Facile Green Preparation of Reduced Graphene Oxide Using Citrus Limetta-Decorated rGO/TiO2 Nanostructures for Glucose Sensing
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of GO
2.3. Preparation of Aqueous Phytoextract
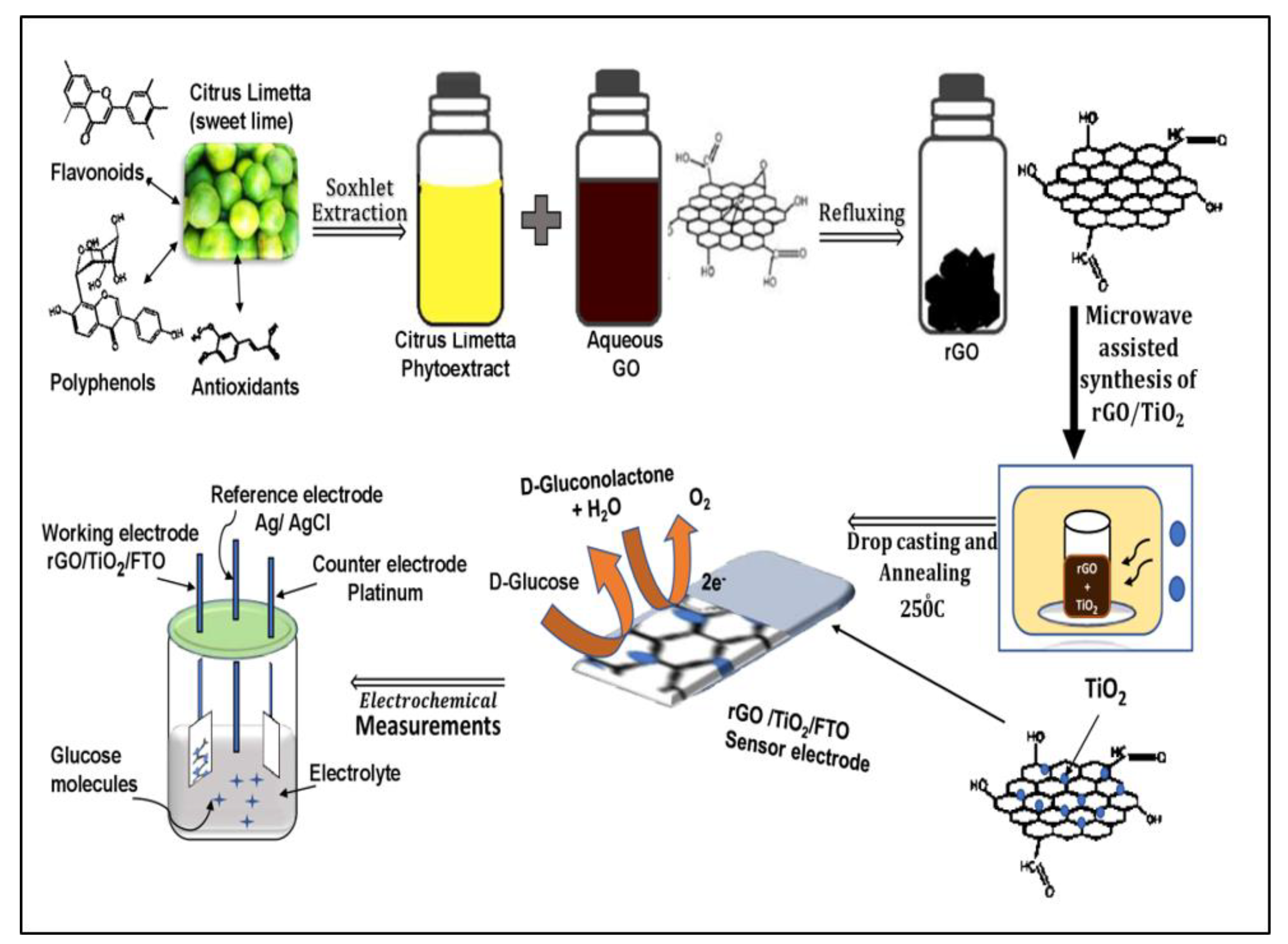
2.4. Mechanism of Reduction of GO by Citrus Limetta Phytoextract
2.5. Reduction of GO Using Aqueous Citrus Limetta Peel Extract
2.6. Synthesis of rGO/TiO2 Nanocomposite
2.7. Glucose Sensing through rGO/TiO2
2.8. Characterization Techniques
3. Results and Discussion
3.1. Structure Elucidation Confirmation
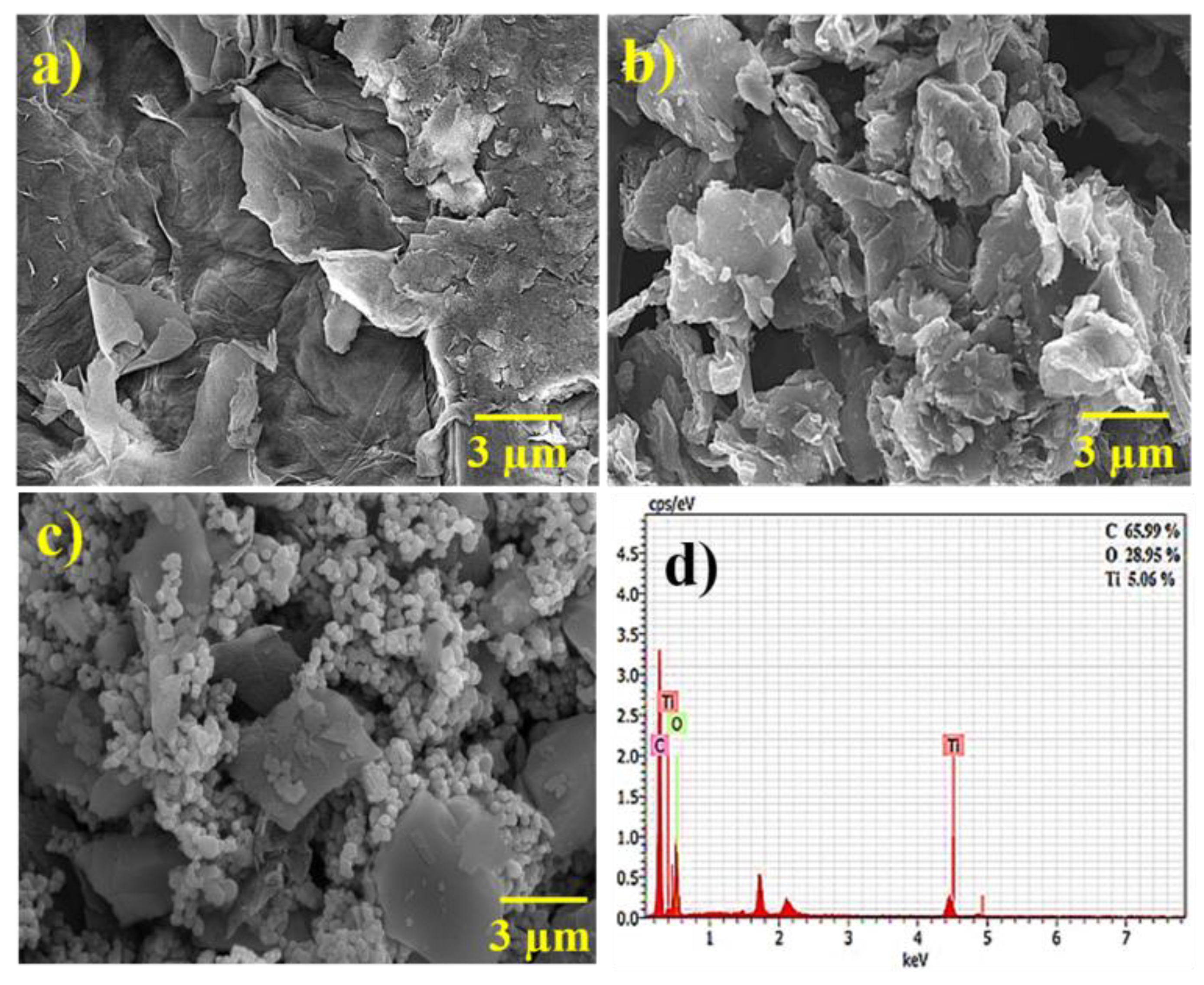
3.2. Morphological Study
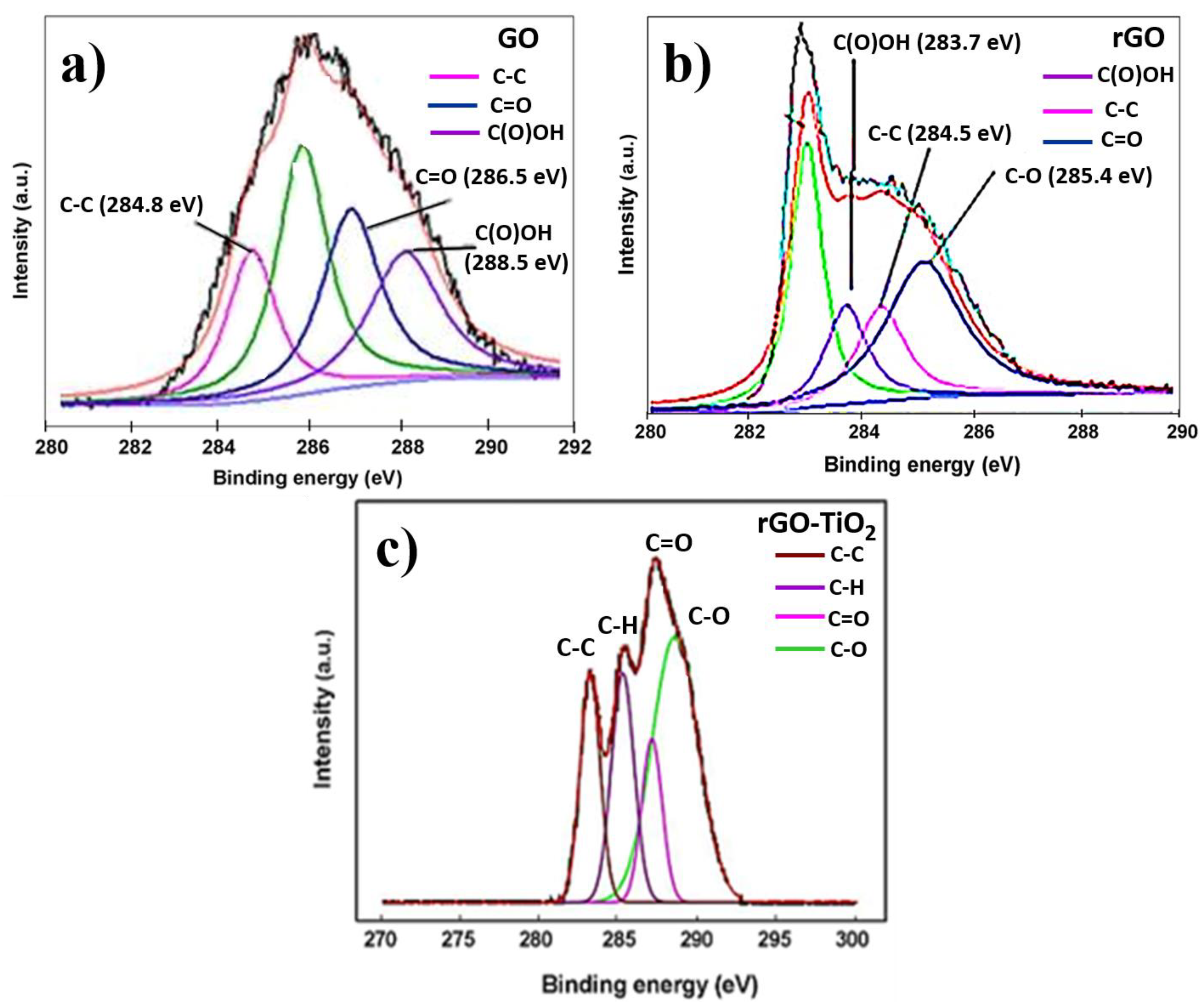
3.3. X-ray Photoelectron Spectroscopy Analysis
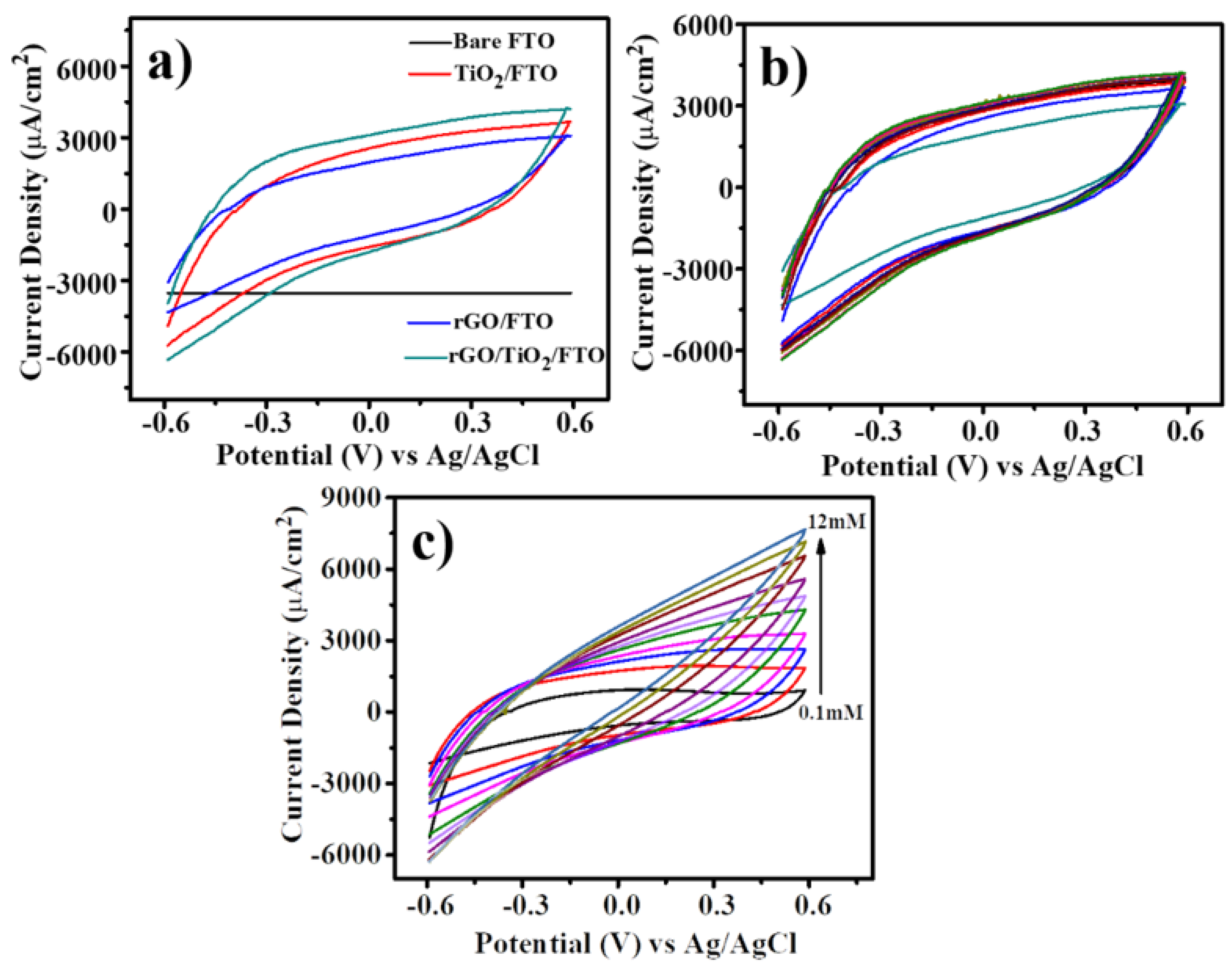
3.4. Electrochemical Non-Enzymatic Detection
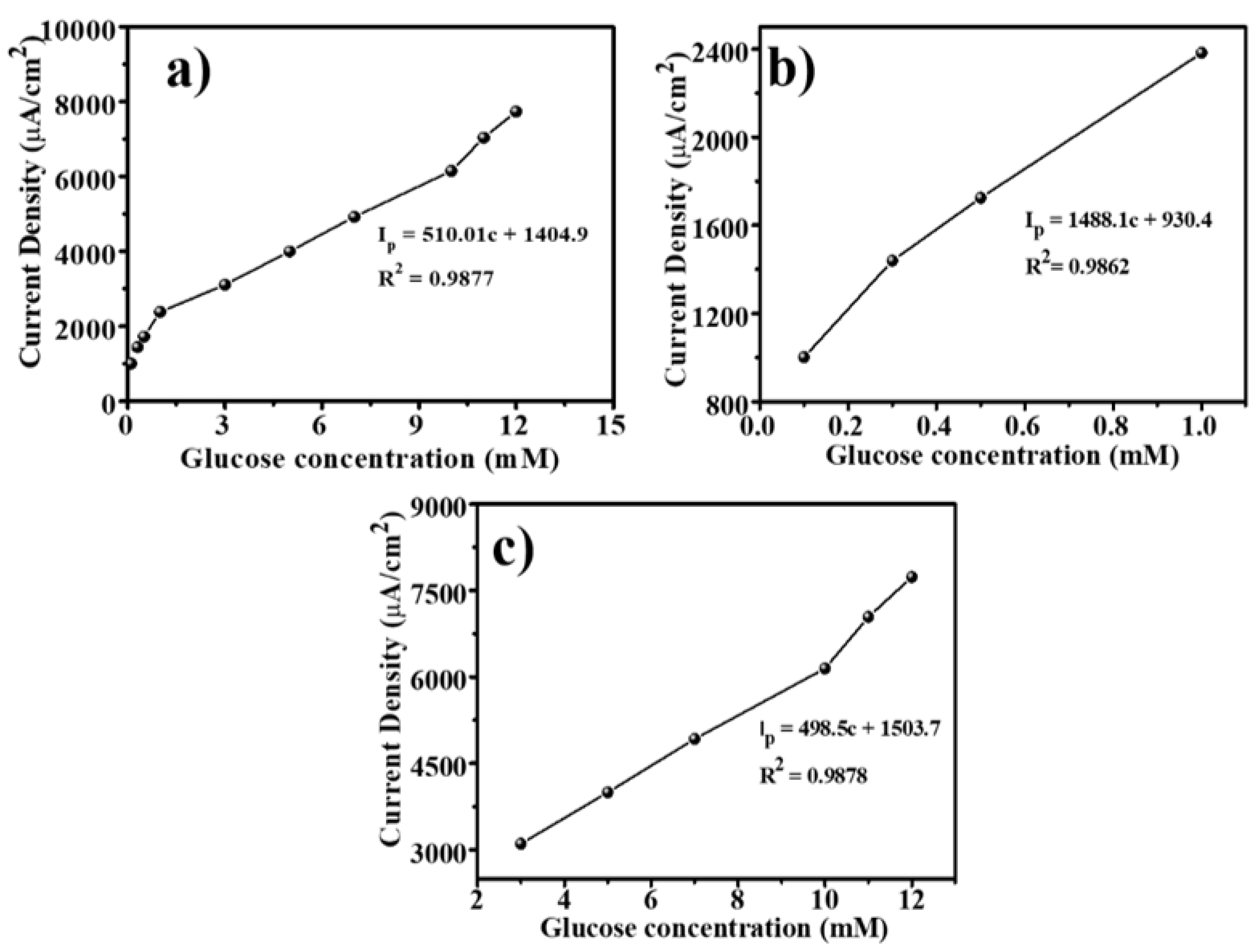
3.5. Calibration of the Sensor
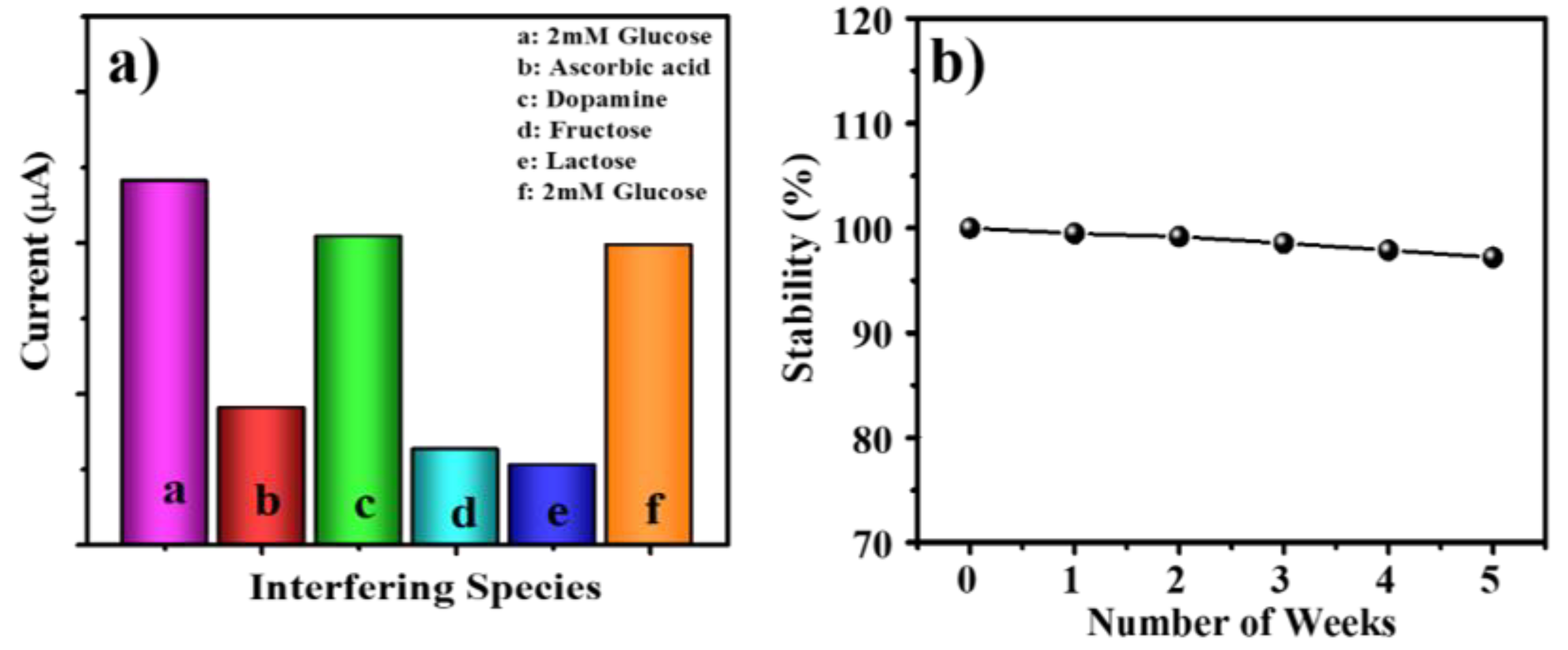
3.6. Selectivity and Stability of the Sensor
3.7. Repeatability of the Sensor
3.8. Investigation of Glucose Level in Blood Serum
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, H.; Li, L.; Zhou, W.; Shao, Z.; Chen, X. Advances in non-enzymatic glucose sensors based on metal oxides. J. Mater. Chem. B 2016, 4, 7333–7349. [Google Scholar] [CrossRef]
- Bruen, D.; Delaney, C.; Florea, L.; Diamond, D. Glucose Sensing for Diabetes Monitoring: Recent Developments. Sensors 2017, 17, 1866. [Google Scholar] [CrossRef]
- Sai-Anand, G.; Gopalan, A.-I.; Kang, S.-W.; Komathi, S.; Lee, K.-P. One Pot Synthesis of New Gold Nanoparticles Dispersed Poly(2-aminophenyl boronic acid) Composites for Fabricating an Affinity Based Electrochemical Detection of Glucose. Sci. Adv. Mater. 2014, 6, 1356–1364. [Google Scholar] [CrossRef]
- Gopalan, A.; Muthuchamy, N.; Lee, K. A novel bismuth oxychloride-graphene hybrid nanosheets based non-enzymatic photoelectrochemical glucose sensing platform for high performances. Biosens. Bioelectron. 2017, 89, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.G.; Amala, G.; Gowtham, S.M. Recent advancements, key challenges and solutions in non-enzymatic electrochemical glucose sensors based on graphene platforms. RSC Adv. 2017, 7, 36949–36976. [Google Scholar] [CrossRef]
- Gopalan, A.; Muthuchamy, N.; Komathi, S.; Lee, K.-P. A novel multicomponent redox polymer nanobead based high performance non-enzymatic glucose sensor. Biosens. Bioelectron. 2016, 84, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Dong, Q.; Ryu, H.; Lei, Y. Metal oxide based non-enzymatic electrochemical sensors for glucose detection. Electrochim. Acta 2021, 370, 137744. [Google Scholar] [CrossRef]
- Manthey, J.A.; Grohmann, K. Phenols in Citrus Peel Byproducts. Concentrations of Hydroxycinnamates and Polymethoxylated Flavones in Citrus Peel Molasses. J. Agric. Food Chem. 2001, 49, 3268–3273. [Google Scholar] [CrossRef]
- Ghule, B.G.; Shinde, N.M.; Raut, S.D.; Gore, S.K.; Shaikh, S.F.; Ekar, S.U.; Ubaidullah, M.; Pak, J.J.; Mane, R.S. Self-assembled α-Fe2O3-GO nanocomposites: Studies on physical, magnetic and ammonia sensing properties. Mater. Chem. Phys. 2022, 278, 125617. [Google Scholar] [CrossRef]
- Balandin, A.A. Thermal properties of graphene and nanostructured carbon materials. Nat. Mater. 2011, 10, 569–581. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, Y.; Li, P.; Zhou, J.; He, J.; Zhang, W.; Guo, Z.; Li, Y.; Dong, M. Modulation of N-bonding configurations and their influence on the electrical properties of nitrogen-doped graphene. RSC Adv. 2016, 6, 92682–92687. [Google Scholar] [CrossRef]
- Shin, H.-J.; Kim, K.K.; Benayad, A.; Yoon, S.-M.; Park, H.K.; Jung, I.-S.; Jin, M.H.; Jeong, H.-K.; Kim, J.M.; Choi, J.-Y.; et al. Efficient Reduction of Graphite Oxide by Sodium Borohydride and Its Effect on Electrical Conductance. Adv. Funct. Mater. 2009, 19, 1987–1992. [Google Scholar] [CrossRef]
- Ren, P.-G.; Yan, D.-X.; Ji, X.; Chen, T.; Li, Z.-M. Temperature dependence of graphene oxide reduced by hydrazine hydrate. Nanotechnology 2010, 22, 055705. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, P.; Liu, C.F.; Zhan, L.; Li, Y.F.; Huang, C.Z. Green and easy synthesis of biocompatible graphene for use as an anticoagulant. RSC Adv. 2012, 2, 2322–2328. [Google Scholar] [CrossRef]
- Mhamane, D.; Ramadan, W.; Fawzy, M.; Rana, A.; Dubey, M.; Rode, C.; Lefez, B.; Hannoyer, B.; Ogale, S. From graphite oxide to highly water dispersible functionalized graphene by single step plant extract-induced deoxygenation. Green Chem. 2011, 13, 1990–1996. [Google Scholar] [CrossRef]
- Salas, E.C.; Sun, Z.; Lüttge, A.; Tour, J.M. Reduction of Graphene Oxide via Bacterial Respiration. ACS Nano 2010, 4, 4852–4856. [Google Scholar] [CrossRef] [PubMed]
- Nasrollahzadeh, M.; Maham, M.; Rostami-Vartooni, A.; Bagherzadeh, M.; Sajadi, S.M. Barberry fruit extract assisted in situ green synthesis of Cu nanoparticles supported on a reduced graphene oxide–Fe3O4nanocomposite as a magnetically separable and reusable catalyst for the O-arylation of phenols with aryl halides under ligand-free conditions. RSC Adv. 2015, 5, 64769–64780. [Google Scholar] [CrossRef]
- Arias, B.; Ramón-Laca, L. Pharmacological properties of citrus and their ancient and medieval uses in the Mediterranean region. J. Ethnopharmacol. 2005, 97, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Manthey, J.A.; Grohmann, K. Concentrations of Hesperidin and Other Orange Peel Flavonoids in Citrus Processing Byproducts. J. Agric. Food Chem. 1996, 44, 811–814. [Google Scholar] [CrossRef]
- Majhi, S.; Mirzaei, A.; Kim, H.; Kim, S. Reduced Graphene Oxide (rGO)-Loaded Metal-Oxide Nanofiber Gas Sensors: An Overview. Sensors 2021, 21, 1352. [Google Scholar] [CrossRef]
- Pradhan, S.; Konwar, K.; Ghosh, T.; Mondal, B.; Sarkar, S.; Deb, P. Multifunctional Iron oxide embedded reduced graphene oxide as a versatile adsorbent candidate for effectual arsenic and dye removal. Colloid Interface Sci. Commun. 2020, 39, 100319. [Google Scholar] [CrossRef]
- Molak, A.; Paluch, M.; Pawlus, S.; Klimontko, J.; Ujma, Z.; Gruszka, I. Electric modulus approach to the analysis of electric relaxation in highly conducting (Na0.75Bi0.25)(Mn0.25Nb0.75)O3 ceramics. J. Phys. D Appl. Phys. 2005, 38, 1450–1460. [Google Scholar] [CrossRef]
- Pradhan, S.S.; Ghosh, T.N.; Marik, A.; Raul, K.K.; Sarkar, S.K. Magnetodielectric effects in three reduced graphene oxide–polymer nanocomposites. Bull. Mater. Sci. 2020, 43, 208. [Google Scholar] [CrossRef]
- Ghule, B.G.; Shinde, N.M.; Raut, S.D.; Shaikh, S.F.; Al-Enizi, A.M.; Kim, K.H.; Mane, R.S. Porous metal-graphene oxide nanocomposite sensors with high ammonia detectability. J. Colloid Interface Sci. 2021, 589, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Sun, H.; Liu, S.; Wang, S. Graphene facilitated visible light photodegradation of methylene blue over titanium dioxide photocatalysts. Chem. Eng. J. 2013, 214, 298–303. [Google Scholar] [CrossRef]
- Luo, L.; Zhu, L.; Wang, Z. Nonenzymatic amperometric determination of glucose by CuO nanocubes–graphene nanocomposite modified electrode. Bioelectrochemistry 2012, 88, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Hao, X.; Chen, S.; Ma, Z.; Wang, W.; Wang, C.; Yue, L.; Sun, H.; Shao, Q.; Murugadoss, V.; et al. Long-term antibacterial stable reduced graphene oxide nanocomposites loaded with cuprous oxide nanoparticles. J. Colloid Interface Sci. 2019, 533, 13–23. [Google Scholar] [CrossRef] [PubMed]
- AAT Bioquest, Inc. Quest Calculate™ PBS (Phosphate Buffered Saline) (1X, pH 7.4) Preparation and Recipe. AAT Bioquest. Available online: https://www.aatbio.com/resources/buffer-preparations-and-recipes/pbs-phosphate-buffered-saline (accessed on 7 December 2022).
- Komathi, S.; Muthuchamy, N.; Lee, K.-P.; Gopalan, A.-I. Fabrication of a novel dual mode cholesterol biosensor using titanium dioxide nanowire bridged 3D graphene nanostacks. Biosens. Bioelectron. 2016, 84, 64–71. [Google Scholar] [CrossRef]
- Zaaba, N.I.; Foo, K.L.; Hashim, U.; Tan, S.J.; Liu, W.W.; Voon, C.H. Synthesis of Graphene Oxide using Modified Hummers Method: Solvent Influence. Procedia Eng. 2017, 184, 469–477. [Google Scholar] [CrossRef]
- Zhang, L.; Hai, X.; Xia, C.; Chen, X.-W.; Wang, J.-H. Growth of CuO nanoneedles on graphene quantum dots as peroxidase mimics for sensitive colorimetric detection of hydrogen peroxide and glucose. Sens. Actuators B Chem. 2017, 248, 374–384. [Google Scholar] [CrossRef]
- Shaikh, S.F.; Mane, R.S.; Min, B.K.; Hwang, Y.J.; Joo, O.-S. D-sorbitol-induced phase control of TiO2 nanoparticles and its application for dye-sensitized solar cells. Sci. Rep. 2016, 6, 20103. [Google Scholar] [CrossRef]
- Nipane, S.V.; Lee, S.-W.; Gokavi, G.S.; Kadam, A.N. In situ one pot synthesis of nanoscale TiO2-anchored reduced graphene oxide (RGO) for improved photodegradation of 5-fluorouracil drug. J. Mater. Sci. Mater. Electron. 2018, 29, 16553–16564. [Google Scholar] [CrossRef]
- Atchudan, R.; Edison, T.N.J.I.; Perumal, S.; Karthikeyan, D.; Lee, Y.R. Effective photocatalytic degradation of anthropogenic dyes using graphene oxide grafting titanium dioxide nanoparticles under UV-light irradiation. J. Photochem. Photobiol. A Chem. 2017, 333, 92–104. [Google Scholar] [CrossRef]
- Ghosh, T.N.; Pradhan, S.S.; Sarkar, S.K.; Bhunia, A.K. On the incorporation of the various reduced graphene oxide into poly(vinyl alcohol) nano-compositions: Comparative study of the optical, structural properties and magnetodielectric effect. J. Mater. Sci. Mater. Electron. 2021, 32, 19157–19178. [Google Scholar] [CrossRef]
- Kołodziej, A.; Długoń, E.; Świętek, M.; Ziąbka, M.; Dawiec, E.; Gubernat, M.; Michalec, M.; Wesełucha-Birczyńska, A. A Raman Spectroscopic Analysis of Polymer Membranes with Graphene Oxide and Reduced Graphene Oxide. J. Compos. Sci. 2021, 5, 20. [Google Scholar] [CrossRef]
- Zhu, J.; Zeng, G.; Nie, F.; Xu, X.; Chen, S.; Han, Q.; Wang, X. Decorating graphene oxide with CuO nanoparticles in a water–isopropanol system. Nanoscale 2010, 2, 988–994. [Google Scholar] [CrossRef]
- Song, J.; Wang, X.; Chang, C.-T. Preparation and Characterization of Graphene Oxide. J. Nanomater. 2014, 2014, 276143. [Google Scholar] [CrossRef]
- Qianqian, Z.; Tang, B.; Guoxin, H. High photoactive and visible-light responsive graphene/titanate nanotubes photocatalysts: Preparation and characterization. J. Hazard. Mater. 2011, 198, 78–86. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyen, S.T.; Ruoff, R.S. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- Jana, M.; Saha, S.; Khanra, P.; Murmu, N.C.; Srivastava, S.K.; Kuila, T.; Lee, J.H. Bio-reduction of graphene oxide using drained water from soaked mung beans (Phaseolus aureus L.) and its application as energy storage electrode material. Mater. Sci. Eng. B 2014, 186, 33–40. [Google Scholar] [CrossRef]
- Mao, Q.; Jing, W.; Zhou, F.; Liu, S.; Gao, W.; Wei, Z.; Jiang, Z. Depositing reduced graphene oxide on ZnO nanorods to improve the performance of enzymatic glucose sensors. Mater. Sci. Semicond. Process. 2021, 121, 105391. [Google Scholar] [CrossRef]
- Shen, Z.; Gao, W.; Li, P.; Wang, X.; Zheng, Q.; Wu, H.; Ma, Y.; Guan, W.; Wu, S.; Yu, Y.; et al. Highly sensitive nonenzymatic glucose sensor based on nickel nanoparticle–attapulgite-reduced graphene oxide-modified glassy carbon electrode. Talanta 2016, 159, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Xu, J.; Liu, H.; Shao, S. Fabrication of RGO-NiCo2O4 nanorods composite from deep eutectic solvents for nonenzymatic amperometric sensing of glucose. Talanta 2018, 185, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Alghazzawi, W.; Danish, E.; Alnahdi, H.; Salam, M.A. Rapid microwave-assisted hydrothermal green synthesis of rGO/NiO nanocomposite for glucose detection in diabetes. Synth. Met. 2020, 267, 116401. [Google Scholar] [CrossRef]
- Tang, W.; Li, L.; Zeng, X. A glucose biosensor based on the synergistic action of nanometer-sized TiO2 and polyaniline. Talanta 2015, 131, 417–423. [Google Scholar] [CrossRef]
- Jang, H.D.; Kim, S.K.; Chang, H.; Jo, E.H.; Roh, K.M.; Choi, J.-H.; Choi, J.-W. Synthesis of 3D Silver-Graphene-Titanium Dioxide Composite via Aerosol Spray Pyrolysis for Sensitive Glucose Biosensor. Aerosol Sci. Technol. 2015, 49, 538–546. [Google Scholar] [CrossRef]
- Du, J.; Tao, Y.; Xiong, Z.; Yu, X.; Xie, A.; Luo, S.; Li, X.; Yao, C. Titanium Dioxide–Graphene–Polyaniline Hybrid for Nonenzymatic Detection of Glucose. Nano 2019, 14, 1950093. [Google Scholar] [CrossRef]
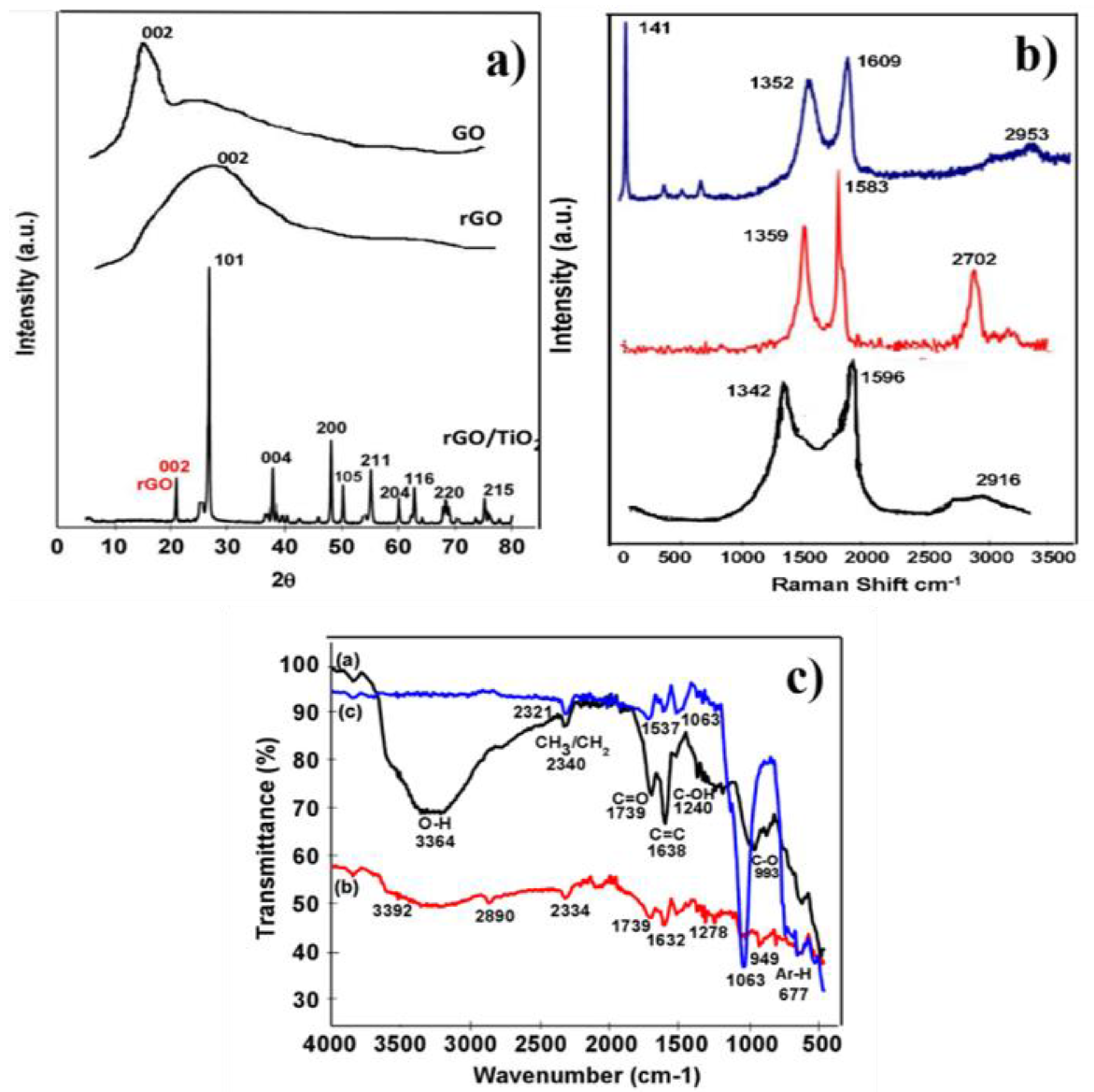
| No | Two Theta (deg.) | (h k l) | Phase | Crystallite Size (D) ± 2 (nm) |
|---|---|---|---|---|
| 1 | 20.9 | 002 | rGO | 19 |
| 2 | 26.7 | 101 | TiO2 | 81 |
| 3 | 37.89 | 004 | TiO2 | 14 |
| 4 | 48.14 | 200 | TiO2 | 41 |
| 5 | 49.89 | 105 | TiO2 | 46 |
| 6 | 55.13 | 211 | TiO2 | 41 |
| 7 | 60.05 | 204 | TiO2 | 22 |
| 8 | 62.77 | 116 | TiO2 | 22 |
| 9 | 68.31 | 220 | TiO2 | 23 |
| 10 | 75.15 | 215 | TiO2 | 47 |
| Electrode | Detection Potential (V) | Sensitivity μA/mM1cm2 | Linear Range/mM | Detection Limit/μM | Reference |
|---|---|---|---|---|---|
| rGO/TiO2/FTO | −0.25 | 1425 | 0.1–12 | 0.32 | This work |
| Nafion/GOx/rGO-6/ZnONRs/Au/PET | −0.8 to +0.8 | 2.26 | 11.5 | 37.5 | [42] |
| Ni Ps/ATP/r-GO | 0.0 to −1.5 | 1414.4 μA | 1–710 μM | 0.37 | [43] |
| NiCo2O4-rGO | -- | 548.9 | 1–25 | 0.35 | [44] |
| rGO/NiO | −0.6 | 6.2 | 1–15 | 19.35 | [45] |
| GOx/n-TiO2/PANI/GCE | -- | 6.31 | 0.02–6 | 18 | [46] |
| 3D Ag-GR-TiO2 | -- | 12 | -- | -- | [47] |
| TiO2–r-GO–PANI | -- | -- | 10–180 μM | 7.46 | [48] |
| Serum Sample | Glucose Added (mM) | Glucose Obtained (mM) | Recovery (%) | RSD (%) |
|---|---|---|---|---|
| 1 | 2.00 | 1.97 | 98.5 | 1.5 |
| 2 | 4.00 | 3.92 | 98.8 | 1.2 |
| 3 | 6.00 | 5.87 | 97.5 | 2.2 |
| 4 | 8.00 | 7.89 | 98.6 | 1.4 |
| 5 | 10.00 | 9.71 | 97.1 | 2.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gijare, M.; Chaudhari, S.; Ekar, S.; Shaikh, S.F.; Mane, R.S.; Pandit, B.; Siddiqui, M.U.H.; Garje, A. Facile Green Preparation of Reduced Graphene Oxide Using Citrus Limetta-Decorated rGO/TiO2 Nanostructures for Glucose Sensing. Electronics 2023, 12, 294. https://doi.org/10.3390/electronics12020294
Gijare M, Chaudhari S, Ekar S, Shaikh SF, Mane RS, Pandit B, Siddiqui MUH, Garje A. Facile Green Preparation of Reduced Graphene Oxide Using Citrus Limetta-Decorated rGO/TiO2 Nanostructures for Glucose Sensing. Electronics. 2023; 12(2):294. https://doi.org/10.3390/electronics12020294
Chicago/Turabian StyleGijare, Medha, Sharmila Chaudhari, Satish Ekar, Shoyebmohamad F. Shaikh, Rajaram S. Mane, Bidhan Pandit, Muhammad Usman Hassan Siddiqui, and Anil Garje. 2023. "Facile Green Preparation of Reduced Graphene Oxide Using Citrus Limetta-Decorated rGO/TiO2 Nanostructures for Glucose Sensing" Electronics 12, no. 2: 294. https://doi.org/10.3390/electronics12020294
APA StyleGijare, M., Chaudhari, S., Ekar, S., Shaikh, S. F., Mane, R. S., Pandit, B., Siddiqui, M. U. H., & Garje, A. (2023). Facile Green Preparation of Reduced Graphene Oxide Using Citrus Limetta-Decorated rGO/TiO2 Nanostructures for Glucose Sensing. Electronics, 12(2), 294. https://doi.org/10.3390/electronics12020294








