.P.; Tabuti, T.G.; Triboni, E.R.; Osajima, J.A.; et al. A Brief Photocatalytic Study of Zno Containing Cerium towards Ibuprofen Degradation. Materials 2021, 14, 5891. [Google Scholar] [CrossRef]
- Konstantinou, I.K.; Albanis, T.A. TiO2-Assisted Photocatalytic Degradation of Azo Dyes in Aqueous Solution: Kinetic and Mechanistic Investigations: A Review. Appl. Catal. B Environ. 2004, 49, 1–14. [Google Scholar] [CrossRef]
- Selvinsimpson, S.; Eva Gnana Dhana Rani, S.; Ganesh Kumar, A.; Rajaram, R.; Sharmila Lydia, I.; Chen, Y. Photocatalytic Activity of SnO2/Fe3O4 Nanocomposites and the Toxicity Assessment of Vigna Radiata, Artemia Salina and Danio Rerio in the Photodegraded Solution. Environ. Res. 2021, 195, 110787. [Google Scholar] [CrossRef]
- Souza, R.P.; Freitas, T.K.F.S.; Domingues, F.S.; Pezoti, O.; Ambrosio, E.; Ferrari-Lima, A.M.; Garcia, J.C. Photocatalytic Activity of TiO2, ZnO and Nb2O5 Applied to Degradation of Textile Wastewater. J. Photochem. Photobiol. A Chem. 2016, 329, 9–17. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
França, R.; Araujo, F.P.; Neves, L.; Melo, A.; Lins, A.; Soares, A.S.; Osajima, J.A.; Guerra, Y.; Almeida, L.C.; Peña-Garcia, R.R. Photoresponsive Activity of the Zn0.94Er0.02Cr0.04O Compound with Hemisphere-like Structure Obtained by Co-Precipitation. Materials 2023, 16, 1446. https://doi.org/10.3390/ma16041446
França R, Araujo FP, Neves L, Melo A, Lins A, Soares AS, Osajima JA, Guerra Y, Almeida LC, Peña-Garcia RR. Photoresponsive Activity of the Zn0.94Er0.02Cr0.04O Compound with Hemisphere-like Structure Obtained by Co-Precipitation. Materials. 2023; 16(4):1446. https://doi.org/10.3390/ma16041446
Chicago/Turabian StyleFrança, Robson, Francisca Pereira Araujo, Luan Neves, Arthur Melo, Alexsandro Lins, Adriano Santana Soares, Josy Anteveli Osajima, Yuset Guerra, Luciano Costa Almeida, and Ramón Raudel Peña-Garcia. 2023. "Photoresponsive Activity of the Zn0.94Er0.02Cr0.04O Compound with Hemisphere-like Structure Obtained by Co-Precipitation" Materials 16, no. 4: 1446. https://doi.org/10.3390/ma16041446
APA StyleFrança, R., Araujo, F. P., Neves, L., Melo, A., Lins, A., Soares, A. S., Osajima, J. A., Guerra, Y., Almeida, L. C., & Peña-Garcia, R. R. (2023). Photoresponsive Activity of the Zn0.94Er0.02Cr0.04O Compound with Hemisphere-like Structure Obtained by Co-Precipitation. Materials, 16(4), 1446. https://doi.org/10.3390/ma16041446








