A State-of-the-Art Review on the Alternatives to Animal Testing for the Safety Assessment of Cosmetics
Abstract
:1. Introduction
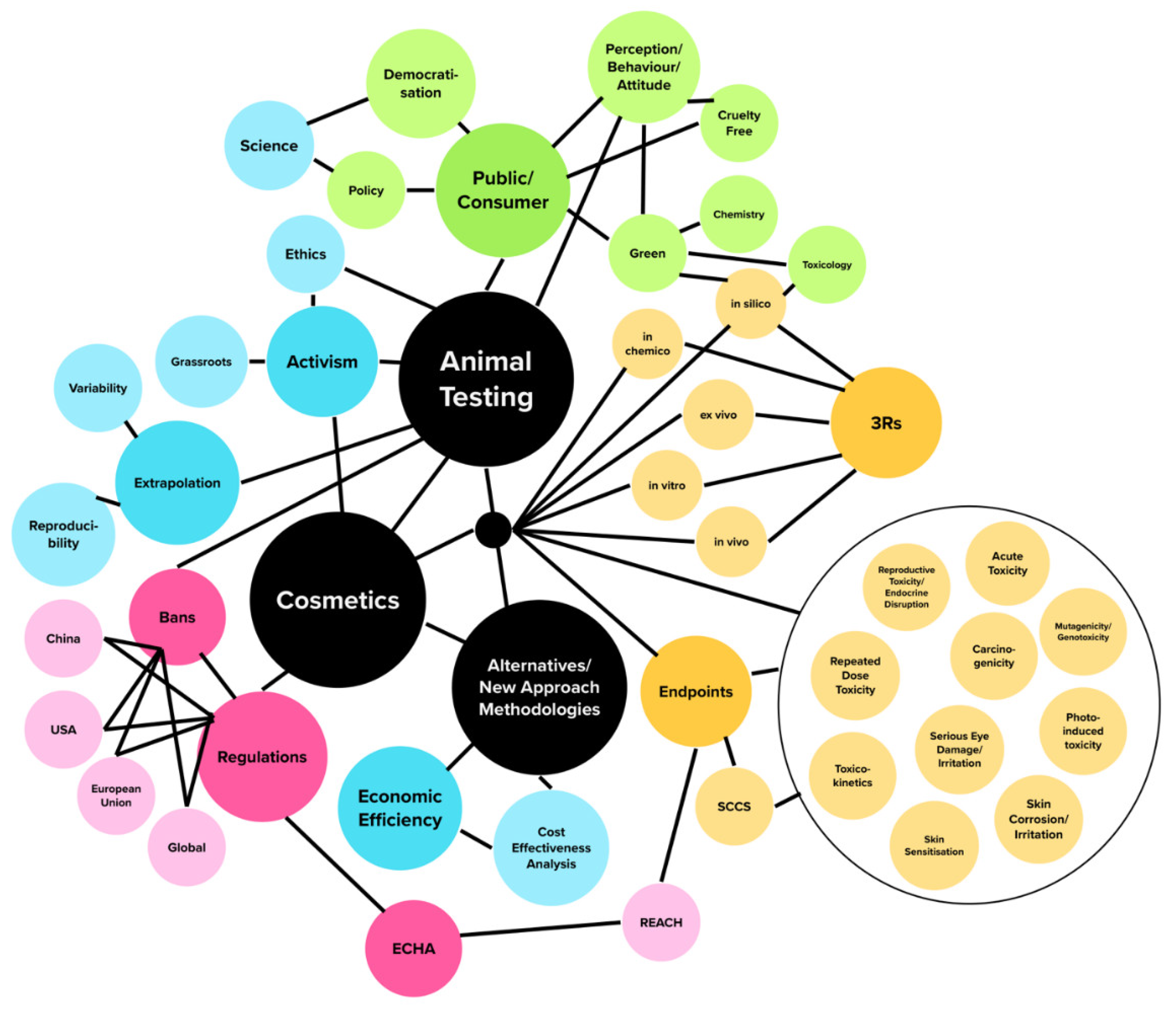
2. Methodology
3. Drivers for the Phasing-Out of Animal Testing in Cosmetics
3.1. Ethical Considerations
3.2. The Lack of Effective Extrapolation
3.3. Economic Efficiency
4. Global Regulatory Responses
4.1. European Union
REACH vs. Regulation EC 1223/2009
4.2. United States
4.3. China
4.4. Rest of the World
5. New Approach Methodologies (NAMs)
| Toxicological Endpoint | Name | Testing Method Title | Year Received | Responsible Organisation | Type | OECD Guideline |
|---|---|---|---|---|---|---|
| Acute toxicity, Basal Cytotoxicity | NRU cytotoxicity | Neutral red uptake for starting doses for acute oral toxicity | 2003 | ICCVAM/NICEATM | in vitro | OECD GD 129 [58] |
| 3T3 NRU | 3T3 Neutral Red Uptake cytotoxicity assay | 2007 | EURL ECVAM | in vitro | N/A | |
| Carcinogenicity | SHE 6.7 CTA | Syrian hamster embryo cell transformation assay at pH 6.7 | 2005 | EURL ECVAM | in vitro | OECD GD 214 [59] |
| Carcinogenicity | SHE 7.0 CTA | Syrian hamster embryo cell transformation assay at pH 7.0 | 2005 | EURL ECVAM | in vitro | |
| Bhas 42 CTA | Cell Transformation Assay Based on the Bhas 42 Cell Line | 2012 | EURL ECVAM | in vitro | OECD GD 231 [60] | |
| Skin Corrosion | TER | Rat skin transcutaneous electrical resistance test 1 | 1995 | EURL ECVAM | ex vivo | OECD TG 430 [61] |
| EpiDerm™ | EpiDerm™ skin corrosivity test | 1998 | EURL ECVAM | in vitro | OECD TG 431 [62] | |
| Epics SCT | Epidermal Skin Corrosion Test 1000 | 2007 | EURL ECVAM | in vitro | ||
| LabCyte EPI-MODEL24 SCT | LabCyte EPI-MODEL24 skin corrosion test | 2017 | JaCVAM | in vitro | ||
| EpiSkin™ | EpiSkin™ skin corrosivity test | 1998 | EURL ECVAM | in vitro | ||
| Corrositex® | In Vitro Membrane Barrier Test Method for Skin Corrosion | 1998 | EURL ECVAM | in vitro | OECD TG 435 [63] | |
| Skin Irritation | LabCyte EPI-MODEL24 SIT | LabCyte EPI-MODEL24 skin irritation test | 2008 | JaCVAM | in vitro | OECD TG 439 [64] |
| SkinEthic RHE | Skinethic Skin Irritation Test-42bis | 2008 | EURL ECVAM | in vitro | ||
| Modified EpiDerm SIT | Modified EpiDerm Skin Irritation Test for Hazard Identification and Labelling of Chemicals According to EU Classification System | 2008 | EURL ECVAM | in vitro | ||
| epiCS SIT | epiCS Skin Irritation Test | 2009 | EURL ECVAM | in vitro | ||
| Skin Sensitisation | Murine Local Lymph Node Assay | Murine Local Lymph Node Assay 2 | 1998 | ICCVAM/NICEATM | in vivo | OECD TG 442B [65] |
| DPRA | Direct Peptide Reactivity Assay | 2009 | EURL ECVAM | in chemico | OECD TG 442C [66] | |
| ADRA for skin sensitization | In Chemico Skin Sensitisation: Amino acid Derivative Reactivity Assay | 2016 | JaCVAM | in chemico | ||
| KeratinoSens | KeratinoSens assay for the testing of skin sensitizers | 2010 | EURL ECVAM | in vitro | OECD TG 442D [67] | |
| LuSens | LuSens Assay | 2011 | EURL ECVAM | in vitro | ||
| U-SENS™ | U-SENS™—Myeloid U937 Skin Sensitisation Test | 2013 | EURL ECVAM | in vitro | OECD TG 442E [68] | |
| hCLAT | Human Cell Line Activation Test | 2008 | EURL ECVAM | in vitro | ||
| IL-8 Luciferase assay for skin sensitization | IL-8 Luciferase assay for skin sensitization | 2016 | JaCVAM | in vitro | ||
| Phototoxicity | EpiDerm-PT | Human 3-D Epidermis Model in Vitro Phototoxicity Test | 1997 | EURL ECVAM | in vitro | OECD TG 498 [69] |
| Ros Assay | Ros (Reactive Oxygen Species) Assay for Photoreactivity | 2013 | JaCVAM | in chemico | OECD TG 495 [70] | |
| Genotoxicity/ Mutagenicity | MNvit | In vitro mammalian cell micronucleus test | 2005 | EURL ECVAM | in vitro | OECD TG 487 [71] |
| Reproductive toxicity, Endocrine disruption | ER-STTA assay | Stably Transfected Human Estrogen Receptor-α Transactivation Assay using the (h) ERα-HeLa-9903 cell line | 2008 | JaCVAM | in vitro | OECD TG 455 [72] |
| ERalpha-CALUX | Prediction of the in vivo estrogenic activity of chemicals ERalpha-CALUX | 2009 | EURL ECVAM | in vitro | ||
| AR-CALUX | Transactivation assay for detection of androgenic activity of chemicals | 2010 | EURL ECVAM | in vitro | OECD TG 458 [73] | |
| AR-Ecoscreen test | Androgen Receptor TransActivation Assay using the stably transfected human AR-EcoScreen™ cell line | 2012 | JaCVAM | in vitro | ||
| VM7Luc ER TA | The VM7Luc ER TA test method: An In Vitro Assay for Identifying Human Estrogen Receptor Agonist and Antagonist Activity of Chemicals | 2016 | ICCVAM/NICEATM | in vitro | OECD TG 457 [74] | |
| ToxCast ER Pathway Model | The ToxCast Estrogen Receptor Agonist Pathway Model | 2016 | ICCVAM/NICEATM | in silico | N/A | |
| Serious eye damage/ Eye irritation | BCOP | Bovine corneal opacity and permeability test method | 2003 | ICCVAM/NICEATM | ex vivo | OECD TG 437 [75] |
| ICE | Isolated Chicken Eye | 2003 | ICCVAM/NICEATM | ex vivo | OECD TG 438 [76] | |
| FL | The fluorescein leakage test method | 2004 | EURL ECVAM | in vitro | OECD TG 460 [77] | |
| Ocular Irritection | Ocular Irritection | 2006 | EURL ECVAM | in vitro | OECD TG 496 [78] | |
| STE | Short Time Exposure Test | 2008 | ICCVAM/NICEATM | in vitro | OECD TG 491 [79] | |
| EpiOcular EIT | EpiOcular™ Human Cell Construct EIT | 2008 | EURL ECVAM | in vitro | OECD TG 492 [80] | |
| LabCyteCORNEAMODEL24 EIT | LabCyteCORNEAMODEL24 eye irritation test | 2015 | JaCVAM | in vitro | ||
| SkinEthic HCE EIT | SkinEthic™ HCE Eye Irritation Test | 2015 | EURL ECVAM | in vitro | ||
| Vitrigel-Eye Irritancy Test | Vitrigel-Eye Irritancy Test | 2011 | JaCVAM | in vitro | OECD TG 494 [81] |
5.1. Non-Testing Methods: In Silico Toxicology
5.2. Endpoint Specific NAMs
5.2.1. Acute Toxicity
5.2.2. Skin Corrosion/Irritation
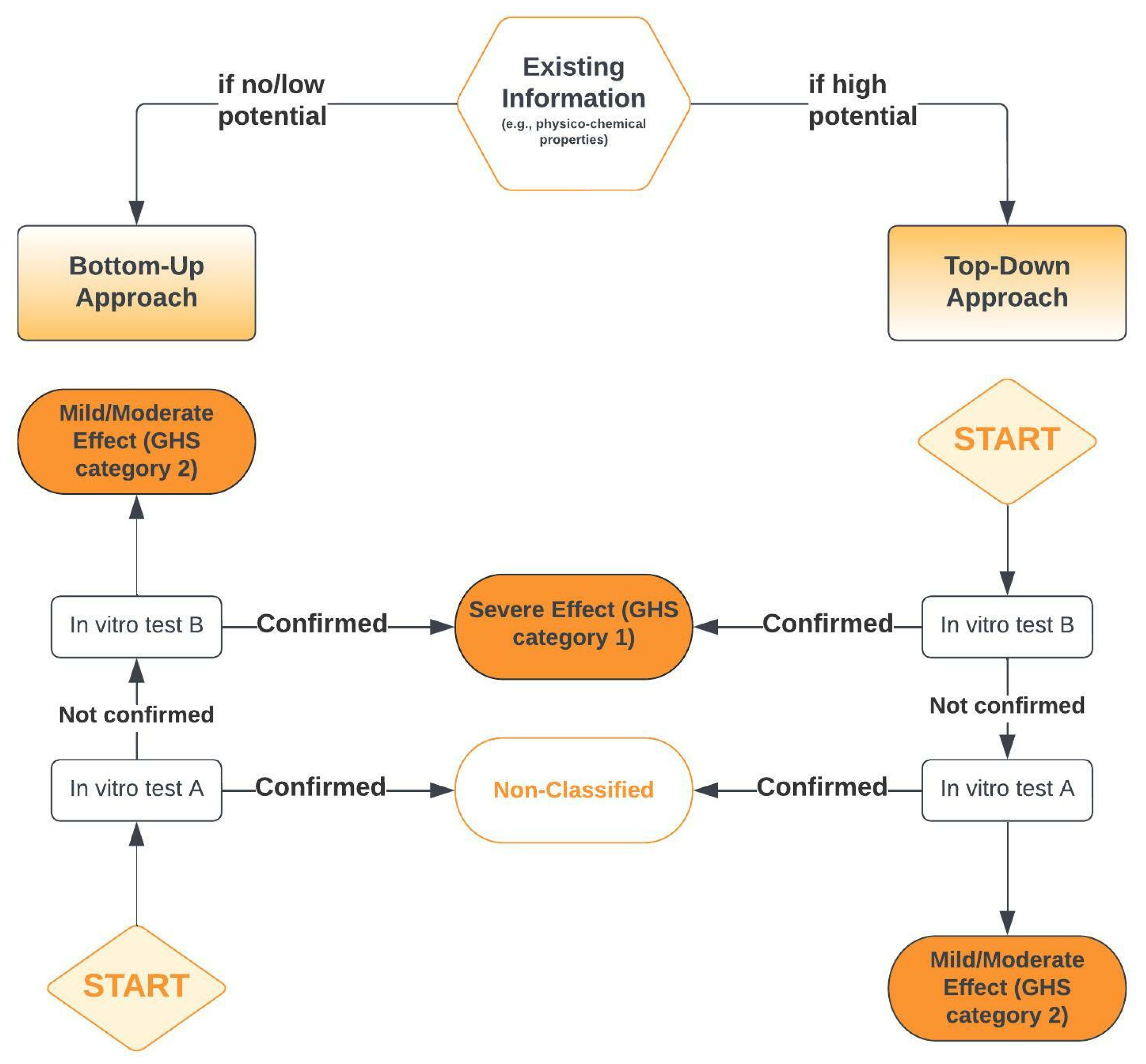
5.2.3. Serious Eye Damage/Irritation
5.2.4. Skin Sensitisation
5.2.5. Repeated Dose Toxicity
5.2.6. Reproductive Toxicity/Endocrine Disruption
5.2.7. Mutagenicity/Genotoxicity
5.2.8. Carcinogenicity
5.2.9. Photo-Induced Toxicity
5.2.10. Toxicokinetics
6. Public Perception of Animal Testing in Cosmetics
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lamb, R.D. American Chamber of Horrors: The Truth about Food and Drugs; Farrar & Rinehart, Incorporated: New York, NY, USA, 1936. [Google Scholar]
- Zurlo, J.; Rudacille, D.; Goldberg, A.M. Animals and Alternatives in Testing: History, Science, and Ethics; Mary Ann Liebert: New York, NY, USA, 1994. [Google Scholar]
- Bottini, A.A.; Hartung, T. Food for thought... on the economics of animal testing. ALTEX 2009, 26, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Russel, W.M.S.; Burch, R.L. The Principles of Humane Experimental Technique; Universities Federation For Animal Welfare (UFAW): Hertfordshire, UK, 1959. [Google Scholar]
- Meigs, L.; Smirnova, L.; Rovida, C.; Leist, M.; Hartung, T. Animal testing and its alternatives—The most important omics is economics. ALTEX 2018, 35, 275–305. [Google Scholar] [CrossRef] [PubMed]
- Rogiers, V.; Benfenati, E.; Bernauer, U.; Bodin, L.; Carmichael, P.; Chaudhry, Q.; Coenraads, P.J.; Cronin, M.T.D.; Dent, M.; Dusinska, M.; et al. The way forward for assessing the human health safety of cosmetics in the EU—Workshop proceedings. Toxicology 2020, 436, 152421. [Google Scholar] [CrossRef] [PubMed]
- ICCVAM. A Strategic Roadmap for Establishing New Approaches to Evaluate the Safety of Chemicals and Medical Products in the United States. Available online: https://ntp.niehs.nih.gov/go/iccvam-rdmp (accessed on 29 April 2022). [CrossRef]
- SCCS. SCCS Notes of Guidance for the Testing of Cosmetic Ingredients and their Safety Evaluation, 11th ed.; SCCS/1628/21; SCCS: 2021. Available online: https://ec.europa.eu/health/system/files/2021-04/sccs_o_250_0.pdf (accessed on 29 April 2022).
- Adler, S.; Basketter, D.; Creton, S.; Pelkonen, O.; van Benthem, J.; Zuang, V.; Andersen, K.E.; Angers-Loustau, A.; Aptula, A.; Bal-Price, A.; et al. Alternative (non-animal) methods for cosmetics testing: Current status and future prospects-2010. Arch. Toxicol. 2011, 85, 367–485. [Google Scholar] [CrossRef]
- Culliney, K. ‘Unique’ for Cosmetics: Animal-Free Safety Assessment New Science Programme to Launch in 2022. Available online: https://www.cosmeticsdesign-europe.com/Article/2021/06/15/Cosmetics-industry-unveils-animal-free-safety-assessment-New-Science-Programme-set-to-launch-2022 (accessed on 22 May 2022).
- Culliney, K. Beauty Majors Sign Joint Statement Claiming EU Animal Testing Ban is ‘Being Undermined’ by ECHA. Available online: https://www.cosmeticsdesign-europe.com/article/2020/11/10/humane-international-society-and-beauty-brands-claim-echa-is-undermining-eu-animal-testing-ban (accessed on 28 April 2022).
- Culliney, K. Cosmetics industry Calls on EU Institutions to Uphold Animal Testing Ban ‘As Intended’. Available online: https://www.cosmeticsdesign-europe.com/article/2020/12/02/eu-ban-on-animal-testing-for-cosmetics-must-be-upheld-by-european-commission-parliament-and-council-says-industry (accessed on 28 April 2022).
- Barthe, M.; Bavoux, C.; Finot, F.; Mouche, I.; Cuceu-Petrenci, C.; Forreryd, A.; Chérouvrier Hansson, A.; Johansson, H.; Lemkine, G.F.; Thénot, J.-P. Safety Testing of Cosmetic Products: Overview of Established Methods and New Approach Methodologies (NAMs). Cosmetics 2021, 8, 50. [Google Scholar] [CrossRef]
- Sheehan, K.B.; Lee, J. What’s cruel about cruelty free: An exploration of consumers, moral heuristics, and public policy. J. Anim. Ethics 2014, 4, 1–15. [Google Scholar] [CrossRef]
- IPSOS. Public Attitudes to Animal Research in 2018. Available online: https://www.ipsos.com/en-uk/public-attitudes-animal-research-2018 (accessed on 21 May 2022).
- Wohlin, C. Guidelines for Snowballing in Systematic Literature Studies and a Replication in Software Engineering; ACM: New York, NY, USA, 2014; pp. 1–10. [Google Scholar] [CrossRef]
- Humane Society of the United States Timeline: Cosmetics Testing on Animals. Available online: https://www.humanesociety.org/resources/timeline-cosmetics-testing-animals (accessed on 28 May 2022).
- Doke, S.K.; Dhawale, S.C. Alternatives to animal testing: A review. Saudi Pharm. J. 2015, 23, 223. [Google Scholar] [CrossRef]
- Singer, P. Animal Liberation; New York Review: New York, NY, USA, 1975. [Google Scholar]
- Singer, P. Ethics into Action: Learning from a Tube of Toothpaste; Rowman & Littlefield: Lanham, MD, USA, 1998. [Google Scholar]
- Voisin, E.M.; Ruthsatz, M.; Collins, J.M.; Hoyle, P.C. Extrapolation of animal toxicity to humans: Interspecies comparisons in drug development. Regul. Toxicol. Pharmacol. 1990, 12, 107–116. [Google Scholar] [CrossRef]
- Sharpe, R. The Draize test—Motivations for change. Food Chem. Toxicol. 1985, 23, 139–143. [Google Scholar] [CrossRef]
- Ram, R. Extrapolation of Animal Research Data to Humans: An Analysis of the Evidence. In Animal Experimentation: Working Towards a Paradigm Change; Herrmann, K., Jayne, K., Eds.; Brill: Boston, MA, USA, 2019; Volume 22, pp. 341–375. [Google Scholar]
- Draize, J.H.; Woodard, G.; Calvery, H.O. Methods for the study of irritation and toxicity of substances applied topically to the skin and mucous membranes. J. Pharmacol. Exp. Ther. 1944, 82, 377–390. [Google Scholar]
- Imai, K.; Ariumi, H.; Matsushita, T.; Hasegawa, T.; Yoshiyama, Y. History and Social Duties in Future of the AATEX: Alternatives to Animal Testing and Experimentation. AATEX 2013, 18, 53–57. [Google Scholar]
- Ukelis, U.; Kramer, P.; Olejniczak, K.; Mueller, S.O. Replacement of in vivo acute oral toxicity studies by in vitro cytotoxicity methods: Opportunities, limits and regulatory status. Regul. Toxicol. Pharmacol. 2008, 51, 108–118. [Google Scholar] [CrossRef] [PubMed]
- OECD Test No. 420: Acute Oral Toxicity—Fixed Dose Procedure. In OECD Guidelines for the Testing of Chemicals, Section 4; OECD Publishing: Paris, France, 2002; Available online: https://www.oecd-ilibrary.org/environment/test-no-420-acute-oral-toxicity-fixed-dose-procedure_9789264070943-en (accessed on 19 April 2022). [CrossRef]
- OECD Test No. 423: Acute Oral toxicity—Acute Toxic Class Method. In OECD Guidelines for the Testing of Chemicals, Section 4; OECD Publishing: Paris, France, 2002; Available online: https://www.oecd-ilibrary.org/environment/test-no-423-acute-oral-toxicity-acute-toxic-class-method_9789264071001-en (accessed on 19 April 2022). [CrossRef]
- OECD Test No. 425: Acute Oral Toxicity: Up-and-Down Procedure. In OECD Guidelines for the Testing of Chemicals; OECD Publishing: Paris, France, 2008; Available online: https://www.oecd-ilibrary.org/environment/test-no-425-acute-oral-toxicity-up-and-down-procedure_9789264071049-en (accessed on 19 April 2022). [CrossRef]
- Norlen, H.; Worth, A.P.; Gabbert, S. A tutorial for analysing the cost-effectiveness of alternative methods for assessing chemical toxicity: The case of acute oral toxicity prediction. Altern. Lab. Anim. 2014, 42, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Andrew, D.J. Acute Systemic Toxicity: Oral, Dermal and Inhalation Exposures. In Reducing, Refining and Replacing the Use of Animals in Toxicity Testing; Allen, D.G., Waters, M.D., Eds.; The Royal Society of Chemistry: London, UK, 2014; pp. 183–214. [Google Scholar]
- Gabbert, S.; von Ierland, E.C. Cost-Effectiveness Analysis of Chemical Testing for Decision-Support: How to Include Animal Welfare? Hum. Ecol. Risk Assess. Int. J. 2010, 16, 603–620. [Google Scholar] [CrossRef]
- U.S. Congress United States Code: Federal Food, Drug, and Cosmetic Act, 21 U.S.C. §§ 301–392 Suppl. 5. Available online: https://www.loc.gov/item/uscode1934-006021009/ (accessed on 24 April 2022).
- Stephens, M.L.; Mak, N.S. History of the 3Rs in Toxicity Testing: From Russell and Burch to 21st Century Toxicology. In Reducing, Refining and Replacing the Use of Animals in Toxicity Testing; Allen, D.G., Waters, M.D., Eds.; The Royal Society of Chemistry: London, UK, 2014; pp. 1–43. [Google Scholar]
- EC Council Directive 86/609/EEC of 24 November 1986 on the approximation of laws, regulations and administrative provisions of the Member States regarding the protection of animals used for experimental and other scientific purposes. Off. J. Eur. Union L 1986, 358, 1–28.
- Halder, M.; Hartung, T. European Centre for the Validation of Alternative Methods (ECVAM): Its Role and Contribution. Available online: https://publications.jrc.ec.europa.eu/repository/handle/JRC44990 (accessed on 25 April 2022).
- EC JRC TSAR—Tracking System for Alternative Methods towards Regulatory Acceptance. Available online: https://tsar.jrc.ec.europa.eu/ (accessed on 25 April 2022).
- EC Directive 2003/15/EC of the European Parliament and of the Council of 27 February 2003 amending Council Directive 76/768/EEC on the approximation of the laws of the Member States relating to cosmetic products (Text with EEA relevance). Off. J. Eur. Union L 2003, 66, 26–35.
- EC History of the Adoption Process for the New Chemicals Legislation. Available online: https://ec.europa.eu/environment/chemicals/reach/background/index_en.htm (accessed on 27 April 2022).
- Culliney, K. EC Stands by ECHA Rulings for Animal Data on Cosmetic Ingredients under REACH. Available online: https://www.cosmeticsdesign-europe.com/Article/2020/12/04/European-Commission-stands-by-ECHA-Board-of-Appeal-Symrise-rulings-for-animal-data-under-REACH (accessed on 28 April 2022).
- Knight, J.; Rovida, C.; Reinhard, K.; Zhu, C.; Knudsen, M.; Hartung, T. Continuing animal tests on cosmetic ingredients for REACH in the EU. ALTEX 2021, 38, 653–668. [Google Scholar] [CrossRef]
- US EPA, Toxicology Testing in the 21st Century (Tox21). Available online: https://www.epa.gov/chemical-research/toxicology-testing-21st-century-tox21 (accessed on 29 April 2022).
- Thomas, R.S.; Paules, R.S.; Simeonov, A.; Fitzpatrick, S.C.; Crofton, K.M.; Casey, W.M.; Mendrick, D.L. The US Federal Tox21 Program: A strategic and operational plan for continued leadership. ALTEX 2018, 35, 163–168. [Google Scholar] [CrossRef]
- NTP about ICCVAM. Available online: https://ntp.niehs.nih.gov/whatwestudy/niceatm/iccvam/index.html#:~:text=The%20Interagency%20Coordinating%20Committee%20on,federal%20regulatory%20and%20research%20agencies (accessed on 29 April 2022).
- Schlabach, J. 900 Companies Support Humane Cosmetics Act to End Animal Testing. Available online: https://www.gcimagazine.com/brands-products/news/news/21874267/900-companies-support-humane-cosmetics-act-to-end-animal-testing (accessed on 29 April 2022).
- CIRS The Technical Safety Standards for Cosmetics (2015) Will Come Into Effect by Dec 2016. Available online: https://www.cirs-group.com/en/cosmetics/the-technical-safety-standards-for-cosmetics-2015-will-come-into-effect-by-dec-2016 (accessed on 2 May 2022).
- Luo, F.; Su, Z.; Wu, J.; Zhang, F.; Xing, S.; Wang, G.; Lu, Y. The current status of alternative methods for cosmetics safety assessment in China. ALTEX 2019, 36, 136–139. [Google Scholar] [CrossRef]
- Kern, J. Bulldog Skincare to Become First-ever Leaping Bunny-approved Brand in China. Available online: https://www.gcimagazine.com/consumers-markets/news/21871820/bulldog-skincare-to-become-first-ever-leaping-bunny-approved-brand-in-china (accessed on 2 May 2022).
- Lim, A. China Animal Testing: Exemptions for Testing on ‘Ordinary’ Cosmetics Start in May. Available online: https://www.cosmeticsdesign-europe.com/Article/2021/03/08/China-animal-testing-Exemptions-for-testing-on-ordinary-cosmetics-start-in-May-officials (accessed on 13 May 2022).
- Fang, V.; Petino, A.; Zhang, J. Update on New Regulations and Animal Testing Exemption for Cosmetics | EU SME Centre: China Market Research, Training, Advice. Available online: https://www.eusmecentre.org.cn/event/2022-04-12/update-new-regulations-and-animal-testing-exemption-cosmetics (accessed on 2 May 2022).
- EC International Cooperation on Alternative Test Methods (ICATM). Available online: https://joint-research-centre.ec.europa.eu/eu-reference-laboratory-alternatives-animal-testing/alternative-methods-toxicity-testing/advisory-and-consultation-bodies/international-cooperation-alternative-test-methods-icatm_en (accessed on 29 April 2022).
- EC Ban on Animal Testing. Available online: https://ec.europa.eu/growth/sectors/cosmetics/ban-animal-testing_en (accessed on 27 April 2022).
- EC Regulation (EC) No 1223/2009 of the European Parliament and of the Council of 30 November 2009 on cosmetic products (Text with EEA relevance). Off. J. Eur. Union L 2009, 342, 59.
- Sreedhar, D.; Manjula, N.; Pise, A.; Pise, S.; Ligade, V.S. Ban of cosmetic testing on animals: A brief overview. Int. J. Curr. Res. Rev. 2020, 12, 113–116. [Google Scholar]
- Trevor, V.; Rossi, A.; Dias, R.; Frutuoso, M. Estados Proíbem Utilização de Animais em Testes de Cosméticos. JOTA. 2022. Available online: https://www.jota.info/opiniao-e-analise/colunas/coluna-do-tracking/estados-proibem-utilizacao-de-animais-em-testes-de-cosmeticos-14062022 (accessed on 10 July 2022).
- Raunio, H. In Silico Toxicology—Non-Testing Methods. Front. Pharmacol. 2011, 2, 1–8. [Google Scholar] [CrossRef] [PubMed]
- ECHA Guidance on the Compilation of Safety Data Sheets. Available online: https://echa.europa.eu/documents/10162/2324906/sds_en.pdf/01c29e23-2cbe-49c0-aca7-72f22e101e20?t=1608126237610 (accessed on 5 May 2022).
- OECD Guidance Document on Using Cytotoxicity Tests to Estimate Starting Doses for Acute Oral Systemic Toxicity Tests; OECD Series on Testing and Assessment, No. 129; OECD Publishing: Paris, France, 2010; Available online: https://www.oecd.org/officialdocuments/publicdisplaydocumentpdf/?cote=env/jm/mono(2010)20&doclanguage=en (accessed on 5 May 2022).
- OECD No. 214: Guidance Document on the In Vitro Syrian Hamster Embryo (SHE) Cell Transformation Assay. In OECD Environment, Health and Safety Publications, Series on Testing and Assessment; OECD Publishing: Paris, France, 2015; Available online: https://www.oecd.org/env/ehs/testing/Guidance-Document-on-the-in-vitro-Syrian-Hamster-Embryo-Cell-Transformation-Assay.pdf (accessed on 5 May 2022).
- OECD No. 231: Guidance document on the in vitro BHAS 42 Cell Transformation Assay. In OECD Environment, Health and Safety Publications, Series on Testing and Assessment; OECD Publishing: Paris, France, 2017; Available online: https://www.oecd.org/officialdocuments/publicdisplaydocumentpdf/?cote=ENV/JM/MONO(2016)1&doclanguage=en (accessed on 5 May 2022).
- OECD Test No. 430: In Vitro Skin Corrosion: Transcutaneous Electrical Resistance Test Method (TER). In OECD Guidelines for the Testing of Chemicals, Section 4; OECD Publishing: Paris, France, 2015. [CrossRef]
- OECD Test No. 431: In vitro skin corrosion: Reconstructed human epidermis (RHE) test method. In OECD Guidelines for the Testing of Chemicals, Section 4; OECD Publishing: Paris, France, 2019. [CrossRef]
- OECD Test No. 435: In Vitro Membrane Barrier Test Method for Skin Corrosion. In OECD Guidelines for the Testing of Chemicals, Section 4; OECD Publishing: Paris, France, 2015. [CrossRef]
- OECD Test No. 439: In Vitro Skin Irritation: Reconstructed Human Epidermis Test Method. In OECD Guidelines for the Testing of Chemicals, Section 4; OECD Publishing: Paris, France, 2021. [CrossRef]
- OECD Test No. 442B: Skin Sensitization: Local Lymph Node Assay: BrdU-ELISA or -FCM. In OECD Guidelines for the Testing of Chemicals, Section 4; OECD Publishing: Paris, France, 2018. [CrossRef]
- OECD Test No. 442C: In Chemico Skin Sensitisation: Assays addressing the Adverse Outcome Pathway key event on covalent binding to proteins. In OECD Guidelines for the Testing of Chemicals, Section 4; OECD Publishing: Paris, France, 2021. [CrossRef]
- OECD Test No. 442D: In Vitro Skin Sensitisation: ARE-Nrf2 Luciferase Test Method. In OECD Guidelines for the Testing of Chemicals, Section 4; OECD Publishing: Paris, France, 2018. [CrossRef]
- OECD Test No. 442E: In Vitro Skin Sensitisation: In Vitro Skin Sensitisation assays addressing the Key Event on activation of dendritic cells on the Adverse Outcome Pathway for Skin Sensitisation. In OECD Guidelines for the Testing of Chemicals, Section 4; OECD Publishing: Paris, France, 2018. [CrossRef]
- OECD Test No. 498: In vitro Phototoxicity—Reconstructed Human Epidermis Phototoxicity test method. In OECD Guidelines for the Testing of Chemicals, Section 4; OECD Publishing: Paris, France, 2021. [CrossRef]
- OECD Test No. 495: Ros (Reactive Oxygen Species) Assay for Photoreactivity. In OECD Guidelines for the Testing of Chemicals, Section 4; OECD Publishing: Paris, France, 2019. [CrossRef]
- OECD Test No. 487: In Vitro Mammalian Cell Micronucleus Test. In OECD Guidelines for the Testing of Chemicals, Section 4; OECD Publishing: Paris, France, 2016. [CrossRef]
- OECD Test No. 455: Performance-Based Test Guideline for Stably Transfected Transactivation In Vitro Assays to Detect Estrogen Receptor Agonists and Antagonists. In OECD Guidelines for the Testing of Chemicals, Section 4; OECD Publishing: Paris, France, 2021. [CrossRef]
- OECD Test No. 458: Stably Transfected Human Androgen Receptor Transcriptional Activation Assay for Detection of Androgenic Agonist and Antagonist Activity of Chemicals. In OECD Guidelines for the Testing of Chemicals, Section 4; OECD Publishing: Paris, France, 2020. [CrossRef]
- OECD Test No. 457: BG1Luc Estrogen Receptor Transactivation Test Method for Identifying Estrogen Receptor Agonists and Antagonists. In OECD Guidelines for the Testing of Chemicals, Section 4; OECD Publishing: Paris, France, 2012. [CrossRef]
- OECD Test No. 437: Bovine Corneal Opacity and Permeability Test Method for Identifying i) Chemicals Inducing Serious Eye Damage and ii) Chemicals Not Requiring Classification for Eye Irritation or Serious Eye Damage. In OECD Guidelines for the Testing of Chemicals, Section 4; OECD Publishing: Paris, France, 2020. [CrossRef]
- OECD Test No. 438: Isolated Chicken Eye Test Method for Identifying i) Chemicals Inducing Serious Eye Damage and ii) Chemicals Not Requiring Classification for Eye Irritation or Serious Eye Damage. In OECD Guidelines for the Testing of Chemicals, Section 4; OECD Publishing: Paris, France, 2018. [CrossRef]
- OECD Test No. 460: Fluorescein Leakage Test Method for Identifying Ocular Corrosives and Severe Irritants. In OECD Guidelines for the Testing of Chemicals, Section 4; OECD Publishing: Paris, France, 2017. [CrossRef]
- OECD Test No. 496: In vitro Macromolecular Test Method for Identifying Chemicals Inducing Serious Eye Damage and Chemicals Not Requiring Classification for Eye Irritation or Serious Eye Damage. In OECD Guidelines for the Testing of Chemicals, Section 4; OECD Publishing: Paris, France, 2019. [CrossRef]
- OECD Test No. 491: Short Time Exposure In Vitro Test Method for Identifying i) Chemicals Inducing Serious Eye Damage and ii) Chemicals Not Requiring Classification for Eye Irritation or Serious Eye Damage. In OECD Guidelines for the Testing of Chemicals, Section 4; OECD Publishing: Paris, France, 2020. [CrossRef]
- OECD Test No. 492: Reconstructed human Cornea-like Epithelium (RhCE) test method for identifying chemicals not requiring classification and labelling for eye irritation or serious eye damage. In OECD Guidelines for the Testing of Chemicals, Section 4; OECD Publishing: Paris, France, 2019. [CrossRef]
- OECD Test No. 494: Vitrigel-Eye Irritancy Test Method for Identifying Chemicals Not Requiring Classification and Labelling for Eye Irritation or Serious Eye Damage. In OECD Guidelines for the Testing of Chemicals, Section 4; OECD Publishing: Paris, France, 2021. [CrossRef]
- OECD Guideline No. 497: Defined Approaches on Skin Sensitisation. In OECD Guidelines for the Testing of Chemicals, Section 4; OECD Publishing: Paris, France, 2021. [CrossRef]
- Hemmerich, J.; Ecker, G.F. In silico toxicology: From structure–activity relationships towards deep learning and adverse outcome pathways. WIREs Comput. Mol. Sci. 2020, 10, e1475. [Google Scholar] [CrossRef]
- Gellatly, N.; Sewell, F. Regulatory acceptance of in silico approaches for the safety assessment of cosmetic-related substances. Comput. Toxicol. 2019, 11, 82–89. [Google Scholar] [CrossRef]
- Rovida, C.; Escher, S.E.; Herzler, M.; Bennekou, S.H.; Kamp, H.; Kroese, D.E.; Maslankiewicz, L.; Moné, M.J.; Patlewicz, G.; Sipes, N.; et al. NAM-supported read-across: From case studies to regulatory guidance in safety assessment. ALTEX 2021, 38, 140–150. [Google Scholar] [CrossRef] [PubMed]
- ECHA Practical Guide. How to Use and Report (Q)SARs. Available online: https://echa.europa.eu/documents/10162/17250/pg_report_qsars_en.pdf/407dff11-aa4a-4eef-a1ce-9300f8460099 (accessed on 18 May 2022).
- Madden, J.C.; Enoch, S.J.; Paini, A.; Cronin, M.T.D. A Review of In Silico Tools as Alternatives to Animal Testing: Principles, Resources and Applications. Altern. Lab. Anim. 2020, 48, 146–172. [Google Scholar] [CrossRef] [PubMed]
- ECHA Guidance on Information Requirements and Chemical Safety Assessment, Chapter R.7b: Endpoint Specific Guidance. Available online: https://echa.europa.eu/documents/10162/17224/information_requirements_r7b_en.pdf/1a551efc-bd6a-4d1f-b719-16e0d3a01919?t=1498476047712 (accessed on 25 May 2022).
- Aleksunes, L.M.; Eaton, D.L. Principles of Toxicology. In Casarett and Doull’s Toxicology: The Basic Science of Poisons, 9th ed.; Klaassen, C.D., Ed.; McGraw-Hill Education: New York, NY, USA, 2019; pp. 25–64. [Google Scholar]
- ECHA Guidance on Information Requirements and Chemical Safety Assessment, Chapter R.7a: Endpoint Specific Guidance. Available online: https://echa.europa.eu/documents/10162/17224/information_requirements_r7a_en.pdf/e4a2a18f-a2bd-4a04-ac6d-0ea425b2567f?t=1500286622893 (accessed on 25 May 2022).
- Myers, D.K.; Goldberg, A.M.; Poth, A.; Wolf, M.F.; Carraway, J.; McKim, J.; Coleman, K.P.; Hutchinson, R.; Brown, R.; Krug, H.F.; et al. From in vivo to in vitro: The medical device testing paradigm shift. ALTEX 2017, 34, 479–500. [Google Scholar] [CrossRef]
- Marx, U.; Andersson, T.B.; Bahinski, A.; Beilmann, M.; Beken, S.; Cassee, F.R.; Cirit, M.; Daneshian, M.; Fitzpatrick, S.; Frey, O.; et al. Biology-inspired microphysiological system approaches to solve the prediction dilemma of substance testing. ALTEX 2016, 33, 272–321. [Google Scholar] [CrossRef]
- OECD Guidance Document on Considerations for Waiving or Bridging of Mammalian Acute Toxicity Tests; OECD Series on Testing and Assessment, No. 237; OECD Publishing: Paris, France, 2017. [CrossRef]
- Scott, L.; Eskes, C.; Hoffmann, S.; Adriaens, E.; Alepée, N.; Bufo, M.; Clothier, R.; Facchini, D.; Faller, C.; Guest, R.; et al. A proposed eye irritation testing strategy to reduce and replace in vivo studies using Bottom–Up and Top–Down approaches. Toxicol. In Vitro 2010, 24, 1–9. [Google Scholar] [CrossRef]
- EC Commission Regulation (EU) 2019/1390 of 31 July 2019 Amending, for the Purpose of Its Adaptation to Technical Progress, the Annex to Regulation (EC) No 440/2008 Laying Down Test Methods Pursuant to Regulation (EC) No 1907/2006 of the European Parliament and of the Council on the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) (Text with EEA Relevance). Available online: https://www.legislation.gov.uk/eur/2019/1390/annex/division/B.46# (accessed on 9 May 2022).
- Achberger, K.; Haderspeck, J.C.; Kleger, A.; Liebau, S. Stem cell-based retina models. Adv. Drug Deliv. Rev. 2019, 140, 33–50. [Google Scholar] [CrossRef]
- Fluhr, J.W.; Darlenski, R.; Angelova-Fischer, I.; Tsankov, N.; Basketter, D. Skin Irritation and Sensitization: Mechanisms and New Approaches for Risk Assessment, 1. Skin Irritation. Skin Pharmacol. Physiol. 2008, 21, 124–135. [Google Scholar] [CrossRef] [PubMed]
- Basketter, D.; Darlenski, R.; Fluhr, J.W. Skin irritation and sensitization: Mechanisms and new approaches for risk assessment, 2. Skin sensitization. Skin Pharmacol. Physiol. 2008, 21, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Gilmour, N.; Kern, P.S.; Alépée, N.; Boislève, F.; Bury, D.; Clouet, E.; Hirota, M.; Hoffmann, S.; Kühnl, J.; Lalko, J.F.; et al. Development of a next generation risk assessment framework for the evaluation of skin sensitisation of cosmetic ingredients. Regul. Toxicol. Pharmacol. 2020, 116, 104721. [Google Scholar] [CrossRef] [PubMed]
- Prieto, P.; Baird, A.W.; Blaauboer, B.J.; Castell Ripoll, J.V.; Corvi, R.; Dekant, W.; Dietl, P.; Gennari, A.; Gribaldo, L.; Griffin, J.L.; et al. The assessment of repeated dose toxicity in vitro: A proposed approach. The report and recommendations of ECVAM workshop 56. Altern. Lab. Anim. 2006, 34, 315–341. [Google Scholar] [CrossRef]
- Zuang, V.; Schäffer, M.; Kirmizidis, G.; Bremer, S.; Corvi, R.; Worth, A.; Casati, S.; Amcoff, P.; Bernasconi, C.; Castello, P.; et al. EURL ECVAM Progress Report on the Development, Validation and Regulatory Acceptance of Alternative Methods (2010–2013): Prepared in the Framework of Directive 76/768/EEC and Regulation (EC) No 1223/2009 on Cosmetic Products; Joint Research Centre, Institute for Health and Consumer Protection: Ispra, Italy, 2013. [Google Scholar]
- Bessems, J.; Coecke, S.; Gouliarmou, V.; Whelan, M.; Worth, A. EURL ECVAM Strategy for Achieving 3Rs Impact in the Assessment of Toxicokinetics and Systemic Toxicity; Publications Office of the European Union: Luxembourg, 2015. [Google Scholar]
- Zuang, V.; Viegas Barroso, J.F.; Belz, S.; Berggren, E.; Bernasconi, C.; Bopp, S.; Bouhifd, M.; Bowe, G.; Campia, I.; Casati, S.; et al. EURL ECVAM Status Report on the Development, Validation and Regulatory Acceptance of Alternative Methods and Approaches; Publications Office of the European Union: Luxembourg, 2017. [Google Scholar]
- Mennecozzi, M.; Landesmann, B.; Harris, G.; Liska, R.; Whelan, M. Hepatotoxicity Screening Taking a Mode-Of-Action Approach Using HepaRG Cells and HCA. In Proceedings of the 8th World Congress on Alternatives and Animal Use in the Life Sciences, Montreal, QC, Canada, 21–25 August 2011; pp. 193–204. [Google Scholar]
- Desprez, B.; Birk, B.; Blaauboer, B.; Boobis, A.; Carmichael, P.; Cronin, M.T.D.; Curie, R.; Daston, G.; Hubesch, B.; Jennings, P.; et al. A mode-of-action ontology model for safety evaluation of chemicals: Outcome of a series of workshops on repeated dose toxicity. Toxicol. In Vitro 2019, 59, 44–50. [Google Scholar] [CrossRef]
- Berggren, E.; White, A.; Ouedraogo, G.; Paini, A.; Richarz, A.; Bois, F.Y.; Exner, T.; Leite, S.; Grunsven, L.A.V.; Worth, A.; et al. Ab initio chemical safety assessment: A workflow based on exposure considerations and non-animal methods. Comput. Toxicol. 2017, 4, 31–44. [Google Scholar] [CrossRef]
- Gocht, T.; Schwarz, M. Foreword. In SEURAT-1 Report: Towards the Replacement of In Vivo Repeated Dose Systemic Toxicity Testing; Gocht, T., Schwarz, M., Eds.; Coach Consortium: Evry, France, 2016; Volume 6, p. 3. [Google Scholar]
- Evans, T.J. Reproductive Toxicity and Endocrine Disruption. In Veterinary Toxicology, 3rd ed.; Academic Press: London, UK, 2018; Chapter 17; pp. 273–316. [Google Scholar]
- Hareng, L.; Pellizzer, C.; Bremer, S.; Schwarz, M.; Hartung, T. The Integrated Project ReProTect: A novel approach in reproductive toxicity hazard assessment. Reprod. Toxicol. 2005, 20, 441–452. [Google Scholar] [CrossRef]
- Bremer, S.; Balduzzi, D.; Cortvrindt, R.; Daston, G.; Eletti, B.; Galli, A.; Huhtaniemi, I.; Laws, S.; Lazzari, G.; Liminga, U.; et al. The effects of chemicals on mammalian fertility. The report and recommendations of ECVAM Workshop 53—The first strategic workshop of the EU ReProTect Project. Altern. Lab. Anim. 2005, 33, 391–416. [Google Scholar] [CrossRef]
- Lorenzetti, S.; Altieri, I.; Arabi, S.; Balduzzi, D.; Bechi, N.; Cordelli, E.; Galli, C.; Ietta, F.; Modina, S.C.; Narciso, L.; et al. Innovative non-animal testing strategies for reproductive toxicology: The contribution of Italian partners within the EU project ReProTect. Ann. Ist. Super. Sanita 2011, 47, 429–444. [Google Scholar] [CrossRef]
- Zuang, V.; Dura, A.; Asturiol Bofill, D.; Batista Leite, S.; Berggren, E.; Bopp, S.; Carpi, D.; Casati, S.; Coecke, S.; Corvi, R.; et al. JRC Science for Policy Report: Non-Animal Methods in Science and Regulation; Publications Office of the European Union: Luxembourg, 2021. [Google Scholar]
- COM Guidance on a Strategy for Genotoxicity Testing of Chemicals. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1043249/strategy-for-genotoxicity-testing-of-chemicals-guidance.pdf (accessed on 16 May 2022).
- Eastmond, D.A.; Hartwig, A.; Anderson, D.; Anwar, W.A.; Cimino, M.C.; Dobrev, I.; Douglas, G.R.; Nohmi, T.; Phillips, D.H.; Vickers, C. Mutagenicity testing for chemical risk assessment: Update of the WHO/IPCS Harmonized Scheme. Mutagenesis 2009, 24, 341–349. [Google Scholar] [CrossRef]
- OECD Test No.471: Bacterial Reverse Mutation Test. In OECD Guidelines for the Testing of Chemicals, Section 4; OECD Publishing: Paris, France, 2020; Available online: https://www.oecd-ilibrary.org/environment/test-no-471-bacterial-reverse-mutation-test_9789264071247-en (accessed on 19 April 2022). [CrossRef]
- Zuang, V.; Dura, A.; Ahs Lopez, E.; Barroso, J.; Batista Leite, S.; Berggren, E.; Bopp, S.; Campia, I.; Carpi, D.; Casati, S.; et al. EURL ECVAM Status Report 2021: Non-Animal Methods in Science and Regulation; Publications Office of the European Union: Luxembourg, 2022. [Google Scholar]
- Pfuhler, S.; Albertini, S.; Fautz, R.; Herbold, B.; Madle, S.; Utesch, D.; Poth, A. Genetic Toxicity Assessment: Employing the Best Science for Human Safety Evaluation Part IV: Recommendation of a Working Group of the Gesellschaft fuer Umwelt-Mutationsforschung (GUM) for a Simple and Straightforward Approach to Genotoxicity Testing. Toxicol. Sci. 2007, 97, 237–240. [Google Scholar] [CrossRef] [PubMed]
- Corvi, R.; Madia, F.; Guyton, K.Z.; Kasper, P.; Rudel, R.; Colacci, A.; Kleinjans, J.; Jennings, P. Moving forward in carcinogenicity assessment: Report of an EURL ECVAM/ESTIV workshop. Toxicol. In Vitro 2017, 45, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Mascolo, M.G.; Perdichizzi, S.; Vaccari, M.; Rotondo, F.; Zanzi, C.; Grilli, S.; Paparella, M.; Jacobs, M.N.; Colacci, A. The transformics assay: First steps for the development of an integrated approach to investigate the malignant cell transformation in vitro. Carcinogenesis 2018, 39, 955–967. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, M.N.; Colacci, A.; Corvi, R.; Vaccari, M.; Aguila, M.C.; Corvaro, M.; Delrue, N.; Desaulniers, D.; Ertych, N.; Jacobs, A.; et al. Chemical carcinogen safety testing: OECD expert group international consensus on the development of an integrated approach for the testing and assessment of chemical non-genotoxic carcinogens. Arch. Toxicol. 2020, 94, 2899–2923. [Google Scholar] [CrossRef] [PubMed]
- EURL ECVAM Recommendation of 14th March 2012 on Three Cell Transformation Assays Using Syrian Hamster Embryo Cells (SHE) and the BALB/c 3T3 Mouse Fibroblast Cell Line for In Vitro Carcinogenicity Testing. Available online: https://tsar.jrc.ec.europa.eu/system/files/Published/EURL%20ECVAM%20Recommendation.%20Annex%201%20-%20ESAC%20Opinion.%20Annex%202%20-%20EURL%20ECVAM%20request%20for%20ESAC%20advice_1.pdf (accessed on 17 May 2022).
- Jacobs, M.N.; Colacci, A.; Louekari, K.; Luijten, M.; Hakkert, B.C.; Paparella, M.; Vasseur, P. International regulatory needs for development of an IATA for non-genotoxic carcinogenic chemical substances. ALTEX 2016, 33, 359–392. [Google Scholar] [CrossRef] [PubMed]
- Madia, F.; Pillo, G.; Worth, A.; Corvi, R.; Prieto, P. Integration of data across toxicity endpoints for improved safety assessment of chemicals: The example of carcinogenicity assessment. Arch. Toxicol. 2021, 95, 1971–1993. [Google Scholar] [CrossRef]
- Kim, K.; Park, H.; Lim, K. Phototoxicity: Its Mechanism and Animal Alternative Test Methods. Toxicol. Res. 2015, 31, 97–104. [Google Scholar] [CrossRef] [Green Version]
- OECD Test No. 432: In Vitro 3T3 NRU Phototoxicity Test. In OECD Guidelines for the Testing of Chemicals, Section 4; OECD Publishing: Paris, France, 2019. [CrossRef]
- Lasarow, R.M.; Isseroff, R.R.; Gomez, E.C. Quantitative In Vitro Assessment of Phototoxicity by a Fibroblast-Neutral Red Assay. J. Investig. Dermatol. 1992, 98, 725–729. [Google Scholar] [CrossRef]
- Onoue, S.; Seto, Y.; Sato, H.; Nishida, H.; Hirota, M.; Ashikaga, T.; Api, A.M.; Basketter, D.; Tokura, Y. Chemical photoallergy: Photobiochemical mechanisms, classification, and risk assessments. J. Dermatol. Sci. 2017, 85, 4–11. [Google Scholar] [CrossRef]
- ICH Guideline S10 Guidance on Photosafety Evaluation of Pharmaceuticals; International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use: London, UK, 2012.
- Lee, Y.S.; Yi, J.; Lim, H.R.; Kim, T.S.; Ahn, I.Y.; Ko, K.; Kim, J.; Park, H.; Sohn, S.J.; Lee, J.K. Phototoxicity Evaluation of Pharmaceutical Substances with a Reactive Oxygen Species Assay Using Ultraviolet A. Toxicol. Res. 2017, 33, 43–48. [Google Scholar] [CrossRef]
- COM Statement on Photogenotoxicity Testing. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/315914/statement_on_photogenotoxicity.pdf (accessed on 18 May 2022).
- ICH Guideline S10, Photosafety Evaluation of Pharmaceuticals, Step 4; International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use: London, UK, 2013.
- Brendler-Schwaab, S.; Czich, A.; Epe, B.; Gocke, E.; Kaina, B.; Muller, L.; Pollet, D.; Utesch, D. Photochemical genotoxicity: Principles and test methods Report of a GUM task force. Mutat. Res. 2004, 566, 65–91. [Google Scholar] [CrossRef]
- Thompson, C.V.; Firman, J.W.; Goldsmith, M.R.; Grulke, C.M.; Tan, Y.; Paini, A.; Penson, P.E.; Sayre, R.R.; Webb, S.; Madden, J.C. A Systematic Review of Published Physiologically-based Kinetic Models and an Assessment of their Chemical Space Coverage. Altern. Lab. Anim. 2021, 49, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Aldhous, P.; Coghlan, A.; Copley, J. Let the people speak. New Sci. 1999, 162, 26–31. [Google Scholar] [PubMed]
- FRAME Fact or Fiction? Mapping Perceptions of Animal Testing. Survey Report. Available online: https://frame.org.uk/wp-content/uploads/2020/06/FRAME-report_final.pdf (accessed on 18 May 2022).
- Pifer, R.; Shimizu, K.; Pifer, L. Public Attitudes Toward Animal Research: Some International Comparisons. Soc. Anim. 1994, 2, 95–113. [Google Scholar] [CrossRef]
- Schuppli, C.A.; Weary, D.M. Attitudes towards the use of genetically modified animals in research. Public Underst. Sci. 2010, 19, 686–697. [Google Scholar] [CrossRef]
- Mills, K.E.; Han, Z.; Robbins, J.; Weary, D.M. Institutional transparency improves public perception of lab animal technicians and support for animal research. PLoS ONE 2018, 13, e0193262. [Google Scholar] [CrossRef] [Green Version]
- Mendez, J.C.; Perry, B.A.L.; Heppenstall, R.J.; Mason, S.; Mitchell, A.S. Openness about animal research increases public support. Nat. Neurosci. 2022, 25, 401–403. [Google Scholar] [CrossRef]
- Evans, G.; Durant, J. The relationship between knowledge and attitudes in the public understanding of science in Britain. Public Underst. Sci. 1995, 4, 57–74. [Google Scholar] [CrossRef]
- Ormandy, E.H.; Schuppli, C.A.; Weary, D.M. Public Attitudes toward the Use of Animals in Research: Effects of Invasiveness, Genetic Modification and Regulation. Anthrozoös 2013, 26, 165–184. [Google Scholar] [CrossRef]
- L’Oréal Milestones in Non-Animal Safety Testing. Available online: https://www.loreal.com/en/commitments-and-responsibilities/for-the-planet/for-beauty-with-no-animal-testing/milestones-in-the-safety-assessment-without-animal/ (accessed on 18 May 2022).
- Fentem, J.H.; Archer, G.E.; Balls, M.; Botham, P.A.; Curren, R.D.; Earl, L.K.; Esdaile, D.J.; Holzhütter, H.G.; Liebsch, M. The ECVAM International Validation Study on In Vitro Tests for Skin Corrosivity. 2. Results and Evaluation by the Management Team. Toxicol. In Vitro 1998, 12, 483–524. [Google Scholar] [CrossRef]
- Lush. The Lush Prize. Available online: https://lushprize.org/ (accessed on 22 May 2022).
- Bhatia, M.; Jain, A. Green Marketing: A Study of Consumer Perception and Preferences in India. Electron. Green J. 2013, 36, 1–19. [Google Scholar] [CrossRef]
- Patel, C.; Chugan, P.K. The Influence of Consumer Perception Towards Green Advertising on Green Purchase Intention. Int. J. Entrep. Bus. Environ. Perspect. 2015, 4, 1865–1873. [Google Scholar]
- Ihemezie, E.J.; Ukwuaba, I.C.; Nnaji, A.P. Impact of ‘Green’ Product Label Standards on Consumer Behaviour: A Systematic Review Analysis. Int. J. Acad. Res. Bus. Soc. Sci. 2018, 8, 666–684. [Google Scholar] [CrossRef]
- Franca, C.C.V.; Ueno, H.M. Green cosmetics: Perspectives and challenges in the context of green chemistry. Desenvolv. Meio Ambiente 2020, 53, 133–150. [Google Scholar] [CrossRef]
- Barlow, S.M.; Boobis, A.R.; Bridges, J.; Cockburn, A.; Dekant, W.; Hepburn, P.; Houben, G.F.; König, J.; Nauta, M.J.; Schuermans, J.; et al. The role of hazard- and risk-based approaches in ensuring food safety. Trends Food Sci. Technol. 2015, 46, 176–188. [Google Scholar] [CrossRef]
- Freudenburg, W.R. Perceived Risk, Real Risk: Social Science and the Art of Probabilistic Risk Assessment. Science 1988, 242, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Crawford, S.E.; Hartung, T.; Hollert, H.; Mathes, B.; van Ravenzwaay, B.; Steger-Hartmann, T.; Studer, C.; Krug, H.F. Green Toxicology: A strategy for sustainable chemical and material development. Environ. Sci. Eur. 2017, 29, 16. [Google Scholar] [CrossRef] [Green Version]
- Ormandy, E.H.; Schuppli, C.A. Public Attitudes toward Animal Research: A Review. Animals 2014, 4, 391–408. [Google Scholar] [CrossRef] [Green Version]
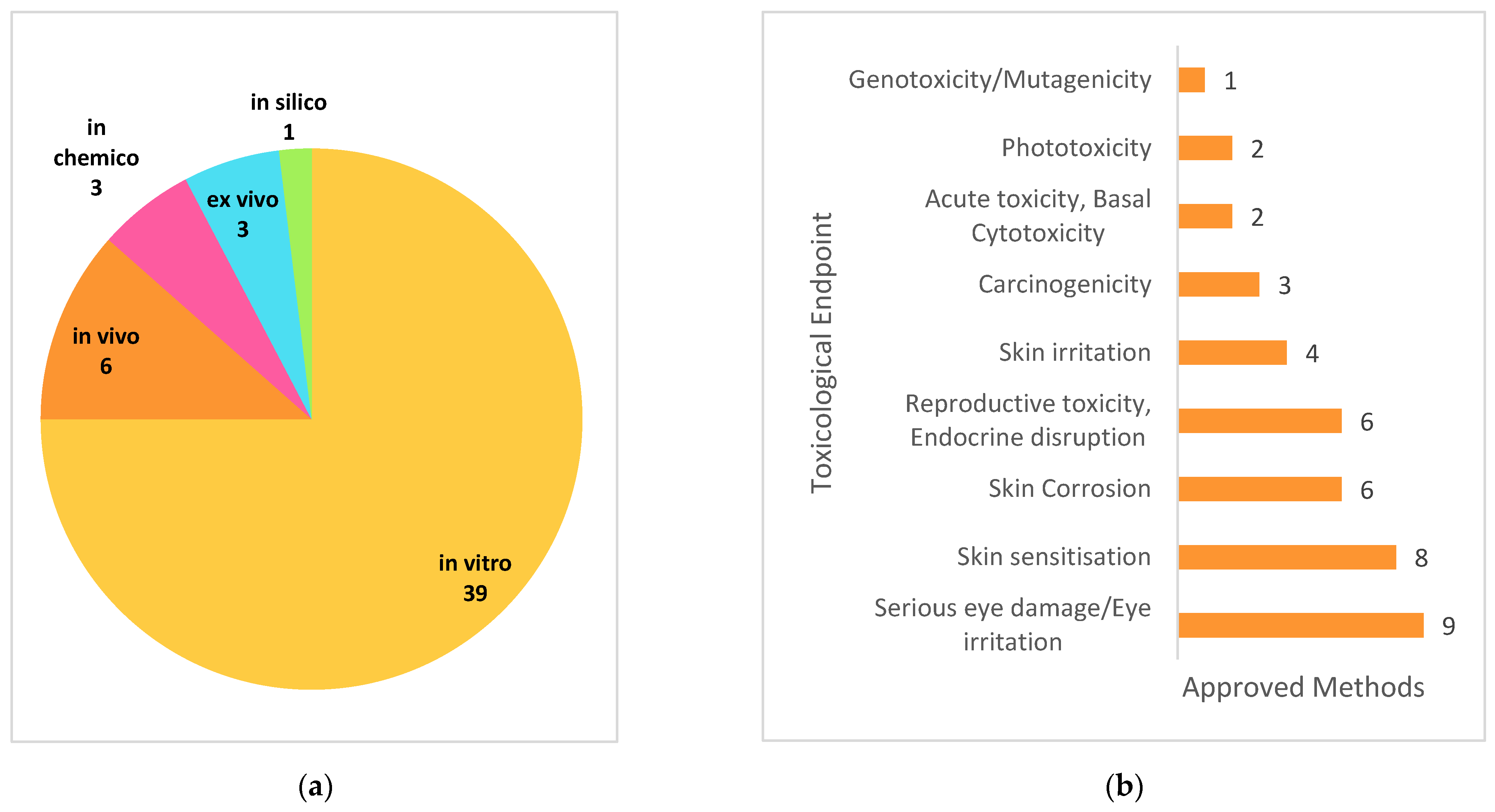
| Format | Source |
|---|---|
| Tier 1: Academic Papers |
|
| Tier 2: Articles |
|
| Tier 3: Reports |
|
| Country | Product Testing Ban a | Ingredient Testing Ban b | Marketing Ban c |
|---|---|---|---|
| United Kingdom | 1998 | 1998 | 2009/13 (as part of EU Regulation EC 1223/2009) |
| EU/EFTA | 2004 | 2009 | 2009/13 (depending on the toxicological endpoint) |
| Brazil (São Paulo) | 2014 | ||
| India | 2014 | ||
| New Zealand | 2015 | 2015 | |
| Turkey | 2015 (where a validated alternative exists) | 2015 (where a validated alternative exists) | 2015 (where a validated alternative exists) |
| South Korea | 2015 (where a validated alternative exists) | 2015 (where a validated alternative exists) | 2015 (where a validated alternative exists) |
| Guatemala | 2017 | ||
| Brazil (Paraná) | 2015 | ||
| Brazil (Pará) | 2016 | ||
| Brazil (Rio de Janeiro) | 2017 | 2017 | |
| Brazil (Minas Gerais) | 2018 | ||
| Brazil (Pernambuco) | 2018 | ||
| USA (California) | 2018 | ||
| Australia | 2019 | 2019 | |
| USA (Illinois, and Nevada) | 2019 | ||
| Taiwan | 2019 | 2019 | |
| Mexico | 2021 | 2021 | |
| Colombia | 2020 | 2020 | |
| Brazil (Santa Catarina) | 2020 | ||
| Brazil (Federal District) | 2020 | ||
| USA (Virginia, Maryland, Maine, Hawaii, and New Jersey) | 2021 | ||
| Brazil (Paraíba) | 2022 |
| Toxicological Endpoint | Test Methods |
|---|---|
| Skin Corrosion (OECD TG 431) |
|
| Skin Irritation (OECD TG 439) |
|
| Group | Test Methods |
|---|---|
| Organotypic tests Able to identify chemicals that induce serious eye damage (GHS Ca-tegory 1), and chemicals that do not require classification | Bovine Cornea Opacity Permeability (BCOP, OECD TG 437) [75] Determines a test chemical’s ability to cause opacity and permeability in an isolated bovine cornea. Isolated Chicken Eye (ICE, OECD TG 438) [76] Determines a test chemical’s ability to induce toxicity in an enucleated chicken eye. |
| Cytotoxicity and cell function-based in vitrotests Able to identify chemicals that induce serious eye damage (GHS Ca-tegory 1), and chemicals that do not require classification | Short Time Exposure (STE, OECD 491) [79] Evaluates eye irritation potential of a test chemical by measuring its cytotoxic effect on a rabbit corneal cell line Fluorescein Leakage (FL, OECD TG 460) [77] Evaluates the toxic effects of a short exposure to a substance by measuring sodium fluorescein permeability through an epithelial monolayer of MDCK kidney cells. |
| Reconstructed human tissue (RhT)-based tests Able to identify chemicals that do not require classification | Reconstructed Human Cornea-like Epithelium (RhCE, OECD TG 492) [80] Determines a test chemical’s ability to induce cytotoxicity. Currently four commercially available RhCE models have been adopted: EpiOcular™ EIT, SkinEthicTM Human Corneal Epithelium (HCE) EIT, LabCyte CORNEA-MODEL 24 EIT, and MCTT HCETM EIT. Vitrigel-EIT (OECD TG 494) [81] Determines eye irritation potential of a test chemical by evaluating its ability to induce damage to the barrier function of a hCE model fabricated in a Collagen Vitrigel Membrane (CVM) chamber, by measuring relative changes in transepithelial electrical resistance (TEER) over time. |
| In vitro macromolecular test Recommended only as part of a tiered testing strategy, with specific limitations | Ocular Irritection® (OI) assay (OECD TG 496) [78] A biochemical assay that determines ocular toxicity through the premise that eye irritation and corneal opacity result from the denaturation or perturbation of corneal proteins. |
| Defined Approach (DA) | Information Sources | Capability (Hazard and/or Potency) |
|---|---|---|
| ‘2 out of 3’ (2o3) DA Able to distinguish chemicals that induce skin sensitisation (GHS Category 1) from chemicals that do not require classification. | KE1 (protein binding): Direct peptide Reactivity assay (DPRA; OECD TG 442C) [66] KE2 (keratinocyte activation): KeratinoSens™ (OECD TG 442D) [67] K3 (dendritic cell activation): Human Cell Line Activation Test (h-CLAT; OECD TG 442E) [68] | Hazard |
| Integrated Testing Strategy (ITS) DA Able to distinguish chemicals that induce skin sensitisation (GHS Category 1) from chemicals that do not require classification and allocate skin sensitizers into GHS sub-categories (1A or 1B). | KE1 (protein binding): Direct Peptide Reactivity assay (DPRA; OECD TG 442C) [66] K3 (dendritic cell activation): Human Cell Line Activation Test (h-CLAT; OECD TG 442E) [68] in silico prediction: Derek Nexus v6.1.0 (ITSv1) or OECD QSAR Toolbox v4.5 (ITSv2) | Hazard, Potency |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, R.J.; Tamburic, S. A State-of-the-Art Review on the Alternatives to Animal Testing for the Safety Assessment of Cosmetics. Cosmetics 2022, 9, 90. https://doi.org/10.3390/cosmetics9050090
Silva RJ, Tamburic S. A State-of-the-Art Review on the Alternatives to Animal Testing for the Safety Assessment of Cosmetics. Cosmetics. 2022; 9(5):90. https://doi.org/10.3390/cosmetics9050090
Chicago/Turabian StyleSilva, Rita José, and Slobodanka Tamburic. 2022. "A State-of-the-Art Review on the Alternatives to Animal Testing for the Safety Assessment of Cosmetics" Cosmetics 9, no. 5: 90. https://doi.org/10.3390/cosmetics9050090
APA StyleSilva, R. J., & Tamburic, S. (2022). A State-of-the-Art Review on the Alternatives to Animal Testing for the Safety Assessment of Cosmetics. Cosmetics, 9(5), 90. https://doi.org/10.3390/cosmetics9050090







