Feature Selection Using Intelligent Agents for Time Improvement in Medical Diagnosis Systems
Abstract
1. Introduction
2. Materials and Methods
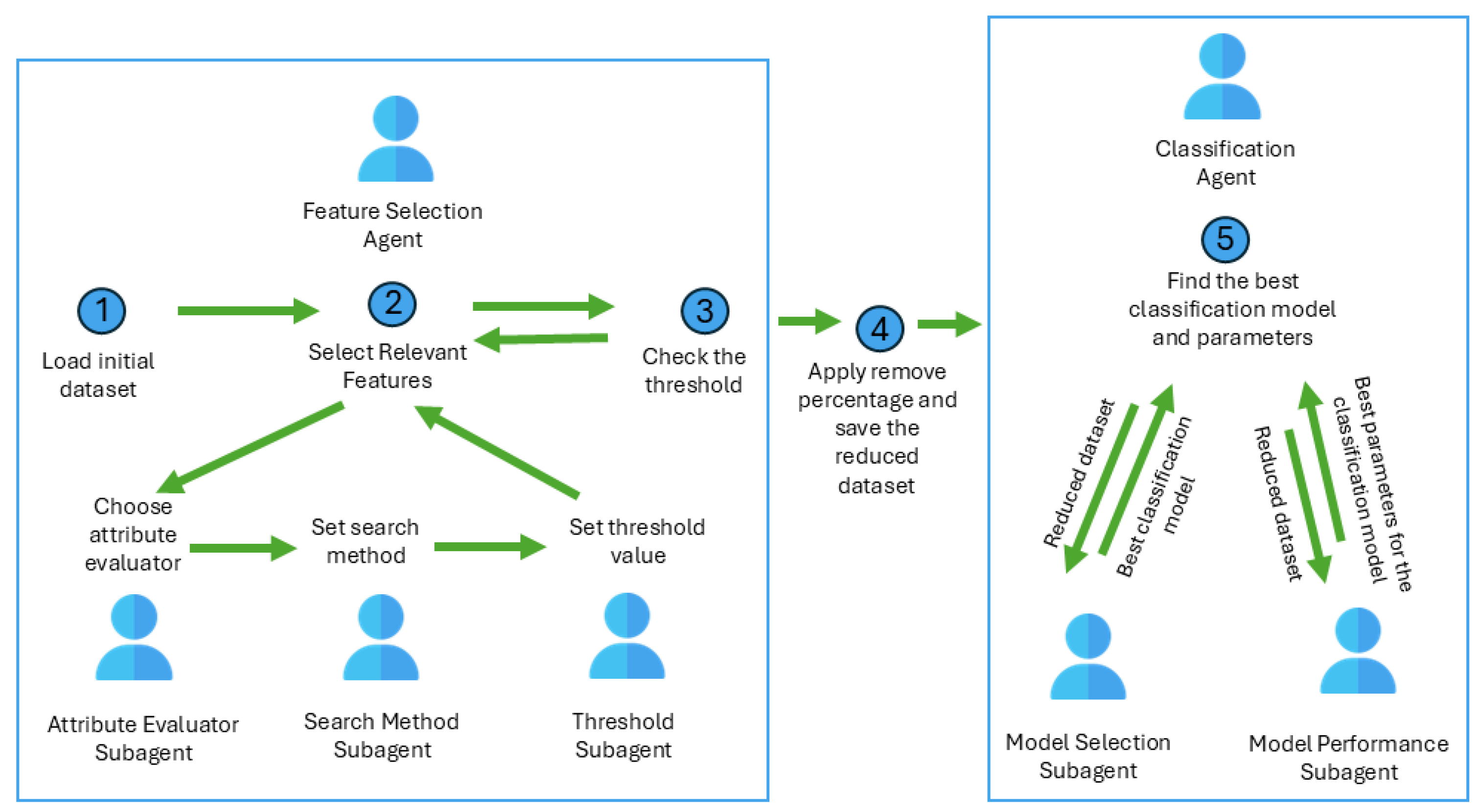
2.1. Intelligent Agents Based on Feature Selection for Time Improvement
- Loads the initial medical dataset;
- Applies different evaluation methods to compute the relevance of an attribute (InfoGain, GainRation, correlation, RelieF);
- Finds the optimum subset in terms of accuracy and time taken to build models (using the Ranker search method);
- Sends the reduced dataset to the Classification Agent.
- Receives the reduced dataset from the Feature Selection Agent;
- Finds the best model and the best model configuration;
- Sends the final model to the chatbot to be used for medical diagnosis.
- Applies Wrapper Subset Evaluation method to compute the relevance of an attribute.
- Uses search method (GreedyStepwise) for finding the relevant features that will be included in a specific subset of data.
- Divides the attributes into strong attributes and weak attributes;
- Evaluates each attribute as a class attribute;
- Applies different threshold values to decide if the attribute is relevant and will be kept in the subset;
- Deletes the weak attributes (below the threshold value).
- Learns the medical data with different models (Decision Trees, Naïve Bayes, Deep Learning);
- Chooses the best model considering the model performance in terms of accuracy and time.
- Finds the best model configuration;
- Send the optimum model to the system’s chatbot to be used in medical diagnosis.
- Behavior Attribute Selection
- load the dataset
- receive inform messages from the Threshold Subagent
- declare and instantiate a new InfoGain attribute selector
- declare and instantiate a new NaiveBayes classifier
- declare and instantiate the AttributeSelection object
- select the optimum discovered subset of attributes
- save the reduced dataset considering the threshold value
- send the inform message and reduced dataset to the Classification Agent
- Behavior Optimum Subset
- load the dataset
- receive inform messages from the Search Method Subagent
- declare and instantiate a new GreedyStepwise() search method
- declare and instantiate a new WrapperSubsetEval() evaluator
- declare and instantiate a new NaiveBayes classifier
- declare and instantiate the AttributeSelection object
- set the evaluator of AttributeSelection
- set the search method of AttributeSelection
- divide the attributes into strong attributes and weak attributes considering a threshold value
- evaluate each attribute as a class
- delete the weak attributes
- select the optimum discovered subset of attributes
- send inform message to the Feature Selection Agent
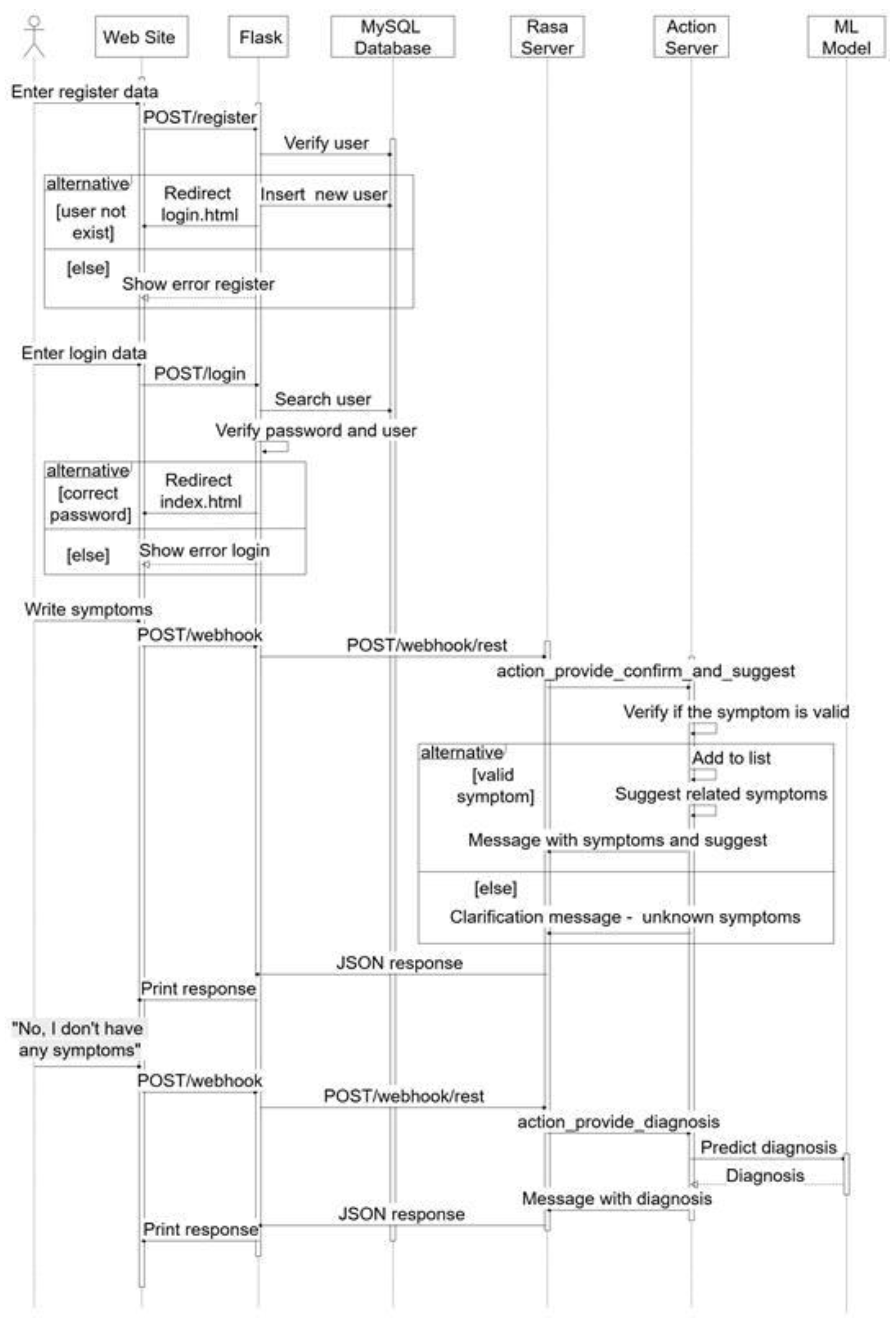
2.2. Medical Diagnosis System Using the Best Discovered Learning Model
- The user sends the symptoms via POST/webhook;
- The Flask Server forwards the POST/webhook/rest to the Rasa Server;
- Rasa Server runs action_provide_diagnosis_and_suggest:
- ○
- Checks if the symptom is valid.
- ✓
- If it is valid, add it to the list and suggest other symptoms.
- ✓
- Otherwise, it returns a message that the symptom has not been recognized.
- A JSON response is sent to the website with the symptoms and symptom suggestions.
- The user sends the message “No, I don’t have any symptoms” via POST/webhook.
- A new POST/webhook/rest call is made to the Rasa Server.
- action_provide_diagnosis shall be executed, that
- ○
- Sends the data to the model for prediction.
- ○
- Gets a diagnosis.
- ○
- Replies with a JSON message containing the diagnostic results.
3. Experimental Results
3.1. Symptom–Disease Prediction Dataset Description
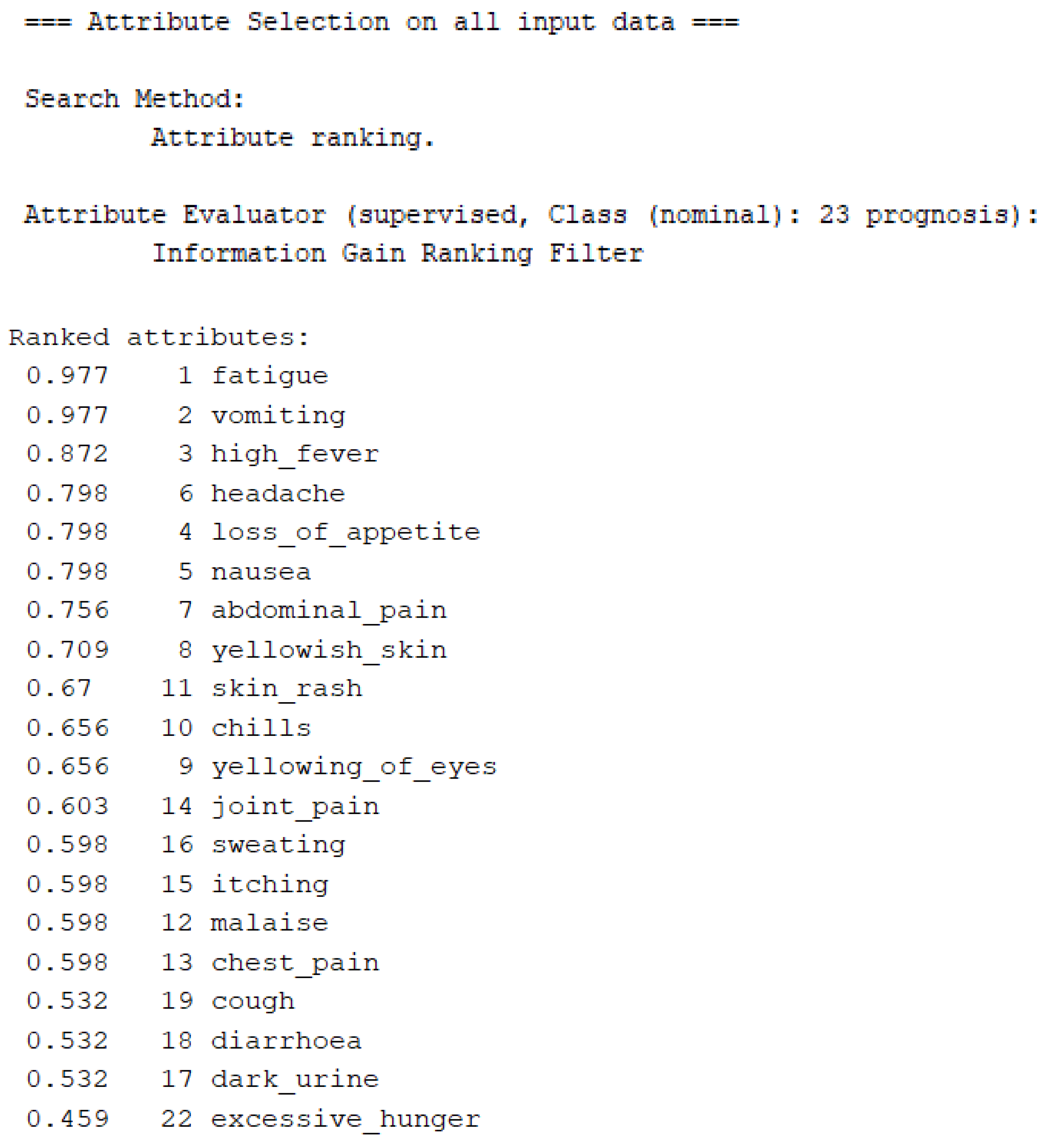
3.2. Feature Selection and Classification
- Attribute subset evaluator (supervised, class: 133 prognosis): Wrapper Subset Evaluator;
- Learning scheme: NaiveBayes;
- Subset evaluation: classification accuracy;
- Number of folds for accuracy estimation: 5;
- Search method: GreedyStepwise (forward);
- Start set: no attributes;
- Merit of best subsets found: 1;
- Validation method: 10-fold cross-validation (stratified);
- Seed: 1.
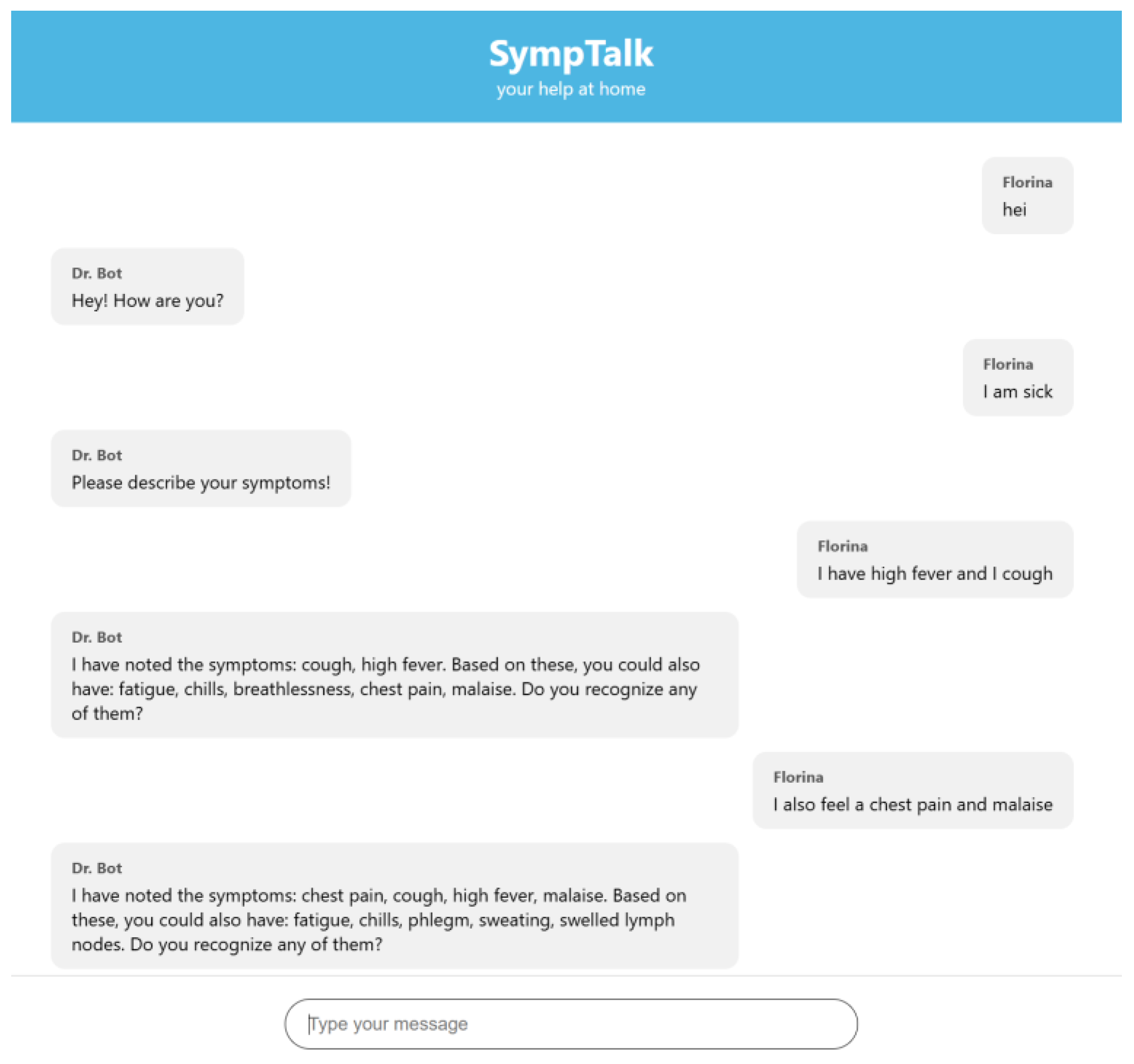
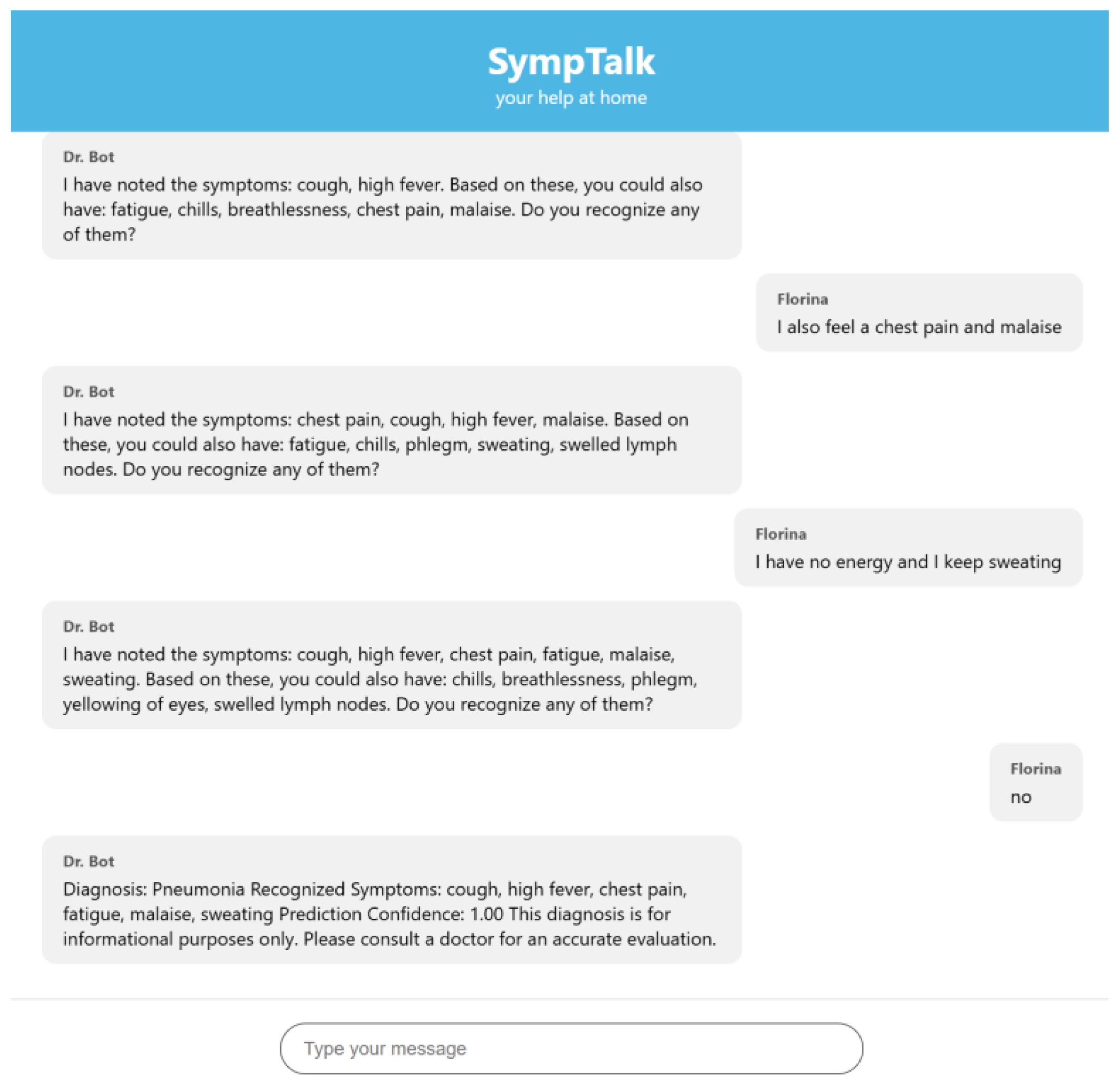
3.3. Testing the Chatbot-Based Naïve Bayes Classifier
- The user sends a message in the interface.
- The JavaScript in the index.html makes a POST to/webhook with the message.
- Flask takes the message and sends it to Rasa.
- Rasa analyzes the message and executes actions.
- Rasa sends a response (JSON) to Flask.
- Flask returns the response to the frontend.
- The frontend displays it in chat as a bot message.
4. Discussion
5. Conclusions and Future Developments
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A

References
- Almadhor, A.; Sampedro, G.A.; Abisado, M.; Abbas, S. Efficient Feature-Selection-Based Stacking Model for Stress Detection Based on Chest Electrodermal Activity. Sensors 2023, 23, 6664. [Google Scholar] [CrossRef] [PubMed]
- Haq, A.U.; Li, J.P.; Khan, J.; Memon, M.H.; Nazir, S.; Ahmad, S.; Khan, G.A.; Ali, A. Intelligent Machine Learning Approach for Effective Recognition of Diabetes in E-Healthcare Using Clinical Data. Sensors 2020, 20, 2649. [Google Scholar] [CrossRef] [PubMed]
- Płudowski, J.; Mulawka, J. Application of Artificial Intelligence in Support of NAFLD Diagnosis. Appl. Sci. 2024, 14, 10237. [Google Scholar] [CrossRef]
- Alyasin, E.I.; Ata, O.; Mohammedqasim, H.; Mohammedqasem, R. Enhancing Self-Care Prediction in Children with Impairments: A Novel Framework for Addressing Imbalance and High Dimensionality. Appl. Sci. 2024, 14, 356. [Google Scholar] [CrossRef]
- Qtaish, A.; Albashish, D.; Braik, M.; Alshammari, M.T.; Alreshidi, A.; Alreshidi, E.J. Memory-Based Sand Cat Swarm Optimization for Feature Selection in Medical Diagnosis. Electronics 2023, 12, 2042. [Google Scholar] [CrossRef]
- Bravo, F.P.; García, A.A.D.B.; Russo, L.M.S.; Ayala, J.L. SOFIA: Selection of Medical Features by Induced Alterations in Numeric Labels. Electronics 2020, 9, 1492. [Google Scholar] [CrossRef]
- Pathan, M.S.; Nag, A.; Pathan, M.M.; Dev, S. Analyzing the Impact of Feature Selection on the Accuracy of Heart Disease Prediction. Healthc. Anal. 2022, 2, 100060. [Google Scholar] [CrossRef]
- Ghourabi, M.; Mourad-Chehade, F.; Chkeir, A. Eye Recognition by YOLO for Inner Canthus Temperature Detection in the Elderly Using a Transfer Learning Approach. Sensors 2023, 23, 1851. [Google Scholar] [CrossRef] [PubMed]
- Ilieva, G. Extension of Interval-Valued Hesitant Fermatean Fuzzy TOPSIS for Evaluating and Benchmarking of Generative AI Chatbots. Electronics 2025, 14, 555. [Google Scholar] [CrossRef]
- Muntean, M.V. Real-Time Detection of IoT Anomalies and Intrusion Data in Smart Cities Using Multi-Agent System. Sensors 2024, 24, 7886. [Google Scholar] [CrossRef] [PubMed]
- Muntean, M.V. Multi-Agent System for Intelligent Urban Traffic Management Using Wireless Sensor Networks Data. Sensors 2022, 22, 208. [Google Scholar] [CrossRef] [PubMed]
- Sales Mendes, A.F.; Sánchez San Blas, H.; Pérez Robledo, F.; De Paz Santana, J.F.; Villarrubia González, G. A Novel Multiagent System for Cervical Motor Control Evaluation and Individualized Therapy: Integrating Gamification and Portable Solutions. Multimed. Syst. 2024, 30, 131. [Google Scholar] [CrossRef]
- Sánchez San Blas, H.; Sales Mendes, A.; de la Iglesia, D.H.; Silva, L.A.; Villarrubia González, G. A Multiagent Platform for Promoting Physical Activity and Learning through Interactive Educational Games Using the Depth Camera Recognition System. Entertain. Comput. 2024, 49, 100629. [Google Scholar] [CrossRef]
- Chen, X.; Yi, H.; You, M.; Liu, W.; Wang, L.; Li, H.; Zhang, X.; Guo, Y.; Fan, L.; Chen, G.; et al. Enhancing diagnostic capability with multi-agents conversational large language models. npj Digit. Med. 2025, 8, 159. [Google Scholar] [CrossRef] [PubMed]
- The Weka Workbench. Available online: https://ml.cms.waikato.ac.nz/weka (accessed on 19 May 2025).
- Frank, E.; Hall, M.A.; Witten, I.H. The WEKA Workbench. Online Appendix for “Data Mining: Practical Machine Learning Tools and Techniques”, 4th ed.; Morgan Kaufmann: San Francisco, CA, USA, 2016. [Google Scholar]
- Java Agent DEvelopment Framework. Available online: https://jade.tilab.com/ (accessed on 19 May 2025).
- Muntean, M.; Vălean, H.; Joldeș, R.; Ceuca, E. Feature Selection for Classifier Accuracy Improvement; No. 26/2011; Acta Universitatis Apulensis: Alba Iulia, Romania, 2011; pp. 203–216. [Google Scholar]
- Rasa. Available online: https://legacy-docs-oss.rasa.com/docs/rasa/ (accessed on 19 May 2025).
- MySQL. Available online: https://www.mysql.com/ (accessed on 19 May 2025).
- Tucker, J. “SymbiPredict”, Mendeley Data, V1. 2024. Available online: https://data.mendeley.com/datasets/dv5z3v2xyd/1 (accessed on 19 May 2025).
- Kira, K.; Rendell, L.A. A Practical Approach to Feature Selection. In Proceedings of the Ninth International Workshop on Machine Learning, Aberdeen, UK, 1–3 July 1992; pp. 249–256. [Google Scholar]
- Robnik-Sikonja, M.; Kononenko, I. An adaptation of Relief for attribute estimation in regression. In Proceedings of the Fourteenth International Conference on Machine Learning, Nashville, TN, USA, 8–12 July 1997; pp. 296–304. [Google Scholar]
- Flask. Available online: https://flask.palletsprojects.com/en/stable/ (accessed on 19 May 2025).
- Mama, E.; Sheri, L.; Aperstein, Y.; Apartsin, A. From Fuzzy Speech to Medical Insight: Benchmarking LLMs on Noisy Patient Narratives. arXiv 2025, arXiv:2509.11803. [Google Scholar] [CrossRef]
- Ferhi, L.A.; Amar, M.B.; Masmoudi, A.; Choubani, F.; Bouallegue, R. Improving Symptom-Based Medical Diagnosis Using Ensemble Learning Approaches. Syst. Res. Behav. Sci. 2025, 42, 1294–1321. [Google Scholar]
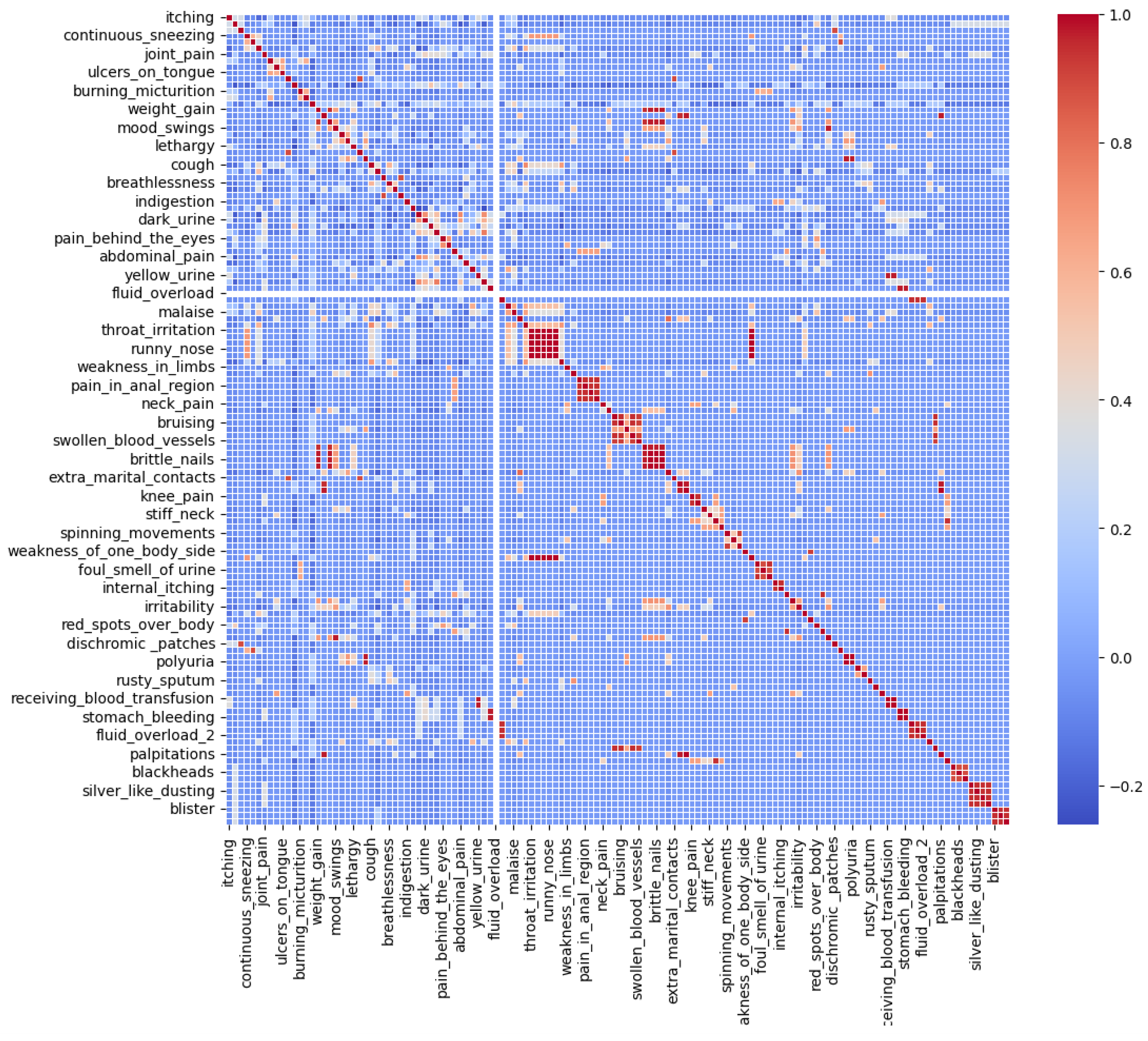


| Class Attribute | Related Attributes | Attribute Importance | Attribute Label |
|---|---|---|---|
| itching | nodal_skin_eruptions | 100% | strong |
| skin_rash | nodal_skin_eruptions, pus_filled_pimples, skin_peeling, blister | 100% | strong |
| nodal_skin_eruptions | - | 0% | weak |
| cough | chills | 100% | strong |
| chills | cough, yellowish_skin, malaise | 100% | strong |
| joint_pain | acute_liver_failure, skin_peeling | 100% | strong |
| stomach_pain | - | 0% | weak |
| muscle_wasting | - | 0% | weak |
| vomiting | yellowing_of_eyes, nausea, abdominal_pain | 100% | strong |
| dark_urine | bladder_discomfort | 100% | strong |
| fatigue | weight_gain, weight_loss, acute_liver_failure, malaise, cramps | 100% | strong |
| weight_gain | - | 0% | weak |
| weight_loss | - | 0% | weak |
| high_fever | chills, muscle_wasting, acute_liver_failure, blister | 100% | strong |
| malaise | 90% | ||
| skin_rash, stomach_pain, fatigue, breathlessness, headache, yellowish_skin, blurred_and_distorted_vision | 10% | ||
| yellowing_of_eyes | vomiting | 100% | strong |
| breathlessness | - | 0% | weak |
| sweating | breathlessness | 100% | strong |
| excessive_hunger | headache | 100% | strong |
| headache | chills, blurred_and_distorted_vision, loss_of_balance | 100% | strong |
| weight_loss | 70% | ||
| breathlessness, neck_pain | 60% | ||
| sweating, excessive_hunger, nausea, diarrhoea, stiff_neck | 40% | ||
| yellowish_skin | 30% | ||
| joint_pain | 20% | ||
| itching | 10% | ||
| yellowish_skin | chills, excessive_hunger, abdominal_pain | 100% | strong |
| nausea | joint_pain, yellowish_skin, loss_of_appetite | 100% | strong |
| loss_of_appetite | joint_pain, yellowish_skin, nausea, mild_fever, malaise | 100% | strong |
| abdominal_pain | yellowish_skin, acute_liver_failure | 100% | strong |
| diarrhoea | yellowing_of_eyes | 100% | strong |
| mild_fever | - | 0% | weak |
| acute_liver_failure | - | 0% | weak |
| malaise | diarrhoea, mild_fever | 100% | strong |
| blurred_and_distorted_vision | - | 0% | weak |
| chest_pain | breathlessness | 100% | strong |
| neck_pain | - | 0% | weak |
| dizziness | vomiting, weight_gain, nausea, loss_of_balance | 100% | strong |
| cramps | - | 0% | weak |
| muscle_weakness | - | 0% | weak |
| stiff_neck | - | 0% | weak |
| loss_of_balance | fatigue, weight_gain, dizziness | 100% | strong |
| bladder_discomfort | - | 0% | weak |
| pus_filled_pimples | - | 0% | weak |
| skin_peeling | - | 0% | weak |
| blister | - | 0% | weak |
| Attribute Selector | Threshold | Accuracy (%) | Precision | Recall | F-Score |
|---|---|---|---|---|---|
| CorrelationAttributeEval | 0.09 | 87.643 | - | 0.876 | - |
| InfoGainAttributeEval | 0.02 | 98.508 | 0.987 | 0.985 | 0.985 |
| ReliefFAttributeEval | 0.06 | 98.508 | 0.987 | 0.985 | 0.985 |
| Classifier | Accuracy on Initial Dataset (ID) | Accuracy on Reduced Dataset (RD) |
|---|---|---|
| J48 (Decision Tree) | 95% | 93% |
| RandomForest | 95% | 98% |
| NaïveBayes | 98% | 98% |
| Dl4MlpClassifier (Deep Learning) | 95% | 98% |
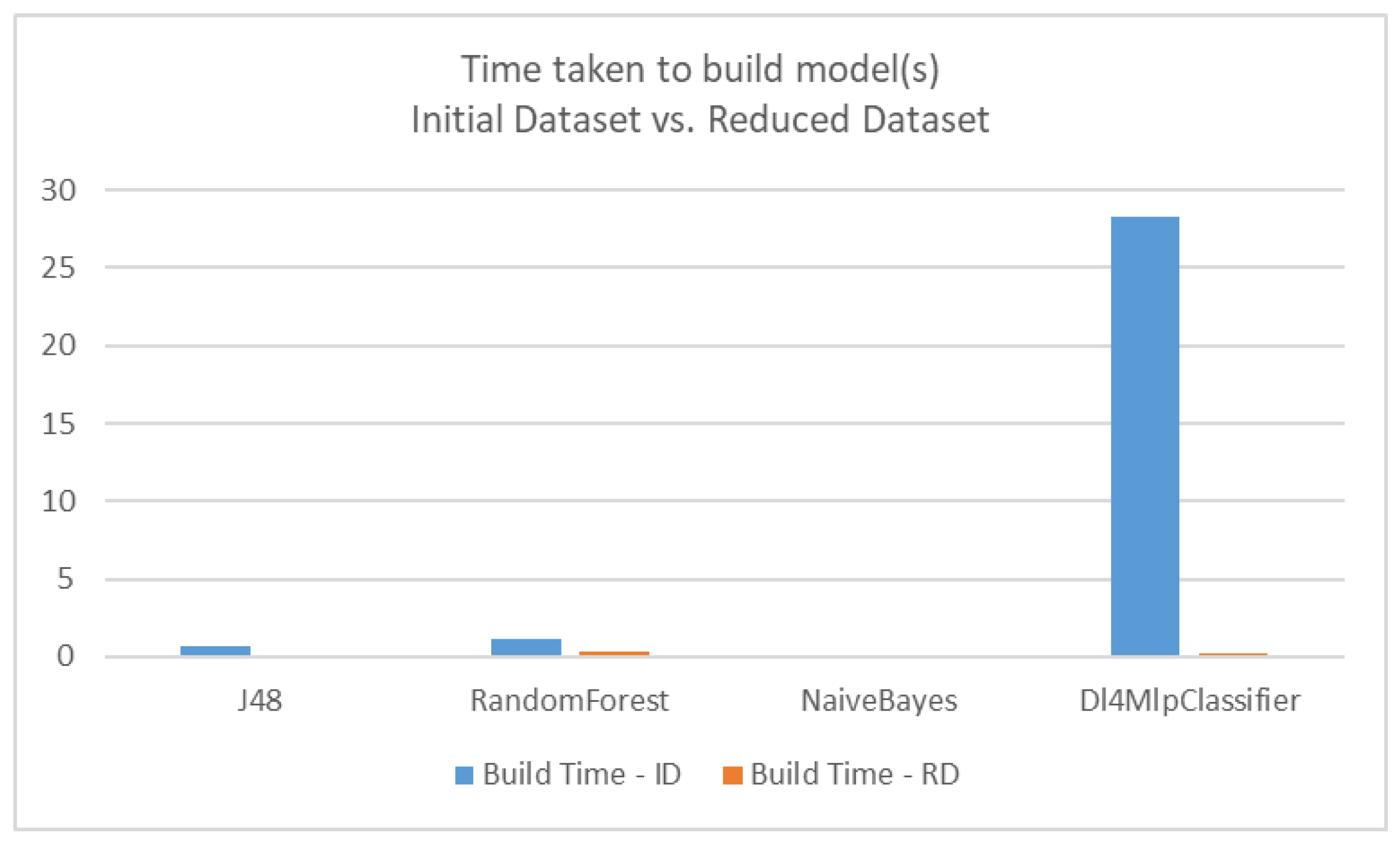
| Classifier | Time for Initial Dataset (ID)—Seconds | Time for Reduced Dataset (RD)—Seconds |
|---|---|---|
| J48 (Decision Tree) | 0.71 | 0.08 |
| RandomForest | 1.13 | 0.33 |
| NaïveBayes | 0.1 | 0.03 |
| Dl4MlpClassifier (Deep Learning) | 28.26 | 0.24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muntean, M.V.; Hîrceagă, A.F.; Căpîlnaș, M.V. Feature Selection Using Intelligent Agents for Time Improvement in Medical Diagnosis Systems. Electronics 2025, 14, 4419. https://doi.org/10.3390/electronics14224419
Muntean MV, Hîrceagă AF, Căpîlnaș MV. Feature Selection Using Intelligent Agents for Time Improvement in Medical Diagnosis Systems. Electronics. 2025; 14(22):4419. https://doi.org/10.3390/electronics14224419
Chicago/Turabian StyleMuntean, Maria Viorela, Andreea Florina Hîrceagă, and Matei Vasile Căpîlnaș. 2025. "Feature Selection Using Intelligent Agents for Time Improvement in Medical Diagnosis Systems" Electronics 14, no. 22: 4419. https://doi.org/10.3390/electronics14224419
APA StyleMuntean, M. V., Hîrceagă, A. F., & Căpîlnaș, M. V. (2025). Feature Selection Using Intelligent Agents for Time Improvement in Medical Diagnosis Systems. Electronics, 14(22), 4419. https://doi.org/10.3390/electronics14224419






