Effect of Mg Doping on the Electrical Performance of a Sol-Gel-Processed SnO2 Thin-Film Transistor
Abstract
1. Introduction
2. Materials and Methods
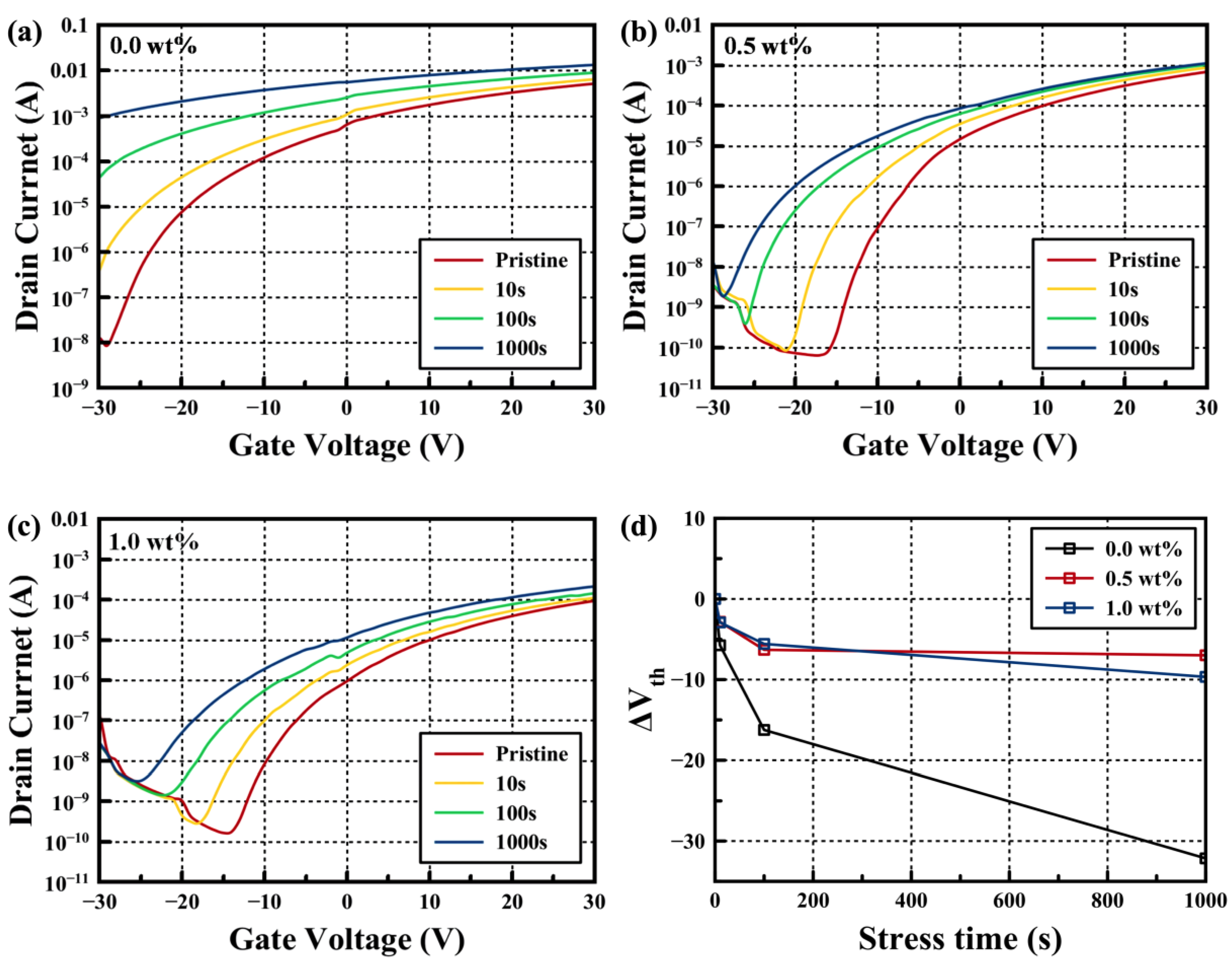
3. Results
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Nomura, K.; Ohta, H.; Takagi, A.; Kamiya, T.; Hirano, M.; Hosono, H. Room-temperature fabrication of transparent flexible thin-film transistors using amorphous oxide semiconductors. Nature 2004, 432, 488–492. [Google Scholar] [CrossRef] [PubMed]
- Yabuta, H.; Sano, M.; Abe, K.; Aiba, T.; Den, T.; Kumomi, H.; Nomura, K.; Kamiya, T.; Hosono, H. High-mobility thin-film transistor with amorphous InGaZnO4 channel fabricated by room temperature rf-magnetron sputtering. Appl. Phys. Lett. 2006, 89, 112123. [Google Scholar] [CrossRef]
- Tomai, S.; Nishimura, M.; Itose, M.; Matuura, M.; Kasami, M.; Matsuzaki, S.; Kawashima, H.; Utsuno, F.; Yano, K. Highperformance thin-film transistor with amorphous In2O3–SnO2–ZnO channel layer. Jpn. J. Appl. Phys. 2012, 51, 03CB01. [Google Scholar] [CrossRef]
- Ebata, K.; Tomai, S.; Tsuruma, Y.; Iitsuka, T.; Matsuzaki, S.; Yano, K. High-mobility thin-film transistors with polycrystalline in–Ga–O channel fabricated by DC magnetron sputtering. Appl. Phys. Express 2012, 5, 011102. [Google Scholar] [CrossRef]
- Park, J.S.; Kim, K.; Park, Y.G.; Mo, Y.G.; Kim, H.D.; Jeong, J.K. Novel ZrInZnO thin-film transistor with excellent stability. Adv. Mater. 2009, 21, 329–333. [Google Scholar] [CrossRef]
- Jeong, S.; Lee, J.Y.; Lee, S.S.; Choi, Y.; Ryu, B.H. Impact of metal salt precursor on low-temperature annealed solution-derived Ga-doped In2O3 semiconductor for thin-film transistors. J. Phys. Chem. C 2011, 115, 11773–11780. [Google Scholar] [CrossRef]
- Chong, E.; Chun, Y.S.; Lee, S.Y. Amorphous silicon-indium-zinc oxide semiconductor. Appl. Phys. Lett. 2010, 97, 102102. [Google Scholar] [CrossRef]
- Yang, Z.; Meng, T.; Zhang, Q.; Shieh, H.P.D. Stability of amorphous indium tungsten oxide thin-film transistors under various wavelength light illumination. IEEE Electron. Device Lett. 2016, 37, 437–440. [Google Scholar] [CrossRef]
- Haxel, G.B.; Hedrick, J.B.; Orris, G.J. Rare Earth Elements—Critical Resources for High Technology; US Department of the Interior, US Geological Survey, 2002; Volume 87. Available online: https://pubs.usgs.gov/fs/2002/fs087-02/ (accessed on 20 November 2002).
- Ren, J.H.; Li, K.W.; Shen, J.; Sheng, C.; Huang, Y.; Zhang, Q. Effect of rare-earth erbium doping on the electrical performance of tin oxide thin-film transistors. J. Alloy. Compd. 2019, 791, 11–18. [Google Scholar] [CrossRef]
- Jang, J.; Kitsomboonloha, R.; Swisher, S.L.; Park, E.S.; Kang, H.; Subramanian, V. Transparent high-performance thin-film transistors from solution-processed SnO2/ZrO2 gel-like precursors. Adv. Mater. 2013, 25, 1042–1047. [Google Scholar] [CrossRef]
- Jang, J.; Kang, H.; Chakravarthula, H.C.N.; Subramanian, V. Fully inkjet-printed transparent oxide thin-film transistors using a fugitive wettability switch. Adv. Electron. Mater. 2015, 1, 1500086. [Google Scholar] [CrossRef]
- Jang, B.; Kim, T.; Lee, S.; Lee, W.Y.; Kang, H.; Cho, C.S.; Jang, J. High performance ultrathin SnO2 thin-film transistors by sol-gel method. IEEE Electron Device Lett. 2018, 39, 1179–1182. [Google Scholar] [CrossRef]
- Hong, S.; Park, J.W.; Kim, H.J.; Kim, Y.; Kim, H.J. A review of multi-stacked active-layer structures for solution-processed oxide semiconductor thin-film transistors. J. Inf. Disp. 2016, 17, 93–101. [Google Scholar] [CrossRef]
- Jeong, J.K. Photo-bias instability of metal oxide thin-film transistors for advanced active matrix displays. J. Mater. Res. 2013, 28, 2071–2084. [Google Scholar] [CrossRef]
- Liu, P.T.; Choi, Y.T.; Teng, L.F. Environment-dependent metastability of passivation-free indium zinc oxide thin-film transistor after gate bias stress. Appl. Phys. Lett. 2009, 95, 233504. [Google Scholar] [CrossRef]
- Fortunato, E.; Barquinha, P.; Pimentel, A.; Pereira, L.; Gonçalves, G.; Martins, R. Amorphous IZO TFTs with saturation mobilities exceeding 100 cm2/Vs. Phys. Status Solidi (RRL) 2007, 1, R34–R36. [Google Scholar] [CrossRef]
- Dehuff, N.L.; Kettenring, E.S.; Hong, D.; Chiang, H.Q.; Wager, J.F.; Hoffman, R.L.; Park, C.-H.; Keszler, D.A. Transparent thin-film transistors with zinc indium oxide channel layer. J. Appl. Phys. 2005, 97, 064505. [Google Scholar] [CrossRef]
- Wang, L.; Yoon, M.H.; Lu, G.; Yang, Y.; Facchetti, A.; Marks, T.J. High-performance transparent inorganic-organic hybrid thin-film n-type transistors. Nat. Mater. 2006, 5, 893–900. [Google Scholar] [CrossRef]
- Scheideler, W.J.; Jang, J.; Karim, M.A.U.; Kitsomboonloha, R.; Zeumault, A.; Subramanian, V. Gravure-printed sol–gels on flexible glass: A scalable route to additively patterned transparent conductors. ACS Appl. Mater. Interfaces 2015, 7, 12679–12687. [Google Scholar] [CrossRef]
- Mazumder, N.; Saha, A.B.; Sen, D.; Chattopadhyay, K.K. Effect of Mg doping on the electrical properties of SnO2 nanoparticles. Curr. Appl. Phys. 2012, 12, 975–982. [Google Scholar] [CrossRef]
- Lee, S.-H.; Kim, T.; Lee, J.; Avis, C.; Jang, J. Solution processed gadolinium doped indium-oxide thin-film transistors with oxide passivation. Appl. Phys. Lett. 2017, 110, 122102. [Google Scholar] [CrossRef]
- Parthiban, S.; Kwon, J.-Y. Role of dopants as a carrier suppressor and strong oxygen binder in amorphous indium-oxide-based field effect transistor. J. Mater. Res. 2014, 29, 1585–1596. [Google Scholar] [CrossRef]
- Ahn, B.D.; Lim, J.H.; Cho, M.-H.; Park, J.-S.; Chung, K.-B. Thin-film transistor behaviour and the associated physical origin of water-annealed in–Ga–Zn oxide semiconductor. J. Phys. D Appl. Phys. 2012, 45, 415307-1–415307-6. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, W.; Yin, S. Oxygen vacancies confined in SnO2 nanoparticles for desirable electronic structure and enhanced visible light photocatalytic activity. Appl. Surf. Sci. 2017, 420, 399. [Google Scholar] [CrossRef]
- Jang, B.; Kim, T.; Lee, S.; Lee, W.; Jang, J. Schottky Nature of Au/SnO2 Ultrathin Film Diode Fabricated Using Sol-Gel Process. IEEE Electron Device Lett. 2018, 39, 1732–1735. [Google Scholar] [CrossRef]
- Gupta, N.; Tyagi, B.P. On-current modeling of polycrystalline silicon thin-film transistors. Phys. Scripta. 2005, 72, 339. [Google Scholar] [CrossRef]
- Kim, Y.S.; Park, C.H. Rich variety of defects in ZnO via an attractive interaction between O vacancies and Zn interstitials: Origin of n-type doping. Phys. Rev. Lett. 2009, 102, 806403. [Google Scholar] [CrossRef]
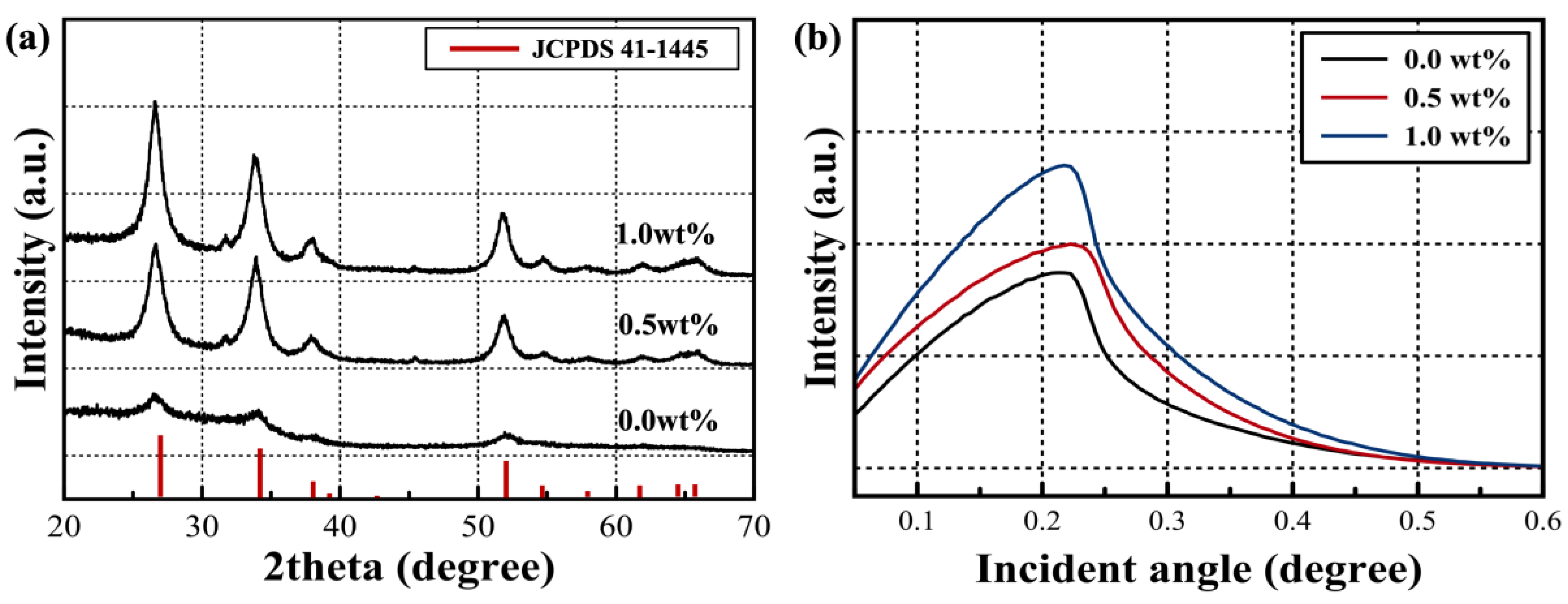
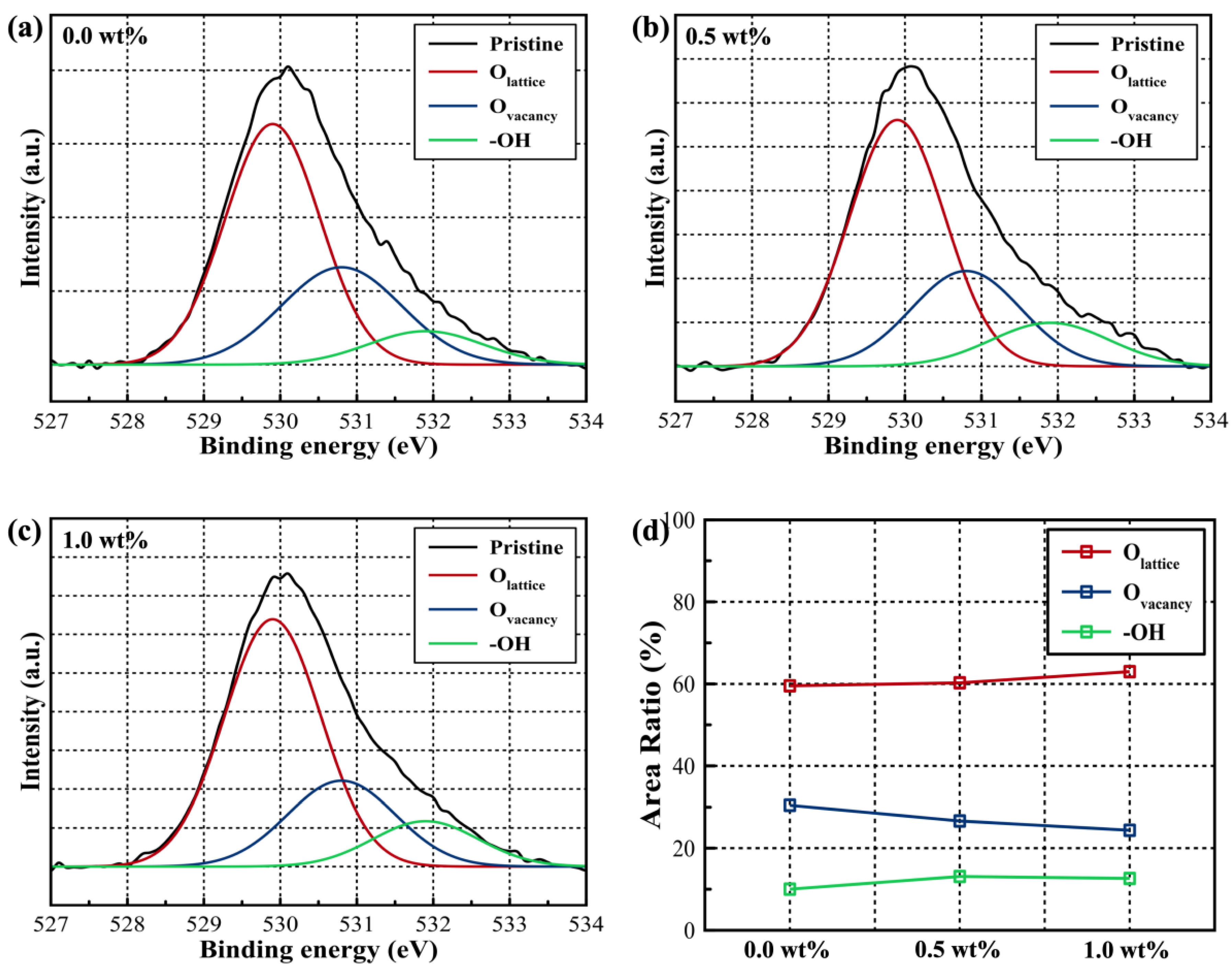

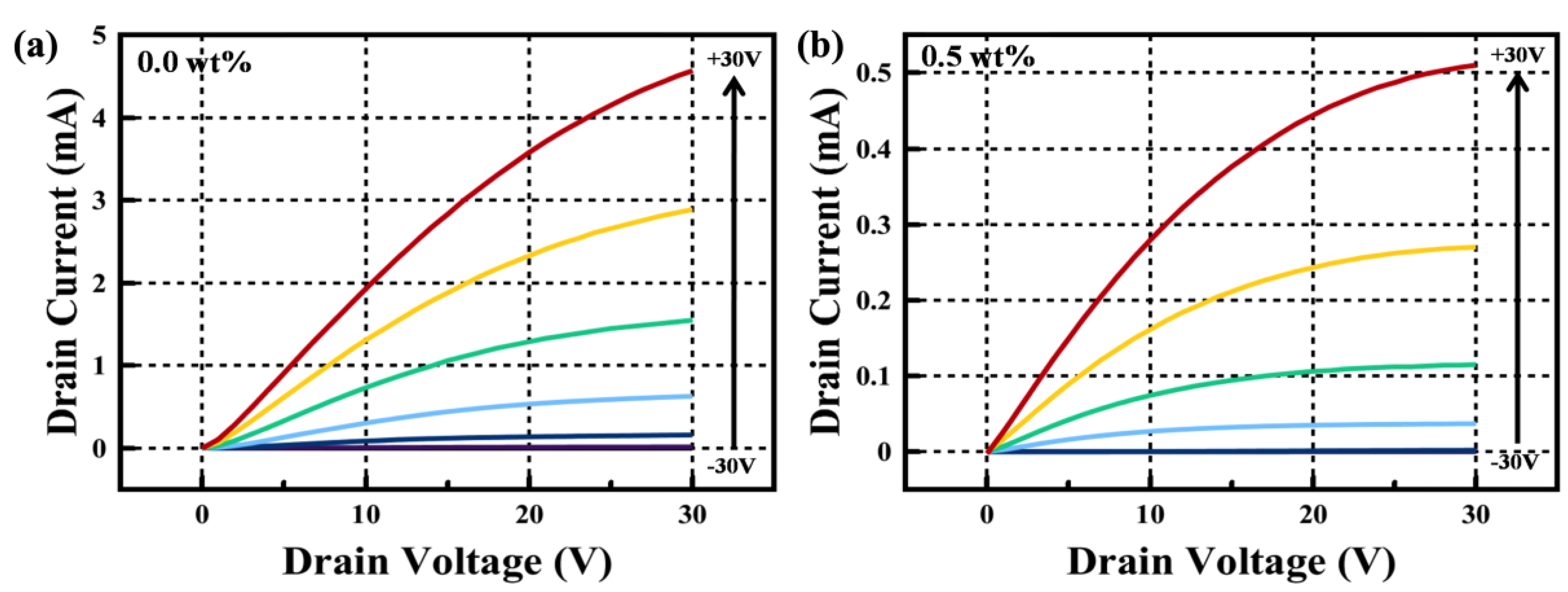
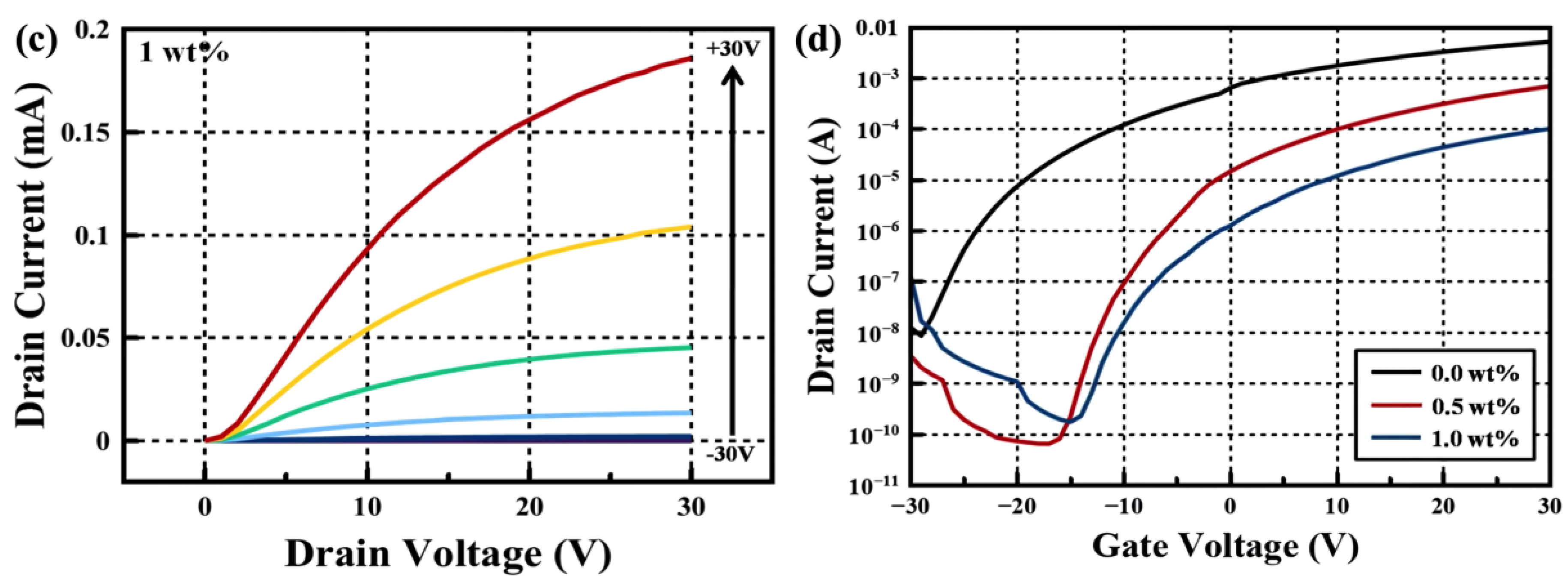



| Critical Angle (Degree) | Density (g/cm3) | Crystallite size (nm) | |
|---|---|---|---|
| 0.0 wt% | 0.261 | 3.70 | 7.68 |
| 0.5 wt% | 0.286 | 4.43 | 7.83 |
| 1.0 wt% | 0.278 | 4.18 | 8.36 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, W.-Y.; Lee, H.; Ha, S.; Lee, C.; Bae, J.-H.; Kang, I.-M.; Kim, K.; Jang, J. Effect of Mg Doping on the Electrical Performance of a Sol-Gel-Processed SnO2 Thin-Film Transistor. Electronics 2020, 9, 523. https://doi.org/10.3390/electronics9030523
Lee W-Y, Lee H, Ha S, Lee C, Bae J-H, Kang I-M, Kim K, Jang J. Effect of Mg Doping on the Electrical Performance of a Sol-Gel-Processed SnO2 Thin-Film Transistor. Electronics. 2020; 9(3):523. https://doi.org/10.3390/electronics9030523
Chicago/Turabian StyleLee, Won-Yong, Hyunjae Lee, Seunghyun Ha, Changmin Lee, Jin-Hyuk Bae, In-Man Kang, Kwangeun Kim, and Jaewon Jang. 2020. "Effect of Mg Doping on the Electrical Performance of a Sol-Gel-Processed SnO2 Thin-Film Transistor" Electronics 9, no. 3: 523. https://doi.org/10.3390/electronics9030523
APA StyleLee, W.-Y., Lee, H., Ha, S., Lee, C., Bae, J.-H., Kang, I.-M., Kim, K., & Jang, J. (2020). Effect of Mg Doping on the Electrical Performance of a Sol-Gel-Processed SnO2 Thin-Film Transistor. Electronics, 9(3), 523. https://doi.org/10.3390/electronics9030523







