Thermal Analysis of a Parallel-Configured Battery Pack (1S18P) Using 21700 Cells for a Battery-Powered Train
Abstract
1. Introduction
2. Electro-Thermal Model of a Lithium-Ion Battery and Battery Pack
2.1. Lithium-Ion Battery Heat Generation
2.2. Heat Dissipation
2.2.1. Forced Convection on Cylinder in Cross Flow
2.2.2. Forced Convection on Flat Plate, Averaged Transfer
3. Experiment Setup
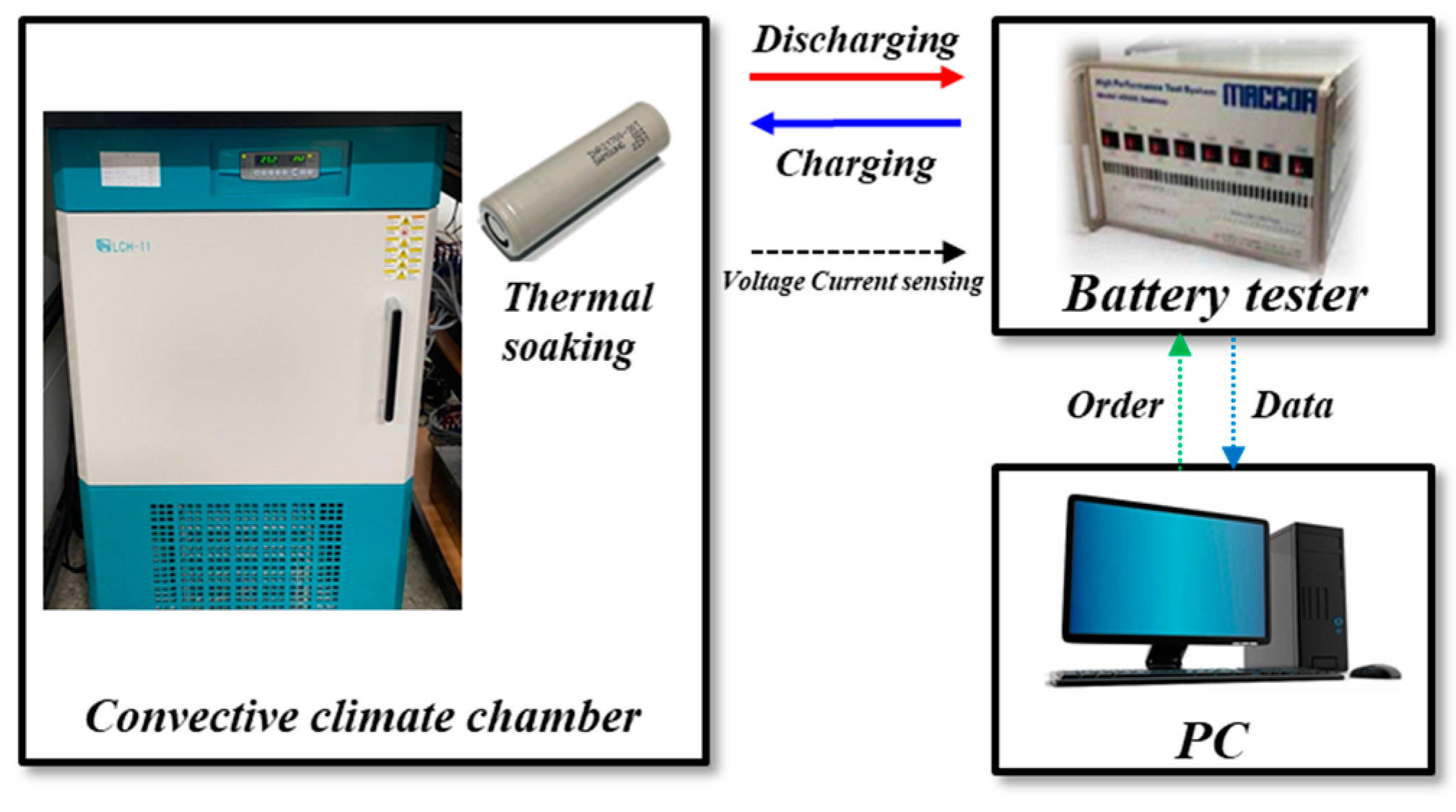
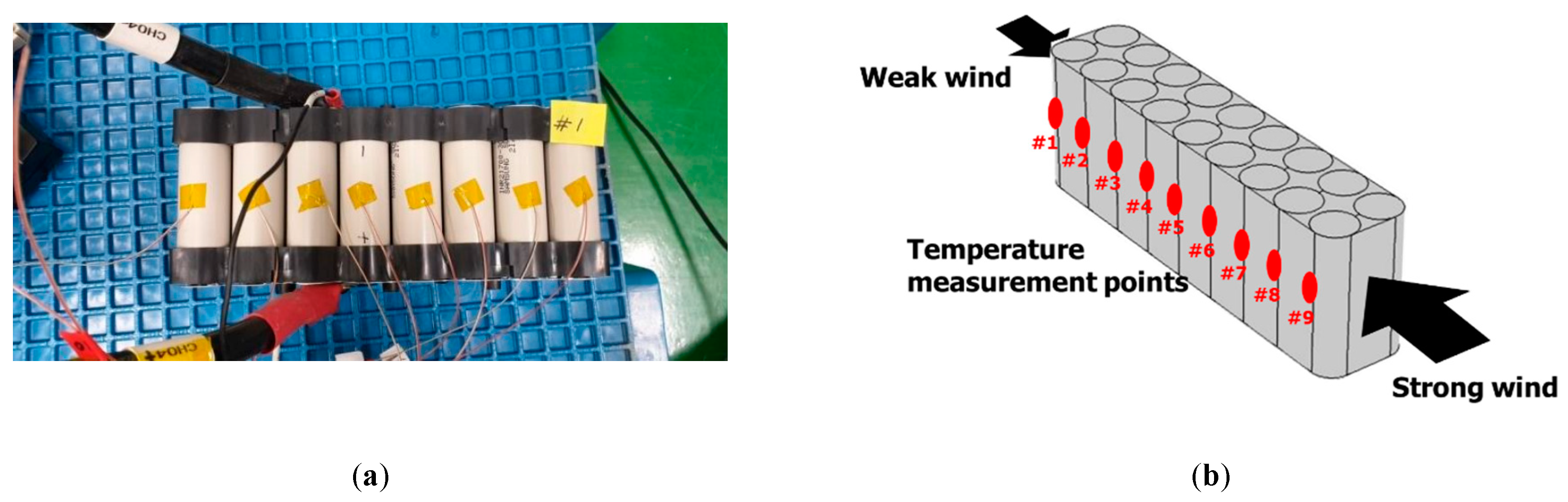
3.1. Cell Level Experiment Setup
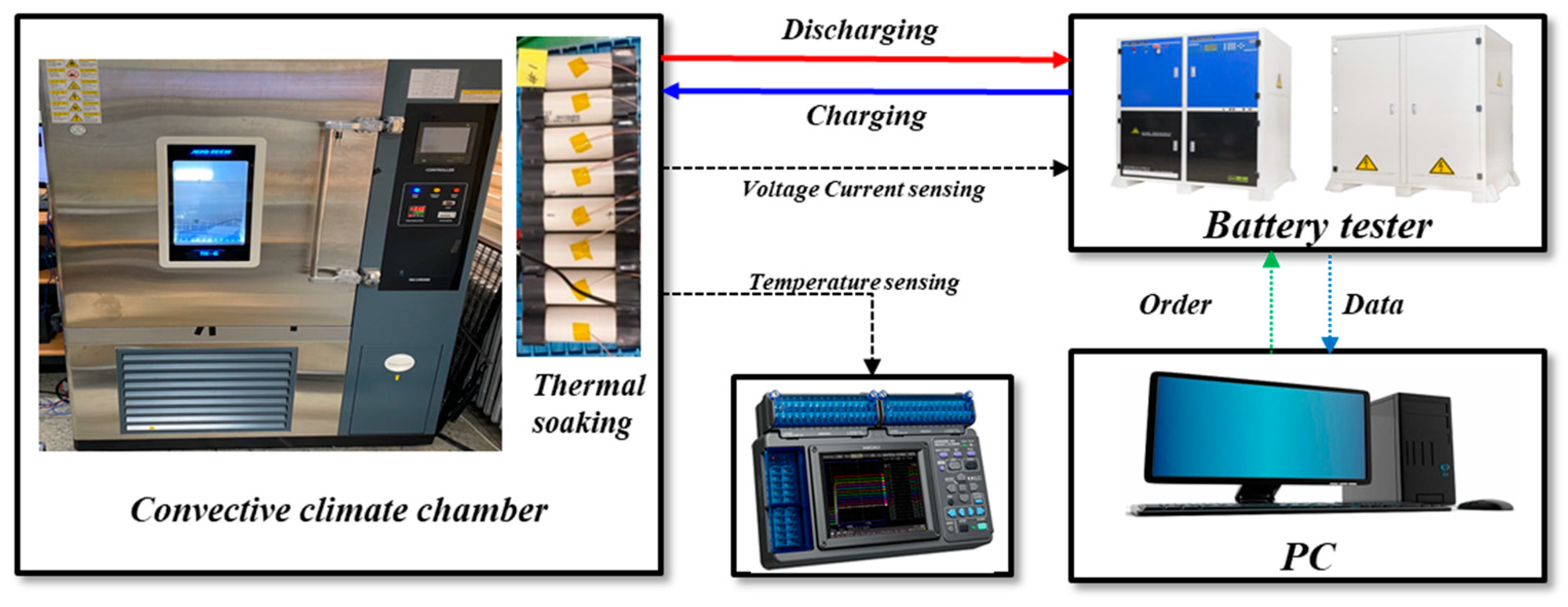
3.2. Pack Level Experiment Setup
4. Electrical Characteristic Test
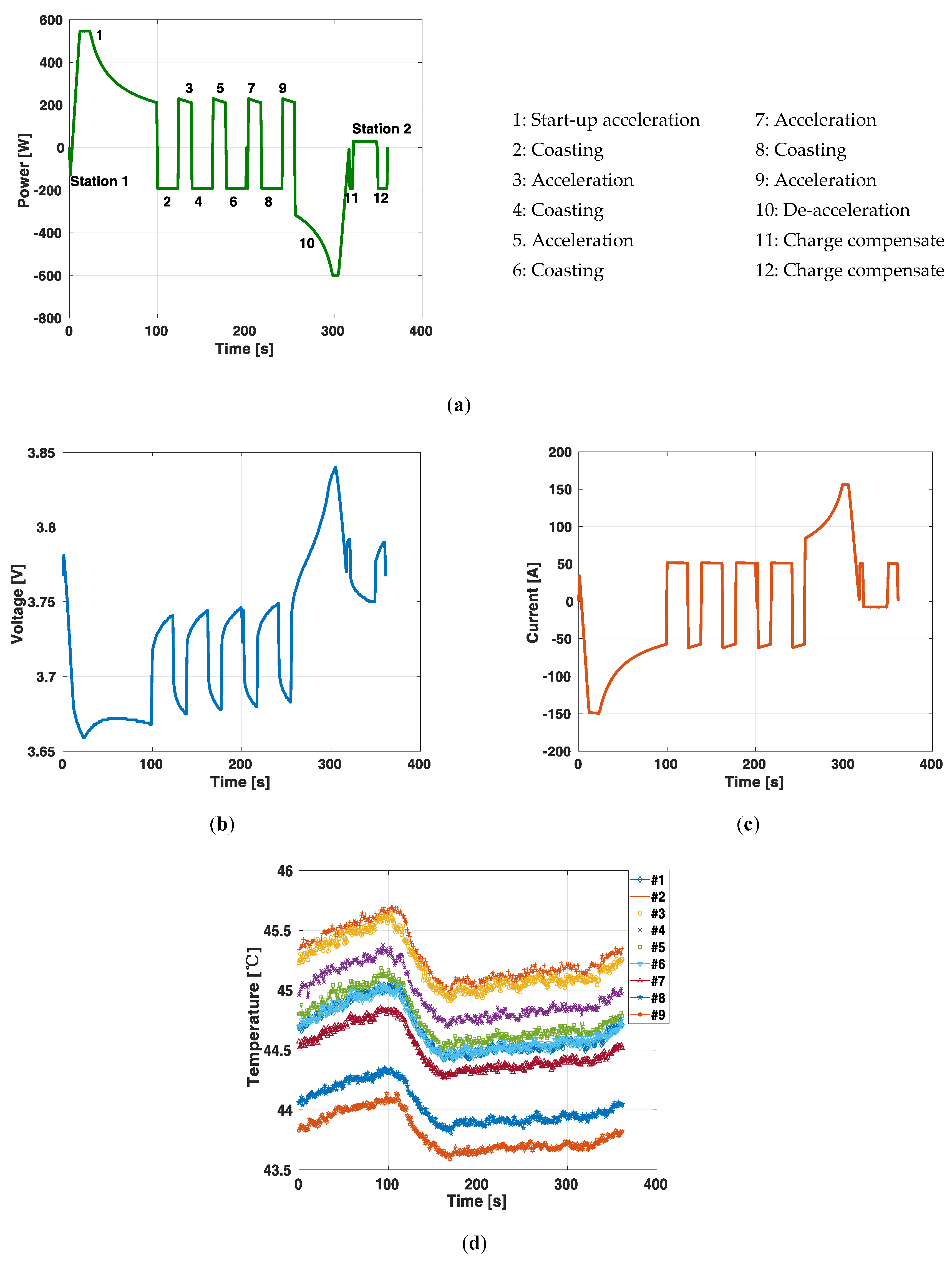
5. Battery Pack Train Propulsion Load Profile Experiment
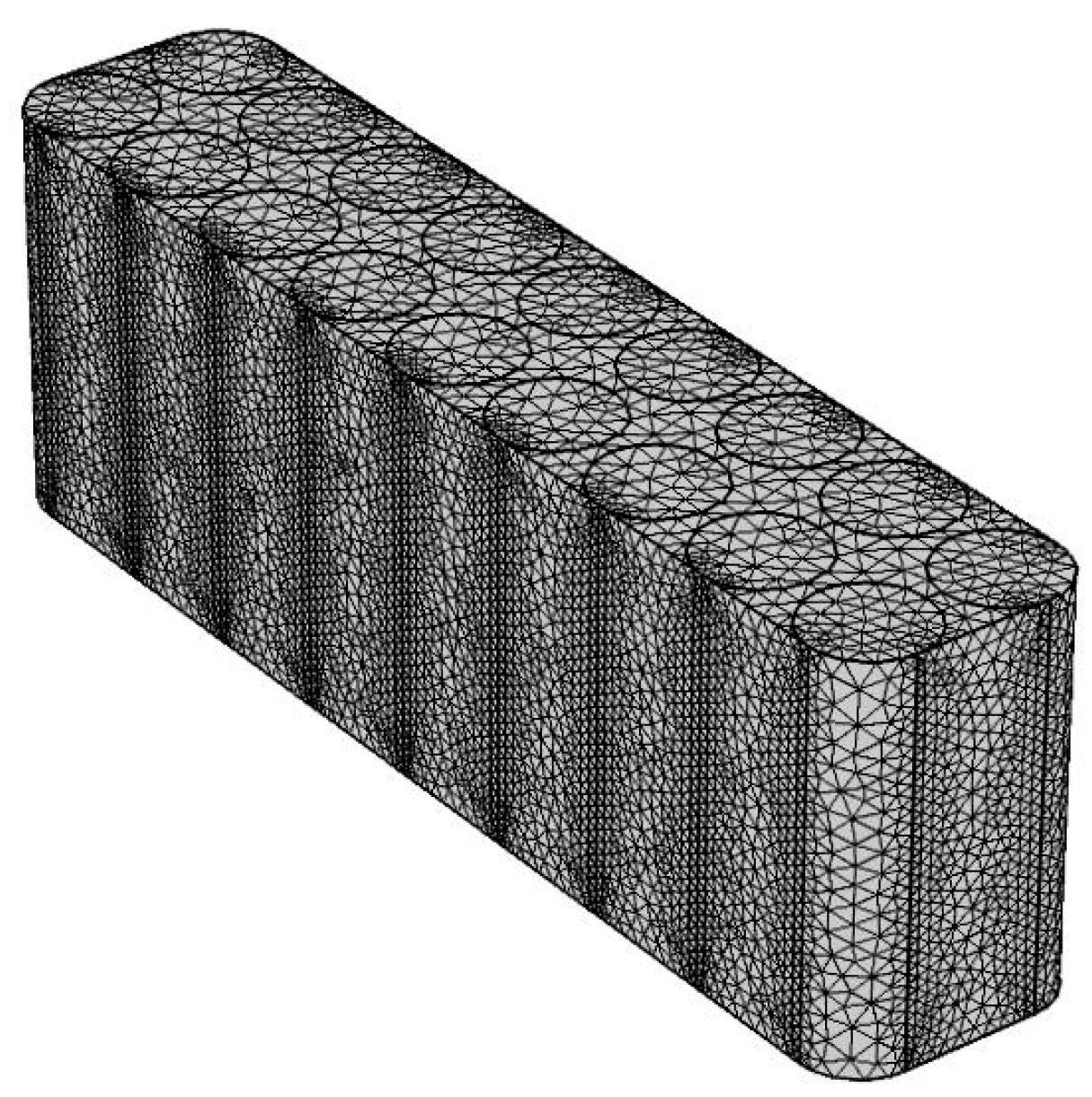
6. Mesh Generation
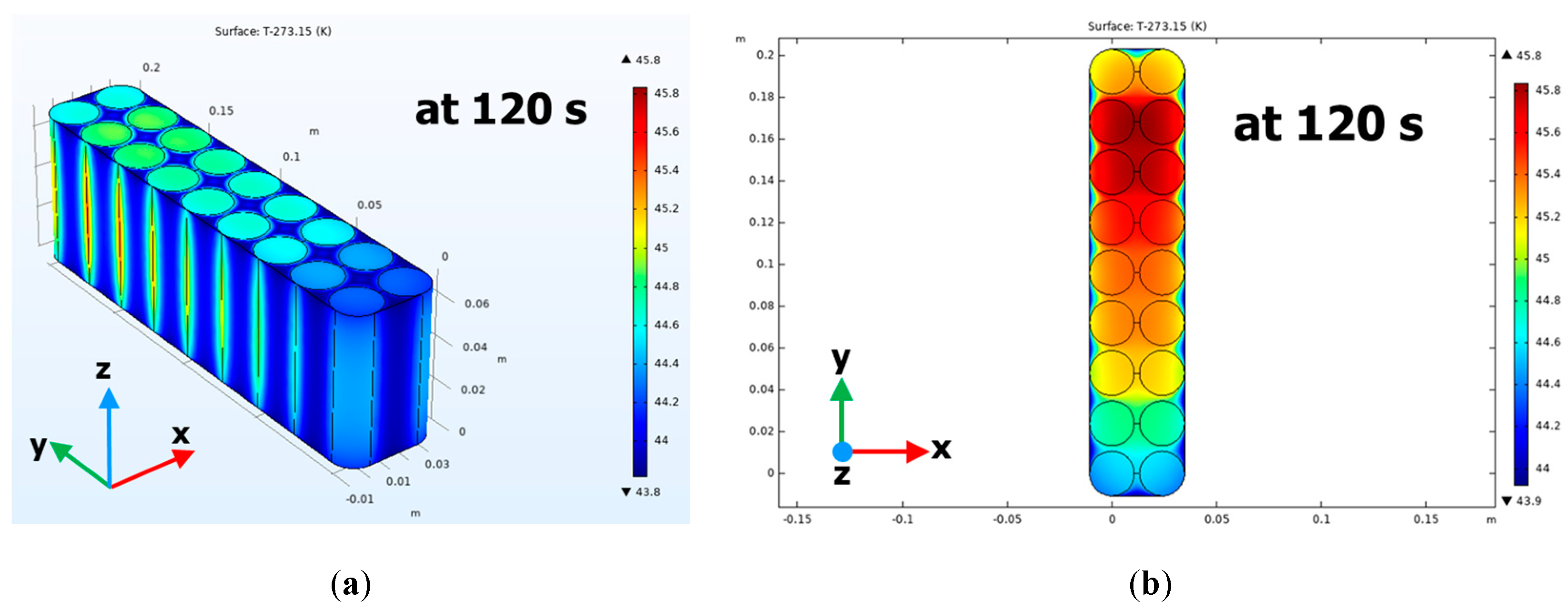
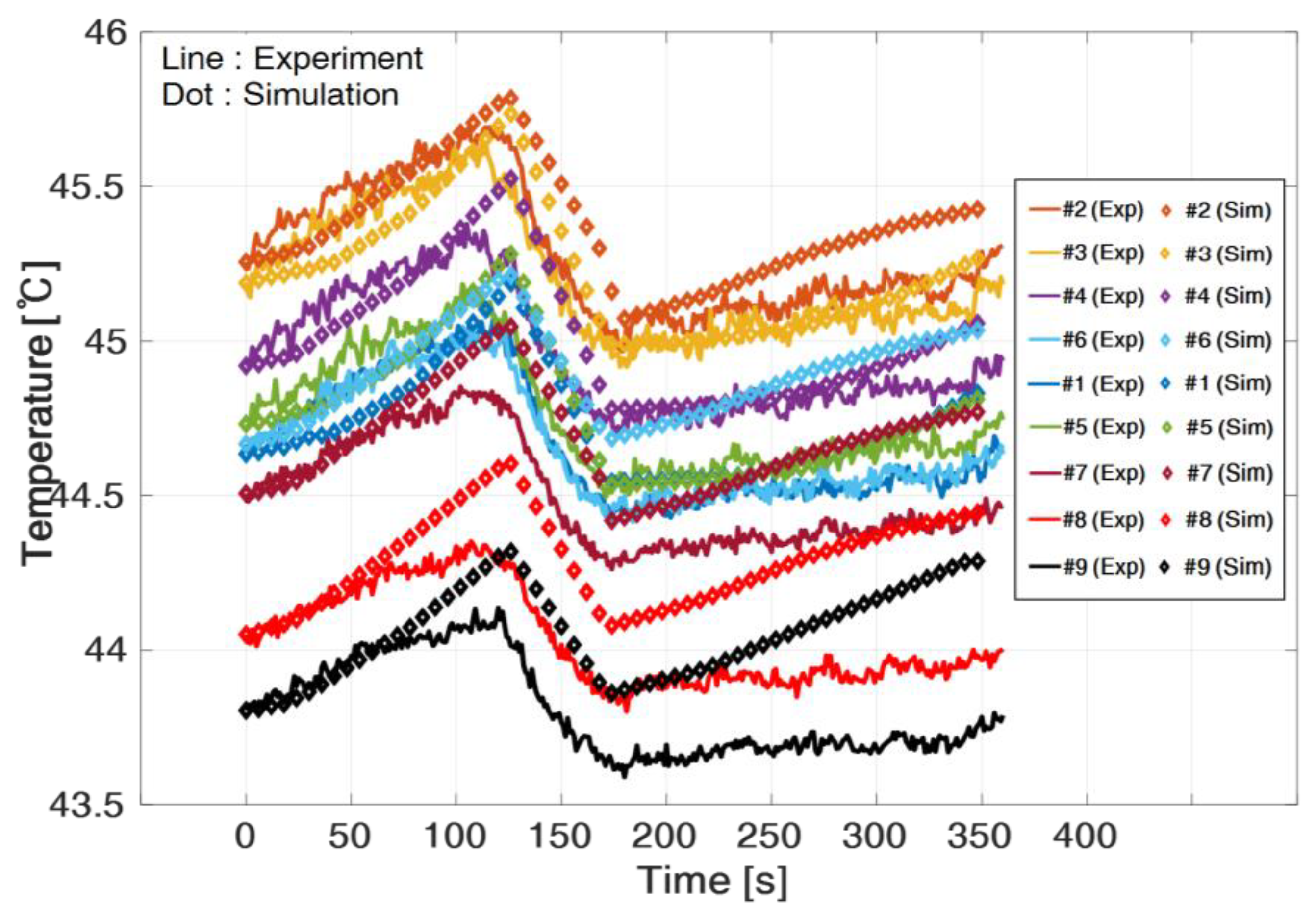
7. Simulation Results and Validation
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sato, N. Thermal behavior analysis of lithium-ion batteries for electric and hybrid vehicles. J. Power Sources 2001, 99, 70–77. [Google Scholar] [CrossRef]
- Bandhauer, T.M.; Garimella, S.; Fuller, T.F. A critical review of thermal issues in lithium-ion batteries. J. Electrochem. Soc. 2011, 3, R1–R25. [Google Scholar] [CrossRef]
- Lisbono, D.; Snee, T. A review of hazards associated with primary lithium and lithium-ion batteries. Process Saf. Environ. Prot. 2011, 89, 434–442. [Google Scholar] [CrossRef]
- Pals, C.R.; Newman, J. Thermal Modeling of the Lithium/Polymer Battery: I. Discharge Behavior of a Single Cell. J. Electrochem. Soc. 1995, 142, 3274. [Google Scholar] [CrossRef]
- Pals, C.R.; Newman, J. Thermal Modeling of the Lithium/Polymer Battery: II. Temperature Profiles in a Cell Stack. J. Electrochem. Soc. 1995, 142, 3282. [Google Scholar] [CrossRef]
- Santhanagopalan, S.; Guo, Q.; Ramadass, P.; White, R.E. Review of models for predicting the cycling performance of lithium ion batteries. J. Power Sources 2006, 152, 620–628. [Google Scholar] [CrossRef]
- Smith, K.; Wang, C.Y. Power and thermal characterization of a lithium-ion battery pack for hybrid-electric vehicles. J. Power Sources 2006, 160, 662–673. [Google Scholar] [CrossRef]
- Onda, K.; Ohshima, T.; Nakayama, M.; Fukuda, K.; Araki, T. Thermal behavior of small lithium-ion battery during rapid charge and discharge cycles. J. Power Sources 2006, 158, 535–542. [Google Scholar] [CrossRef]
- Rao, L.; Newman, J. Heat-generation rate and general energy balance for insertion battery system. J. Electrochem. Soc. 1997, 144, 2697–2704. [Google Scholar] [CrossRef]
- Lin, X.; Stefanopoulou, A.G.; Perez, H.E.; Siegel, J.B.; Li, Y.H.; Anderson, R.D. Quadruple adaptive observer of the core temperature in cylindrical Li-ion batteries and their health monitoring. In Proceedings of the 2012 American Control Conference, Montréal, QC, Canada, 27–29 June 2012; pp. 578–583. [Google Scholar]
- Akhoundzadeh, M.H.; Raahemifar, K.; Panchal, S.; Samadani, E.; Haghi, E.; Fraser, R.; Fowler, M. A conceptualized hydrail powertrain: A case study of the Union Pearson Express route. World Electr. Veh. J. 2019, 10, 32. [Google Scholar] [CrossRef]
- Panchal, S.; Dincer, I.; Agelin-Chaab, M.; Fraser, R.; Fowler, M. Design and simulation of a lithium-ion battery at large C-rate and varying boundary conditions through heat flux distributions. Measurement 2018, 116, 382–390. [Google Scholar] [CrossRef]
- Panchal, S.; Rashid, M.; Long, F.; Mathew, M.; Fraser, R.; Fowler, M. Degradation testing and modeling of 200 Ah LiFePO4 battery. SAE Techn. Pap. 2018. [Google Scholar] [CrossRef]
- Viswanathan, V.V.; Choi, D.; Wang, D.; Xu, W.; Towne, S.; Williford, R.E.; Zhang, J.G.; Liu, J.; Yang, Z. Effect of entropy change of lithium intercalation in cathodes and anodes on Li-ion battery thermal management. J. Power Sources 2010, 195, 3720–3729. [Google Scholar] [CrossRef]
- Lyu, Y.; Siddique, A.R.M.; Majid, S.H.; Biglarbegian, M.; Gadsden, S.A.; Mahmud, S. Electrical vehicle battery thermal management system with thermoelectric cooling. J. Energy Rep. 2019, 5, 822–827. [Google Scholar] [CrossRef]
- Huang, H.H.; Chen, H.Y.; Liao, K.C.; Young, H.T.; Lee, C.F.; Tien, J.Y. Thermal-electrochemical coupled simulations for cell-to-cell imbalances in lithium-iron-phosphate based battery packs. Appl. Therm. Eng. 2017, 123, 584–591. [Google Scholar] [CrossRef]
- Saito, Y.; Kanari, K.; Takano, K. Thermal studies of a lithium-ion battery. J. Power Sources 1997, 68, 451–454. [Google Scholar] [CrossRef]
- Zhao, R.; Gu, J.; Liu, J. An investigation on the significance of reversible heat to the thermal behavior of lithium ion battery through simulations. J. Power Sources 2014, 266, 422–432. [Google Scholar] [CrossRef]
- Lai, Y.; Du, L.; Ai, L.; Cheng, Y.; Tang, T.; Jia, M. Insight into heat generation of lithium ion batteries based on the electrochemical-thermal model at high discharge rates. Int. J. Hydrog. Energy 2015, 40, 13039–13049. [Google Scholar] [CrossRef]
- Davidzon, M.I. Newton’s law of cooling and its interpretation. Int. J. Heat Mass Transf. 2012, 55, 5397–5402. [Google Scholar] [CrossRef]
- Tong, W.; Somasundaram, K.; Bigersson, E.; Mujumdar, A.S.; Yap, C. Thermo-electrochemical model for forced convection air cooling of a lithium-ion battery module. Appl. Therm. Eng. 2016, 99, 672–682. [Google Scholar] [CrossRef]
- Yoo, K.; Kim, J. Thermal behavior of full-scale battery pack based on comprehensive heat-generation model. J. Power Sources 2019, 433, 226715. [Google Scholar] [CrossRef]
- Homan, J.P. Heat Transfer, 7th ed.; McGraw Hill Book Company: New York, NY, USA, 1990; pp. 281–303. [Google Scholar]
- Schlichting, H.; Gersten, K. Boundary Layer Theory, 8th ed.; Springer: Berlin, Germany, 2000; pp. 3–33. [Google Scholar]
- Werner, D.; Loges, A.; Becker, D.J.; Wetzel, T. Thermal conductivity of Li-ion batteries and their electrode configurations—A novel combination modelling and experimental approach. J. Power Sources 2017, 364, 72–83. [Google Scholar] [CrossRef]
- Pennstate College of Engineering. Available online: https://www.me.psu.edu/cimbala/me433/Links/Table_A_9_CC_Properties_of_Air.pdf (accessed on 25 February 2020).
- Drake, S.J. Thermal Conduction and Heat Generation Phenomena in Li-Ion Cells. Ph. D. Thesis, The University of Texas at Arlington, Arlington, TX, USA, December 2014. [Google Scholar]
- Idaho National Laboratory. Available online: https://avt.inl.gov/sites/default/files/pdf/battery/uncertainty_report_vol2_03_03.pdf (accessed on 3 March 2020).
- Imrbatteries. Available online: https://www.imrbatteries.com/content/samsung_30T.pdf (accessed on 26 February 2020).
- Barai, A.; Uddin, K.; Widanage, W.D.; Mcgordon, A.; Jennings, P. A study of the influence of measurement timescale on internal resistance characterization methodologies for lithium-ion cells. Nat. Sci. Rep. 2018, 8, 21. [Google Scholar] [CrossRef] [PubMed]
- Kang, T.; Yoo, K.; Kim, J. Irreversible heat generation based electro-thermal modeling and its experiment validation of the 18,650 cylindrical cells. In Proceedings of the 10th International Conference on Power Electronics-ECCE Asia, BEXCO, Busan, Korea, 27–30 May 2019. [Google Scholar]
- Ruiz, V.; Pfrang, A.; Kriston, A.; Omar, A.; Van den Bossche, P.; Boon-Brett, L. A review of international abuse testing standards and regulations for lithium ion batteries in electric and hybrid electric vehicles. Renew. Sustain. Energy Rev. 2018, 81, 142–145. [Google Scholar] [CrossRef]
- Batterystandards.info. Available online: https://www.batterystandards.info/sites/batterystandards.info/files/general_overview_part1.pdf (accessed on 23 January 2020).
- Batterystandards.info. Available online: https://www.batterystandards.info/sites/batterystandards.info/files/general_overview_part2.pdf (accessed on 23 January 2020).
- Idaho National Laboratory. Available online: https://avt.inl.gov/sites/default/files/pdf/battery/usabc_manual_rev2.pdf (accessed on 23 January 2020).
- United States Environment Protection Agency. Available online: https://www.epa.gov/vehicle-and-fuel-emissions-testing/dynamometer-drive-schedules (accessed on 23 January 2020).
- The International Council on Clean Transportation. Available online: https://theicct.org/sites/default/files/publications/ICCT_EU_fuelconsumption2_workingpaper_2012.pdf (accessed on 23 January 2020).
- The International Council on Clean Transportation. Available online: https://theicct.org/sites/default/files/publications/ICCT_LaboratoryToRoad_2014_Report_English.pdf (accessed on 23 January 2020).


| Abbreviation | Definition |
|---|---|
| EV | Electric vehicle |
| SOC | State of charge |
| NMC | LiNiMnCoO2 |
| HPPC | Hybrid pulse power characterization |
| CC-CV | Constant current-constant voltage |
| DTS | Dynamic stress test |
| UDDS | Urban dynamometer driving schedule |
| NEDC | New European driving cycle |
| 21700 | Cylindrical lithium-ion battery dimension |
| Symbol | Description |
|---|---|
| Heat measured by thermocouple | |
| Qcell | Total generated heat in a lithium ion-battery |
| Joule heating | |
| Qrev | Reversible heating |
| Heat dissipation | |
| i | Load current |
| Req | Equivalent resistance |
| Ohmic resistance | |
| Diffusion resistance | |
| Tcell | Battery temperature |
| ΔS | Entropy change |
| n | Number of electrons |
| F | Faraday constant |
| h | Heat transfer coefficient |
| A | Heat transfer area |
| D | Diameter of cylinder |
| L | Length of plate |
| Nu | Nusselt number |
| Pr | Prandtl number |
| Re | Reynolds number |
| C and r | Unit less correlation variables for Re calculation |
| Re | C | r |
|---|---|---|
| 0.4–4 | 0.989 | 0.330 |
| 4–40 | 0.911 | 0.385 |
| 40–4000 | 0.683 | 0.466 |
| 4000–40,000 | 0.193 | 0.618 |
| 40,000–40,0000 | 0.0266 | 0.805 |
| Parameter | Value | Unit | |
|---|---|---|---|
| Battery | Density | 2615.7 | kg/m3 |
| Specific heat | 1605 | J/kg-K | |
| Thermal conductivity | 3 | W/m-K | |
| Air (at 45 °C) | Kinematic viscosity, v | 19.3979 × 10−6 | m2/s |
| Thermal diffusivity, a | 24.165 × 10−6 | m2/s | |
| Thermal conductivity, k | 27.44 × 10−3 | W/m-K | |
| Velocity (strong wind), Ud | 5 (front), 1 (back) | m/s |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, T.; Park, S.; Lee, P.-Y.; Cho, I.-H.; Yoo, K.; Kim, J. Thermal Analysis of a Parallel-Configured Battery Pack (1S18P) Using 21700 Cells for a Battery-Powered Train. Electronics 2020, 9, 447. https://doi.org/10.3390/electronics9030447
Kang T, Park S, Lee P-Y, Cho I-H, Yoo K, Kim J. Thermal Analysis of a Parallel-Configured Battery Pack (1S18P) Using 21700 Cells for a Battery-Powered Train. Electronics. 2020; 9(3):447. https://doi.org/10.3390/electronics9030447
Chicago/Turabian StyleKang, Taewoo, Seongyun Park, Pyeong-Yeon Lee, In-Ho Cho, Kisoo Yoo, and Jonghoon Kim. 2020. "Thermal Analysis of a Parallel-Configured Battery Pack (1S18P) Using 21700 Cells for a Battery-Powered Train" Electronics 9, no. 3: 447. https://doi.org/10.3390/electronics9030447
APA StyleKang, T., Park, S., Lee, P.-Y., Cho, I.-H., Yoo, K., & Kim, J. (2020). Thermal Analysis of a Parallel-Configured Battery Pack (1S18P) Using 21700 Cells for a Battery-Powered Train. Electronics, 9(3), 447. https://doi.org/10.3390/electronics9030447






