Evaluation of Tolerance and Trichological Efficacy of a Food Supplement in Men and Women with Telogen Effluvium-like Disorder
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Inclusion and Exclusion Criteria
2.3. Test Product
2.4. Study Evaluations
2.5. Study Endpoints
2.6. Statistical Analysis
3. Results
3.1. Demographic and Baseline Characteristics
3.2. Primary Endpoints
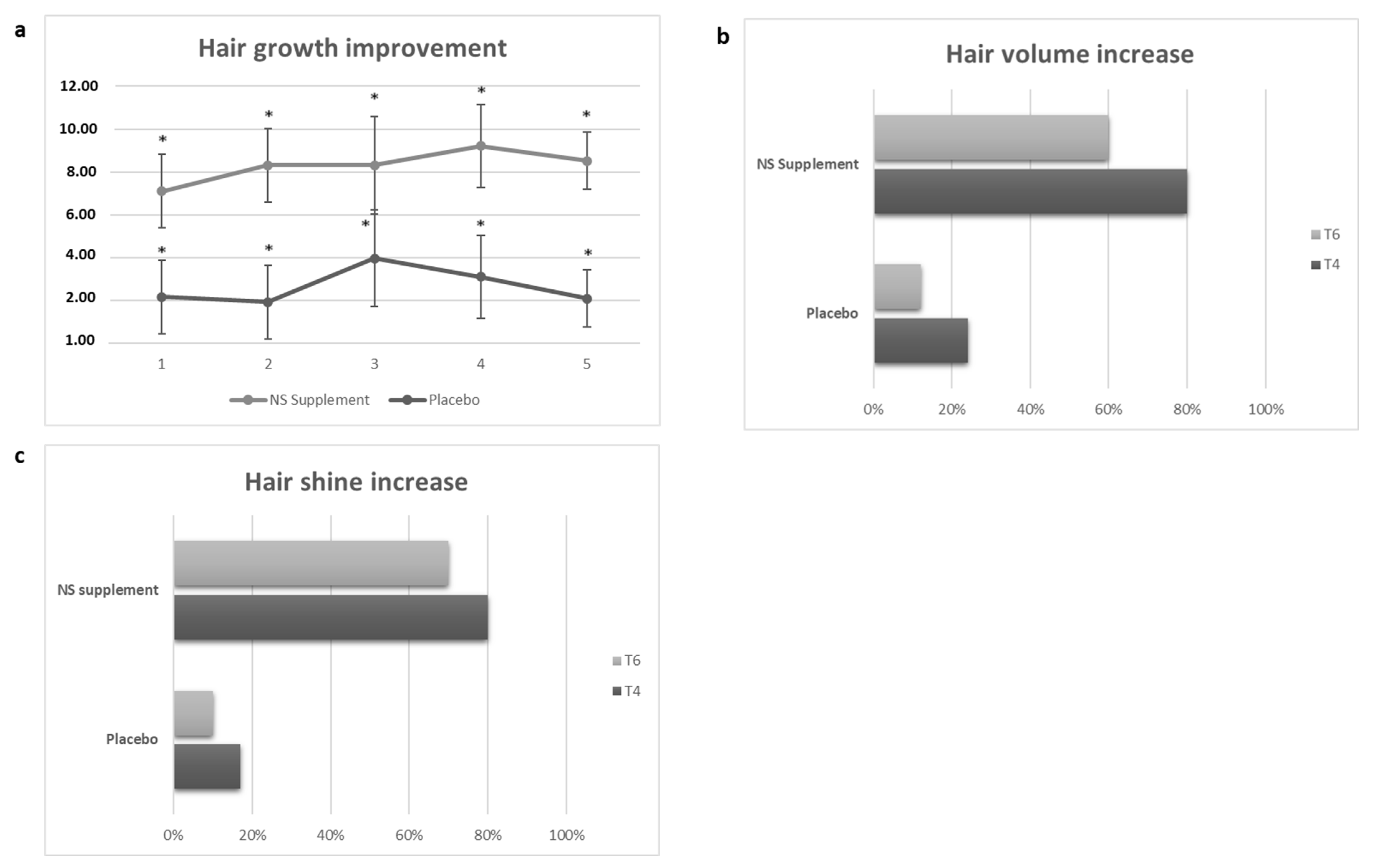
3.3. Secondary Endpoints
3.3.1. Pull Test
3.3.2. Self-Assessment Questionnaire
3.3.3. Safety
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Phillips, T.G.; Slomiany, W.P.; Allison, R. Hair Loss: Common Causes and Treatment. Am. Fam. Physician 2017, 96, 371–378. [Google Scholar] [PubMed]
- Sadick, N.S.; Callender, V.D.; Kircik, L.H.; Kogan, S. New Insight Into the Pathophysiology of Hair Loss Trigger a Paradigm Shift in the Treatment Approach. J. Drugs Derm. 2017, 16, s135–s140. [Google Scholar]
- Cash, T.F.; Price, V.H.; Savin, R.C. Psychological effects of androgenetic alopecia on women: Comparisons with balding men and with female control subjects. J. Am. Acad. Derm. 1993, 29, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Tabolli, S.; Sampogna, F.; di Pietro, C.; Mannooranparampil, T.J.; Ribuffo, M.; Abeni, D. Health status, coping strategies, and alexithymia in subjects with androgenetic alopecia: A questionnaire study. Am. J. Clin. Derm. 2013, 14, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Han, S.H.; Byun, J.W.; Lee, W.S.; Kang, H.; Kye, Y.-C.; Kim, K.-H.; Kim, D.-W.; Kim, M.-B.; Kim, S.-J.; Kim, H.O.; et al. Quality of life assessment in male patients with androgenetic alopecia: Result of a prospective, multicenter study. Ann. Derm. 2012, 24, 311–318. [Google Scholar] [CrossRef]
- Pinto, D.; Giuliani, G.; Marzani, B. 209 Galeopsis segetum Necker extracts for the prevention and treatment of hair loss. J. Investig. Derm. 2016, 136, S196. [Google Scholar] [CrossRef]
- Giammaria, G.; Anna, B.; Barbara, M. Use of a Vegetal Extract for the Prevention and Treatment of Hair Loss. U.S. Patent EP3062636B1, 27 June 2018. [Google Scholar]
- Rashed, M.M.; Shallan, M.; Mohamed, D.A.; Fouda, K.; Hanna, L.M. Biological Evaluation of Anti-androgenic Effect of Some Plant Foods. J. Food Nutr. Res. 2015, 2, 645–651. [Google Scholar] [CrossRef]
- Liu, J.; Shimizu, K.; Kondo, R. Anti-androgenic activity of fatty acids. Chem. Biodivers. 2009, 6, 503–512. [Google Scholar] [CrossRef]
- Le Floc’h, C.; Cheniti, A.; Connétable, S.; Piccardi, N.; Vincenzi, C.; Tosti, A. Effect of a nutritional supplement on hair loss in women. J. Cosmet. Derm. 2015, 14, 76–82. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Wachtel-Galor, S. Herbal Medicine: Biomolecular and Clinical Aspects, 2nd ed.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2011. Available online: https://www.ncbi.nlm.nih.gov/books/NBK92771/ (accessed on 28 June 2022).
- Wang, M.; Simon, J.E.; Aviles, I.F.; He, K.; Zheng, Q.Y.; Tadmor, Y. Analysis of antioxidative phenolic compounds in artichoke (Cynara scolymus L.). J. Agric. Food Chem. 2003, 51, 601–608. [Google Scholar] [CrossRef]
- Jahan, S.; Munir, F.; Razak, S.; Mehboob, A.; Ain, Q.U.; Ullah, H.; Afsar, T.; Shaheen, G.; Almajwal, A. Ameliorative effects of rutin against metabolic, biochemical and hormonal disturbances in polycystic ovary syndrome in rats. J. Ovarian Res. 2016, 9, 86. [Google Scholar] [CrossRef] [PubMed]
- Almohanna, H.M.; Ahmed, A.A.; Tsatalis, J.P.; Tosti, A. The Role of Vitamins and Minerals in Hair Loss: A Review. Derm. Ther. 2019, 9, 51–70. [Google Scholar] [CrossRef] [PubMed]
- Dhaher, S.A.; Yacoub, A.A.; Jacob, A.A. Estimation of Zinc and Iron Levels in the Serum and Hair of Women with Androgenetic Alopecia: Case-control Study. Indian. J. Derm. 2018, 63, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Ramot, Y.; Pietilä, M.; Giuliani, G.; Rinaldi, F.; Alhonen, L.; Paus, R. Polyamines and hair: A couple in search of perfection. Exp. Derm. 2010, 19, 784–790. [Google Scholar] [CrossRef]
- Harrison, S.; Bergfeld, W. Diffuse hair loss: Its triggers and management. Clevel. Clin. J. Med. 2009, 76, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Finner, A.M. Nutrition and hair: Deficiencies and supplements. Dermatol. Clin. 2013, 31, 167–172. [Google Scholar] [CrossRef]
- Piccardi, N.; Manissier, P. Nutrition and nutritional supplementation: Impact on skin health and beauty. Dermatoendocrinol 2009, 1, 271–274. [Google Scholar] [CrossRef]
- Peyravian, N.; Deo, S.; Daunert, S.; Jimenez, J.J. The Inflammatory Aspect of Male and Female Pattern Hair Loss. J. Inflamm. Res. 2020, 13, 879–881. [Google Scholar] [CrossRef]
- Choi, S.; Zhang, B.; Ma, S.; Gonzalez-Celeiro, M.; Stein, D.; Jin, X.; Kim, S.T.; Kang, Y.L.; Besnard, A.; Rezza, A.; et al. Corticosterone inhibits GAS6 to govern hair follicle stem-cell quiescence. Nature 2021, 592, 428–432. [Google Scholar] [CrossRef]
- Breitkopf, T.; Leung, G.; Yu, M.; Wang, E.; McElwee, K.J. The basic science of hair biology: What are the causal mechanisms for the disordered hair follicle? Derm. Clin. 2013, 31, 1–19. [Google Scholar] [CrossRef]
- Belfrit List. Available online: https://ec.europa.eu/growth/tools-databases/tris/en/search/?trisaction=search.detail&year=2017&num=276 (accessed on 28 June 2022).
- Patent number EP2124922B1 (A2): Composition Comprising Rutin and Polyunsaturated Fatty Acid having an Inhibitory Activity on 5 Alpha-Reductase. Available online: https://xueshu.baidu.com/s?wd=paperuri:(8951b897917cb8e6b2e50c9213c155a3)&filter=sc_long_sign&sc_ks_para=q%3DComposi-tion+comprising+rutin+and+polyunsaturated+fatty+acid+having+an+inhibitory+activity+on+5+%CE%B1-reductase&tn=SE_baiduxueshu_c1gjeupa&ie=utf-8&sc_us=13837088404048465021 (accessed on 28 June 2022).
- Naieni, F.F.; Ebrahimi, B.; Vakilian, H.R.; Shahmoradi, Z. Serum iron, zinc, and copper concentration in premature graying of hair. Biol. Trace. Elem. Res. 2012, 146, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Pietila, M.; Alhonen, L.; Halmekyto, M.; Kanter, P.; Janne, J.; Porter, C.W. Activation of polyamine catabolism profoundly alters tissue polyamine pools and affects hair growth and female fertility in transgenic mice overexpressing spermidine/spermine N1-acetyltransferase. J. Biol. Chem. 1997, 272, 18746–18751. [Google Scholar] [CrossRef] [PubMed]
- Pietila, M.; Parkkinen, J.J.; Alhonen, L.; Janne, J. Relation of skin polyamines to the hairless phenotype in transgenic mice overexpressing spermidine/spermine N-acetyltransferase. J. Invest. Derm. 2001, 116, 801–805. [Google Scholar] [CrossRef] [PubMed]
- Soler, A.P.; Gilliard, G.; Megosh, L.C.; O’Brien, T.G. Modulation of murine hair follicle function by alterations in ornithine decarboxylase activity. J. Invest. Derm. 1996, 106, 1108–1113. [Google Scholar] [CrossRef]
- Giammaria, G.; Anna, B.; Antonio, M.; Antonio, L. Dosage Forms for Oral Administration of Active Substances. U.S. Patent EP2988782B1, 2 March 2016. [Google Scholar]
| Demographic | NS Supplement | Placebo |
|---|---|---|
| Men (n, %) | 13 (43%) | 14 (46%) |
| Women (n, %) | 17 (57%) | 16 (54%) |
| Age (y, mean ± DS) | 45.9 ± 11.6 | 43.3 ± 12.8 |
| NS Supplement | Placebo | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T1 | T2 | T3 | T4 | T5 | T6 | T1 | T2 | T3 | T4 | T5 | T6 | |
| Anagen Hair * | 12% a,b | 24% a,b | 29% a,b | 33% a,b | 28% a,b | 17% a,b | 1% | 4% | 0% | 1% | 0% | 0% |
| Hair Density | 12% a,b | 22% a,b | 31% a,b | 35% a,b | 33% a,b | 15% a,b | 1% | 5% | 2% | 11% | 2% | 1% |
| Vellus Hair | −4% a,b | −14% a,b | −21% a,b | −27% a,b | −15% a,b | −9% a,b | 0% | −1% | −3% | −6% | 3% a | 0% |
| NS Supplement | Placebo | |||||
|---|---|---|---|---|---|---|
| T4 | T5 | T6 | T4 | T5 | T6 | |
| Pull Test * | 54% a,b | 42% a,b | 38% a,b | 21% | 22% | 4% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rinaldi, F.; Marzani, B.; Pinto, D. Evaluation of Tolerance and Trichological Efficacy of a Food Supplement in Men and Women with Telogen Effluvium-like Disorder. Cosmetics 2022, 9, 135. https://doi.org/10.3390/cosmetics9060135
Rinaldi F, Marzani B, Pinto D. Evaluation of Tolerance and Trichological Efficacy of a Food Supplement in Men and Women with Telogen Effluvium-like Disorder. Cosmetics. 2022; 9(6):135. https://doi.org/10.3390/cosmetics9060135
Chicago/Turabian StyleRinaldi, Fabio, Barbara Marzani, and Daniela Pinto. 2022. "Evaluation of Tolerance and Trichological Efficacy of a Food Supplement in Men and Women with Telogen Effluvium-like Disorder" Cosmetics 9, no. 6: 135. https://doi.org/10.3390/cosmetics9060135
APA StyleRinaldi, F., Marzani, B., & Pinto, D. (2022). Evaluation of Tolerance and Trichological Efficacy of a Food Supplement in Men and Women with Telogen Effluvium-like Disorder. Cosmetics, 9(6), 135. https://doi.org/10.3390/cosmetics9060135






