1. Introduction
The human skin is known to harbor numerous microorganisms, such as bacteria, viruses and fungi. In total, it must be assumed that in total, 10
11 microbial cells can be found on the skin surface [
1], although their number and composition may vary with the specific conditions provided by the different areas of the skin [
2].
Staphylococcus,
Corynebacterium and
Cutibacterium represent the three dominant bacterial genera on the skin [
3]. It is already known that members of the human microbiota can be influenced by supporting agents, which are frequently used in food and have been shown to positively impact several diseases [
4]. These prebiotic supplements have also gained attention for the use of cosmetic products. Here, they already have proven to promote the growth of beneficial bacteria such as coagulase-negative
Staphylococci, while inhibiting the growth of
Cutibacterium (Propionibacterium) acnes [
5], assuming that an unbalanced skin biota may favour acne, when the pathogen outweighs the beneficial bacteria in terms of quantity [
6]. In general, prebiotics may be used to rebalance the skin microbiota by promoting the growth of beneficial bacteria and inhibit the growth of pathogens [
7]. Putative prebiotics may be chosen from, for example, natural extracts, various sugars, or fermented products of bacteria, latter sometimes also referred to as postbiotics. The present study describes the development of an in vitro model using a leather surface, which may provide a surface similar to human skin. This model was used to validate to function of two commercially available prebiotic actives and has shown to be suitable for generating new insights about the influence of cosmetic ingredients on bacterial growth under realistic conditions.
2. Materials and Methods
2.1. Cultivation of Test Strains
Seven bacterial strains comprising ubiquitous bacteria or typical members of the Gram-positive skin microbiota were chosen as test strains and obtained from the German collection of microorganisms (DSMZ, Braunschweig, Germany). The choice of test strains was also made based on the supposed promoting or inhibiting effects of the tested prebiotic actives on different species.
For preparing the bacterial suspensions, strains were grown in CASO bouillon (Merck KGaA, Darmstadt, Germany) for 24–72 h at 30 or 37 °C and diluted in NaCl to a final concentration of 10
5 CFU/mL. An overview of test strains and the used time/temperature combinations for their cultivation can be found in
Table 1.
2.2. Active Ingredients and Formulas
For the serum, 88.34 g–92.34 g of demineralized water (depending on the concentration of the active ingredient, which ranged from 0% to 4%) was mixed with 3.0 g plant-based glycerol (85%, Mercur Handel GmbH, Düsseldorf, Germany), 1.0 g diglycerol (diglycerin S MB RSPO, Rossow Group, Gennevilliers Cedex, France), 0.3 g glyceryl caprylate (Dermosoft GMCY, Evonik Dr. Straetmans GmbH, Hamburg, Germany) and 3.0 g of a multifunctional ingredient (Dermosoft 1388 ECO, Evonik Dr. Straetmans GmbH, Hamburg, Germany). After homogenisation using a T 50 digital ULTRA-TURRAX (IKA-Werke GmbH & Co. KG, Staufen, Germany), the pH was adjusted to 4.7 to 5.3 with 0.01 g of citric acid. Afterwards, the formula was thickened by adding 0.25 g acacia senegal gum (Solagum AX, Seppic, Puteaux Cedex, France) and 0.1 g xanthan gum (Rhodicare T, Kahl GmbH & Co. KG, Trittau, Germany), before adding the requested concentration of the actives mentioned in
Table 2. Serum without added active ingredients served as the control.
2.3. Determination of General Growth Effects
To determine the general inhibiting or promoting effects of the active ingredients on the bacterial test strains, growth curves were recorded for each strain on standard media in the absence or presence of an active substance, as described in [
5]. Therefore, the respective product was mixed with TSB and 10 μL of the bacterial suspension in a microtiter plate to a total volume of 200 µL, resulting in the concentrations indicated in
Table 2. Afterwards, each plate was incubated at the temperature suitable for the respective test strains (cp.
Table 1), and the optical density (oD) was measured at 600 nm in triplicates for each parameter every 30–60 min using a microplate reader (Varioskan Flash, Thermo Fisher Scientific, Waltham, MA, USA). To ensure legibility and since growth curves were meant to show general trends, no standard deviations were indicated for data points.
2.4. Microbiological Assays on the Leather Skin Model
To investigate the microbiological effects of the cosmetic serum under more realistic conditions, a leather model simulating the skin surface was used, allowing for the testing of bacteria adhered to a surface and for the product to be applied in a realistic scenario. Therefore, untreated, natural finished, vegetable tanned sheep leather of 0.8–1.2 mm thickness (Giese & Bruhm GmbH, Duisburg, Germany) was cut into 1 cm × 1 cm pieces, transferred to a petri dish and sterilized by UV exposure for 1 h per site. A total of 100 µL of a bacterial suspension, prepared as described above, was then pipetted on the smooth surface of the leather pieces and dried for at least 15 min. The bacterial count on the leather surface was determined by the transfer of the pieces to 2 mL reaction tubes filled with 1 mL TSB (30 g/L, Merck KGaA, Darmstadt, Germany), shaking at 1000 rpm for 15 min on an incubation mixer (Eppendorf Thermomixer 5355, Hamburg, Germany), followed by sequential decimal dilution and plating on TSA (40 g/L, Merck KGaA, Darmstadt, Germany). UV-irradiated pieces not inoculated with bacterial suspensions were treated equally and served as the sterilization control. To evaluate the impact of cosmetic formulas on the bacterial strains, 10 µL and 50 µL of the serum formulas containing different concentrations of the active ingredients were transferred to the inoculated and dried leather pieces and were allowed to take effect for 30 min. Afterwards, the bacterial count was determined for each strain and formula as described above.
3. Results and Discussion
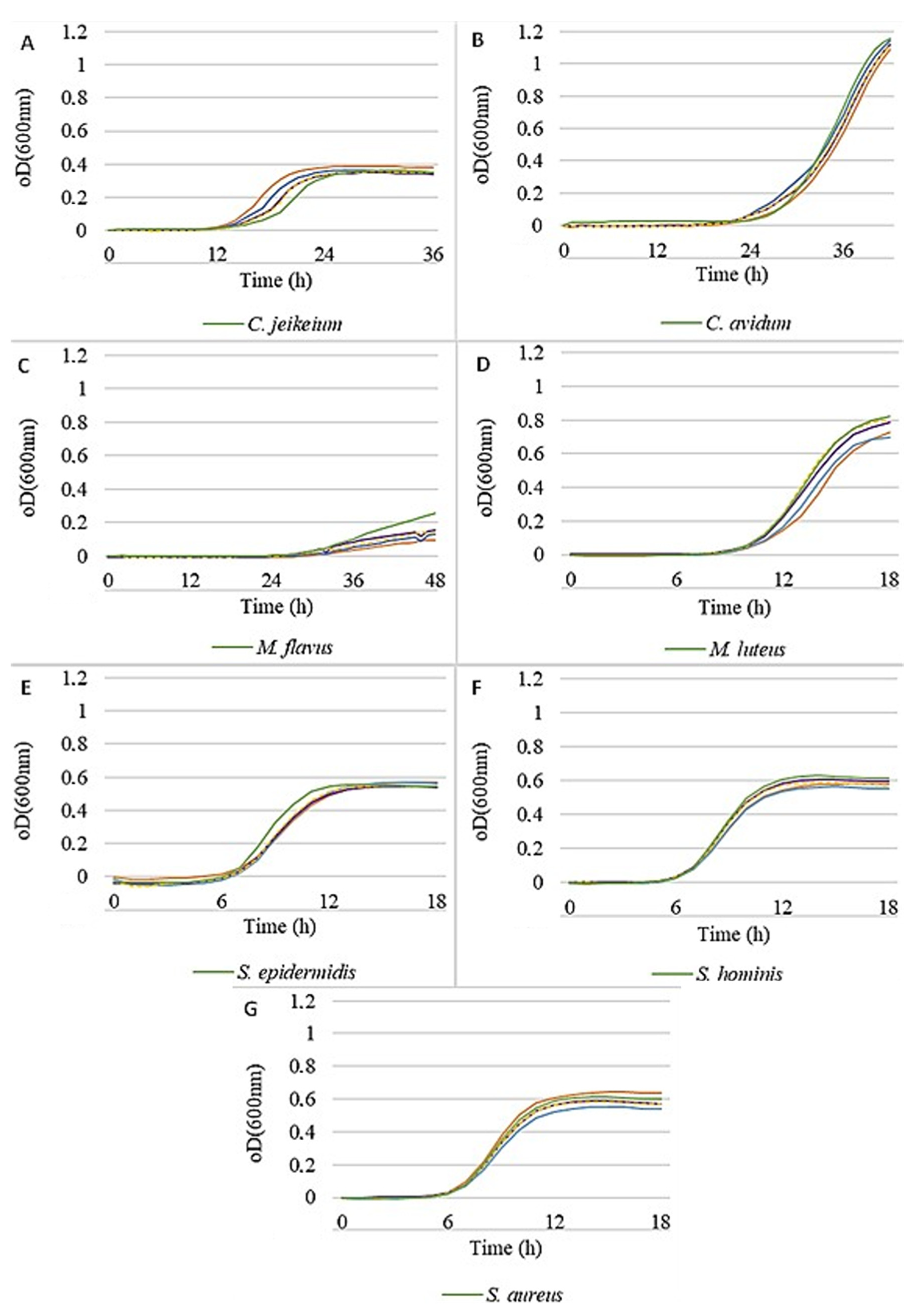
A first indication for promoting or inhibiting effects on the test strains might be observed when they are grown in liquid cultures in the presence of the prebiotic actives at different concentrations.
Figure 1 shows the general effects of active 1 on the seven test strains. A promoting effect must be assumed if the growth curve is shifted to higher oDs at a given time compared to the control. Likewise, if the growth curve is shifted to lower oDs, the action must be considered inhibiting. The effects become particularly obvious during the logarithmic phase. In this regard, a little growth promotion could be determined for
C. jeikeium (
Figure 1A), while the
Micrococci (
Figure 1C,D) were slightly inhibited. All other strains were left virtually unaffected by the active ingredients, even at higher concentrations.
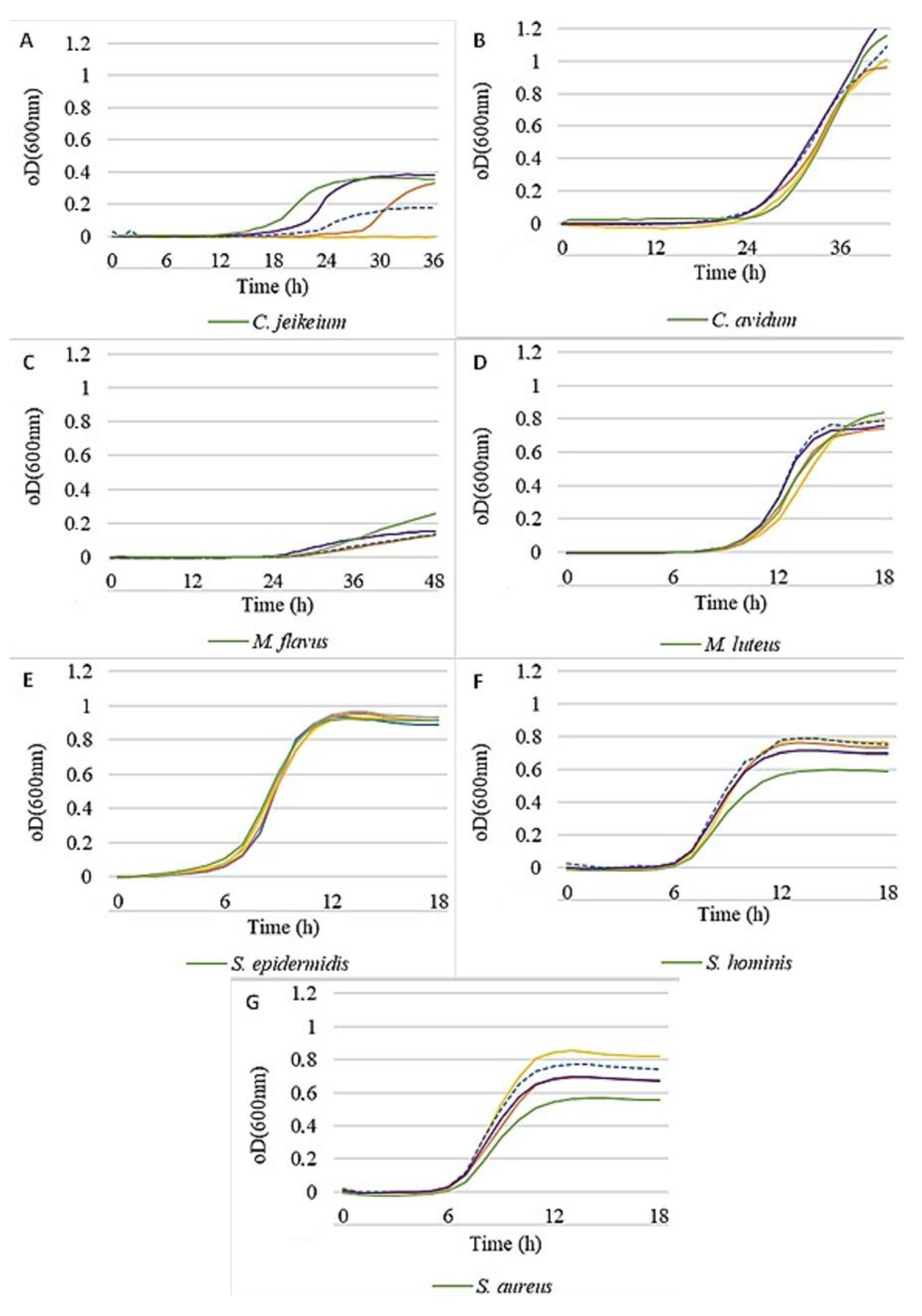
Figure 2 illustrates that all strains were able to grow in the presence of active 2, with only small inhibiting effects, except for
C. jeikeium, which was specifically inhibited by increasing concentrations of this active. However, a clear growth promotion for some
Staphylococci could be observed, being most pronounced for
S. aureus, but also for
S. hominis, while
S. epidermidis remained unaffected.
Despite providing information on the general effect on different bacterial species, the growth-promoting or–inhibiting effects as shown in
Figure 1 and
Figure 2 might not resemble the situation in vivo, where prebiotic actives are acting within a frame formula and—maybe even more important—on a skin surface colonized by bacteria, rather than in a bacterial suspension.
To address this issue, a newly developed leather skin model was used to investigate the influence of cosmetic formulas containing prebiotic actives on natural bacterial colonizers of the skin. Although several in-vitro skin models based on cultivated human epidermal cells are available [
8,
9,
10], these models are difficult to handle and must be considered highly variable due to their biological nature. Moreover, the complexity of the interactions between bacteria and skin cells cannot be simulated in such a cell-culture-based model anyway since it is hardly possible to handle a realistic microbial community in a lab environment. The rather simple leather skin model used in this study permits the analysis of members of the skin microbiota on a surface exhibiting many characteristics of natural human skin, e.g., in terms of bacterial adhesion and absorption of the cosmetic formula, but at the same time remains simple to use and reproduce.
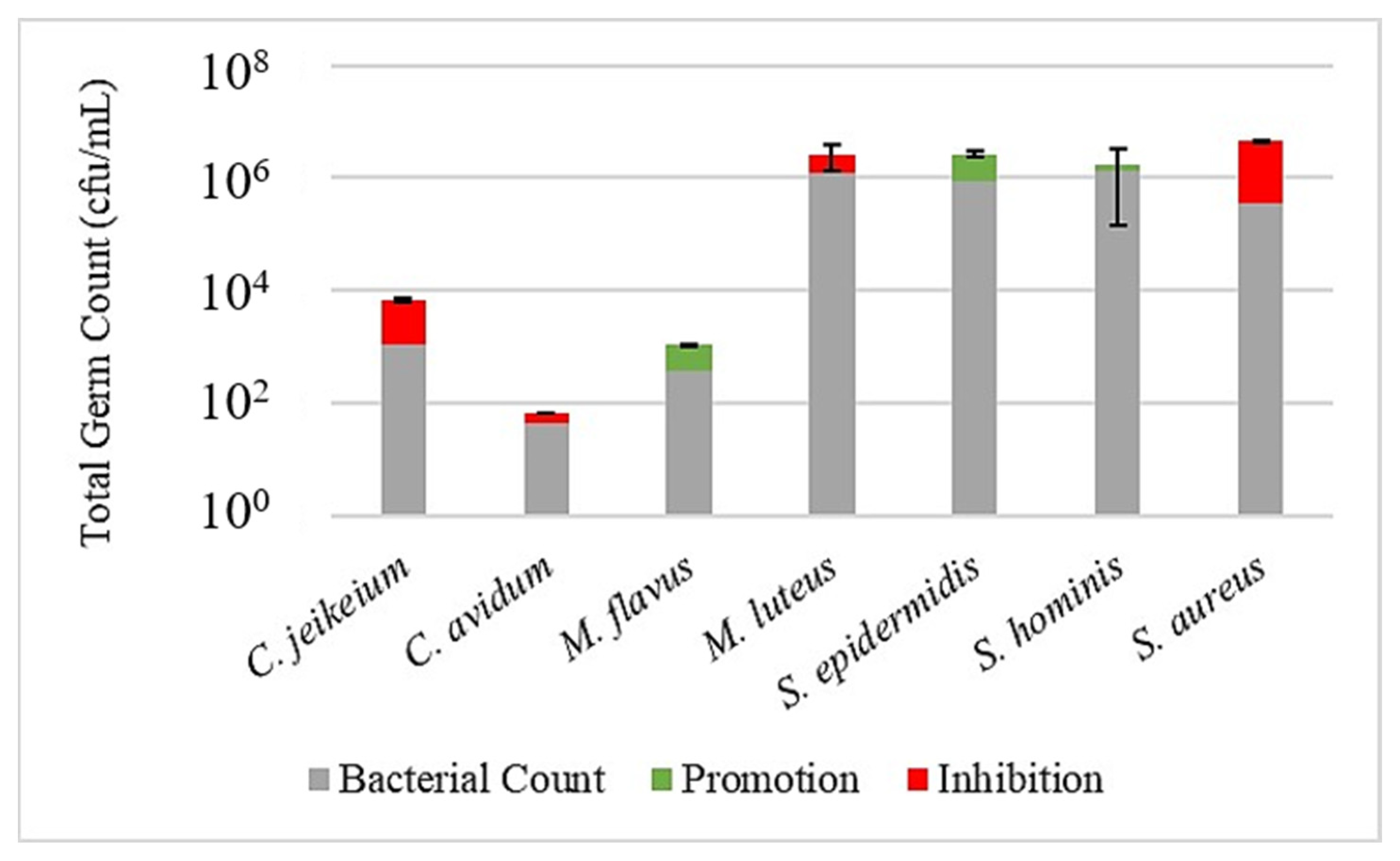
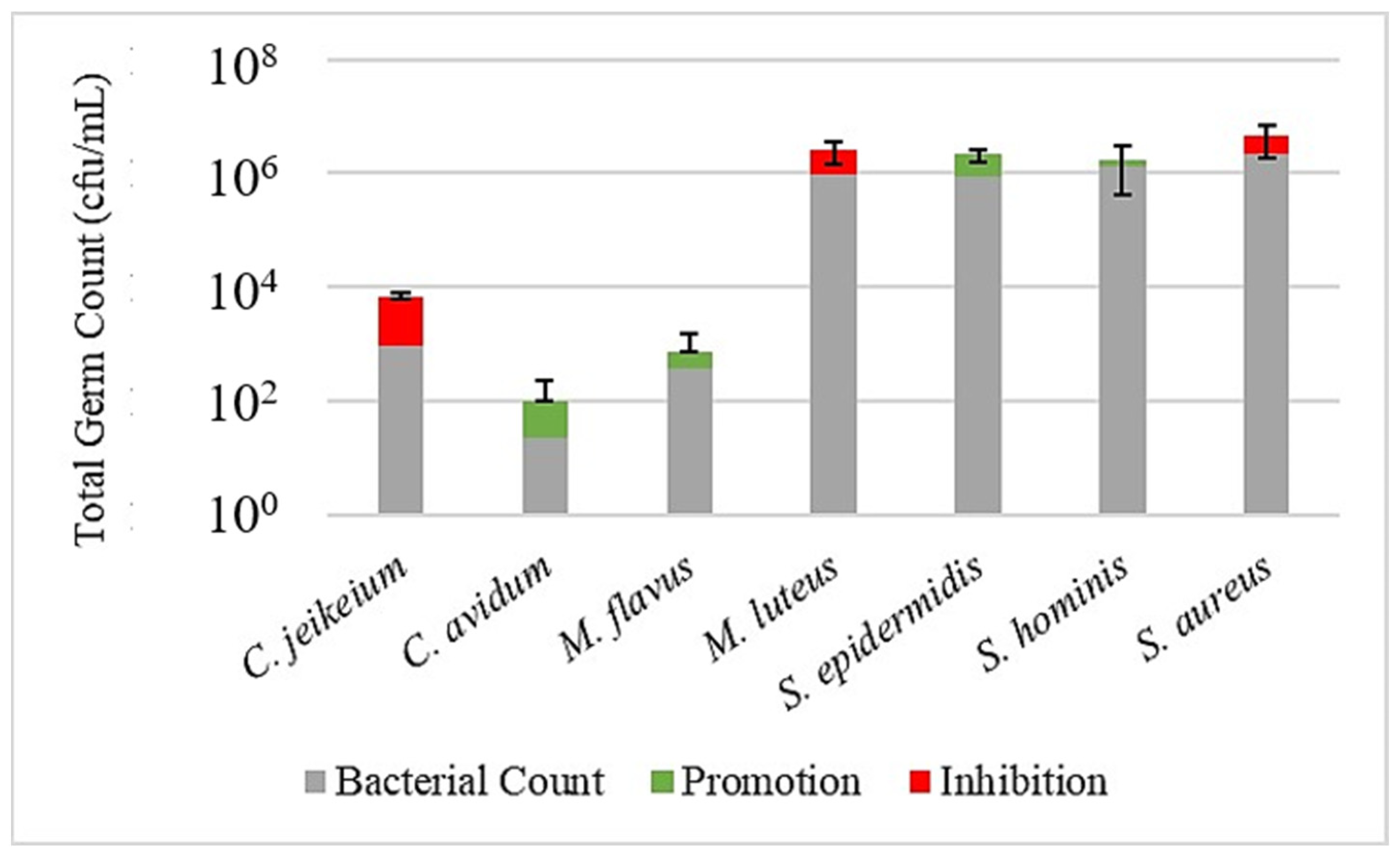
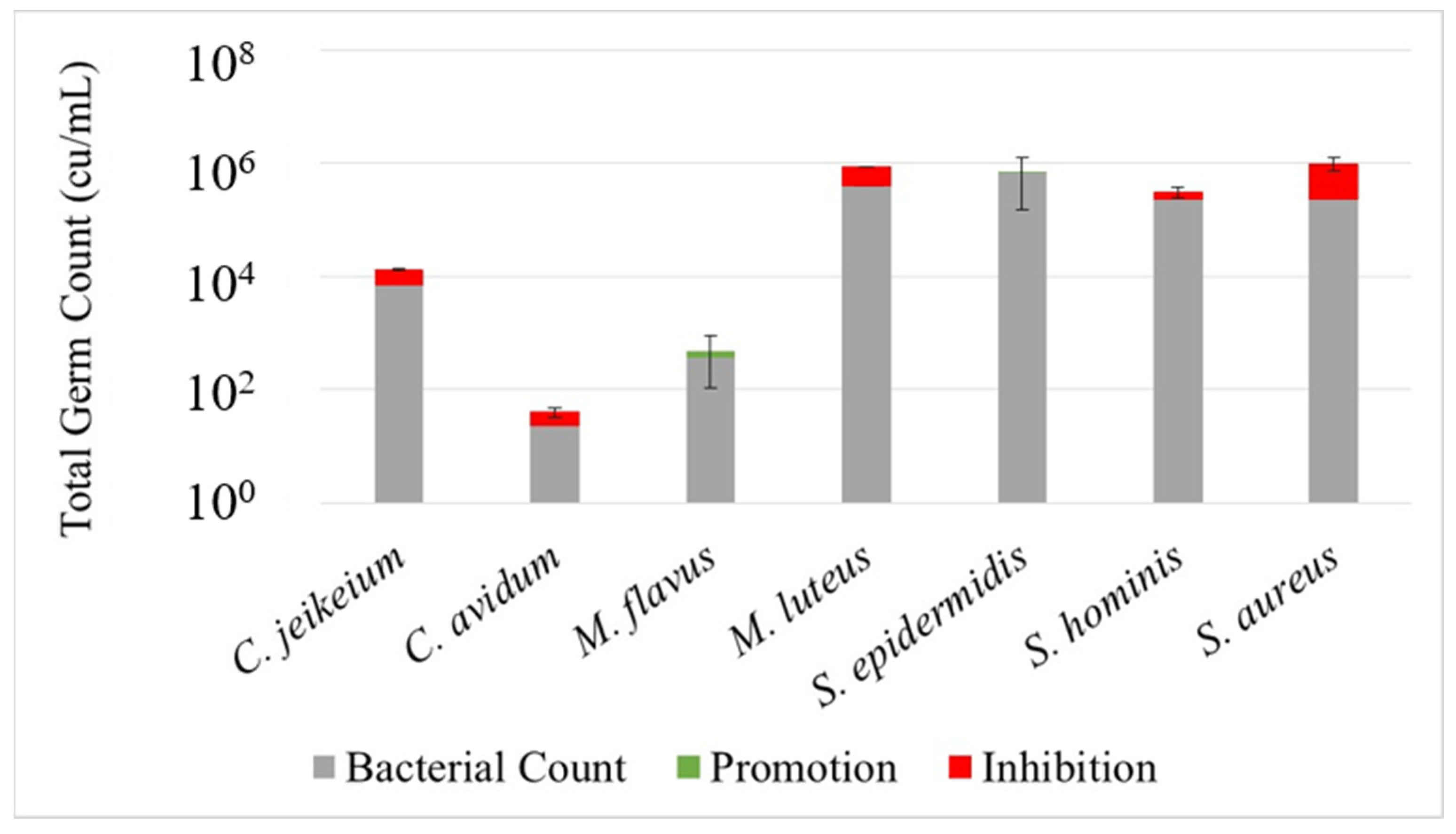
Figure 3,
Figure 4 and
Figure 5 illustrate the effect of the investigated prebiotic actives using the leather skin model. When comparing the bacterial count after a 30 min treatment with the serum to the untreated control, it got obvious that the serum without any added actives has a considerable effect on the bacterial colonization. While the growth of
S. epidermidis,
S. hominis and
M. flavus was slightly promoted, a humble inhibition could be observed for the other strains, being more severe, however, for
S. aureus. This general pattern could be reproduced when using the sera with actives, although with some minor changes and exceptions. Most strikingly, for active 1, the inhibition of
S. aureus was less pronounced, and for active 2, the inhibition of
C. jeikeium was higher than for serum alone. An overview of the promoting or inhibiting effects of the serum control or serum with added actives compared to the untreated control is provided in
Table 3.
Apart from showing that the leather skin model is generally able to act as a realistic in vitro model for investigating the behavior of bacterial colonizers of the skin, data suggest that different members of the skin microbiota are impacted by a cosmetic treatment in a discriminative way. This is even true for different species of the same genera as observed for the coagulase-positive
S. aureus, which was inhibited in the presence of the cosmetic serum, whereas the coagulase-negative
Staphylococci S. epidermidis and
S. hominis were promoted. The effects of the actives compared to the untreated control, as illustrated in
Figure 3,
Figure 4 and
Figure 5, were not significant (except for
M. luteus treated with active 2). Likewise, comparisons between skin models treated with serum alone and serum containing actives did not reveal statistically significant effects (data not shown). Thus, the results must be interpreted very carefully and may only indicate a trend. Moreover, it must be considered that the bacterial strains, which were investigated separately in this study, would normally occur in a complex microbial community, which may influence or modify the effects. Most importantly, the growth curves experiments in the presence of the post- or prebiotic actives do not reveal the same behavior of the investigated strains compared to the results obtained with the leather skin model. Although this might also be due to a combined effect of frame formula and active ingredients, it must also be considered that the realistic environment for bacteria colonizing a skin-like surface is responsible for the different effects of the treatment. It should be noted that the fact that most observed effects were not significant does not mean that the leather skin model is not able to simulate the impact of cosmetics, but that growth effects on skin microorganisms by cosmetic actives may be generally small.
The experiments were conducted with actives that are marketed as prebiotics; however, it should be mentioned that the manufacturers of the actives do not target the same bacterial species as used in this study. Thus, the results are not necessarily in contradiction with the cosmetic effect aimed by the manufacturer. However, data suggest effects that might not have been considered by the manufacturer but should be considered, especially if a cosmetic formula is able to promote pathogens such as
S. aureus. In this regard, the leather skin might provide a suitable in vitro model to comprehensively investigate inhibitory or promoting effects on bacterial members of the skin microbiota, even beyond claimed effects. Moreover, this experimental setup may be further exploited in the future by including more aspects, such as a co-cultivation of bacterial strains or the addition of skin-derived media such as sweat or sebum. Taken together, the leather model might prove as a suitable means to investigate prebiotic effects on skin in an in vivo-like environment. It will be necessary to compare data obtained with this model to other experimental setups, e.g., using cell culture models, as recently used by Le Bourgot et al. [
10]. While these models are rather complex in comparison to the leather model, they might prove to be more close to the in vivo situation, thus providing some other insights.