A Traceable Vaccine Production Supervision System with Embedded IoT Devices Based on Blockchains
Abstract
1. Introduction
- Enhanced Transparency and Traceability in the Vaccine Production Process: The primary goal of this research is to develop a system that can track and record crucial data and events in real time during the vaccine production process. This will significantly enhance the transparency of the production history, providing assurance of vaccine quality and safety throughout the production process.
- Improved Security of Vaccine Production Data: By encrypting vaccine-sensitive data generated during the production process, the system also offers data protection to production facilities without compromising data security due to transparency concerns. Preserving the generated production history securely on the blockchain and the decentralized IPFS [2] ensures that data cannot be maliciously altered. In this paper, the Ethereum blockchain is adopted. The Ethereum blockchain is a secure, decentralized, and distributed ledger platform [3], and it is currently one of the most well-known and widely used open-source blockchain systems.
- Data Consistency in Vaccine Production: The proposed system also ensures consistency in vaccine production by merging and encrypting data generated during a single production cycle with timestamps. This prevents the occurrence of sensitive data swapping or modification during the vaccine production process. Additionally, under the protection of the digital signature, the vaccine enterprise company cannot deny that the modification came from other vaccine companies.
- Nonrepudiation of Production History: To establish the involvement of various production nodes and individuals in the vaccine production process, sensitive vaccine data and events generated at each production stage are accompanied by digital signatures. This ensures that in the event of vaccine-related issues, arbitrators can verify these digital signatures to confirm which parties are involved in the vaccine production process, thereby establishing a direct connection between personnel, machines, and the production history beyond data generation and storage.
2. Related Work
3. Proposed Scheme
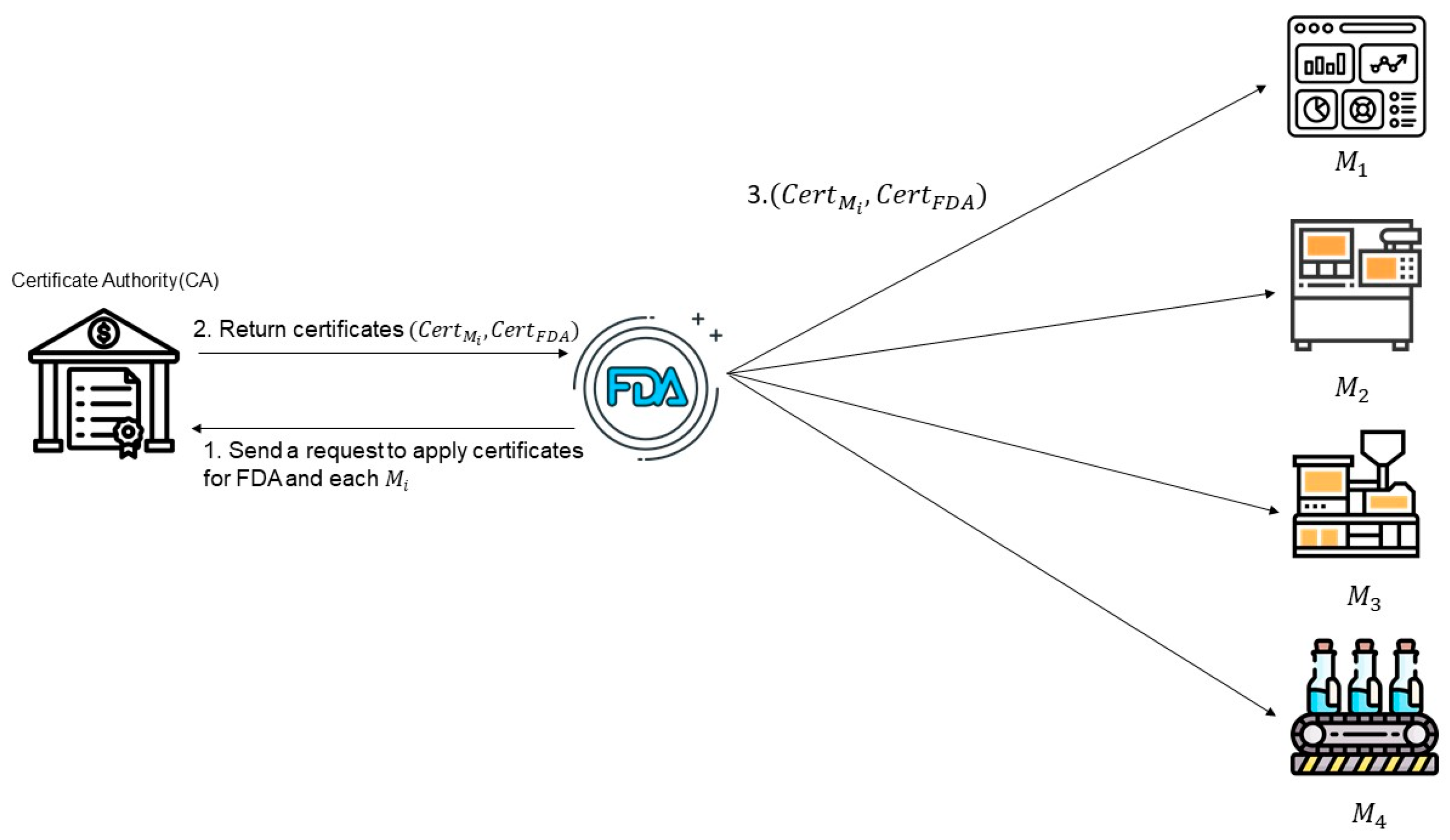
3.1. Setup Phase
3.2. Vaccine Producing Phase
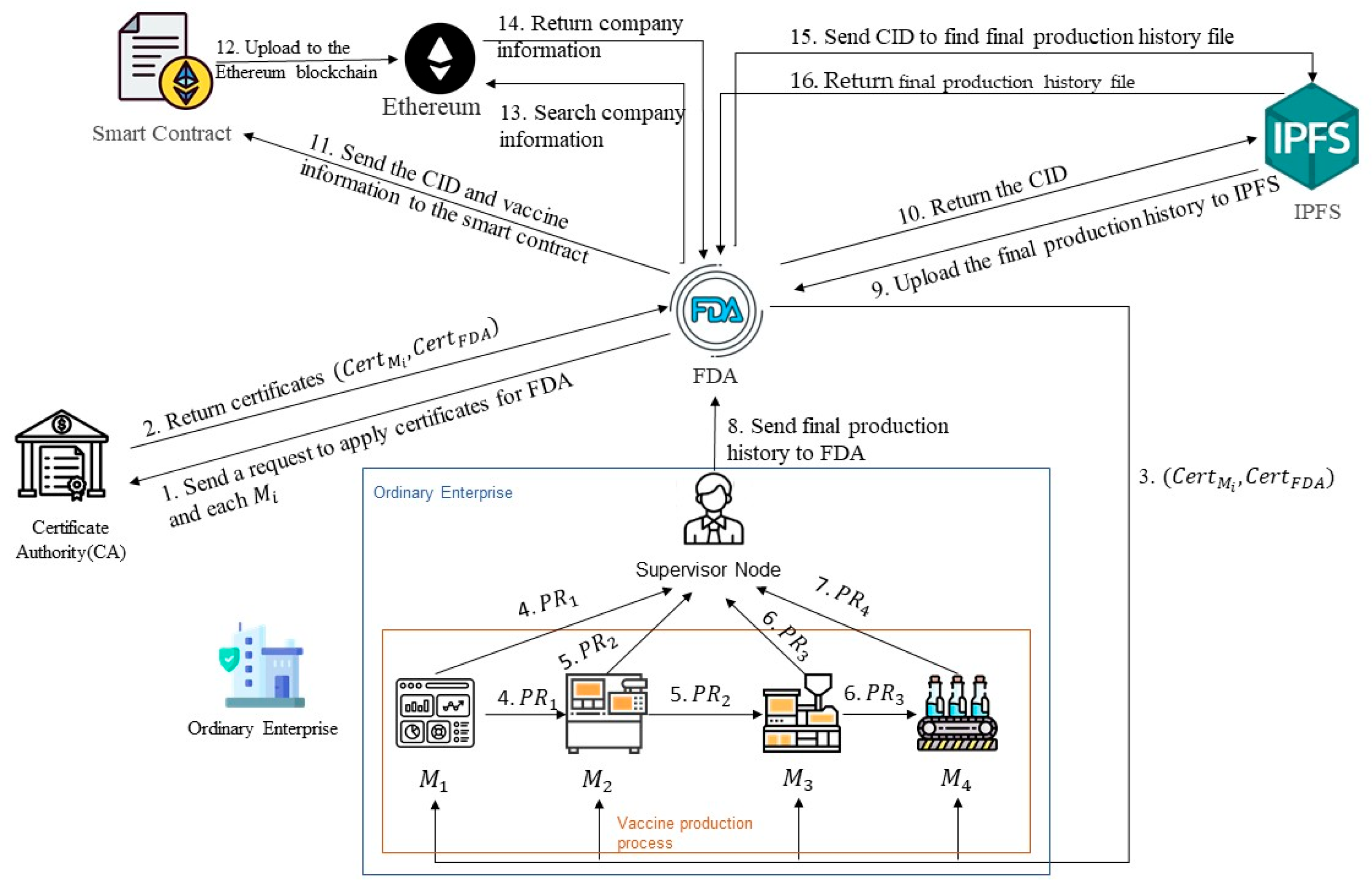
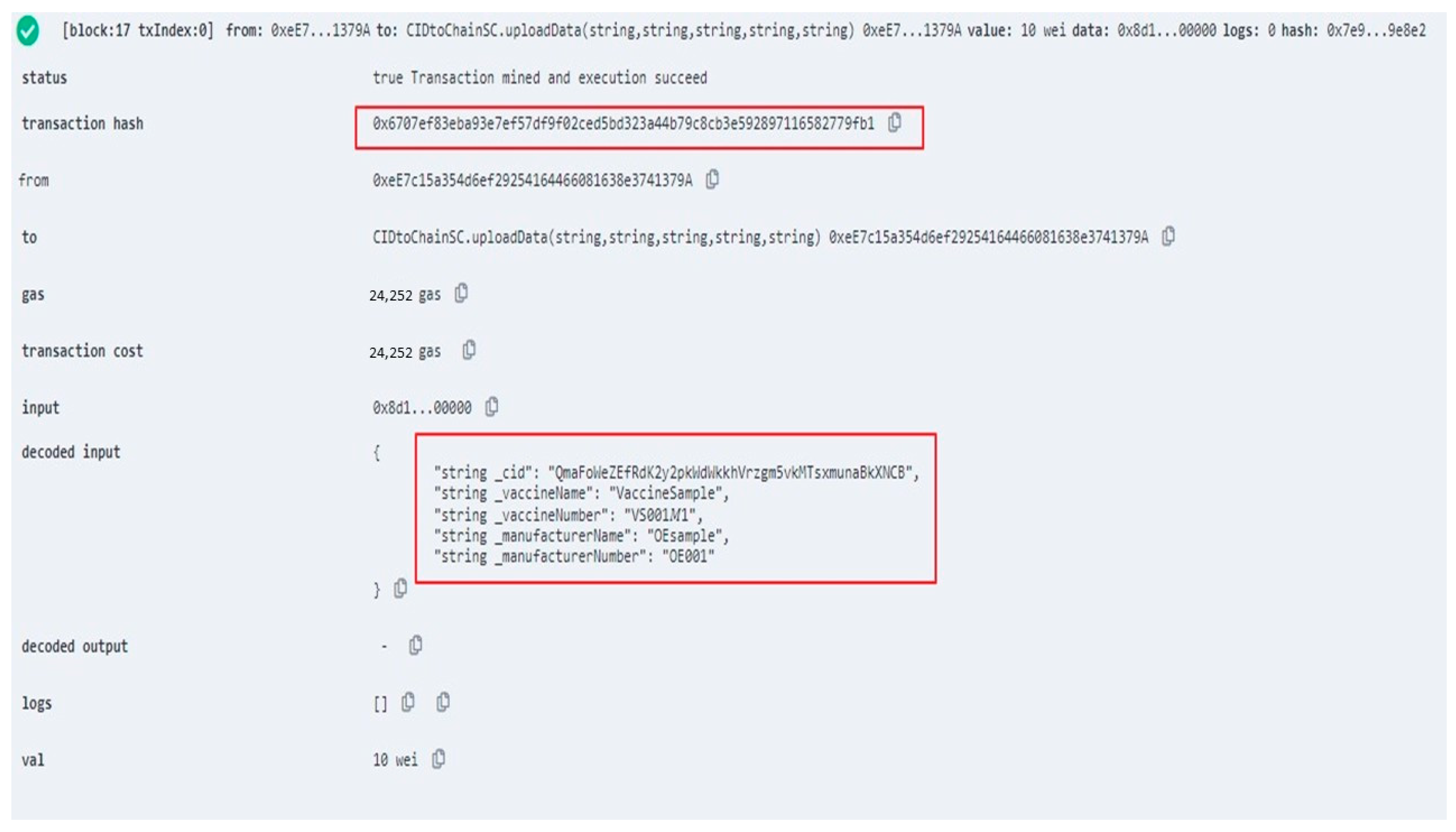
3.3. Vaccine Production History Uploading Phase
3.4. Vaccine Production Traceability Phase
- The FDA can obtain the company name, production number, vaccine name, vaccine number, and CID number of the vaccine to be traced on the Ethereum.
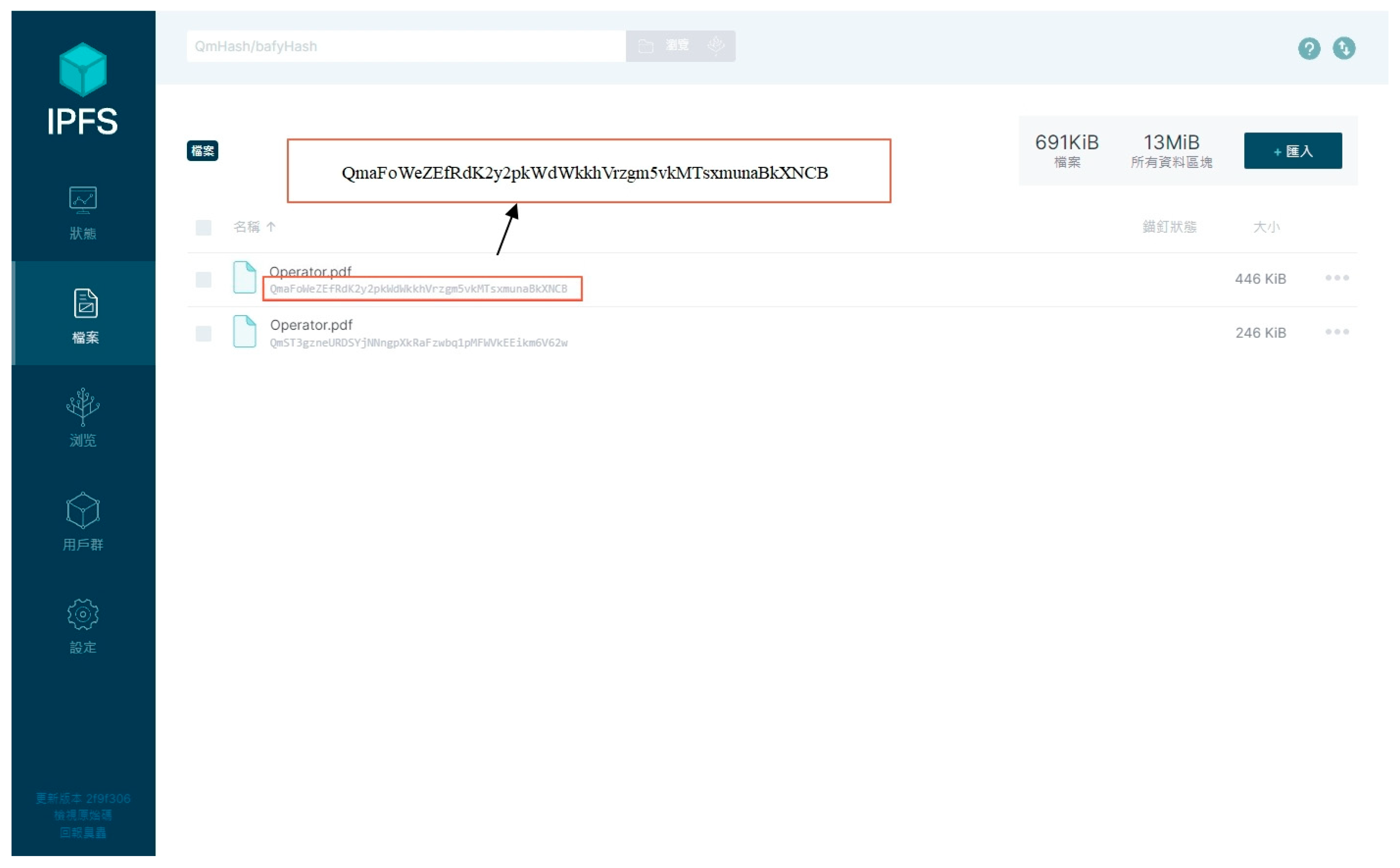
- The obtained CID is then used to retrieve the corresponding final production history file on the IPFS.
- The FDA uses the public key issued by the CA to sequentially verify the digital signature generated by each IoT device using the following formula:to determine if the is authentic.
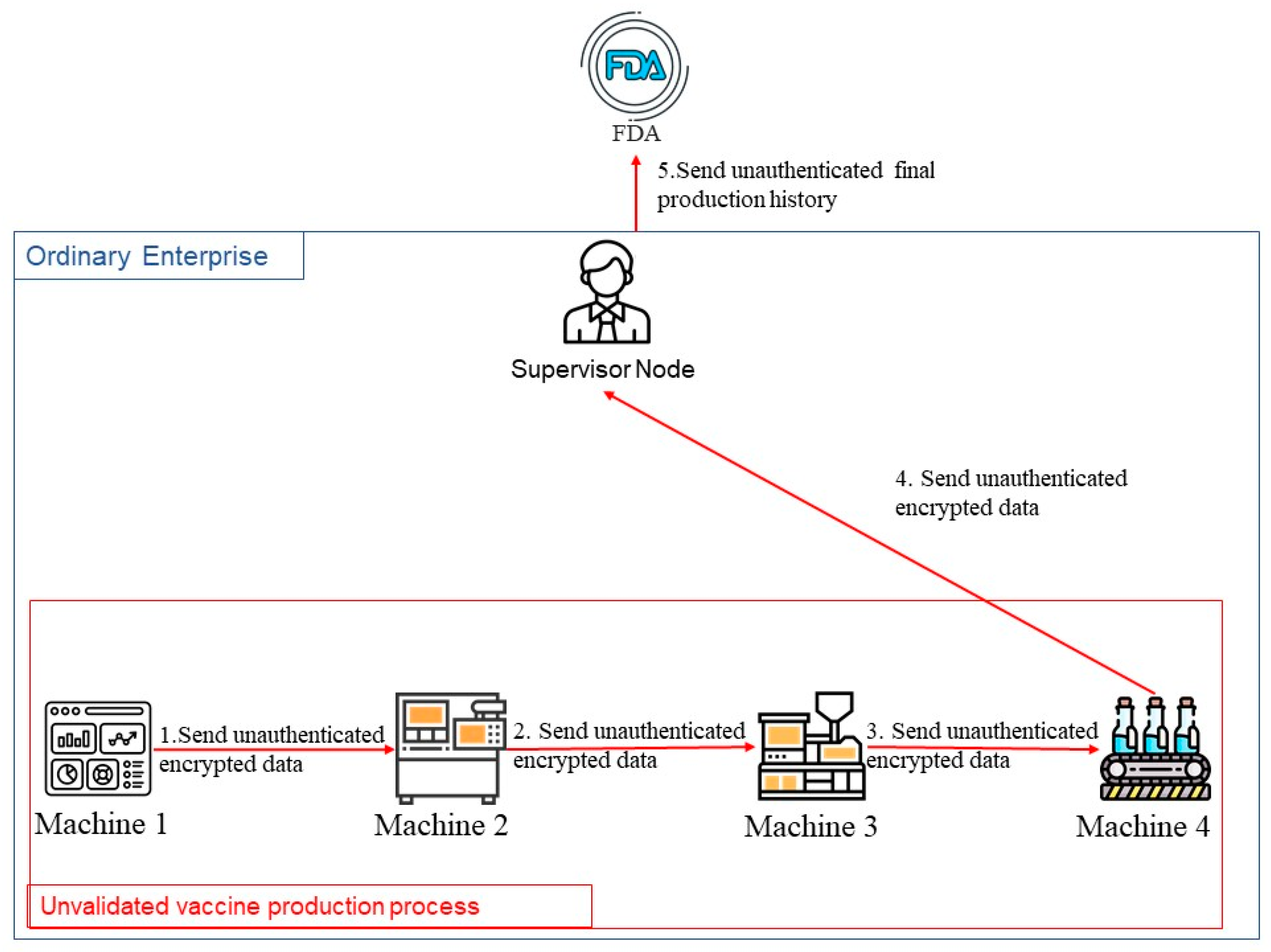
- If a device’s signature fails verification, the system can continue to verify the signatures of subsequent nodes to determine whether the error occurred in the current record or the previous node. Once the signature node with the anomaly is identified, the system can use the hash value and timestamp of that or the previous node to trace the corresponding production equipment and operator to determine the source of the problem.
- The FDA verifies the consistency between the CID stored on the blockchain and the IPFS content and records the verification results in the form of a digest hash in a public audit ledger, which serves as a basis for subsequent administrative investigations and quality audits.
3.5. IoT Device Recovery Phase
4. System Implementation and Efficiency Comparisons
4.1. IPFS and Blockchain System Implementation
4.2. Functionality Comparisons
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Peng, S.; Hu, X.; Zhang, J.; Xie, X.; Long, C.; Tian, Z.; Jiang, H. An efficient double-layer blockchain method for vaccine production supervision. IEEE Trans. Nanobiosci. 2020, 19, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Benet, J. IPFS—Content addressed, versioned, P2P file system. arXiv 2014. [Google Scholar] [CrossRef]
- Wood, G. Ethereum: A secure decentralized generalized transaction ledger. Ethereum Proj. Yellow Pap. 2014, 151, 1–32. [Google Scholar]
- Nakamoto, S. Bitcoin: A peer-to-peer electronic cash system. Decentralized Bus. Rev. 2008. [Google Scholar]
- Gabriel, T.; Cornel-Cristian, A.; Arhip-Calin, M.; Zamfirescu, A. Cloud storage: A comparison between centralized solutions versus decentralized cloud storage solutions using Blockchain technology. In Proceedings of the 2019 54th International Universities Power Engineering Conference (UPEC), Bucharest, Romania, 3–6 September 2019; pp. 1–5. [Google Scholar]
- Xiong, W.; Xiong, L. Data trading certification based on consortium blockchain and smart contracts. IEEE Access 2020, 9, 3482–3496. [Google Scholar] [CrossRef]
- Hastig, G.M.; Sodhi, M.S. Blockchain for supply chain traceability: Business requirements and critical success factors. Prod. Oper. Manag. 2020, 29, 935–954. [Google Scholar] [CrossRef]
- Swan, M. Blockchain: Blueprint for a New Economy; O’Reilly Media: Santa Rosa, CA, USA, 2015. [Google Scholar]
- Cocco, L.; Mannaro, K.; Tonelli, R.; Mariani, L.; Lodi, M.B.; Melis, A.; Simone, M.; Fanti, A. A blockchain-based traceability system in agri-food SME: Case study of a traditional bakery. IEEE Access 2021, 9, 62899–62915. [Google Scholar] [CrossRef]
- Haq, I.; Esuka, O.M. Blockchain technology in pharmaceutical industry to prevent counterfeit drugs. Int. J. Comput. Appl. 2018, 180, 8–12. [Google Scholar] [CrossRef]
- Bandhu, K.C.; Litoriya, R.; Lowanshi, P.; Jindal, M.; Chouhan, L.; Jain, S. Making drug supply chain secure traceable and efficient: A Blockchain and smart contract-based implementation. Multimed. Tools Appl. 2023, 82, 23541–23568. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Wang, J. Undeniable vaccine production protocol with blockchain and IPFS. In Proceedings of the 2022 IEEE/ACIS 23rd International Conference on Software Engineering, Artificial Intelligence, Networking and Parallel/Distributed Computing (SNPD), Taichung, Taiwan, 7–9 December 2022. [Google Scholar] [CrossRef]
- Ng, S.C.H.; Ho, G.T.S.; Wu, C.H. Blockchain-IIoT-big data aided process control and quality analytics. Int. J. Prod. Econ. 2023, 261, 108871. [Google Scholar] [CrossRef]
- Wu, C.; Tang, Y.M.; Kuo, W.T.; Yip, H.T.; Chau, K.Y. Healthcare 5.0: A secure and distributed network for system informatics in medical surgery. Int. J. Med. Inform. 2024, 186, 105415. [Google Scholar] [CrossRef] [PubMed]




| Refers to the embedded system’s serial number of each IoT machine in the vaccine production line and each of the devices equipped with the emergency switch button and MQTT app which could forward the sensing data to the app, where }. | |
| It represents information from all relevant operators in the IoT machine . | |
| It represents crucial vaccine data produced by the IoT machine , where }. | |
| The public key issued by the CA to each IoT machine device, where | |
| The digital signature function which is performed by the IoT machine , when using ski to generate the digital signature and }. | |
| The production record data transmitted from the embedded system of the IoT machine that it includes a digital signature and a vaccine production material plaintext inside, where }. | |
| It defines a hash operation to generate a fixed-length output of bits string. | |
| It generates the time stamps period from current time to the end by each IoT machine , where }. | |
| It represents the certificate authority that issues all certificate pairs for the embedded system and FDA, etc. | |
| FDA | It represents the Food and Drug Administration (FDA), a trustworthy government agency. |
| Img | Photos taken after vaccine production is completed and exterior numbering is conducted. |
| Encrypting function by inputting the vaccine data with using FDA’s public key . |
| uploadData() | Upload Production Data to the Blockchain |
| setAllowedAddress() | Authorize a New Address the Node on the Blockchain |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, M.-T.; Wang, J.-T.; Shih, Y.-Z. A Traceable Vaccine Production Supervision System with Embedded IoT Devices Based on Blockchains. Electronics 2025, 14, 4391. https://doi.org/10.3390/electronics14224391
Chen M-T, Wang J-T, Shih Y-Z. A Traceable Vaccine Production Supervision System with Embedded IoT Devices Based on Blockchains. Electronics. 2025; 14(22):4391. https://doi.org/10.3390/electronics14224391
Chicago/Turabian StyleChen, Ming-Te, Jih-Ting Wang, and Yu-Ze Shih. 2025. "A Traceable Vaccine Production Supervision System with Embedded IoT Devices Based on Blockchains" Electronics 14, no. 22: 4391. https://doi.org/10.3390/electronics14224391
APA StyleChen, M.-T., Wang, J.-T., & Shih, Y.-Z. (2025). A Traceable Vaccine Production Supervision System with Embedded IoT Devices Based on Blockchains. Electronics, 14(22), 4391. https://doi.org/10.3390/electronics14224391










