Identification of Diseases Based on the Use of Inertial Sensors: A Systematic Review
Abstract
1. Introduction
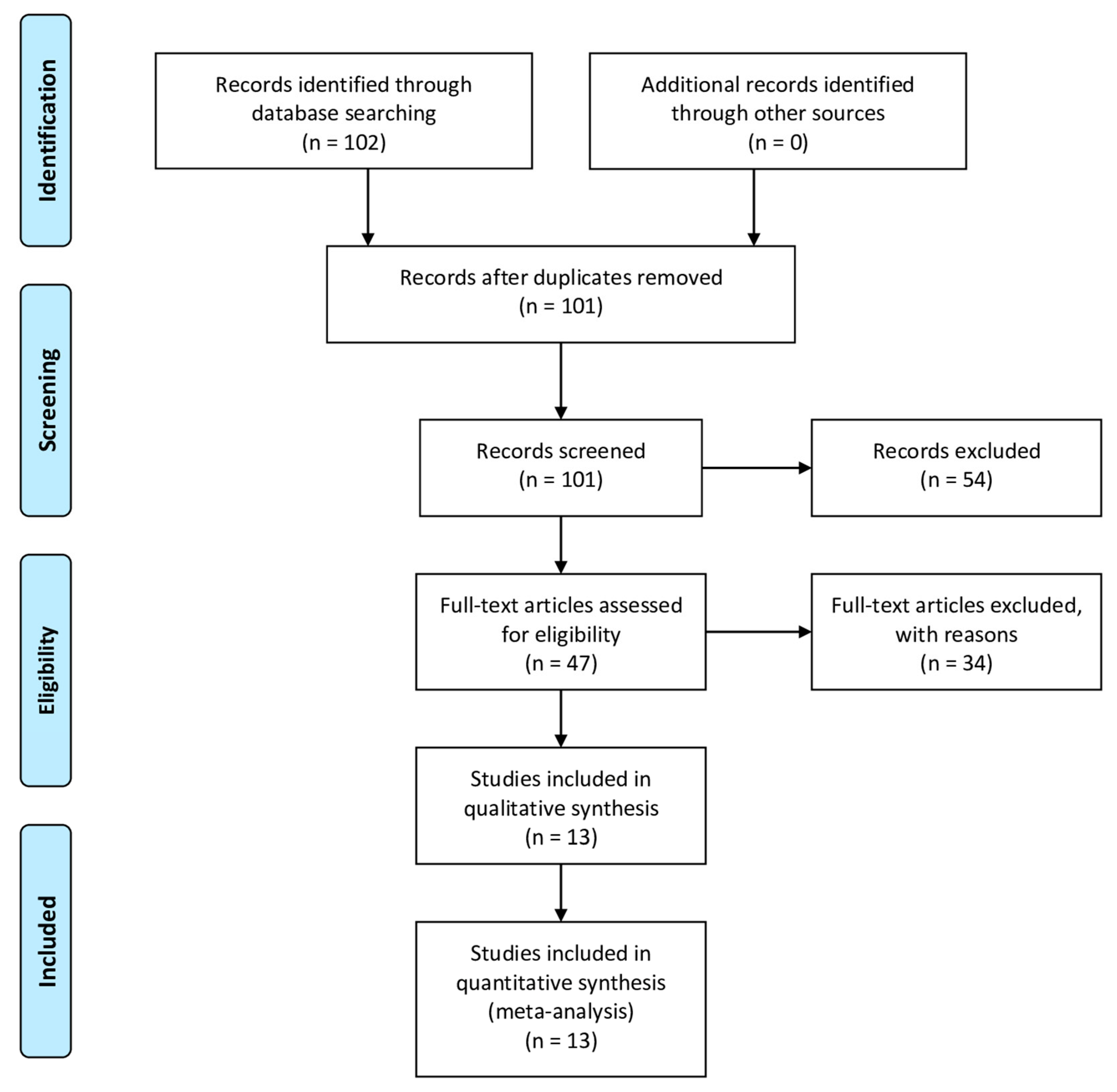
2. Materials and Methods
2.1. Research Questions
2.2. Inclusion Criteria
2.3. Search Strategy
2.4. Extraction of Study Characteristics
3. Results
3.1. Parkinson’s Disease
3.2. Other Diseases
4. Discussion
5. Conclusions
- (RQ1) How many people are involved in the different studies related to the use of the inertial sensors? The number of volunteers involved in the studies analyzed ranged from 5 to 85 (27 ± 22 individuals), where the increasing number of individuals increase the reliability of the study.
- (RQ2) Which diseases can be detected with inertial sensors? Several diseases could be detected using accelerometer sensors such as Parkinson’s, lumbar radiculopathy, and the related ankle dorsiflexion weakness with a noticeable foot drop, epilepsy, bipolar disorder, idiopathic toe walkers, multiple sclerosis, arrhythmia, and sleep apnea.
- (RQ3) Which artificial intelligence methods are used for the identification or recognition of different diseases? The artificial intelligence methods used for disease identification are Random Forest, SVM, Naive Bayes, kNN, C4.5, PART, and BSS, and K-means.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Teixeira, A.A.C.; Nagarajan, N.R.; Silva, S.T. The Impact of Ageing and the Speed of Ageing on the Economic Growth of Least Developed, Emerging and Developed Countries, 1990–2013. Rev. Dev. Econ. 2017, 21, 909–934. [Google Scholar] [CrossRef]
- Nagarajan, N.R.; Teixeira, A.A.C.; Silva, S.T. Ageing Population: Identifying the Determinants of Ageing in the Least Developed Countries. Popul. Res. Policy Rev. 2020, 1–24. [Google Scholar] [CrossRef]
- Tsoi, K.K.F.; Hirai, H.W.; Chan, F.C.H.; Griffiths, S.; Sung, J.J.Y. Predicted Increases in Incidence of Colorectal Cancer in Developed and Developing Regions, in Association with Ageing Populations. Clin. Gastroenterol. Hepatol. 2017, 15, 892–900. [Google Scholar] [CrossRef] [PubMed]
- WORLD FACTS AND STATISTICS ON DISABILITIES AND DISABILITY ISSUES. Available online: www.cdc.gov/ncbddd/disabilityandhealth/dhds.html (accessed on 17 December 2018).
- Population Ages 65 and above (% of Total) Data. Available online: https://data.worldbank.org/indicator/SP.POP.65UP.TO.ZS#fromHistory (accessed on 24 November 2018).
- Office of Disease Prevention and Health Promotion. 2020 Topics & Objectives: Older Adults; ODPHP: Washington, DC, USA, 2020.
- World Health Organization. Ageing and Health. Available online: https://www.who.int/news-room/fact-sheets/detail/ageing-and-health (accessed on 27 December 2019).
- Euromonitor International. The Top 5 Oldest Countries in the World. Available online: https://blog.euromonitor.com/the-top-5-oldest-countries-in-the-world/ (accessed on 30 April 2019).
- Paulo, A.C.; Sampaio, A.; Santos, N.C.; Costa, P.S.; Cunha, P.; Zihl, J.; Cerqueira, J.; Palha, J.A.; Sousa, N. Patterns of Cognitive Performance in Healthy Ageing in Northern Portugal: A Cross-Sectional Analysis. PLoS ONE 2011, 6, e24553. [Google Scholar] [CrossRef]
- Lopes, M.C.; Liberato, D.; Alén, E.; Liberato, P. Social Tourism Development and the Population Ageing: Case Study in Portugal and Spain. In Advances in Tourism, Technology and Smart Systems, Proceedings of the ICOTTS 2019, Buenos Aires, Argentina, 5–7 December 2019; Rocha, Á., Abreu, A., de Carvalho, J.V., Liberato, D., González, E.A., Liberato, P., Eds.; Springer: Singapore, 2020; pp. 527–536. [Google Scholar]
- Kana, M.A.; Correia, S.; Peleteiro, B.; Severo, M.; Barros, H. Impact of the global financial crisis on low birth weight in Portugal: A time-trend analysis. BMJ Glob. Health 2017, 2, e000147. [Google Scholar] [CrossRef]
- Correia, S.; Rodrigues, T.; Montenegro, N.; Barros, H. Critical evaluation of national vital statistics: The case of preterm birth trends in Portugal. Acta Obstet. Gynecol. Scand. 2015, 94, 1215–1222. [Google Scholar] [CrossRef]
- PORDATA, Índice de Dependência de Idosos: Que Países Têm Mais e Menos Idosos por 100 Pessoas em Idade Activa? 2018. Available online: https://www.pordata.pt/Europa/%C3%8Dndice+de+depend%C3%AAncia+de+idosos-1929 (accessed on 31 October 2018).
- Guimarães, H.; Boix, H.; Rodrigues, C.; Tomé, T.; Rocha, G.; Vento, M. Impact of the global financial crisis on newborn care in Portugal and Spain: Perception of health professionals. Acta Paediatr. 2020, 109, 625–627. [Google Scholar] [CrossRef]
- Marques, G.; Pitarma, R.; Garcia, N.M.; Pombo, N. Internet of Things Architectures, Technologies, Applications, Challenges, and Future Directions for Enhanced Living Environments and Healthcare Systems: A Review. Electronics 2019, 8, 1081. [Google Scholar] [CrossRef]
- Colantonio, S.; Coppini, G.; Giorgi, D.; Morales, M.-A.; Pascali, M.A. Computer Vision for Ambient Assisted Living. In Computer Vision for Assistive Healthcare; Elsevier: Amsterdam, The Netherlands, 2018; pp. 147–182. ISBN 978-0-12-813445-0. [Google Scholar]
- Ganchev, I.; Garcia, N.M.; Dobre, C.; Mavromoustakis, C.X.; Goleva, R. Enhanced Living Environments: Algorithms, Architectures, Platforms, and Systems; Lecture Notes in Computer Science; Springer International Publishing: Cham, Switzerland, 2019; Volume 11369, ISBN 978-3-030-10751-2. [Google Scholar]
- Dobre, C.; Mavromoustakis, C.X.; Garcia, N.M.; Mastorakis, G.; Goleva, R.I. Introduction to the AAL and ELE Systems. In Ambient Assisted Living and Enhanced Living Environments; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1–16. ISBN 978-0-12-805195-5. [Google Scholar]
- Botia, J.A.; Villa, A.; Palma, J. Ambient Assisted Living system for in-home monitoring of healthy independent elders. Expert Syst. Appl. 2012, 39, 8136–8148. [Google Scholar] [CrossRef]
- Costa, S.E.P.; Rodrigues, J.J.P.C.; Silva, B.M.C.; Isento, J.N.; Corchado, J.M. Integration of Wearable Solutions in AAL Environments with Mobility Support. J. Med. Syst. 2015, 39, 184. [Google Scholar] [CrossRef]
- Azimi, I.; Rahmani, A.M.; Liljeberg, P.; Tenhunen, H. Internet of things for remote elderly monitoring: A study from user-centered perspective. J. Ambient Intell. Humaniz. Comput. 2016, 8, 273–289. [Google Scholar] [CrossRef]
- Biagetti, G.; Crippa, P.; Curzi, A.; Orcioni, S.; Turchetti, C. Analysis of the EMG Signal During Cyclic Movements Using Multicomponent AM–FM Decomposition. IEEE J. Biomed. Health Inform. 2015, 19, 1672–1681. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Lim, S. A Multilayer Secure Biomedical Data Management System for Remotely Managing a Very Large Number of Diverse Personal Healthcare Devices. BioMed Res. Int. 2015, 2015, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Pak, J. An Integrated Gateway for Various PHDs in U-Healthcare Environments. J. Biomed. Biotechnol. 2012, 2012, 1–7. [Google Scholar] [CrossRef]
- Evans, J.; Papadopoulos, A.; Silvers, C.T.; Charness, N.; Boot, W.R.; Schlachta-Fairchild, L.; Crump, C.; Martinez, M.; Ent, C.B. Remote Health Monitoring for Older Adults and Those with Heart Failure: Adherence and System Usability. Telemed. E-Health 2016, 22, 480–488. [Google Scholar] [CrossRef]
- Liu, L.; Stroulia, E.; Nikolaidis, I.; Miguel-Cruz, A.; Rios Rincon, A. Smart homes and home health monitoring technologies for older adults: A systematic review. Int. J. Med. Inf. 2016, 91, 44–59. [Google Scholar] [CrossRef]
- Luxton, D.D.; McCann, R.A.; Bush, N.E.; Mishkind, M.C.; Reger, G.M. mHealth for mental health: Integrating smartphone technology in behavioral healthcare. Prof. Psychol. Res. Pract. 2011, 42, 505–512. [Google Scholar] [CrossRef]
- Charani, E.; Castro-Sánchez, E.; Moore, L.S.; Holmes, A. Do smartphone applications in healthcare require a governance and legal framework? It depends on the application! BMC Med. 2014, 12, 29. [Google Scholar] [CrossRef]
- Lloret, J.; Canovas, A.; Sendra, S.; Parra, L. A smart communication architecture for ambient assisted living. Commun. Mag. IEEE 2015, 53, 26–33. [Google Scholar] [CrossRef]
- Parra, L.; Sendra, S.; Jiménez, J.; Lloret, J. Multimedia sensors embedded in smartphones for ambient assisted living and e-health. Multimed. Tools Appl. 2015, 75, 1–27. [Google Scholar] [CrossRef]
- Pires, I.M.; Marques, G.; Garcia, N.M.; Flórez-Revuelta, F.; Ponciano, V.; Oniani, S. A Research on the Classification and Applicability of the Mobile Health Applications. J. Pers. Med. 2020, 10, 11. [Google Scholar] [CrossRef] [PubMed]
- Bisio, I.; Lavagetto, F.; Marchese, M.; Sciarrone, A. Smartphone-centric ambient assisted living platform for patients suffering from co-morbidities monitoring. Commun. Mag. IEEE 2015, 53, 34–41. [Google Scholar] [CrossRef]
- Iglesias, R.; Parra, J.; Cruces, C.; de Segura, N.G. Experiencing NFC-based touch for home healthcare. ACM Press 2009, 1–4. [Google Scholar] [CrossRef]
- Kakria, P.; Tripathi, N.K.; Kitipawang, P. A Real-Time Health Monitoring System for Remote Cardiac Patients Using Smartphone and Wearable Sensors. Int. J. Telemed. Appl. 2015, 2015, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Yan, K.; Tracie, B.; Marie-Ève, M.; Jean-Luc, B.; Benoit, T.; St-Onge, M.; Marie, L. Innovation through Wearable Sensors to Collect Real-Life Data among Pediatric Patients with Cardiometabolic Risk Factors. Int. J. Pediatr. 2014, 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Matiko, J.W.; Wei, Y.; Torah, R.; Grabham, N.; Paul, G.; Beeby, S.; Tudor, J. Wearable EEG headband using printed electrodes and powered by energy harvesting for emotion monitoring in ambient assisted living. Smart Mater. Struct. 2015, 24, 125028. [Google Scholar] [CrossRef]
- Zhu, C.; Sheng, W.; Liu, M. Wearable Sensor-Based Behavioral Anomaly Detection in Smart Assisted Living Systems. IEEE Trans. Autom. Sci. Eng. 2015, 12, 1225–1234. [Google Scholar] [CrossRef]
- Aced López, S.; Corno, F.; De Russis, L. Supporting caregivers in assisted living facilities for persons with disabilities: A user study. Univers. Access Inf. Soc. 2015, 14, 133–144. [Google Scholar] [CrossRef]
- Zainal, A.; Razak, F.H.A.; Ahmad, N.A. Older People and the Use of Mobile Phones: An Interview Study. In Proceedings of the 2013 International Conference on Advanced Computer Science Applications and Technologies, Kuching, Malaysia, 22–24 December 2013; pp. 390–395. [Google Scholar]
- Majumder, S.; Deen, M.J. Smartphone Sensors for Health Monitoring and Diagnosis. Sensors 2019, 19, 2164. [Google Scholar] [CrossRef]
- Haghi, M.; Thurow, K.; Stoll, R. Wearable Devices in Medical Internet of Things: Scientific Research and Commercially Available Devices. Healthc. Inform. Res. 2017, 23, 4. [Google Scholar] [CrossRef]
- Guo, J.; Zhou, X.; Sun, Y.; Ping, G.; Zhao, G.; Li, Z. Smartphone-Based Patients’ Activity Recognition by Using a Self-Learning Scheme for Medical Monitoring. J. Med. Syst. 2016, 40, 140. [Google Scholar] [CrossRef] [PubMed]
- Chung, P.-C.; Hsu, Y.-L.; Wang, C.-Y.; Lin, C.-W.; Wang, J.-S.; Pai, M.-C. Gait analysis for patients with Alzheimer’s disease using a triaxial accelerometer. In Proceedings of the IEEE International Symposium on Circuits and Systems (ISCAS) 2012, Seoul, Korea, 20–23 May 2012; pp. 1323–1326. [Google Scholar]
- Ibrahim, A.; Eltawil, A.; Na, Y.; El-Tawil, S. Accuracy Limits of Embedded Smart Device Accelerometer Sensors. IEEE Trans. Instrum. Meas. 2020. [Google Scholar] [CrossRef]
- Trisno, R.; Nair, P.; Martin, D.; Baghini, M.S.; Chung, H.; Pendharkar, G.; Kulkarni, J. Using accelerometer as a diagnostic tool to detect drug-induced parkinsonism (DIP) secondary to first-generation anti-psychotic medications. Australas. Psychiatry 2020. [Google Scholar] [CrossRef] [PubMed]
- Biagetti, G.; Crippa, P.; Falaschetti, L.; Orcioni, S.; Turchetti, C. Human activity monitoring system based on wearable sEMG and accelerometer wireless sensor nodes. Biomed. Eng. OnLine 2018, 17, 132. [Google Scholar] [CrossRef] [PubMed]
- Miao, F.; He, Y.; Liu, J.; Li, Y.; Ayoola, I. Identifying typical physical activity on smartphone with varying positions and orientations. Biomed. Eng. Online 2015, 14, 32. [Google Scholar] [CrossRef]
- Weiss, A.; Sharifi, S.; Plotnik, M.; van Vugt, J.P.P.; Giladi, N.; Hausdorff, J.M. Toward Automated, At-Home Assessment of Mobility Among Patients with Parkinson Disease, Using a Body-Worn Accelerometer. Neurorehabil. Neural Repair 2011, 25, 810–818. [Google Scholar] [CrossRef]
- Poewe, W.; Seppi, K.; Tanner, C.M.; Halliday, G.M.; Brundin, P.; Volkmann, J.; Schrag, A.-E.; Lang, A.E. Parkinson disease. Nat. Rev. Dis. Primer 2017, 3, 1–21. [Google Scholar] [CrossRef]
- Deng, H.; Wang, P.; Jankovic, J. The genetics of Parkinson disease. Ageing Res. Rev. 2018, 42, 72–85. [Google Scholar] [CrossRef]
- Learmonth, Y.C.; Ensari, I.; Motl, R.W. Cognitive Motor Interference in Multiple Sclerosis: Insights from a Systematic Quantitative Review. Arch. Phys. Med. Rehabil. 2017, 98, 1229–1240. [Google Scholar] [CrossRef]
- Kunkle, S.; Christie, G.; Yach, D.; El-Sayed, A.M. The Importance of Computer Science for Public Health Training: An Opportunity and Call to Action. JMIR Public Health Surveill. 2016, 2, e10. [Google Scholar] [CrossRef]
- Molina Recio, G.; García-Hernández, L.; Molina Luque, R.; Salas-Morera, L. The role of interdisciplinary research team in the impact of health apps in health and computer science publications: A systematic review. Biomed. Eng. OnLine 2016, 15, 77. [Google Scholar] [CrossRef] [PubMed]
- Free, C.; Phillips, G.; Galli, L.; Watson, L.; Felix, L.; Edwards, P.; Patel, V.; Haines, A. The Effectiveness of Mobile-Health Technology-Based Health Behaviour Change or Disease Management Interventions for Health Care Consumers: A Systematic Review. PLoS Med. 2013, 10, e1001362. [Google Scholar] [CrossRef] [PubMed]
- Davenport, T.; Kalakota, R. The potential for artificial intelligence in healthcare. Future Healthc. J. 2019, 6, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; The PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Zdravevski, E.; Lameski, P.; Trajkovik, V.; Chorbev, I.; Goleva, R.; Pombo, N.; Garcia, N.M. Automation in Systematic, Scoping and Rapid Reviews by an NLP Toolkit: A Case Study in Enhanced Living Environments. In Enhanced Living Environments; Ganchev, I., Garcia, N.M., Dobre, C., Mavromoustakis, C.X., Goleva, R., Eds.; Lecture Notes in Computer Science; Springer International Publishing: Cham, Switzerland, 2019; Volume 11369, pp. 1–18. ISBN 978-3-030-10751-2. [Google Scholar]
- Viteckova, S.; Krupicka, R.; Dusek, P.; Cejka, V.; Kutilek, P.; Novak, J.; Szabo, Z.; Růžička, E. The repeatability of the instrumented timed Up & Go test: The performance of older adults and parkinson’s disease patients under different conditions. Biocybern. Biomed. Eng. 2020, 40, 363–377. [Google Scholar]
- Sharif Bidabadi, S.; Murray, I.; Lee, G.Y.F.; Morris, S.; Tan, T. Classification of foot drop gait characteristic due to lumbar radiculopathy using machine learning algorithms. Gait Posture 2019, 71, 234–240. [Google Scholar] [CrossRef]
- Stamate, C.; Magoulas, G.D.; Kueppers, S.; Nomikou, E.; Daskalopoulos, I.; Jha, A.; Pons, J.S.; Rothwell, J.; Luchini, M.U.; Moussouri, T.; et al. The cloudUPDRS app: A medical device for the clinical assessment of Parkinson’s Disease. Pervasive Mob. Comput. 2018, 43, 146–166. [Google Scholar] [CrossRef]
- Joshi, D.; Khajuria, A.; Joshi, P. An automatic non-invasive method for Parkinson’s disease classification. Comput. Methods Programs Biomed. 2017, 145, 135–145. [Google Scholar] [CrossRef]
- Ribeiro, E.; Bentes, L.; Cruz, A.; Leitão, G.; Barreto, R.; Silva, V.; Primo, T.; Koch, F. On the use of inertial sensors and machine learning for automatic recognition of fainting and epileptic seizure. In Proceedings of the 18th International Conference on e-Health Networking, Applications and Services, Healthcom, Munich, Germany, 14–17 September 2016. [Google Scholar]
- Djuric-Jovicic, M.D.; Jovicic, N.S.; Radovanovic, S.M.; Stankovic, I.D.; Popovic, M.B.; Kostic, V.S. Automatic identification and classification of freezing of gait episodes in Parkinson’s disease patients. IEEE Trans. Neural Syst. Rehabil. Eng. 2014, 22, 685–694. [Google Scholar] [CrossRef]
- Gruenerbl, A.; Osmani, V.; Bahle, G.; Carrasco, J.C.; Oehler, S.; Mayora, O.; Haring, C.; Lukowicz, P. Using smart phone mobility traces for the diagnosis of depressive and manic episodes in bipolar patients. In ACM International Conference Proceeding Series; Association for Computing Machinery: New York, NY, USA, 2014; pp. 1–8. [Google Scholar]
- Pendharkar, G.; Naik, G.R.; Nguyen, H.T. Using Blind Source Separation on accelerometry data to analyze and distinguish the toe walking gait from normal gait in ITW children. Biomed. Signal Process. Control 2014, 13, 41–49. [Google Scholar] [CrossRef]
- Kugler, P.; Jaremenko, C.; Schlachetzki, J.; Winkler, J.; Klucken, J.; Eskofier, B. Automatic recognition of Parkinson’s disease using surface electromyography during standardized gait tests. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBS, Osaka, Japan, 3–7 July 2013; pp. 5781–5784. [Google Scholar]
- Barth, J.; Sünkel, M.; Bergner, K.; Schickhuber, G.; Winkler, J.; Klucken, J.; Eskofier, B. Combined analysis of sensor data from hand and gait motor function improves automatic recognition of Parkinson’s disease. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBS 2012, San Diego, CA, USA, 28 August–1 September 2012; pp. 5122–5125. [Google Scholar]
- Alaqtash, M.; Yu, H.; Brower, R.; Abdelgawad, A.; Sarkodie-Gyan, T. Application of wearable sensors for human gait analysis using fuzzy computational algorithm. Eng. Appl. Artif. Intell. 2011, 24, 1018–1025. [Google Scholar] [CrossRef]
- Phan, D.H.; Bonnet, S.; Guillemaud, R.; Castelli, E.; Thi, N.Y.P. Estimation of respiratory waveform and heart rate using an accelerometer. In Proceedings of the 30th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBS’08—“Personalized Healthcare through Technology”, Vancouver, BC, Canada, 20–25 August 2008; pp. 4916–4919. [Google Scholar]
- Garcia Ruiz, P.J.; Sanchez Bernardos, V. Evaluation of ActiTrac® (ambulatory activity monitor) in Parkinson’s Disease. J. Neurol. Sci. 2008, 270, 67–69. [Google Scholar] [CrossRef] [PubMed]
- Batista, D.; Silva, H.; Fred, A. Experimental characterization and analysis of the BITalino platforms against a reference device. In Proceedings of the 2017 39th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Seogwipo, Korea, 11–15 July 2017; pp. 2418–2421. [Google Scholar]
- Pires, I.; Felizardo, V.; Pombo, N.; Garcia, N.M. Limitations of energy expenditure calculation based on a mobile phone accelerometer. In Proceedings of the 2017 International Conference on High Performance Computing & Simulation (HPCS), Genoa, Italy, 17–21 July 2017; pp. 124–127. [Google Scholar]
- Pires, I.M.; Garcia, N.M.; Pombo, N.; Flórez-Revuelta, F. Limitations of the Use of Mobile Devices and Smart Environments for the Monitoring of Ageing People. In Proceedings of the 4th International Conference on Information and Communication Technologies for Ageing Well and e-Health (ICT4AWE 2018), Madeira, Portugal, 22–23 March 2018. [Google Scholar]
- Cancela, J.; Pastorino, M.; Arredondo, M.T.; Pansera, M.; Pastor-Sanz, L.; Villagra, F.; Pastor, M.A.; Gonzalez, A.P. Gait assessment in Parkinson’s disease patients through a network of wearable accelerometers in unsupervised environments. In Proceedings of the 2011 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Boston, MA, USA, 30 August–3 September 2011; pp. 2233–2236. [Google Scholar]
- Ponciano, V.; Pires, I.M.; Ribeiro, F.R.; Garcia, N.M.; Pombo, N.; Spinsante, S.; Crisóstomo, R. Smartphone-based automatic measurement of the results of the Timed-Up and Go test. In Proceedings of the 5th EAI International Conference on Smart Objects and Technologies for Social Good, Valencia, Spain, 25–27 September 2019; pp. 239–242. [Google Scholar]
- Ponciano, V.; Pires, I.M.; Ribeiro, F.R.; Garcia, N.M.; Pombo, N. Non-invasive measurement of results of timed-up and go test: Preliminary results. In Proceedings of the Ageing Congress 2019, Coimbra, Portugal, 25–28 May 2019. [Google Scholar]

| Paper | Year of Publication | Population | Purpose of the Study | Sensors | Diseases Detected | Accuracy |
|---|---|---|---|---|---|---|
| Viteckova et al. [58] | 2020 | 26 healthy adults and 25 subjects with Parkinson’s disease | Compare and quantify the results of repeated performance over time and the performance of healthy and sick people with Parkinson’s disease | Accelerometer and Gyroscope | Parkinson | N/A |
| Sharif Bidabadi et al. [59] | 2019 | 30 healthy subjects and 56 patients with Lumbar radiculopathy and related ankle dorsiflexion weakness with observable foot drop | Use of inertial measurement unit using machine learning methods to distinguish gait disturbances | Accelerometer, Gyroscope, and Magnetometer | Lumbar radiculopathy and related to ankle dorsiflexion weakness with observable foot drop | 93.18% |
| Stamate et al. [60] | 2018 | 22 individuals with Parkinson’s disease | Develop an application to create an extensive data set of motor characteristics of individuals with Parkinson’s disease | Accelerometer | Parkinson | 95% |
| Joshi et al. [61] | 2017 | 15 patients with Parkinson’s disease and 16 healthy control subjects | Method to analyze gait variables for Parkinson’s patients | Accelerometer | Parkinson | 90.32% |
| Ribeiro et al. [62] | 2016 | Five volunteers with recent episodes of Epilepsy | Development of a technique using machine learning, to automatically recognize people with epilepsy | Accelerometer | Epilepsy | 99% |
| Djuric-Jovicic et al. [63] | 2014 | 12 patients with idiopathic Parkinson’s disease | Method for the detection of walking disorders for people with Parkinson | Accelerometer and Gyroscope | Parkinson | 98.55% |
| Gruenerbl et al. [64] | 2014 | 12 bipolar disorder patients | Demonstrate how smartphones can be used to aid the diagnosis of people with psychiatric disorders | Accelerometer and GPS receiver | Bipolar | 80% |
| Pendharkar et al. [65] | 2014 | Ten children with idiopathic toe walkers and ten children with a normal gait | Automated classification of heel accelerometer data | Accelerometer | Idiopathic Toe Walkers | 97.9% |
| Kugler et al. [66] | 2013 | Five healthy adults and five subjects with Parkinson’s disease | Make an automatic classification between healthy individuals and people with Parkinson’s disease using walking electromyography | Accelerometer and electromyography (EMG) sensor | Parkinson | N/A |
| Barth et al. [67] | 2012 | 17 healthy adults and 18 subjects with Parkinson’s disease | System to analyze the motor function of the hand and to walk to differentiate healthy people and people with Parkinson’s disease | Accelerometer and Gyroscope | Parkinson | 97% |
| Alaqtash et al. [68] | 2011 | Ten healthy adults and four relapsing-remitting multiple sclerosis patients | Wearable system for the acquisition of gait parameters | Accelerometer | Multiple Sclerosis | N/A |
| Phan et al. [69] | 2008 | 30 subjects with recent symptoms of arrhythmia or sleep apnea | Accelerometer system to compare efficiency in detecting heart disease, compared to traditionally used tools | Accelerometer and electrocardiography (ECG) sensor | Arrhythmia or sleep apnea | N/A |
| Garcia Ruiz et al. [70] | 2008 | 28 patients with idiopathic Parkinson’s disease | Analysis of the utility and correlation of Active Appearance Model (AAM) with timed tests and Unified Parkinson’s Disease Rating Scale (UPDRS) scores with people with Parkinson’s disease | Accelerometer | Parkinson | N/A |
| Paper | Outcomes |
|---|---|
| Viteckova et al. [58] | The authors intended to use the instrumented Timed-Up and Go test, repeatedly in young adults and people with Parkinson’s disease to make comparisons and test the efficiency of the method. Various related features were calculated, with the test time and the other parameters related to walking and angular velocity. An Xbus Mater was used for data acquisition, which includes 5 accelerometers with a sampling rate of 100 Hz. |
| Sharif Bidabadi et al. [59] | The study aimed to investigate disorders related to falls in people with low back problems and used machine learning algorithms. Machine learning was implemented to use an accelerometer to acquire data. The results showed that the performance was better with the use of the three classifiers Random Forest, Support Vector Machine (SVM), and Naive Bayes. In contrast, when the wrapper feature technique was used, the highest accuracy was 93.18% with the Random Forest classifier. The accelerometer used is a three-axis accelerometer to measure the different directions of movement. |
| Stamate et al. [60] | A cloud application called Unified Parkinson’s Disease Rating Scale (UPDRS) was presented as a tool for people with Parkinson’s disease. The system features a workflow compatible with various formats of audio, video, and text media. It consists of an Android application for testing, a cloud system for saving data, and a data mining tool kit for medical intelligence that incorporates quantitative data and semi-structured and longitudinal analyzes, groupings, and classifications. The data was acquired by the accelerometer embedded in 9 different phone models with a sampling rate of 50 Hz. |
| Joshi et al. [61] | The authors implemented a wavelet analysis method combined with the SVM method for Parkinson’s patients. Various parameters related to walking were calculated, namely stride interval, swing interval, and stance interval (from both legs). The results showed an accuracy of 90.32%. The data was acquired by a three-axis accelerometer with specificity of 93.75%. |
| Ribeiro et al. [62] | The study used machine learning methods for the automatic recognition of people with epilepsy. Five machine learning methods were used to determine the most efficient among Naive Bayes, k-Nearest Neighbors (kNN), C4.5, Support Vector Machine (SVM), and Decision Tree-based-method (PART). The results showed that kNN had the highest computational cost, and PART and C4.5 had the lowest. Furthermore, the sensor used by the system was a three-axis accelerometer. |
| Djuric-Jovicic et al. [63] | The authors presented a method to identify the problem of falls in people with Parkinson’s disease. Several types of stride were considered, and some features (namely Shank Movement Displacement, stride duration, and shank transversal orientation) were calculated. The results showed the highest performance of the algorithm was achieved when using a type of FOG stride with 100% accuracy. The data was acquired by a three-axis accelerometer with a minimum specificity of 87.8%. |
| Gruenerbl et al. [64] | The authors intended to use smartphones to help diagnose people with mental disorders such as depression and bipolar disorder. Inertial sensors and Global Positioning System (GPS) traces were used in the developed system. The results showed an accuracy level of 80%. The accelerometer used has a fixed sampling rate of 5Hz. |
| Pendharkar et al. [65] | The authors presented a method called Idiopathic Toe-Walking (ITW) to detect walking problems in children. The sensor used in this system was the accelerometer, with the two signals of horizontal and vertical acceleration decomposed to avoid overlap. The results showed that Blind Source Separation (BSS) techniques combined with a K-means classifier could distinguish gait from foot to normal pace in children with ITW with an accuracy of 97.9%. The sensor used is a dual-axis accelerometer. |
| Kugler et al. [66] | The authors presented a method of automatic recognition of people with Parkinson’s disease. An accelerometer and an electromyography sensor were used to recognize and validate the walking parameters. When cross-validation to leave a subject out was used, the sensitivity and specificity values were the highest at 0.90, the best-rated features were the kurtosis and the mean frequency, and the best features had a significant difference in kurtosis of (p = 0.013). The authors used a three-axis accelerometer with a specificity of 90%. |
| Barth et al. [67] | The study featured a combined hand and leg analysis system for recognizing people with Parkinson’s disease. Pressure sensors were used in conjunction with the accelerometer to analyze the hand. Moreover, gyroscope and accelerometer sensors were used to analyze the foot. The results were crossed between healthy individuals and people with Parkinson’s disease, showing that when the AdaBoost classifier was used, the efficiency of the system reached 97%. These authors used a three-axis accelerometer, reporting a specificity between 88% and 100%. |
| Alaqtash et al. [68] | The authors presented a wearable sensor system for the acquisition of parameters related to walking by using a fuzzy computational algorithm, with healthy individuals and a group of patients with multiple sclerosis. The results showed that this system could be beneficial for the identification of problems related to walking showing the differences between healthy people and people with multiple sclerosis. This experiment used a dual-belt instrumented treadmill, which includes several three-axis accelerometers. |
| Phan et al. [69] | A system using the accelerometer was presented to detect diseases of the respiratory system and the heart. The system was positioned on the chest by using a belt. The study compared the use of traditional sensors such as an electrocardiogram (ECG) with a system implemented using an accelerometer. The results showed that the system provided identical results when the heart rate graph with the QRS complex was presented. This experiment considered the use of dual-axis accelerometer with high sensitivity. |
| Garcia Ruiz et al. [70] | The authors presented a method called ActiTrac for people with Parkinson’s disease. The technique had the right level of efficiency in observing the motor part of the subjects participating in the study. The results showed that the mean activity significantly correlated with the total and the motor UPDRS scores. The accelerometer embedded in the ActiTrac device is a three-axis accelerometer. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ponciano, V.; Pires, I.M.; Ribeiro, F.R.; Marques, G.; Villasana, M.V.; Garcia, N.M.; Zdravevski, E.; Spinsante, S. Identification of Diseases Based on the Use of Inertial Sensors: A Systematic Review. Electronics 2020, 9, 778. https://doi.org/10.3390/electronics9050778
Ponciano V, Pires IM, Ribeiro FR, Marques G, Villasana MV, Garcia NM, Zdravevski E, Spinsante S. Identification of Diseases Based on the Use of Inertial Sensors: A Systematic Review. Electronics. 2020; 9(5):778. https://doi.org/10.3390/electronics9050778
Chicago/Turabian StylePonciano, Vasco, Ivan Miguel Pires, Fernando Reinaldo Ribeiro, Gonçalo Marques, Maria Vanessa Villasana, Nuno M. Garcia, Eftim Zdravevski, and Susanna Spinsante. 2020. "Identification of Diseases Based on the Use of Inertial Sensors: A Systematic Review" Electronics 9, no. 5: 778. https://doi.org/10.3390/electronics9050778
APA StylePonciano, V., Pires, I. M., Ribeiro, F. R., Marques, G., Villasana, M. V., Garcia, N. M., Zdravevski, E., & Spinsante, S. (2020). Identification of Diseases Based on the Use of Inertial Sensors: A Systematic Review. Electronics, 9(5), 778. https://doi.org/10.3390/electronics9050778











