Total Phenolic Content, Antioxidant Capacity and UV Radiation Protection Properties of Marigold (Calendula officinalis), Carrot (Daucus carota), Tomato (Solanum lycopersicum) and Hop (Humulus lupulus) Extracts
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Sample Preparation
2.3. Determination of Total Phenolic Content
2.4. Determination of Antioxidant Capacity by CUPRAC Method
2.5. Assessment of Sun Protection Properties of Plant Extracts by Mathematical Indication Method
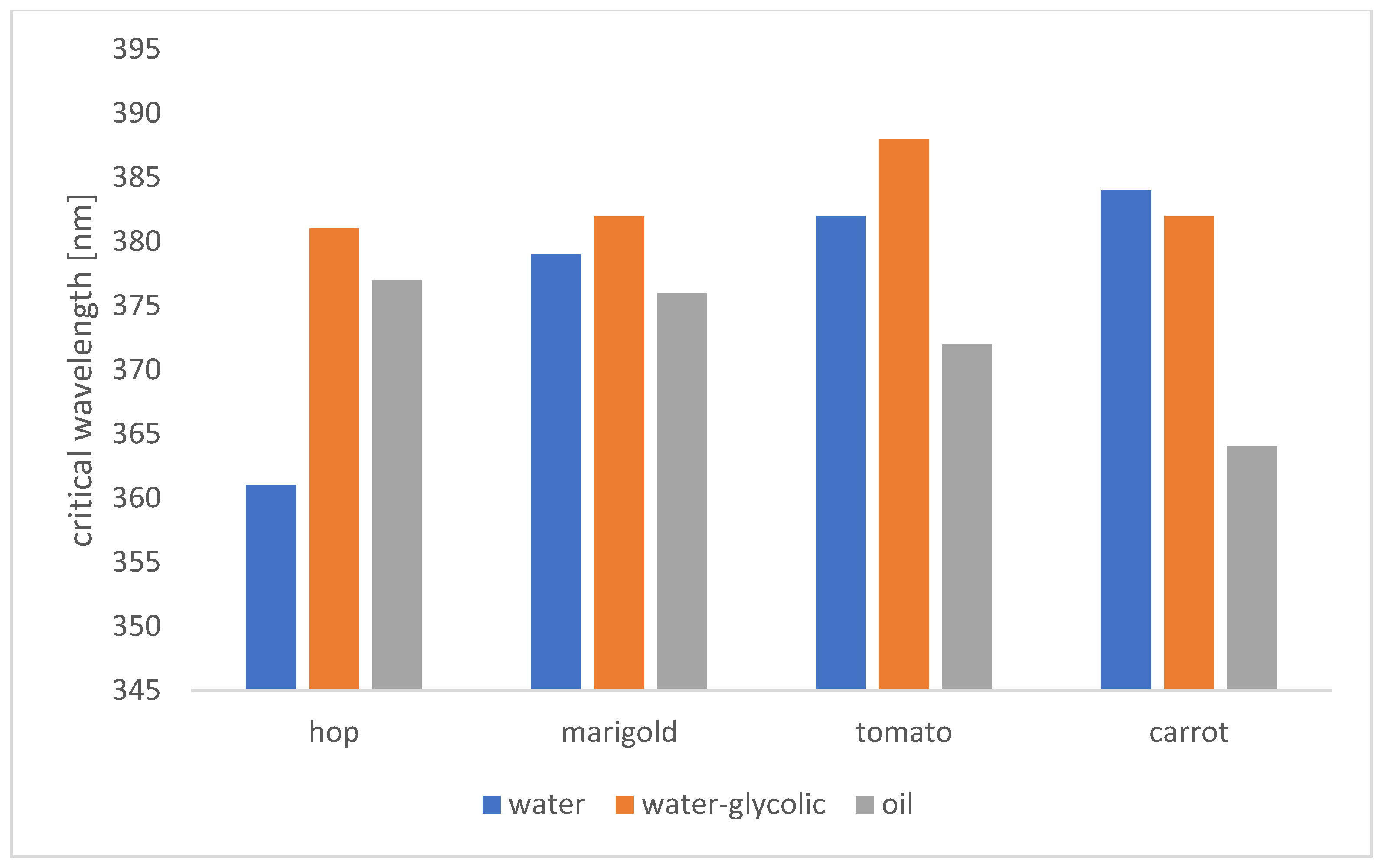
2.5.1. Critical Wavelength
2.5.2. Ratio UVA/UVB
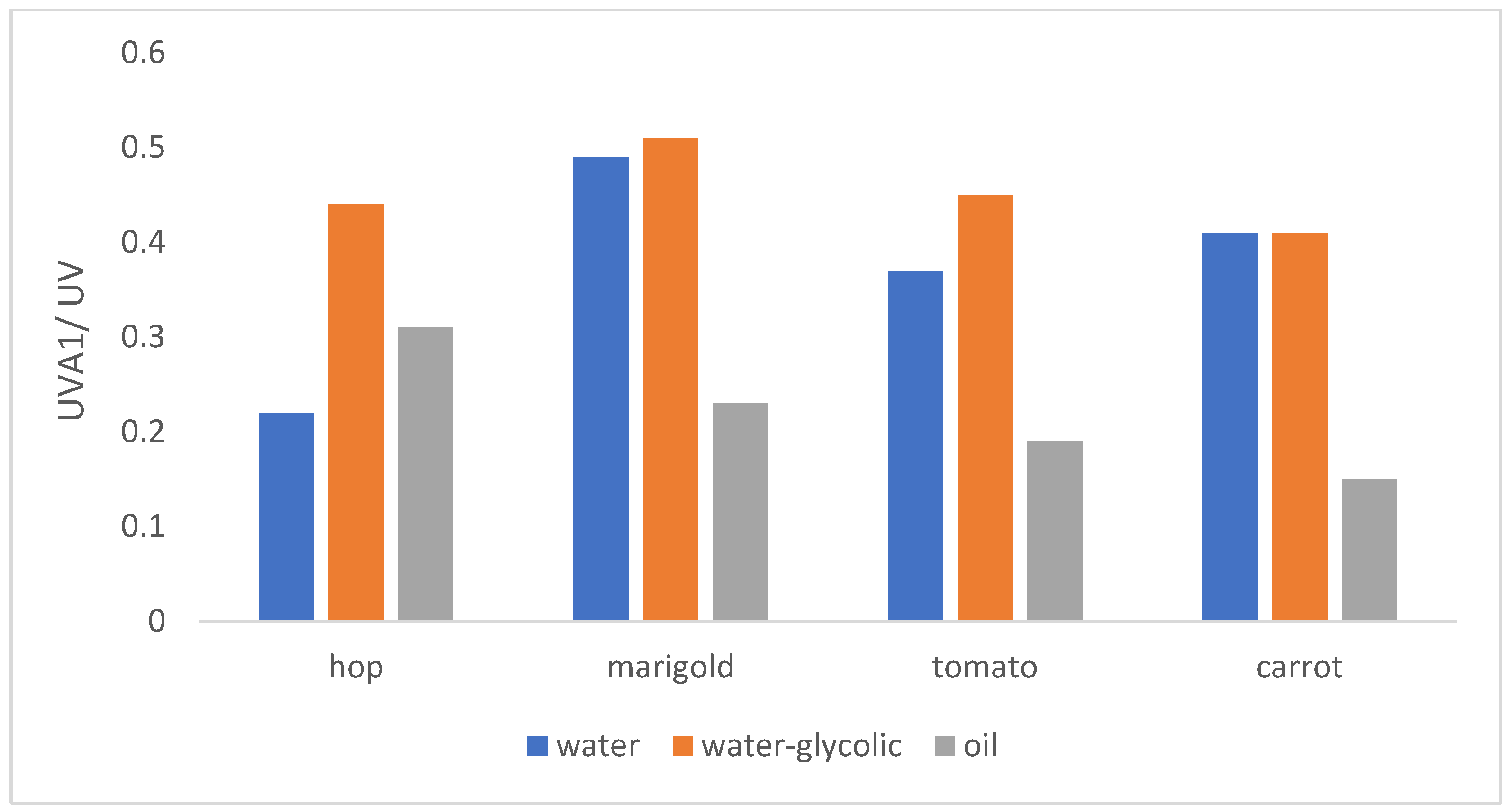
2.5.3. Ratio UVA1/UV
2.5.4. SUI (Spectral Uniformity Index) Parameter
2.5.5. Spectrophotometric Method of Sun Protection Factor (SPF) Determination
3. Results
3.1. Total Phenolic Content
3.2. Antioxidant Activity Determined by CUPRAC Method
3.3. Assessment of Radiation Protection Properties of Selected Extracts by Mathematical Indication Method
3.4. Determination of Spectrophotometric SPF in Examined Extracts
4. Discussion
4.1. Total Phenolic Content
4.2. Antioxidant Capacity
4.3. Method of Mathematical Indication of Radiation Protection
4.4. Determination of Spectrophotometric Sun Protection Factor (SPF)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kiriiri, G.; Mwangi, A.N.; Maru, S.M. Sunscreen products: Rationale for use, formulation development and regulatory con-siderations. Saudi Pharm. J. 2019, 27, 1009–1018. [Google Scholar]
- Schmalwieser, A.W.; Casale, G.R.; Colosimo, A.; Schmalwieser, S.S.; Siani, A.M. Review on Occupational Personal Solar UV Exposure Measurements. Atmosphere 2021, 12, 142. [Google Scholar] [CrossRef]
- Egambaram, O.P.; Pillai, S.K.; Ray, S.S. Materials Science Challenges in Skin UV Protection: A Review. Photochem. Photobiol. 2019, 96, 779–797. [Google Scholar] [CrossRef] [PubMed]
- Gromkowska-Kępka, K.J.; Puścion-Jakubik, A.; Markiewicz-Żukowska, R.; Socha, K. The impact of ultraviolet radiation on skin photoaging—Review of in vitro studies. J. Cosmet. Dermatol. 2021, 20, 3427–3431. [Google Scholar] [CrossRef] [PubMed]
- Lopes, F.C.P.S.; Sleiman, M.G.; Sebastian, K.; Bogucka, R.; Jacobs, E.A.; Adamson, A.S. UV Exposure and the Risk of Cuta-neous Melanoma in Skin of Color a Systematic Review. JAMA Dermatol. 2021, 157, 213–219. [Google Scholar] [CrossRef]
- Scheuer, C.; Pommergaard, H.C.; Rosenberg, J.; Gogenur, J. Melatonin’s Protective Effect Against UV Radiation: A Systematic Review of Clinical and Experimental Studies. Photodermatol. Photoimmunol. Photomed. 2014, 30, 180–188. [Google Scholar] [CrossRef]
- Priyanka, K.G.; Mayuri, S.; Rashmi, S. Natural Sunscreen Agents: A Review. Sch. Acad. J. Pharm. 2013, 2, 458–463. [Google Scholar]
- Sarbak, Z.; Jachymska-Sarbak, B.; Sarbak, A. Chemia w Kosmetyce i Kosmetologii; MedPharm Polska: Wrocław, Poland, 2013. [Google Scholar]
- Schueller, R.; Romanowski, P. Beginning Cosmetic Chemistry, 3rd ed.; Allured Business Media: Carol Stream, IL, USA, 2009. [Google Scholar]
- Lamer-Zarawska, E.; Chwała, C.; Gwardys, A. Rośliny w Kosmetyce i Kosmetologii Przeciwstarzeniowej; Wydawnictwo Lekarskie PZWL: Warszawa, Poland, 2012. [Google Scholar]
- Schueller, R.; Romanowski, P. Multifunctional Cosmetics; Marcel Dekker Inc.: New York, NY, USA, 2001. [Google Scholar]
- Martini, M.-C. Kosmetologia i Farmakologia Skóry; Wydawnictwo Lekarskie PZWL: Warszawa, Poland, 2009. [Google Scholar]
- Sikora, E.; Olszańska, M.; Ogonowski, J. Chemia i Technologia Kosmetyków; Wydawnictwo Politechniki Krakowskiej im. Tadeusza Kościuszki: Kraków, Poland, 2012. [Google Scholar]
- Moyal, D. The development of efficient sunscreens. Indian J. Dermatol. Venereol. Leprol. 2012, 78, 31–34. [Google Scholar] [CrossRef]
- Wang, S.Q.; Tanner, P.R.; Lim, H.W.; Nash, J.F. The evolution of sunscreen products in the United States—A 12-year cross sectional study. Photochem. Photobiol. Sci. 2013, 12, 197–202. [Google Scholar] [CrossRef]
- Nunes, A.R.; Rodrigues, A.L.M.; de Queiróz, D.B.; Vieira, I.G.P.; Neto, J.F.C.; Junior, J.T.C.; Tintino, S.R.; de Morais, S.M.; Coutinho, H.D.M. Photoprotective potential of medicinal plants from Cerrado biome (Brazil) in relation to phenolic content and antioxidant activity. J. Photochem. Photobiol. B Biol. 2018, 189, 119–123. [Google Scholar] [CrossRef]
- Napagoda, M.T.; Malkanthi, B.M.A.S.; Abayawardana, S.A.K.; Qader, M.M.; Jayasinghe, L. Photoprotective potential in some medicinal plants used to treat skin diseases in Sri Lanka. BMC Complement. Altern. Med. 2016, 16, 147. [Google Scholar] [CrossRef]
- Polonini, H.C.; Brandão, M.A.F.; Raposo, N.R.B. A natural broad-spectrum sunscreen formulated from the dried extract of Brazilian Lippia sericea as a single UV filter. RSC Adv. 2014, 4, 62566–62575. [Google Scholar] [CrossRef]
- Cefali, L.C.; Ataide, J.A.; Fernandes, A.R.; Sousa, I.M.D.O.; Gonçalves, F.C.D.S.; Eberlin, S.; Dávila, J.L.; Jozala, A.F.; Chaud, M.V.; Sanchez-Lopez, E.; et al. Flavonoid-Enriched Plant-Extract-Loaded Emulsion: A Novel Phytocosmetic Sunscreen Formulation with Antioxidant Properties. Antioxidants 2019, 8, 443. [Google Scholar] [CrossRef]
- Baldisserotto, A.; Buso, P.; Radice, M.; Dissette, V.; Lampronti, I.; Gambari, R.; Manfredini, S.; Vertuani, S. Moringa oleifera Leaf Extracts as Multifunctional Ingredients for “Natural and Organic” Sunscreens and Photoprotective Preparations. Molecules 2018, 23, 664. [Google Scholar] [CrossRef]
- Schueller, R.; Romanowski, P. Conditioning Agents for Hair and Skin; Marcel Dekker Inc.: New York, NY, USA, 1999. [Google Scholar]
- Cieszyńska, A.; Wieczorek, D. Estimation and Comparison of Ultraviolet Protection Properties of Essential Oil, Infusion and Extracts of Hop (Humulus lupulus L.). Towarozn. Probl. Jakości 2016, 1, 115–125. [Google Scholar]
- Unny, R.; Chauhan, A.K.; Joshi, Y.C. A Review on Potentiality of Medicinal Plants as the Source of New Contraceptive Principles. Phytomedicine 2016, 10, 233–260. [Google Scholar] [CrossRef]
- Tiwari, B.K.; Brunton, N.P.; Brennan, C.S. Handbook of Plant Food Phytochemicals Sources, Stability and Extraction; John Wiley & Sons Ltd.: Oxford, UK, 2013. [Google Scholar]
- Amparo, S.; Chisvert, A. Analysis of Cosmetic Products; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Rawson, A.; Patras, A.; Oomah, B.D.; Campos-Vega, R.; Hossain, M.B. Antioxidant Activity of Phytochemicals and Their Method of Analysis. Adv. Food Sci. Nutr. 2013, 2, 153–256. [Google Scholar] [CrossRef]
- Apak, R.; Güçlü, K.; Özyürek, M.; Celik, S.E. Mechanism of antioxidant capacity assays and the CUPRAC (cupric ion reducing antioxidant capacity) assay. Microchim. Acta 2007, 160, 413–419. [Google Scholar] [CrossRef]
- Timoumi, R.; Salem, I.B.; Amara, I.; Anabi, E.; Abid-Esefi, E. Protective Effects of Fennel Essential Oil Against Oxidative Stress and Genotoxicity Induced by the Insecticide Triflumuron in Human Colon Carcinoma Cells. Environ. Sci. Pollut. Res. Int. 2020, 27, 7957–7966. [Google Scholar] [CrossRef]
- Suva, M.A. Evaluation of Sun Protection Factor of Zingiber Officinale Roscoe Extract by Ultraviolet Spectroscopy Method. J. Pharma Sci. Technol. 2014, 3, 95–97. [Google Scholar]
- Dutra, E.A.; Oliveira, D.A.G.D.C.; Kedor-Hackmann, E.R.M.; Santoro, M.I.R.M. Determination of sun protection factor (SPF) of sunscreens by ultraviolet spectrophotometry. Rev. Bras. Ciências Farm. 2004, 40, 381–385. [Google Scholar] [CrossRef]
- Conde, E.; Moure, A.; Domínguez, H.; Parajó, J. Extraction of natural antioxidants from plant foods. In Separation, Extraction and Concentration Processes in the Food, Beverage and Nutraceutical Industries; Elsevier: Amsterdam, The Netherlands, 2013; pp. 506–594. [Google Scholar] [CrossRef]
- Cannell, R.J.P. Natural Products Isolation; Humana Press lnc.: Totowa, NJ, USA, 1998. [Google Scholar]
- Sun, T.; Simon, P.; Tanumihardjo, S.A. Antioxidant Phytochemicals and Antioxidant Capacity of Biofortified Carrots (Daucus carota L.) of Various Colors. J. Agric. Food Chem. 2009, 57, 4142–4147. [Google Scholar] [CrossRef] [PubMed]
- Bozalan, N.C.; Karadeniz, F. Carotenoid Profile, Total Phenolic Content, and Antioxidant Activity of Carrots. Int. J Food Prop. 2011, 14, 1060–1068. [Google Scholar] [CrossRef]
- Ćetković, G.S.; Djilas, S.M.; Čanadanović-Brunet, J.M.; Tumbas, V.T. Antioxidant properties of marigold extracts. Food Res. Int. 2004, 37, 643–650. [Google Scholar] [CrossRef]
- Gong, Y.; Liu, X.; He, W.-H.; Xu, H.-G.; Yuan, F.; Gao, Y.-X. Investigation into the antioxidant activity and chemical composition of alcoholic extracts from defatted marigold (Tagetes erecta L.) residue. Fitoterapia 2012, 83, 481–489. [Google Scholar] [CrossRef]
- García-Alonso, F.; Navarro-González, I.; Ros, G.; Periago, M. Assessment of the antioxidant properties of tomato extracts: A synergistic approach using in vitro chemical tests and cell-based assays. Acta Aliment. 2015, 44, 297–303. [Google Scholar] [CrossRef]
- Toor, R.K.; Savage, G.P. Antioxidant activity in different fractions of tomatoes. Food Res. Int. 2005, 38, 487–494. [Google Scholar] [CrossRef]
- Erge, H.S.; Karadenism, F. Bioactive compounds and antioxidant activity of tomato cultivars. Int. J Food Prop. 2011, 14, 968–977. [Google Scholar] [CrossRef]
- Bocquet, L.; Sahpaz, S.; Hilbert, J.L.; Rambaud, C.; Rivière, C. Humulus lupulus L., a very popular beer ingredient and medicinal plant: Overview of its phytochemistry, its bioactivity, and its biotechnology. Phytochem. Rev. 2018, 17, 1047–1090. [Google Scholar] [CrossRef]
- Önder, F.C.; Ay, M.; Sarker, S.D. Comparative study of antioxidant properties and total phenolic content of the extracts of Humulus lupulus L. and quantification of bioactive components by LC-MS/MS and GC-MS. J. Agric Food Chem. 2013, 61, 10498–10506. [Google Scholar]
- Maietti, A.; Brighenti, V.; Bonetti, G.; Tedeschi, P.; Prencipe, F.P.; Benvenuti, S. Metabolite profil-Metabolite profiling of flavonols and in vitro antioxidant activity of young shoots of wild Humulus lupulus L. (hop). J. Pharm. Biomed. Anal. 2017, 142, 28–34. [Google Scholar] [CrossRef]
- Abram, V.; Čeh, B.; Vidmar, M.; Hercezi, M.; Lazić, N.; Bucik, V.; Možina, S.S.; Košir, I.J.; Kač, M.; Demšar, L.; et al. A comparison of antioxidant and antimicrobial activity between hop leaves and hop cones. Ind. Crop. Prod. 2015, 64, 124–134. [Google Scholar] [CrossRef]
- Rowe, R.C.; Sheskey, P.J.; Owen, S.C. Handbook of Pharmaceutic Excipients, 6th ed.; Pharmaceutical Press and American Pharmacist Association: London, UK, 2009; pp. 721–722. [Google Scholar]
- Idson, B. Vitamins in Cosmetics, An Update Part II: Vitamin E. Drug Cosmet. Ind. 1990, 147, 20–25. [Google Scholar]
- Pisoschi, A.M.; Pop, A.; Cimpeanu, C.; Predoi, G. Antioxidant Capacity Determination in Plants and Plant-Derived Products: A Review. Oxidative Med. Cell. Longev. 2016, 2016, 9130976. [Google Scholar] [CrossRef]
- Amendola, D.; De Faveri, D.; Spigno, G. Grape marc phenolics: Extraction kinetics, quality and stability of extracts. J. Food Eng. 2010, 97, 384–392. [Google Scholar] [CrossRef]
- Ouchemoukh, S.; Hachoud, S.; Boudraham, H.; Mokrani, A.; Louaileche, H. Antioxidant activities of some dried fruits consumed in Algeria. LWT 2012, 49, 329–332. [Google Scholar] [CrossRef]
- Adnan, L.; Osman, A.; Hamid, A.A. Antioxidant Activity of Different Extracts of Red Pitaya (Hylocereus polyrhizus) Seed. Int. J. Food Prop. 2011, 14, 1171–1181. [Google Scholar] [CrossRef]
- Šamec, D.; Durgo, K.; Grúz, J.; Kremer, D.; Kosalec, I.; Piljac-Žegarac, J.; Salopek-Sondi, B. Genetic and phytochemical variability of six Teucrium arduini L. populations and their antioxidant/prooxidant behaviour examined by biochemical, macromolecule- and cell-based approaches. Food Chem. 2015, 186, 298–305. [Google Scholar] [CrossRef]
- Arora, N.; Pandey-Rai, S. GC-MS analysis of the essential oil of Celastrus paniculatus Willd: Seeds and antioxidant, anti-inflammatory study of its various solvent extracts. Ind. Crops Prod. 2014, 61, 345–351. [Google Scholar] [CrossRef]
- Weber, N.; Biehler, K.; Schwabe, K.; Haarhaus, B.; Quirin, K.-W.; Frank, U.; Schempp, C.M.; Wölfle, U. Hop Extract Acts as an Antioxidant with Antimicrobial Effects against Propionibacterium acnes and Staphylococcus aureus. Molecules 2019, 24, 223. [Google Scholar] [CrossRef]
- Sinha, P.; Srivastava, S.; Mishra, N.; Yadav, N.P. New Perspectives on Antiacne Plant Drugs: Contribution to Modern Therapeutics. BioMed Res. Int. 2014, 2014, 301304. [Google Scholar] [CrossRef] [PubMed]
- Lupo, M.P. Antioxidants and vitamins in cosmetics. Clin. Dermatol. 2001, 19, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Hoang, H.T.; Moon, J.-Y.; Lee, Y.-C. Natural Antioxidants from Plant Extracts in Skincare Cosmetics: Recent Applications, Challenges and Perspectives. Cosmetics 2021, 8, 106. [Google Scholar] [CrossRef]


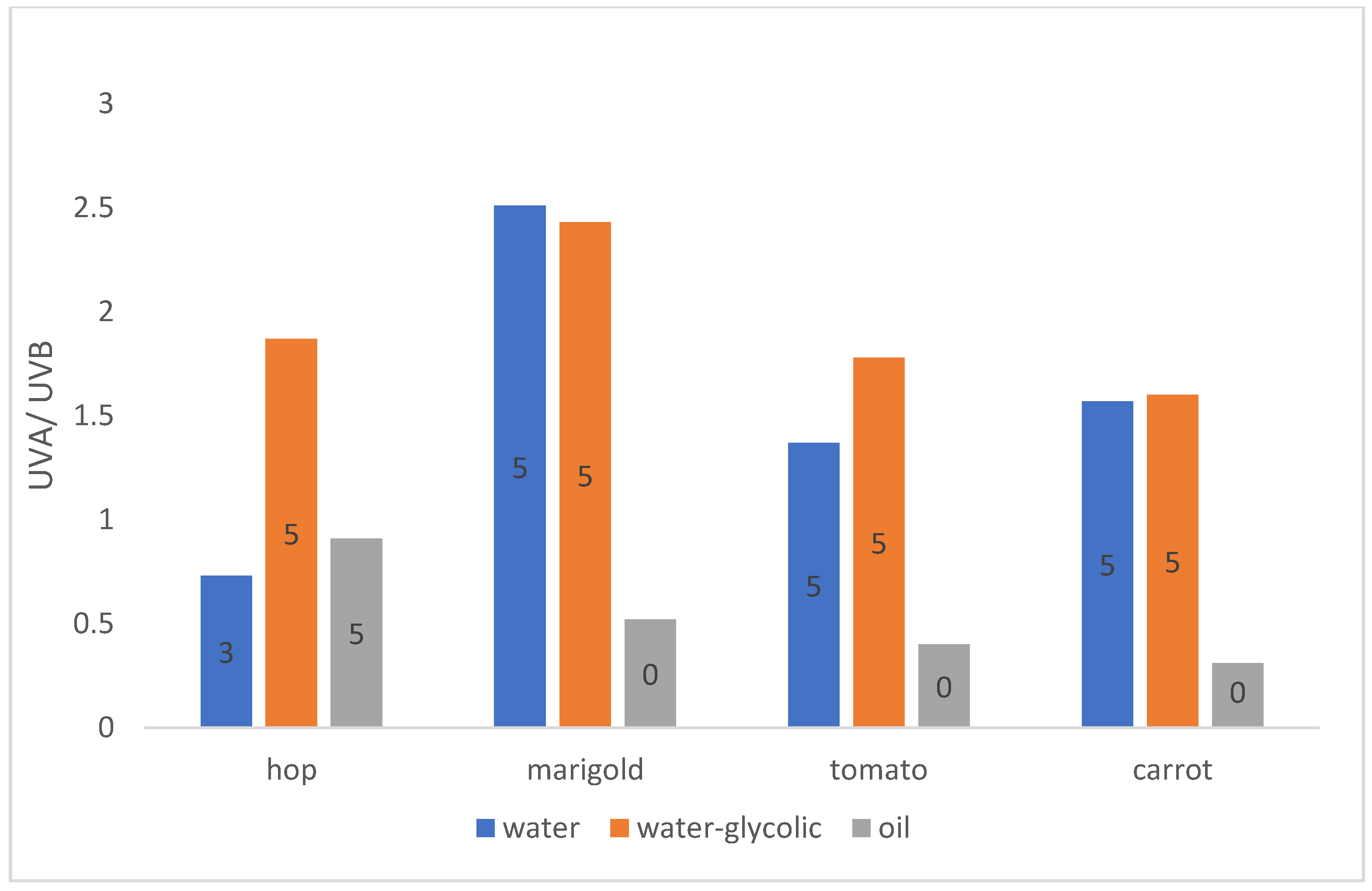

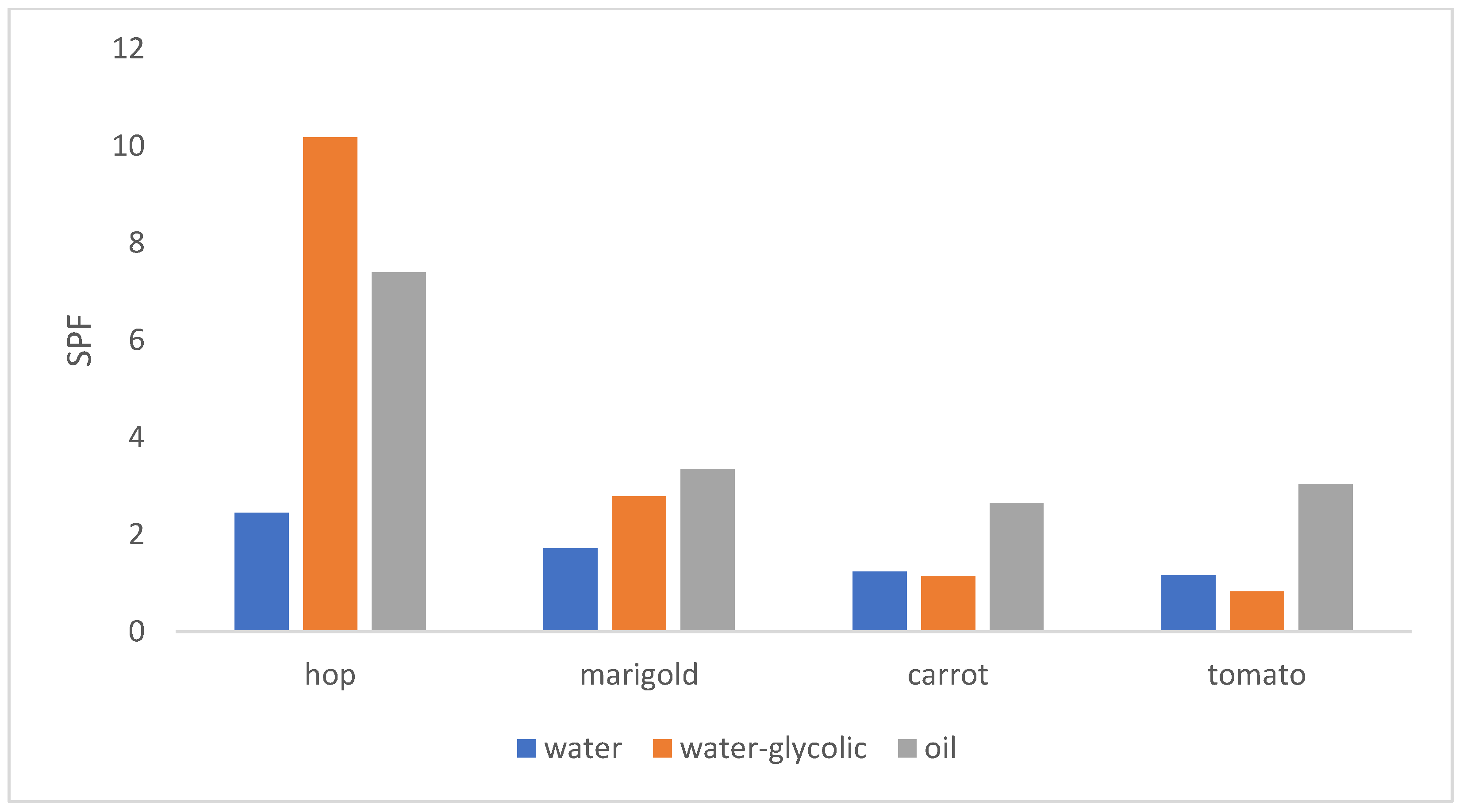
| Type of Extracts | TPC in 100 g Dried Plant [mg/100 g] CAE * |
|---|---|
| Water extract of marigold (unground petals) | 368.51 ± 58.94 |
| Water extract of marigold (powdered petald) | 1140.53 A ± 55.47 |
| Water-glycolic extract of marigold | 1214.24 B ± 28.88 |
| Water extract of hop cones | 1009.58 A ± 31.60 |
| Water-glycolic extract of hop cones | 4017.41 B ± 131.56 |
| Water extract of carrot | 188.97 B ± 7.68 |
| Water-glycolic extract of carrot | 164.81 A ± 2.01 |
| Water extract of tomato | 314.41 A ± 8.65 |
| Water-glycolic extract of tomato | 386.28 B ± 3.46 |
| Type of Extracts | Antioxidant Activity [mg/100 g] CAE * |
|---|---|
| Water extract of marigold (unground petals) | 75.76 ± 9.80 |
| Water extract of marigold (powdered petals) | 218.76 A ± 11.96 |
| Water-glycolic extract of marigold | 232.62 A ± 17.90 |
| Water extract of hop cones | 232.45 A ± 6.33 |
| Water-glycolic extract of hop cones | 1395.97 B ± 22.33 |
| Water extract of carrot | 32.30 A ± 2.38 |
| Water-glycolic extract of carrot | 38.59 B ± 0.02 |
| Water extract of tomato | 79.81 A ± 2.37 |
| Water-glycolic extract of tomato | 127.49 B ± 4.43 |
| Parameters | ||||
|---|---|---|---|---|
| UVA/UVB | UVA1/UV | Critical Wavelength [nm] | SUI | |
| Type of extract and its concentration | Number of stars assigned to cosmetic product | Protection level | Protection level | Protection level |
| Marigold water extract (0.4; 0.8; 1.6 mg/mL) | 5 | medium | maximum | high |
| Marigold water-glycolic extract (0.4; 0.8; 1.6 mg/mL) | 5 | medium | maximum | very high |
| Marigold oil extract (0.2; 0.4; 0.8; 1.6 mg/mL) | 0 | low | maximum | low |
| Hop cones water extract (0.2; 0.4; 0.8 mg/mL) | 3/4- for concentration 0.8 mg/mL | low | excellent | low/medium- for concentration 0.8 mg/ml |
| Hop cones water-glycolic extract (0.1; 0.2; 0.4 mg/mL) | 5 | medium | maximum | high |
| Hop cones oil extract (0.2; 0.4; 0.8; 1.6 mg/mL) | 5/4- for concentration 0.8 mg/mL | low | maximum | medium |
| Carrot water extract (0.2; 0.4; 0.8; 2,0; 4.0 mg/mL) | 5 | medium | maximum | medium |
| Carrot water-glycoli extract (0.4; 0.8; 2.0; 4.0 mg/mL) | 5 | Low- for concentration 0.8; 2.0 mg/mL, medium- for concentration 0.4; 4.0 mg/ml | maximum | medium |
| Carrot oil extract (0.2; 0.4; 0.8; 1.6 mg/mL) | 0 | Low- for concentration 0.2 mg/mL, no protection for the rest | Maximum- for concentration 0.2 mg/mL, excellent for concentration 0.4; 0.8 mg/mL, good for concentration 1.6 mg/mL | low |
| Tomato water extract (0.2; 0.4; 0.8; 2.0; 4.0 mg/mL) | 5 | low | maximum | medium |
| Tomato water-glycolic extract (0.4; 0.8; 1.6; 2.4; 3.2 mg/mL) | 5 | Medium- for concentration 0.4; 0.8; 1.6 mg/mL, low- for rest | maximum | High- for concentration 0.4 mg/mL, medium- for the rest |
| Tomato oil extract (0.2; 0.4; 0.8; 1.6 mg/mL) | 3- for concetration 0.2 mg/mL 0- for the rest | Low- for concentration 0.2 mg/mL, no protection for the rest | Maximum- for concentration 0.2; 0.4 mg/mL, excellent- for 0.8; 1.6 mg/mL | low |
| Type of Extract | Water (1 g) | Water-Glycolic | Oil | |
|---|---|---|---|---|
| Type of Plant | Concentration [mg Dried Plants/mL Extractant] | SPF | SPF | SPF |
| Marigold | 0.2 | - | - | 1.59 |
| 0.4 | 1.72 | 2.78 | 3.35 | |
| 0.8 | 3.70 | 4.93 | 6.79 | |
| 1.6 | 7.79 | 9.84 | 12.62 | |
| Hop | 0.1 | - | 3.18 | - |
| 0.2 | 1.27 | 5.80 | 4.30 | |
| 0.4 | 2.45 | 10.18 | 7.40 | |
| 0.8 | 5.58 | - | 14.19 | |
| 1.6 | - | - | 21.05 | |
| Carrot | 0.20 | 0.95 | - | 1.54 |
| 0.40 | 1.23 | 1.14 | 2.65 | |
| 0.80 | 1.87 | 1.31 | 5.54 | |
| 1.60 | - | - | 10.52 | |
| 2.00 | 4.10 | 2.33 | - | |
| 4.00 | 6.83 | 3.95 | - | |
| Tomato | 0.2 | 0.90 | - | 1.95 |
| 0.4 | 1.16 | 0.82 | 3.03 | |
| 0.8 | 1.58 | 1.28 | 6.24 | |
| 1.6 | - | 2.21 | 11.61 | |
| 2.0 | 2.99 | - | - | |
| 2.4 | - | 3.18 | - | |
| 3.2 | - | 3.56 | - | |
| 4.0 | 5.46 | - | - | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurzawa, M.; Wilczyńska, E.; Brudzyńska, P.; Sionkowska, A. Total Phenolic Content, Antioxidant Capacity and UV Radiation Protection Properties of Marigold (Calendula officinalis), Carrot (Daucus carota), Tomato (Solanum lycopersicum) and Hop (Humulus lupulus) Extracts. Cosmetics 2022, 9, 134. https://doi.org/10.3390/cosmetics9060134
Kurzawa M, Wilczyńska E, Brudzyńska P, Sionkowska A. Total Phenolic Content, Antioxidant Capacity and UV Radiation Protection Properties of Marigold (Calendula officinalis), Carrot (Daucus carota), Tomato (Solanum lycopersicum) and Hop (Humulus lupulus) Extracts. Cosmetics. 2022; 9(6):134. https://doi.org/10.3390/cosmetics9060134
Chicago/Turabian StyleKurzawa, Marzanna, Emilia Wilczyńska, Patrycja Brudzyńska, and Alina Sionkowska. 2022. "Total Phenolic Content, Antioxidant Capacity and UV Radiation Protection Properties of Marigold (Calendula officinalis), Carrot (Daucus carota), Tomato (Solanum lycopersicum) and Hop (Humulus lupulus) Extracts" Cosmetics 9, no. 6: 134. https://doi.org/10.3390/cosmetics9060134
APA StyleKurzawa, M., Wilczyńska, E., Brudzyńska, P., & Sionkowska, A. (2022). Total Phenolic Content, Antioxidant Capacity and UV Radiation Protection Properties of Marigold (Calendula officinalis), Carrot (Daucus carota), Tomato (Solanum lycopersicum) and Hop (Humulus lupulus) Extracts. Cosmetics, 9(6), 134. https://doi.org/10.3390/cosmetics9060134







