Recent Green Technologies in Natural Stilbenoids Production and Extraction: The Next Chapter in the Cosmetic Industry
Abstract
:1. Introduction
2. Stilbenoids
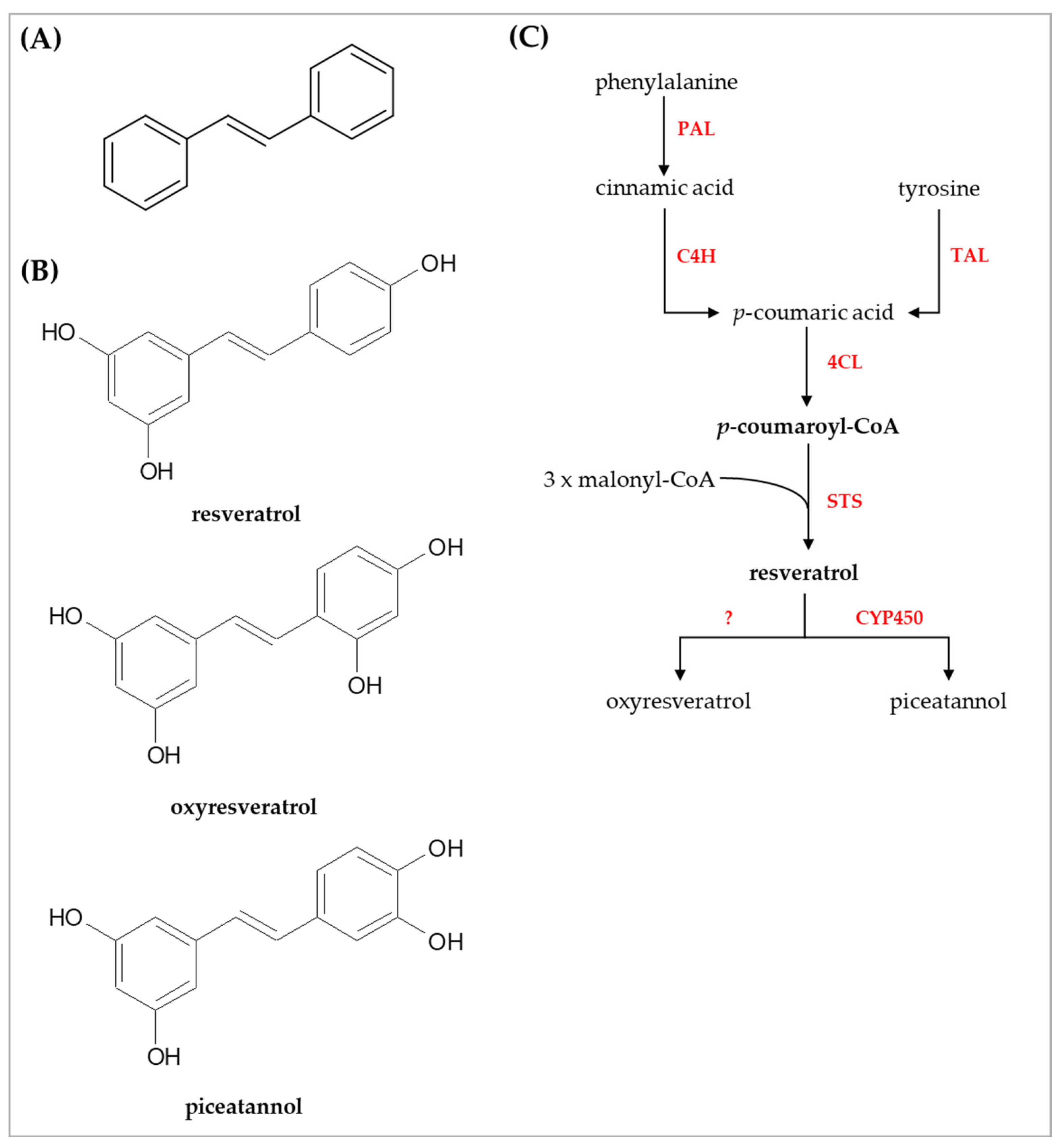
2.1. Chemical Structures and Biosynthesis
2.2. Plant Sources
2.3. Bioactivities
2.4. Safety
3. Current Green Technologies for Stilbenoids Production
3.1. Green Solvent Extraction
3.2. Plant Cell Cultures
4. Future Research Challenges and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alves, T.F.R.; Morsink, M.; Batain, F.; Chaud, M.V.; Almeida, T.; Fernandes, D.A.; da Silva, C.F.; Souto, E.B.; Severino, P. Applications of Natural, Semi-Synthetic, and Synthetic Polymers in Cosmetic Formulations. Cosmetics 2020, 7, 75. [Google Scholar] [CrossRef]
- He, H.; Li, A.; Li, S.; Tang, J.; Li, L.; Xiong, L. Natural Components in Sunscreens: Topical Formulations with Sun Protection Factor (SPF). Biomed. Pharmacother. 2021, 134, 111161. [Google Scholar] [CrossRef]
- Kostyuk, V.; Potapovich, A.; Albuhaydar, A.R.; Mayer, W.; De Luca, C.; Korkina, L. Natural Substances for Prevention of Skin Photoaging: Screening Systems in the Development of Sunscreen and Rejuvenation Cosmetics. Rejuvenation Res. 2018, 21, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Barbulova, A.; Apone, F.; Colucci, G. Plant Cell Cultures as Source of Cosmetic Active Ingredients. Cosmetics 2014, 1, 94–104. [Google Scholar] [CrossRef]
- Juliano, C.; Magrini, G.A. Cosmetic Ingredients as Emerging Pollutants of Environmental and Health Concern. A Mini-Review. Cosmetics 2017, 4, 11. [Google Scholar] [CrossRef]
- Guillerme, J.-B.; Couteau, C.; Coiffard, L. Applications for Marine Resources in Cosmetics. Cosmetics 2017, 4, 35. [Google Scholar] [CrossRef]
- Morais, T.; Cotas, J.; Pacheco, D.; Pereira, L. Seaweeds Compounds: An Ecosustainable Source of Cosmetic Ingredients? Cosmetics 2021, 8, 8. [Google Scholar] [CrossRef]
- Xi, X.; Li, J.; Guo, S.; Li, Y.; Xu, F.; Zheng, M.; Cao, H.; Cui, X.; Guo, H.; Han, C. The Potential of Using Bee Pollen in Cosmetics: A Review. J. Oleo Sci. 2018, 67, 1071–1082. [Google Scholar] [CrossRef]
- Farràs, A.; Cásedas, G.; Les, F.; Terrado, E.M.; Mitjans, M.; López, V. Evaluation of Anti-Tyrosinase and Antioxidant Properties of Four Fern Species for Potential Cosmetic Applications. Forests 2019, 10, 179. [Google Scholar] [CrossRef]
- Akinwumi, B.C.; Bordun, K.-A.M.; Anderson, H.D. Biological Activities of Stilbenoids. Int. J. Mol. Sci. 2018, 19, 792. [Google Scholar] [CrossRef] [Green Version]
- Zillich, O.V.; Schweiggert-Weisz, U.; Eisner, P.; Kerscher, M. Polyphenols as Active Ingredients for Cosmetic Products. Int. J. Cosmet. Sci. 2015, 37, 455–464. [Google Scholar] [CrossRef] [PubMed]
- de Lima Cherubim, D.J.; Buzanello Martins, C.V.; Oliveira Fariña, L.; da Silva de Lucca, R.A. Polyphenols as Natural Antioxidants in Cosmetics Applications. J. Cosmet. Dermatol. 2020, 19, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Likhitwitayawuid, K. Oxyresveratrol: Sources, Productions, Biological Activities, Pharmacokinetics, and Delivery Systems. Molecules 2021, 26, 4212. [Google Scholar] [CrossRef]
- Nagapan, T.S.; Ghazali, A.R.; Basri, D.F.; Lim, W.N. Photoprotective Effect of Stilbenes and Its Derivatives Against Ultraviolet Radiation-Induced Skin Disorders. Biomed. Pharmacol. J. 2018, 11, 1199–1208. [Google Scholar] [CrossRef]
- Mattio, L.M.; Catinella, G.; Dallavalle, S.; Pinto, A. Stilbenoids: A Natural Arsenal against Bacterial Pathogens. Antibiotics 2020, 9, 336. [Google Scholar] [CrossRef]
- Soural, I.; Vrchotová, N.; Tříska, J.; Balík, J.; Horník, Š.; Cuřínová, P.; Sýkora, J. Various Extraction Methods for Obtaining Stilbenes from Grape Cane of Vitis Vinifera L. Molecules 2015, 20, 6093–6112. [Google Scholar] [CrossRef]
- Kanda, H.; Oishi, K.; Machmudah, S.; Wahyudiono; Goto, M. Ethanol-Free Extraction of Resveratrol and Its Glycoside from Japanese Knotweed Rhizome by Liquefied Dimethyl Ether without Pretreatments. Asia-Pacific J. Chem. Eng. 2021, 16, e2600. [Google Scholar] [CrossRef]
- Feng, C.; Chen, J.; Ye, W.; Liao, K.; Wang, Z.; Song, X.; Qiao, M. Synthetic Biology-Driven Microbial Production of Resveratrol: Advances and Perspectives. Front. Bioeng. Biotechnol. 2022, 10, 833920. [Google Scholar] [CrossRef]
- Karimi, A.; Krähmer, A.; Herwig, N.; Schulz, H.; Hadian, J.; Meiners, T. Variation of Secondary Metabolite Profile of Zataria Multiflora Boiss. Populations Linked to Geographic, Climatic, and Edaphic Factors. Front. Plant Sci. 2020, 11, 969. [Google Scholar] [CrossRef]
- Aneklaphakij, C.; Saigo, T.; Watanabe, M.; Naake, T.; Fernie, A.R.; Bunsupa, S.; Satitpatipan, V.; Tohge, T. Diversity of Chemical Structures and Biosynthesis of Polyphenols in Nut-Bearing Species. Front. Plant Sci. 2021, 12, 440. [Google Scholar] [CrossRef]
- Tohge, T.; Wendenburg, R.; Ishihara, H.; Nakabayashi, R.; Watanabe, M.; Sulpice, R.; Hoefgen, R.; Takayama, H.; Saito, K.; Stitt, M.; et al. Characterization of a Recently Evolved Flavonol-Phenylacyltransferase Gene Provides Signatures of Natural Light Selection in Brassicaceae. Nat. Commun. 2016, 7, 12399. [Google Scholar] [CrossRef] [PubMed]
- Vermerris, W.; Nicholson, R. Phenolic Compound Biochemistry; Springer Dordrecht: Dordrecht, The Netherlands, 2006; ISBN 978-1-4020-5163-0. [Google Scholar]
- Milke, L.; Aschenbrenner, J.; Marienhagen, J.; Kallscheuer, N. Production of Plant-Derived Polyphenols in Microorganisms: Current State and Perspectives. Appl. Microbiol. Biotechnol. 2018, 102, 1575–1585. [Google Scholar] [CrossRef] [PubMed]
- Afendi, F.M.; Okada, T.; Yamazaki, M.; Hirai-Morita, A.; Nakamura, Y.; Nakamura, K.; Ikeda, S.; Takahashi, H.; Amin, A.U.M.; Darusman, L.K.; et al. KNApSAcK Family Databases: Integrated Metabolite–Plant Species Databases for Multifaceted Plant Research. Plant Cell Physiol. 2012, 53, e1. [Google Scholar] [CrossRef] [PubMed]
- Ratz-Łyko, A.; Arct, J. Resveratrol as an Active Ingredient for Cosmetic and Dermatological Applications: A Review. J. Cosmet. Laser Ther. 2019, 21, 84–90. [Google Scholar] [CrossRef]
- Salehi, B.; Mishra, A.P.; Nigam, M.; Sener, B.; Kilic, M.; Sharifi-Rad, M.; Fokou, P.V.; Martins, N.; Sharifi-Rad, J. Resveratrol: A Double-Edged Sword in Health Benefits. Biomedicines 2018, 6, 91. [Google Scholar] [CrossRef]
- Tian, B.; Liu, J. Resveratrol: A Review of Plant Sources, Synthesis, Stability, Modification and Food Application. J. Sci. Food Agric. 2020, 100, 1392–1404. [Google Scholar] [CrossRef]
- Maneechai, S.; Likhitwitayawuid, K.; Sritularak, B.; Palanuvej, C.; Ruangrungsi, N.; Sirisa-Ard, P. Quantitative Analysis of Oxyresveratrol Content in Artocarpus lakoocha and “Puag-Haad”. Med. Princ. Pract. 2009, 18, 223–227. [Google Scholar] [CrossRef]
- Aneklaphakij, C.; Bunsupa, S.; Sirichamorn, Y.; Bongcheewin, B.; Satitpatipan, V. Taxonomic Notes on the ‘Mahat’ (Artocarpus lacucha and A. thailandicus, Moraceae) Species Complex in Thailand. Plants 2020, 9, 391. [Google Scholar] [CrossRef]
- Wongon, M.; Limpeanchob, N. Inhibitory Effect of Artocarpus Lakoocha Roxb and Oxyresveratrol on α-Glucosidase and Sugar Digestion in Caco-2 Cells. Heliyon 2020, 6, e03458. [Google Scholar] [CrossRef]
- Zhou, J.; Li, S.-X.; Wang, W.; Guo, X.-Y.; Lu, X.-Y.; Yan, X.-P.; Huang, D.; Wei, B.-Y.; Cao, L. Variations in the Levels of Mulberroside A, Oxyresveratrol, and Resveratrol in Mulberries in Different Seasons and during Growth. Sci. World J. 2013, 2013, 380692. [Google Scholar] [CrossRef] [Green Version]
- Inyai, C.; Yusakul, G.; Komaikul, J.; Kitisripanya, T.; Likhitwitayawuid, K.; Sritularak, B.; Putalun, W. Improvement of Stilbene Production by Mulberry Morus alba Root Culture via Precursor Feeding and Co-Elicitation. Bioprocess Biosyst. Eng. 2021, 44, 653–660. [Google Scholar] [CrossRef]
- Lu, H.-P.; Jia, Y.-N.; Peng, Y.-L.; Yu, Y.; Sun, S.-L.; Yue, M.-T.; Pan, M.-H.; Zeng, L.-S.; Xu, L. Oxyresveratrol, a Stilbene Compound from Morus alba L. Twig Extract Active Against Trichophyton Rubrum. Phyther. Res. 2017, 31, 1842–1848. [Google Scholar] [CrossRef] [PubMed]
- Kershaw, J.; Kim, K.-H. The Therapeutic Potential of Piceatannol, a Natural Stilbene, in Metabolic Diseases: A Review. J. Med. Food 2017, 20, 427–438. [Google Scholar] [CrossRef] [PubMed]
- Viñas, P.; Martínez-Castillo, N.; Campillo, N.; Hernández-Córdoba, M. Directly Suspended Droplet Microextraction with in Injection-Port Derivatization Coupled to Gas Chromatography-Mass Spectrometry for the Analysis of Polyphenols in Herbal Infusions, Fruits and Functional Foods. J. Chromatogr. A 2011, 1218, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Matsui, Y.; Sugiyama, K.; Kamei, M.; Takahashi, T.; Suzuki, T.; Katagata, Y.; Ito, T. Extract of Passion Fruit (Passiflora edulis) Seed Containing High Amounts of Piceatannol Inhibits Melanogenesis and Promotes Collagen Synthesis. J. Agric. Food Chem. 2010, 58, 11112–11118. [Google Scholar] [CrossRef]
- Rimando, A.M.; Kalt, W.; Magee, J.B.; Dewey, A.J.; Ballington, J.R. Resveratrol, Pterostilbene, and Piceatannol in Vaccinium Berries. J. Agric. Food Chem. 2004, 52, 4713–4719. [Google Scholar] [CrossRef]
- Kammeyer, A.; Luiten, R.M. Oxidation Events and Skin Aging. Ageing Res. Rev. 2015, 21, 16–29. [Google Scholar] [CrossRef]
- Afaq, F.; Adhami, V.M.; Mukhtar, H. Photochemoprevention of Ultraviolet B Signaling and Photocarcinogenesis. Mutat. Res. Mol. Mech. Mutagen. 2005, 571, 153–173. [Google Scholar] [CrossRef]
- Malhotra, A.; Bath, S.; Elbarbry, F. An Organ System Approach to Explore the Antioxidative, Anti-Inflammatory, and Cytoprotective Actions of Resveratrol. Oxidative Med. Cell. Longev. 2015, 2015, 803971. [Google Scholar] [CrossRef]
- Lorenz, P.; Roychowdhury, S.; Engelmann, M.; Wolf, G.; Horn, T.F.W. Oxyresveratrol and Resveratrol Are Potent Antioxidants and Free Radical Scavengers: Effect on Nitrosative and Oxidative Stress Derived from Microglial Cells. Nitric Oxide 2003, 9, 64–76. [Google Scholar] [CrossRef]
- Aftab, N.; Likhitwitayawuid, K.; Vieira, A. Comparative Antioxidant Activities and Synergism of Resveratrol and Oxyresveratrol. Nat. Prod. Res. 2010, 24, 1726–1733. [Google Scholar] [CrossRef] [PubMed]
- Krambeck, K.; Oliveira, A.; Santos, D.; Pintado, M.M.; Baptista Silva, J.; Sousa Lobo, J.M.; Amaral, M.H. Identification and Quantification of Stilbenes (Piceatannol and Resveratrol) in Passiflora edulis By-Products. Pharmaceuticals 2020, 13, 73. [Google Scholar] [CrossRef] [PubMed]
- Piotrowska, H.; Kucinska, M.; Murias, M. Biological Activity of Piceatannol: Leaving the Shadow of Resveratrol. Mutat. Res. Mutat. Res. 2012, 750, 60–82. [Google Scholar] [CrossRef] [PubMed]
- Huertas, A.C.M.; Schmelzer, C.E.H.; Hoehenwarter, W.; Heyroth, F.; Heinz, A. Molecular-Level Insights into Aging Processes of Skin Elastin. Biochimie 2016, 128–129, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Varani, J.; Warner, R.L.; Gharaee-Kermani, M.; Phan, S.H.; Kang, S.; Chung, J.H.; Wang, Z.Q.; Datta, S.C.; Fisher, G.J.; Voorhees, J.J. Vitamin A Antagonizes Decreased Cell Growth and Elevated Collagen-Degrading Matrix Metalloproteinases and Stimulates Collagen Accumulation in Naturally Aged Human Skin. J. Investig. Dermatol. 2000, 114, 480–486. [Google Scholar] [CrossRef]
- Ralf Paus, L.; Berneburg, M.; Trelles, M.; Friguet, B.; Ogden, S.; Esrefoglu, M.; Kaya, G.; Goldberg, D.J.; Mordon, S.; Calderhead, R.G.; et al. How Best to Halt and/or Revert UV-Induced Skin Ageing: Strategies, Facts and Fiction. Exp. Dermatol. 2008, 17, 228–229. [Google Scholar] [CrossRef]
- Gelse, K.; Pöschl, E.; Aigner, T. Collagens--Structure, Function, and Biosynthesis. Adv. Drug Deliv. Rev. 2003, 55, 1531–1546. [Google Scholar] [CrossRef]
- Brennan, M.; Bhatti, H.; Nerusu, K.C.; Bhagavathula, N.; Kang, S.; Fisher, G.J.; Varani, J.; Voorhees, J.J. Matrix Metalloproteinase-1 Is the Major Collagenolytic Enzyme Responsible for Collagen Damage in UV-Irradiated Human Skin. Photochem. Photobiol. 2003, 78, 43–48. [Google Scholar] [CrossRef]
- Zhang, S.; Duan, E. Fighting against Skin Aging: The Way from Bench to Bedside. Cell Transplant. 2018, 27, 729–738. [Google Scholar] [CrossRef]
- Lee, J.-E.; Oh, J.; Song, D.; Lee, M.; Hahn, D.; Boo, Y.C.; Kang, N.J. Acetylated Resveratrol and Oxyresveratrol Suppress UVB-Induced MMP-1 Expression in Human Dermal Fibroblasts. Antioxidants 2021, 10, 1252. [Google Scholar] [CrossRef]
- Chuang, S.-Y.; Lin, C.-H.; Fang, J.-Y. Natural Compounds and Aging: Between Autophagy and Inflammasome. BioMed Res. Int. 2014, 2014, 297293. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.J.; Lim, Y. Resveratrol Ameliorates Hepatic Metaflammation and Inhibits NLRP3 Inflammasome Activation. Metabolism 2014, 63, 693–701. [Google Scholar] [CrossRef] [PubMed]
- Pietrocola, F.; Mariño, G.; Lissa, D.; Vacchelli, E.; Malik, S.A.; Niso-Santano, M.; Zamzami, N.; Galluzzi, L.; Maiuri, M.C.; Kroemer, G. Pro-Autophagic Polyphenols Reduce the Acetylation of Cytoplasmic Proteins. Cell Cycle 2012, 11, 3851–3860. [Google Scholar] [CrossRef] [PubMed]
- Yokozawa, T.; Kim, Y.J. Piceatannol inhibits melanogenesis by its antioxidative actions. Biol. Pharm. Bull. 2007, 30, 2007–2011. [Google Scholar] [CrossRef]
- Nichols, J.A.; Katiyar, S.K. Skin Photoprotection by Natural Polyphenols: Anti-Inflammatory, Antioxidant and DNA Repair Mechanisms. Arch. Dermatol. Res. 2010, 302, 71–83. [Google Scholar] [CrossRef]
- Liu, Y.; Chan, F.; Sun, H.; Yan, J.; Fan, D.; Zhao, D.; An, J.; Zhou, D. Resveratrol Protects Human Keratinocytes HaCaT Cells from UVA-Induced Oxidative Stress Damage by Downregulating Keap1 Expression. Eur. J. Pharmacol. 2011, 650, 130–137. [Google Scholar] [CrossRef]
- D’Orazio, J.; Jarrett, S.; Amaro-Ortiz, A.; Scott, T. UV Radiation and the Skin. Int. J. Mol. Sci. 2013, 14, 12222–12248. [Google Scholar] [CrossRef]
- Sirerol, J.A.; Feddi, F.; Mena, S.; Rodriguez, M.L.; Sirera, P.; Aupí, M.; Pérez, S.; Asensi, M.; Ortega, A.; Estrela, J.M. Topical Treatment with Pterostilbene, a Natural Phytoalexin, Effectively Protects Hairless Mice against UVB Radiation-Induced Skin Damage and Carcinogenesis. Free Radic. Biol. Med. 2015, 85, 1–11. [Google Scholar] [CrossRef]
- Taylor, E.J.M.; Yu, Y.; Champer, J.; Kim, J. Resveratrol Demonstrates Antimicrobial Effects Against Propionibacterium acnes In Vitro. Dermatol. Ther. 2014, 4, 249–257. [Google Scholar] [CrossRef]
- Sheng, J.-Y.; Chen, T.-T.; Tan, X.-J.; Chen, T.; Jia, A.-Q. The Quorum-Sensing Inhibiting Effects of Stilbenoids and Their Potential Structure-Activity Relationship. Bioorg. Med. Chem. Lett. 2015, 25, 5217–5220. [Google Scholar] [CrossRef]
- Kumar, M.R. Chromobacterium violaceum: A Rare Bacterium Isolated from a Wound over the Scalp. Int. J. Appl. Basic Med. Res. 2012, 2, 70–72. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Fang, F.; Sun, D.; Yang, S.; Zhang, X.; Yu, X.; Yang, L. Piceatannol Inhibits P. acnes-Induced Keratinocyte Proliferation and Migration by Downregulating Oxidative Stress and the Inflammatory Response. Inflammation 2020, 43, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Basset, C.; Rodrigues, A.M.S.; Eparvier, V.; Silva, M.R.R.; Lopes, N.P.; Sabatier, D.; Fonty, E.; Espindola, L.S.; Stien, D. Secondary Metabolites from Spirotropis longifolia (DC) Baill and Their Antifungal Activity against Human Pathogenic Fungi. Phytochemistry 2012, 74, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Shaito, A.; Posadino, A.M.; Younes, N.; Hasan, H.; Halabi, S.; Alhababi, D.; Al-Mohannadi, A.; Abdel-Rahman, W.M.; Eid, A.H.; Nasrallah, G.K.; et al. Potential Adverse Effects of Resveratrol: A Literature Review. Int. J. Mol. Sci. 2020, 21, 2084. [Google Scholar] [CrossRef]
- Park, K.-T.; Kim, J.-K.; Lim, Y.-H. Evaluation on Skin Irritation and Sensitization of Oxyresveratrol and Oxyresveratrol-3-O-Glucoside Produced by Biotransformation of Morus Alba Extract. Korean J. Food Sci. Technol. 2012, 44, 251–256. [Google Scholar] [CrossRef]
- Medrano-Padial, C.; Prieto, A.I.; Puerto, M.; Pichardo, S. Toxicological Evaluation of Piceatannol, Pterostilbene, and ε-Viniferin for Their Potential Use in the Food Industry: A Review. Foods 2021, 10, 592. [Google Scholar] [CrossRef]
- Bradu, P.; Biswas, A.; Nair, C.; Sreevalsakumar, S.; Patil, M.; Kannampuzha, S.; Mukherjee, A.G.; Wanjari, U.R.; Renu, K.; Vellingiri, B.; et al. Recent Advances in Green Technology and Industrial Revolution 4.0 for a Sustainable Future. Environ. Sci. Pollut. Res. 2022, 1–32. [Google Scholar] [CrossRef]
- Hikmawanti, N.P.E.; Ramadon, D.; Jantan, I.; Mun’im, A. Natural Deep Eutectic Solvents (NADES): Phytochemical Extraction Performance Enhancer for Pharmaceutical and Nutraceutical Product Development. Plants 2021, 10, 2091. [Google Scholar] [CrossRef]
- Putnik, P.; Lorenzo, J.M.; Barba, F.J.; Roohinejad, S.; Režek Jambrak, A.; Granato, D.; Montesano, D.; Bursać Kovačević, D. Novel Food Processing and Extraction Technologies of High-Added Value Compounds from Plant Materials. Foods 2018, 7, 106. [Google Scholar] [CrossRef]
- Carreira-Casais, A.; Lourenço-Lopes, C.; Otero, P.; Rodríguez, M.; González Pereira, A.; Echave Álvarez, J.; Soria López, A.; Rivo, F.N.; Simal-Gandara, J.; Prieto Lage, M. Application of Green Extraction Techniques for Natural Additives Production. In Natural Food Additives; Lage, M.Á.Á.P., Otero, P., Eds.; IntechOpen: London, UK, 2021; Available online: https://doi.org/10.5772/intechopen.100320 (accessed on 28 August 2022).
- Benoit, C.; Virginie, C.; Boris, V. Chapter Twelve—The Use of NADES to Support Innovation in the Cosmetic Industry. In Eutectic Solvents and Stress in Plants; Verpoorte, R., Witkamp, G.-J., Choi, Y.H., Eds.; Academic Press: Cambridge, MA, USA, 2021; Volume 97, pp. 309–332. [Google Scholar]
- Goyal, N.; Jerold, F. Biocosmetics: Technological Advances and Future Outlook. Environ. Sci. Pollut. Res. 2021, 1–22. [Google Scholar] [CrossRef]
- Ivanović, M.I.; Islamčević Razboršek, M.; Kolar, M. Innovative Extraction Techniques Using Deep Eutectic Solvents and Analytical Methods for the Isolation and Characterization of Natural Bioactive Compounds from Plant Material. Plants 2020, 9, 1428. [Google Scholar] [CrossRef]
- Sun, B.; Zheng, Y.-L.; Yang, S.-K.; Zhang, J.-R.; Cheng, X.-Y.; Ghiladi, R.; Ma, Z.; Wang, J.; Deng, W.-W. One-Pot Method Based on Deep Eutectic Solvent for Extraction and Conversion of Polydatin to Resveratrol from Polyg cuspidatum. Food Chem. 2021, 343, 128498. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-D.; Fu, L.-N.; Wang, L.-T.; Cai, Z.-H.; Wang, Y.-Q.; Yang, Q.; Fu, Y.-J. Simultaneous Transformation and Extraction of Resveratrol from Polygonum Cuspidatum Using Acidic Natural Deep Eutectic Solvent. Ind. Crop. Prod. 2021, 173, 114140. [Google Scholar] [CrossRef]
- Syahdi, R.R.; Nadyana, R.; Putri, R.H.; Santi, R.; Mun’im, A. Application of Green Extraction Methods to Resveratrol Extraction from Peanut (Arachis hypogaea L.) Skin. Int. J. Appl. Pharm. 2020, 12, 38–42. [Google Scholar] [CrossRef]
- Chen, J.; Jiang, X.; Yang, G.; Bi, Y.; Liu, W. Green and Efficient Extraction of Resveratrol from Peanut Roots Using Deep Eutectic Solvents. J. Chem. 2018, 2018, 4091930. [Google Scholar] [CrossRef]
- Aryati, W.D.; Azka, K.M.; Mun’im, A. Ultrasonic-Assisted Extraction Using a Betaine-Based Natural Deep Eutectic Solvent for Resveratrol Extraction from Melinjo (Gnetum Gnemon) Seeds. Int. J. Appl. Pharm. 2020, 12, 26–31. [Google Scholar] [CrossRef]
- Komaikul, J.; Mangmool, S.; Putalun, W.; Kitisripanya, T. Preparation of Readily-to-Use Stilbenoids Extract from Morus alba Callus Using a Natural Deep Eutectic Solvent. Cosmetics 2021, 8, 91. [Google Scholar] [CrossRef]
- Alishlah, T.; Mun’im, A.; Jufri, M. Optimization of Urea-Glycerin Based NADES-UAE for Oxyresveratrol Extraction from Morus alba Roots for Preparation of Skin Whitening Lotion. J. Young Pharm. 2019, 11, 155–160. [Google Scholar] [CrossRef]
- Donnez, D.; Jeandet, P.; Clément, C.; Courot, E. Bioproduction of Resveratrol and Stilbene Derivatives by Plant Cells and Microorganisms. Trends Biotechnol. 2009, 27, 706–713. [Google Scholar] [CrossRef]
- Kiselev, K.V. Perspectives for Production and Application of Resveratrol. Appl. Microbiol. Biotechnol. 2011, 90, 417–425. [Google Scholar] [CrossRef]
- Medina-Bolivar, F.; Condori, J.; Rimando, A.M.; Hubstenberger, J.; Shelton, K.; O’Keefe, S.F.; Bennett, S.; Dolan, M.C. Production and Secretion of Resveratrol in Hairy Root Cultures of Peanut. Phytochemistry 2007, 68, 1992–2003. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, S.-Y.; Park, S.U. Resveratrol Production in Hairy Root Culture of Peanut, Arachis hypogaea L. Transformed with Different Agrobacterium Rhizogenes Strains. African J. Biotechnol. 2010, 7, 3788–3790. [Google Scholar]
- Yang, T.; Fang, L.; Nopo-Olazabal, C.; Condori, J.; Nopo-Olazabal, L.; Balmaceda, C.; Medina-Bolivar, F. Enhanced Production of Resveratrol, Piceatannol, Arachidin-1, and Arachidin-3 in Hairy Root Cultures of Peanut Co-Treated with Methyl Jasmonate and Cyclodextrin. J. Agric. Food Chem. 2015, 63, 3942–3950. [Google Scholar] [CrossRef] [PubMed]
- Abbott, J.A.; Medina-Bolivar, F.; Martin, E.M.; Engelberth, A.S.; Villagarcia, H.; Clausen, E.C.; Carrier, D.J. Purification of Resveratrol, Arachidin-1, and Arachidin-3 from Hairy Root Cultures of Peanut (Arachis hypogaea) and Determination of Their Antioxidant Activity and Cytotoxicity. Biotechnol. Prog. 2010, 26, 1344–1351. [Google Scholar] [CrossRef]
- Ku, K.-L.; Chang, P.-S.; Cheng, Y.-C.; Lien, C.-Y. Production of Stilbenoids from the Callus of Arachis hypogaea: A Novel Source of the Anticancer Compound Piceatannol. J. Agric. Food Chem. 2005, 53, 3877–3881. [Google Scholar] [CrossRef]
- Bunchom, N.; Phadungkit, M.; Saijuntha, W.; Thanonkeo, P.; Thanonkeo, S. Production of Resveratrol from Callus Cultures of Artocarpus lacucha Buch.-Ham. Asia-Pacific J. Sci. Technol. 2017, 19, 262–267. [Google Scholar]
- Kouakou, T.H.; Téguo, P.W.; Valls, J.; Kouadio, Y.J.; Decendit, A.; Mérillon, J.-M. First Evidence of trans-Resveratrol Production in Cell Suspension Cultures of Cotton (Gossypium hirsutum L.). Plant Cell. Tissue Organ Cult. 2006, 86, 405–409. [Google Scholar] [CrossRef]
- Pongkitwitoon, B.; Simpan, K.; Chobsri, T.; Sritularak, B.; Putalun, W. Combined UV-C Irradiation and Precursor Feeding Enhances Mulberroside A Production in Morus alba L. Cell Suspension Cultures. ScienceAsia 2020, 46, 679. [Google Scholar] [CrossRef]
- Komaikul, J.; Kitisripanya, T.; Likhitwitayawuid, K.; Sritularak, B.; Tanaka, H.; Putalun, W. Improvement of Stilbenoid Production by 2-Hydroxypropyl-β-Cyclodextrin in White Mulberry (Morus alba L.) Callus Cultures. Nat. Prod. Res. 2019, 33, 2762–2769. [Google Scholar] [CrossRef]
- Kiselev, K.; Dubrovina, A.; Veselova, M.; Bulgakov, V.; Fedoreyev, S.; Zhuravlev, Y. The RolB Gene-Induced Overproduction of Resveratrol in Vitis amurensis Transformed Cells. J. Biotechnol. 2007, 128, 681–692. [Google Scholar] [CrossRef]
- Chen, J.; Hall, D.E.; Murata, J.; De Luca, V. L-Alanine Induces Programmed Cell Death in V. labrusca Cell Suspension Cultures. Plant Sci. 2006, 171, 734–744. [Google Scholar] [CrossRef]
- Belhadj, A.; Telef, N.; Saigne, C.; Cluzet, S.; Barrieu, F.; Hamdi, S.; Mérillon, J.-M. Effect of Methyl Jasmonate in Combination with Carbohydrates on Gene Expression of PR Proteins, Stilbene and Anthocyanin Accumulation in Grapevine Cell Cultures. Plant Physiol. Biochem. 2008, 46, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Tassoni, A.; Fornalè, S.; Franceschetti, M.; Musiani, F.; Michael, A.J.; Perry, B.; Bagni, N. Jasmonates and Na-Orthovanadate Promote Resveratrol Production in Vitis Vinifera Cv. Barbera Cell Cultures. New Phytol. 2005, 166, 895–905. [Google Scholar] [CrossRef] [PubMed]
- Donnez, D.; Kim, K.-H.; Antoine, S.; Conreux, A.; De Luca, V.; Jeandet, P.; Clément, C.; Courot, E. Bioproduction of Resveratrol and Viniferins by an Elicited Grapevine Cell Culture in a 2L Stirred Bioreactor. Process Biochem. 2011, 46, 1056–1062. [Google Scholar] [CrossRef]
- Belchí-Navarro, S.; Almagro, L.; Lijavetzky, D.; Bru, R.; Pedreño, M.A. Enhanced Extracellular Production of trans-Resveratrol in Vitis Vinifera Suspension Cultured Cells by Using Cyclodextrins and Methyljasmonate. Plant Cell Rep. 2012, 31, 81–89. [Google Scholar] [CrossRef]
- Yue, X.; Zhang, W.; Deng, M. Hyper-Production of 13C-Labeled trans-Resveratrol in Vitis Vinifera Suspension Cell Culture by Elicitation and in Situ Adsorption. Biochem. Eng. J. 2011, 53, 292–296. [Google Scholar] [CrossRef]
- Jeong, Y.J.; Park, S.H.; Park, S.-C.; Kim, S.; Kim, T.H.; Lee, J.; Kim, S.W.; Ryu, Y.B.; Jeong, J.C.; Kim, C.Y. Induced Extracellular Production of Stilbenes in Grapevine Cell Culture Medium by Elicitation with Methyl Jasmonate and Stevioside. Bioresour. Bioprocess. 2020, 7, 38. [Google Scholar] [CrossRef]
- Komaikul, J.; Kitisripanya, T.; Inyai, C.; Likhitwitayawuid, K.; Sritularak, B.; Tanaka, H.; Putalun, W. Phytostilbenoid Production in White Mulberry (Morus alba L.) Cell Culture Using Bioreactors and Simple Deglycosylation by Endogenous Enzymatic Hydrolysis. Vitr. Cell. Dev. Biol.-Plant 2019, 55, 199–208. [Google Scholar] [CrossRef]
- Maneechai, S.; De-Eknamkul, W.; Umehara, K.; Noguchi, H.; Likhitwitayawuid, K. Flavonoid and Stilbenoid Production in Callus Cultures of Artocarpus lakoocha. Phytochemistry 2012, 81, 42–49. [Google Scholar] [CrossRef]
- Jeandet, P.; Clément, C.; Courot, E. Resveratrol Production at Large Scale Using Plant Cell Suspensions. Eng. Life Sci. 2014, 14, 622–632. [Google Scholar] [CrossRef]
- Jeandet, P.; Clément, C.; Tisserant, L.-P.; Crouzet, J.; Courot, É. Use of Grapevine Cell Cultures for the Production of Phytostilbenes of Cosmetic Interest. Comptes Rendus Chim. 2016, 19, 1062–1070. [Google Scholar] [CrossRef] [Green Version]
| Chemical Names | Family | Plant Species |
|---|---|---|
| Resveratrol | Acoraceae | Acorus calamu L. |
| Agavaceae | Yucca periculosa Baker | |
| Cyperaceae | Scirpus fluviatilis (Torr.) A.Gray (synonym of Bolboschoenus fluviatilis (Torr.) Soják) | |
| Scirpus maritimus L.(synonym of Bolboschoenus maritimus (L.) Palla) | ||
| Dipterocarpaceae | Hopea utilis (Bedd.) Bole | |
| Vatica rassak Blume | ||
| Ericaceae | Vaccinium alaskaense Howell | |
| Vaccinium angustifolium Aiton | ||
| Fabaceae | Amorpha nana C.Fraser | |
| Arachis hypogaea L. | ||
| Bauhinia racemosa Lam. | ||
| Caesalpinia decapetala (Roth) Alston | ||
| Caragana tibetica Kom. | ||
| Cassia dentata Vogel (synonym of Chamaecrista dentata (Vogel) H.S.Irwin & Barneby) | ||
| Haplormosia monophyla (Harms) Harms | ||
| Intsia bijuga (Colebr.) Kuntze | ||
| Maackia amurensis Rupr. | ||
| Pterolobium hexapetallum (Roth) Santapau & Wagh (synonym of Pterolobium hexapetalum (Roth) Santapau & Wagh) | ||
| Trifolium campestre Schreb. | ||
| Trifolium dubium Sibth. | ||
| Vicia faba L. | ||
| Gnetum gnemon L. | ||
| Gnetum latifolium Blume | ||
| Gnetum parvifolium (Warb.) W.C.Cheng | ||
| Gnetum pendulum C.Y.Cheng | ||
| Gnetum venosum Spruce ex Benth. | ||
| Hyacinthaceae | Scilla nervosa (Burch.) J.P.Jessop (synonym of Schizocarphus nervosus (Burch.) van der Merwe) | |
| Iridaceae | Belamcanda chinensis (L.) DC. (synonym of Iris domestica (L.) Goldblatt & Mabb.) | |
| Melanthiaceae | Veratrum album L. | |
| Veratrum grandiflorum (Maxim. ex Miq.) O.Loes. | ||
| Veratrum nigrum var.ussuriense O.Loes. (Synonym of Veratrum nigrum L.) | ||
| Moraceae | Artocarpus chaplasha Roxb.(synonym of Artocarpus chama Buch.-Ham.) | |
| Artocarpus dadah Miq. | ||
| Artocarpus lakoocha Roxb. (synonym of Artocarpus lacucha Buch.-Ham.) | ||
| Broussonetia papyrifera (L.) L’Hér. ex Vent. | ||
| Cudrania javanensis Trécul (synonym of Maclura cochinchinensis (Lour.) Corner) | ||
| Myrtaceae | Corymbia haematoxylon (Maiden) K.D.Hill & L.A.S.Johnson | |
| Corymbia papuana (F.Muell.) K.D.Hill & L.A.S.Johnson | ||
| Eucalyptus abergiana F.Muell. (synonym of Corymbia abergiana (F.Muell.) K.D.Hill & L.A.S.Johnson) | ||
| Eucalyptus astringens (Maiden) Maiden | ||
| Eucalyptus caesia Benth. | ||
| Eucalyptus calophylla R.Br. ex Lindl. (synonym of Corymbia calophylla (R.Br. ex Lindl.) K.D.Hill & L.A.S.Johnson) | ||
| Eucalyptus campaspe S.Moore | ||
| Eucalyptus citriodora Hook. (synonym of Corymbia citriodora (Hook.) K.D.Hill & L.A.S.Johnson) | ||
| Eucalyptus crebra F.Muell. | ||
| Eucalyptus decorticans (F.M.Bailey) Maiden | ||
| Eucalyptus dichromophloia F.Muell. (synonym of Corymbia dichromophloia (F.Muell.) K.D.Hill & L.A.S.Johnson) | ||
| Eucalyptus eremophila (Diels) Maiden | ||
| Eucalyptus erythrophloia Blakely (synonym of Corymbia erythrophloia (Blakely) K.D.Hill & L.A.S.Johnson) | ||
| Eucalyptus ficifolia F.Muell. (synonym of Corymbia ficifolia (F.Muell.) K.D.Hill & L.A.S.Johnson) | ||
| Eucalyptus gardneri Maiden | ||
| Eucalyptus griffithsii Maiden | ||
| Eucalyptus grossa F.Muell. ex Benth. | ||
| Eucalyptus gummifera (Gaertn.) Hochr. (synonym of Corymbia gummifera (Gaertn.) K.D.Hill & L.A.S.Johnson) | ||
| Eucalyptus intermedia F.Muell. ex R.T.Baker (synonym of Corymbia intermedia (F.Muell. ex R.T.Baker) K.D.Hill & L.A.S.Johnson) | ||
| Eucalyptus maculata Hook. (synonym of Corymbia maculata (Hook.) K.D.Hill & L.A.S.Johnson) | ||
| Eucalyptus x nowraensis Maiden | ||
| Eucalyptus nutans F.Muell. | ||
| Eucalyptus platypus Hook.f. | ||
| Eucalyptus polycarpa F.Muell. (synonym of Corymbia polycarpa (F.Muell.) K.D.Hill & L.A.S.Johnson) | ||
| Eucalyptus pruinose Turcz. (synonym of Eucalyptus pyriformis Turcz.) | ||
| Eucalyptus sargentii Maiden | ||
| Eucalyptus sideroxylon A.Cunn. ex Woolls | ||
| Eucalyptus stricklandii Maiden | ||
| Eucalyptus trachyphloia F.Muell. (synonym of Corymbia trachyphloia (F.Muell.) K.D.Hill & L.A.S.Johnson) | ||
| Eucalyptus wandoo Blakely | ||
| Eucalyptus woodwardia Maiden | ||
| Palmae | Phoenix dactylifera L. | |
| Pinaceae | Picea abies (L.) H.Karst. | |
| Picea bicolor (Maxim.) Mayr (synonym of Picea alcoquiana (H.J.Veitch ex Lindl.) Carrière) | ||
| Picea excelsa Wender. (synonym of Abies alba Mill.) | ||
| Picea glehnii (F.Schmidt) Mast. | ||
| Picea jezoensis (Siebold & Zucc.) Carrière | ||
| Picea koraiensis Nakai | ||
| Picea koyamae Shiras. | ||
| Picea obovate Ledeb. | ||
| Picea torano (Siebold ex K.Koch) Koehne | ||
| Pinus sibirica (Ledeb.) Turcz. (synonym of Abies sibirica Ledeb.) | ||
| Poaceae | Festuca argentina (Speg.) Parodi | |
| Festuca arundinacea Lilj (synonym of Scolochloa festucacea (Willd.) Link) | ||
| Festuca versuta Beal | ||
| Hordeum bogdanii Wilensky | ||
| Hordeum brachyantherum Nevski | ||
| Poa alsodes A.Gray | ||
| Stipa robusta (Vasey) Scribn. | ||
| Pleuropterus ciliinervis Nakai (synonym of Reynoutria ciliinervis (Nakai) Moldenke) | ||
| Polygonum cuspidatum Siebold & Zucc. (synonym of Reynoutria japonica Houtt.) | ||
| Polygonum multiflorum Thunb. (synonym of Reynoutria multiflora (Thunb.) Moldenke) | ||
| Rheum rhaponticum L. | ||
| Rosaceae | Rubus idaeus Vell. | |
| Rubus occidentalis L. | ||
| Spiraea formosana Hayata | ||
| Smilacaceae | Smilax aspera subsp. mauritanica (Poir.) Arcang. (synonym of Smilax aspera L.) | |
| Smilax bracteate C.Presl | ||
| Smilax menispermoidea A.DC. | ||
| Vitaceae | Ampelopsis brevipedunculata (Maxim.) Trautv. | |
| Ampelopsis japonica (Thunb.) Makino | ||
| Cissus Antarctica Vent. | ||
| Cissus quadrangularis L. | ||
| Parthenocissus quinquefolia (L.) Planch. | ||
| Parthenocissus tricuspidata (Siebold & Zucc.) Planch. | ||
| Rhoicissus rhomboidea (E. Mey. ex Harv.) Planch. | ||
| Vitis coignetiae Pulliat ex Planch. | ||
| Vitis riparia Michx. (synonym of Vitis vulpina L.) | ||
| Vitis rupestris Scheele | ||
| Vitis vinifera L. | ||
| Oxyresveratrol | Fabaceae | Erythrina variegata L. |
| Melanthiaceae | Schoenocaulon officinale (Schltdl. & Cham.) A.Gray | |
| Veratrum album L. | ||
| Veratrum grandiflorum (Maxim. ex Miq.) O.Loes. | ||
| Moraceae | Artocarpus chaplasha Roxb.(synonym of Artocarpus chama Buch.-Ham.) | |
| Artocarpus dadah Miq. | ||
| Artocarpus gomezianus Wall. ex Trécul | ||
| Artocarpus lakoocha Roxb. (synonym of Artocarpus lacucha Buch.-Ham.) | ||
| Artocarpus reticulatus Miq. | ||
| Chlorophora excelsa (Welw.) Benth. (synonym of Milicia excelsa (Welw.) C.C.Berg) | ||
| Chlorophora regia A.Chev. (synonym of Milicia regia (A.Chev.) C.C.Berg) | ||
| Cudrania javanensis Trécul (synonym of Maclura cochinchinensis (Lour.) Corner) | ||
| Maclura pomifera (Raf.) C.K.Schneid. | ||
| Morus alba L. | ||
| Morus bombycis Koidz. (synonym of Morus australis Poir.) | ||
| Morus indica L. | ||
| Morus laevigata Wall. ex Brandis (synonym of Morus macroura Miq.) | ||
| Morus rubra L. | ||
| Morus serrata Roxb. | ||
| Piceatannol | Cyperaceae | Scirpus fluviatilis (Torr.) A.Gray (synonym of Bolboschoenus fluviatilis (Torr.) Soják) |
| Scirpus maritimus L. (synonym of Bolboschoenus maritimus (L.) Palla) | ||
| Fabaceae | Caragana tibetica Kom. | |
| Cassia dentata Vogel (synonym of Chamaecrista dentata (Vogel) H.S.Irwin & Barneby) | ||
| Cassia garrettiana Craib (synonym of Senna garrettiana (Craib) H.S.Irwin & Barneby) | ||
| Cassia marginata Roxb. (synonym of Cassia roxburghii DC.) | ||
| Centrolobium robustum (Vell.) Benth. | ||
| Intsia bijuga (Colebr.) Kuntze | ||
| Laburnum alpinum (Mill.) Bercht. & J.Presl | ||
| Laburnum anagyroides Medik. | ||
| Maackia amurensis Rupr. | ||
| Pericopsis angolensis (Baker) Meeuwen | ||
| Pericopsis elata (Harms) Meeuwen | ||
| Schotia brachypetala Sond. | ||
| Vouacapoua americana Aubl. | ||
| Vouacapoua macropetala Sandwith | ||
| Pinaceae | Picea abies (L.) H.Karst. | |
| Picea engelmannii Parry ex Engelm. | ||
| Picea excelsa Wender. (synonym of Abies alba Mill.) | ||
| Picea glauca (Moench) Voss | ||
| Picea glehnii (F.Schmidt) Mast. | ||
| Picea jezoensis (Siebold & Zucc.) Carrière | ||
| Picea mariana (Mill.) Britton, Sterns & Poggenb. | ||
| Picea obovate Ledeb. | ||
| Picea rubens Sarg. | ||
| Picea sitchensis (Bong.) Carrière | ||
| Poaceae | Saccharum officinarum L. | |
| Polygonaceae | Eskemukerjea megacarpum (H.Hara) H.Hara (synonym of Fagopyrum megacarpum H.Hara) | |
| Rheum rhaponticum L. | ||
| Vitaceae | Cissus quadrangularis L. |
| Compounds | Biological Activities | Molecular Mechanism | Type of Cell Cultures | References |
|---|---|---|---|---|
| Resveratrol | antioxidative stress | ↑ GST and SOD activities | HaCat | [56] |
| MMP inhibition | ↓phosphorylation of MAPKs and Akt/mTOR signaling pathways | HDF | [51] | |
| antioxidant | ↑ SOD, and GSH-Px activities; ↓ lipid peroxidation | HaCat | [57] | |
| anti-tyrosinase | ↓ melanin pigmentation | B16 F10 melanoma cells | [58] | |
| Oxyresveratrol | suppression of UV-B-induced MMP-1 | ↓phosphorylation of MAPKs and Akt/mTOR signaling pathways | HDF | [51] |
| Piceatannol | antioxidant | ↑ GSH activity; ↓ intracellular ROS level | HaCat | [59] |
| Compound | Plant Species (Part) | Method Extract Condition (Temperature, Time, Soli-Liquid Ratio) | Solvent (Molar Ratio) | Yield (Mean) | References |
|---|---|---|---|---|---|
| Resveratrol | P. cuspidatum(root) | One-pot method based on DES 85 °C 80 min 1:50 g/mL | 70% of tetrabutylammonium chloride: ethylene glycol (1:3) mixed with 30% of water | 12.26 mg/g | [75] |
| P. cuspidatum (root) | NADES with ultrasound-assisted extraction (UAE) 75 °C 80 min 1:50 g/mL ultrasonic power 250 W | 70% of choline chloride: oxalic acid (1:1) mixed with 30% of water | 12.31 mg/g | [76] | |
| A. hypogaea (skin) | NADES with UAE room temperature 15 min 1:20 g/mL | choline chloride: oxalic acid (1:1) | 0.049 mg/g dry weight | [77] | |
| A. hypogaea (root) | DES with UAE 55 °C 40 min 1:30 g/mL | 60% of choline chloride: 1,4-butanediol (1:1) mixed with 40% of water | 38.91 mg/kg | [78] | |
| G. gnemon (seed) | NADES with UAE 10 min 1:10 g/mL | 40% of betaine: lactic acid (1:1) mixed with 60% of water | 0.227 mg/g | [79] | |
| Oxyresveratrol | M. alba (callus) | NADES with UAE (40 kHz) 30 min 0.6:9 g/mL | 70% of choline chloride: glycerol (1:2) mixed with 30% of water | 0.13 mg/g dry weight | [80] |
| M. alba (root) | NADES with UAE 15 min 1:20 g/mL | urea-glycerin (1:3) | 2.42 mg/g dry powder | [81] |
| Compound | Plant Species | Types of Culture | Elicitors/Inducers | Quantity | References |
|---|---|---|---|---|---|
| Resveratrol | A. hypogaea | hairy root | - | <0.002 mg/g of extract (dry medium) | [84] |
| hairy root | sodium acetate | 0.05–0.098 mg/g of extract (dry medium) | [84] | ||
| hairy root | - | 0.8–1.5 mg/g dry weight of hairy root | [85] | ||
| hairy root | MeJA, CD | 16,716 nmol/g(dry medium) | [86] | ||
| hairy root | sodium acetate | 12 μg/mg of extract (dry medium) | [87] | ||
| cell suspension | UV-C irradiation | 3.14–6.93 μg/g of callus | [88] | ||
| callus | UV-C irradiation | 0.25–11.97 μg/g of callus | [88] | ||
| A. lacucha | callus | - | 0.66–0.79 mg/g of dry weight of callus | [89] | |
| Gossypium hirsutum L. (Coker 312) | cell suspension | - | 2.44 ± 0.15 to 7.2 ± 0.19 µg/g of dry weight of cell suspension | [90] | |
| M. alba | root | - | 41.6 ± 5.84 µg/g of dry weight of root | [32] | |
| root | MeJA, yeast extract | 10.2 ± 0.53 µg/g of dry weight of root | [32] | ||
| cell suspension | UV-C irradiation | 0.044 ± 0.002 mg/g dry weight of cell suspension | [91] | ||
| cell suspension | UV-C irradiation, 0.05 mM L-phenylalanine and/or 0.03 mM L-tyrosine | 0.007 ± 0.003 to 0.025 ± 0.001 mg/g dry weight of cell suspension | [91] | ||
| callus | 2-hydroxypropyl-β-cyclodextrin | Non-immobilization: 3.95 ± 1.03 to 15.29 ± 0.53 mg/L of media Immobilization: 3.31 ± 0.16 to 5.81 ± 0.31 mg/L of media | [92] | ||
| V. amurensis Rupr. | callus | - | 0.004 ± 0.002 to 0.026 ± 0.010 %dry weight of callus | [93] | |
| callus | MeJA, salicylic acid, sodium orthovanadate, sodium nitroprusside, phenylalanine | 0.017–0.15 %dry weight of callus | [93] | ||
| Vitis labrusca L. | cell suspension | L-alanine | 60 nmol/50 mL of media 20 nmol/g of fresh weight | [94] | |
| V. vinifera | cell suspension | MeJA | 52 nmol/g of fresh weight | [95] | |
| cell suspension | sucrose | 52 nmol/g of fresh weight | [95] | ||
| cell suspension | MeJA, sucrose | 120 nmol/g of fresh weight | [95] | ||
| cell suspension | jasmonic acid | 15 nmol/g of dry weight (intracellular) 15 nmol/g of dry weight (extracellular) | [96] | ||
| cell suspension | MeJA | 100 nmol/g of dry weight (intracellular) 37 nmol/g of dry weight (extracellular) | [96] | ||
| cell suspension | 0.1 nM sodium orthovanadate | 115 nmol/g of dry weight (intracellular) 98 nmol/g of dry weight (extracellular) | [96] | ||
| cell suspension | 1 nM sodium orthovanadate | 90 nmol/g of dry weight (intracellular) 80 nmol/g of dry weight (extracellular) | [96] | ||
| cell suspension | MeJA | 150 mg/L resveratrol (flasks) 209 mg/L resveratrol (bioreactor) | [97] | ||
| cell suspension | MeJA, CD | 1447.8 ± 60.4 μmol/g dry weight | [98] | ||
| cell suspension | jasmonic acid, salicylic acid and HP2 MGL (adsorbent) | 2666.7 mg/L | [99] | ||
| cell suspension | MeJA, stevioside | 12.2 mg/L | [100] | ||
| cell suspension | MeJA, Methyl-β-cyclodextrin (MeβCD) | 371.9 mg/L | [100] | ||
| Oxyresveratrol | M. alba | root | - | 136 ± 5.05 µg/g of dry weight of root | [32] |
| root | MeJA, yeast extract | 68.6 ± 3.53 µg/g of dry weight of root | [32] | ||
| cell suspension | incubation at 50 °C for 1 h | 8.06 ± 0.14 μmol/g of dry weight | [101] | ||
| callus | 2-hydroxypropyl-β-cyclodextrin | Non-immobilization: 12.3 ± 2.71 to 190.41 ± 48.24 mg/L of media Immobilization: 2.9 ± 0.09 to 43.86 ± 6.25 mg/L of media | [92] | ||
| Oxyresveratrol(prenylated) | A. lacucha | callus | - | - | [102] |
| Piceatannol | A. hypogaea | hairy root | MeJA, CD | 1909.92 nmol/g (dry medium) | [86] |
| callus | UV irradiation | 2.17 to 5.31 μg/g of callus | [88] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aneklaphakij, C.; Chamnanpuen, P.; Bunsupa, S.; Satitpatipan, V. Recent Green Technologies in Natural Stilbenoids Production and Extraction: The Next Chapter in the Cosmetic Industry. Cosmetics 2022, 9, 91. https://doi.org/10.3390/cosmetics9050091
Aneklaphakij C, Chamnanpuen P, Bunsupa S, Satitpatipan V. Recent Green Technologies in Natural Stilbenoids Production and Extraction: The Next Chapter in the Cosmetic Industry. Cosmetics. 2022; 9(5):91. https://doi.org/10.3390/cosmetics9050091
Chicago/Turabian StyleAneklaphakij, Chaiwat, Phatthilakorn Chamnanpuen, Somnuk Bunsupa, and Veena Satitpatipan. 2022. "Recent Green Technologies in Natural Stilbenoids Production and Extraction: The Next Chapter in the Cosmetic Industry" Cosmetics 9, no. 5: 91. https://doi.org/10.3390/cosmetics9050091
APA StyleAneklaphakij, C., Chamnanpuen, P., Bunsupa, S., & Satitpatipan, V. (2022). Recent Green Technologies in Natural Stilbenoids Production and Extraction: The Next Chapter in the Cosmetic Industry. Cosmetics, 9(5), 91. https://doi.org/10.3390/cosmetics9050091






