1. Introduction
Phusang waterfall or “warm waterfall” is located within Phusang National Park, Phayao Province, Thailand. The water of the warm waterfall is sourced from hot springs and has low contents of sodium and other minerals [
1]. Phusang waterfall is the only place in Thailand where water has temperatures between 35–36 °C. Surprisingly, the Phusang waterfall originates from the Phusang warm pond (Phusang hot spring), which has a temperature of approximately 36–38 °C and was discovered approximately ten years ago. National Park officials and the community pay attention to natural hot springs in Thailand and try to develop them as tourist attractions and for various applications. People still believe in the magic of the hot springs for good health and their ability to treat diseases. However, Thailand has never studied the safety and properties of domestic hot springs or Phusang hot spring. Hot springs in Thailand are widely available to the public but have no scientific references. People tend to ignore basic knowledge about water sources because they think they are not directly related to poor health. However, the water source is actually a reservoir for many pathogens (drug-resistant bacteria, pathogenic bacteria and viruses) that can be transmitted to humans if these sources are used without basic knowledge and caution.
However, the advantages of hot springs are evident. Hot spring water may be used in balneotherapy, rehabilitation of dermatological diseases and treatment of immunoinflammatory diseases, chronic pain syndromes, chronic cardiac diseases, metabolic syndromes, neurological diseases [
2], and atopic dermatitis [
3]. For example, Avène thermal spring water (France) exerts antiradical, anti-inflammatory and immune effects. Avène water induces keratinocyte differentiation [
4]. Silent spring water (Italy) reduces the frequency and severity of symptoms of patients with knee arthritis [
5]. Water from Mageumsan hot spring (Korea) also suppresses inflammation in individuals with atopic dermatitis [
6]. Bathing in Suanbo hot springs (Korea) reduces modified atopic dermatitis (SCORAD) index scores, transepidermal water loss (TEWL), epidermal hyperplasia, and inflammatory cell infiltration in individuals with atopic dermatitis [
7]. Avène thermal spring water has been shown to decrease IL-6 levels. Interestingly, IL-10 production by CD4+ T cells was induced by Avène thermal spring water [
8]. For clarity, the balneotherapeutic mechanism of Phusang hot spring water will be evaluated.
Our previous study detected trace elements such as sodium (Na), calcium (Ca), and strontium (Sr), which might be advantageous for drinking water applications [
1]. However, the result must be evaluated using other methods.
The temperature of Phusang hot spring water ranges from 36–38 °C, and a previous study reported many types of pathogenic bacteria in Phusang water samples [
1]. Moreover, viral infectious diseases that are transmitted by water, food (norovirus and rotavirus) and contact/secretions (human papillomavirus [HPV] and Epstein–Barr virus [EBV]) are more common today [
9,
10,
11,
12,
13,
14]. These viruses are inherent in nature and may be unexpected; therefore, people may remain unprotected. In addition to pathogenic viruses, archaeal viruses from high-temperature environments were discovered after the discovery of bacteriophages. Bacteriophages infecting enteric bacteria have been identified in over 1500 drinking water samples from Israel and Spain [
11]. Consequently, we must consider these findings before using hot spring water to prevent viral infections [
15,
16]. In many pond water samples (Bangladesh), the average total viable count (TVC) ranged from 2.7 × 10
5 to 4.4 × 10
13 colony forming units (CFUs)/mL [
17]. Surprisingly, the Phusang hot spring contains approximately 250 CFUs/mL of total microorganisms, and the temperature is suitable for bacterial growth [
1]. Regulators that maintain the natural balance with microorganism may exist, such as other substances (trace elements etc.), antimicrobial effect or possibly bacteriophages. Bacteriophages are viruses that infect and lyse bacteria [
18]. As bacterial viruses, bacteriophages (phages) are the most widely distributed and abundant organisms in the biosphere [
19]. Previous metagenomic studies have identified bacteriophages in samples from Manikaran hot springs (India) [
20]. The potential for phage-based bacterial control in water exists [
21]. Bacteriophages play important roles in the regulation and evolution of microbial communities in most ecosystems [
22]. The study of specific phages would also help control environmental pathogens [
23]. Bacteriophages are the only pathogens of microbes in hot springs (concentration of one million phages/mL) [
24]. Circumneutral thermal springs (Mexico) contain bacteriophages within the order
Caudovirales [
25], along with springs in the Valley of Geysers (Russia) [
26]. His1-like viruses have been detected in South African hot springs [
27]. The application of bacteriophages to control bacterial populations has extended from medicine (antibiotics) to agriculture, the food industry [
28] and veterinary practice [
29,
30]. Therefore, the existence of bacteriophages must be determined, where relevant. In this study, the antimicrobial effect will be evaluated to confirm the antimicrobial properties of hot spring water (derived from trace elements or other substances).
Previous studies have shown that a number of minerals that may be useful as cosmetic ingredients are present in hot springs [
1], such as mineral water sprays with active ingredients such as curcumin. Curcumin (CUR) is a phyto-antioxidant from turmeric that has been reported to exert powerful antioxidant activity. Several studies have suggested that CUR possesses potential biological and physiological activities [
31]. Additional research and development of formulations containing CUR is still needed to support this hypothesis. Because of the biological and physiological activities of CUR, several researchers have attempted to formulate it into suitable dosage forms for human use. Liposomal curcuminoids have been revealed to be stable and are soluble in aqueous solution.
The advantages and disadvantages of Phusang hot spring water in terms of the safety of its use must be determined to benefit from and apply this resource in government, community and industry (cosmetics and medicine). Therefore, this study aimed to evaluate the characteristics of Phusang hot spring water by clarifying the microorganisms and viral pathogens present in this water sample. Cytotoxicity, irritation, liposome uptake, antimicrobial and anti-inflammatory effects were evaluated. The levels of trace elements such as Na, Ca, and Sr were confirmed. The effects of Phusang hot spring water (prebiotics) on colorectal bacteria, such as Escherichia coli (E. coli) and lactic acid bacteria (LAB), were studied for application in the screening of mineral drinking water. The safety and benefits of the Phusang hot spring water were evaluated.
2. Materials and Methods
2.1. Sample Collection
Water samples were collected from the Phusang hot spring and Phusang waterfall located in Phusang National Park, Phayao Province, Thailand (19°39′50.2″ N 100°22′35.6″ E). For analysis, Phusang hot spring water was obtained under the surface of the pool near the water inlet and from the middle of the waterfall. Twenty liters (L) of Phusang hot spring water were used for metagenomics, and 2 L were used for viral DNA detection. One-twenty liters were used for the dry weight analysis, and 1 L was used for cell cultures and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) tetrazolium reduction assay (MTT assays). Afterward, the samples were placed in sterile glass containers and stored on ice for future microbial analyses.
2.2. Dry Weight
One-twenty liters of Phusang hot spring water and the same volume of water from the Phusang waterfall were incubated at 180 °C until dry. The dry weights of the Phusang hot spring water and Phusang waterfall water samples were recorded.
2.3. Scanning Electron Microscopy and Energy Dispersive X-ray Spectrometry (SEM–EDS)
Phusang hot spring powder, Phusang waterfall powder and traditional powder were used for SEM and EDS analyses. Low vacuum Au sputter coating was performed (Oxford Instrument Co., Oxfordshire, UK). SEM–EDS was used to characterize microstructural features and confirm the presence of trace elements using methods described in a previous study [
1]. The SEM–EDS technique provides information and identifies the microstructural location and morphology of trace elements.
2.4. Cell Cultures
Human dermal fibroblasts, adult (HDFa) (Gibco, Life Technologies, Carlsbad, CA, USA), a mouse fibroblast L-929 cells (L-929 cells) and peripheral blood mononuclear cells (PBMCs) were incubated in a 37 °C, 5% CO2/95% air, humidified cell culture incubator. Cells were sub-passaged at 2–3 days. The HDFa and L-929 cells used in this study were grown to >95% confluence, as visualized under a microscope. HDFa and L-929 cells were used for MTT assays. HDFa cells and PBMCs were used for cell treatment experiments. Experiments using PBMCs were approved by the University of Phayao Human Ethics Committee (UP_HEC 1.2/040/64), and the study conformed to the following recognized standards: Declaration of Helsinki, the Belmont Report, the Council for International Organizations of Medical Sciences (CIOMS) guidelines and International Conference on Harmonization in Good Clinical Practice (ICH-GCP).
2.5. MTT Assays
HDFa and L-929 cells were seeded in a 96-well plate at a density of 1.5 × 104 cells and incubated overnight (total volume of 100 μL/well). Water samples (Phusang hot spring water, Phusang waterfall water, Phusang hot spring powder, a control [phosphate-buffered saline or PBS] and an untreated control) were prepared by dilution to six different concentrations in complete media and added to the wells containing the cells (100 μL/well) for cell treatments. The supernatants were removed, complete medium containing water samples or dimethyl sulfoxide (DMSO) (vehicle control) was added, and the samples were incubated for 24 h. Cytotoxicity was measured by adding the MTT solution (5 mg/mL, 10 μL/well) and incubating the plate at 37 °C for 4 h in a CO2 incubator. Purple formazan crystals were dissolved with isopropanol containing HCl (100 μL/well). The absorbance was measured at 540 nm using a microplate reader. Viable cells (%) = (abs sample − abs blank)/(abs control − abs blank) × 100. Powder was re-hydrated (deionized water [DI]) before use.
2.6. Treatment of PBMCs
PBMCs were cultured in supplemented Dulbecco’s modified Eagle’s medium (DMEM) in the absence of antibiotics or antimycotics (Gibco, Life Technology, Grand Island, NY, USA). PBMCs (3 × 105 cells/mL) were subcultured, treated with water samples (Phusang hot spring water, Phusang waterfall water, Phusang hot spring powder, a control [PBS] and an untreated control) and used after reaching >95% confluence at 24 h, as visualized under a microscope. These cells were cultured at 37 °C in a 5% CO2 atmosphere. PBMCs were used for RNA extraction and semi quantitative reverse transcription polymerase chain reaction (semi qRT-PCR). The corresponding supernatant from cultured PBMCs were used for enzyme-linked immunosorbent assay (ELISA).
2.7. RNA Extraction and Semi qRT-PCR Analysis
RNA was extracted from PBMCs using a TRIzol reagent kit (Life Technologies, Carlsbad, CA, USA) according to the manufacturer’s instructions. Cells were mixed with 1 mL of TRIzol reagent, homogenized and incubated for 5 min at 15–30 °C. Chloroform (0.2 mL) was added to each homogenized solution, and then the sample tubes were shaken for 2–3 min at 15–30 °C and centrifuged at 12,000×
g for 15 min at 2–8 °C. The aqueous phase from each sample was transferred to a fresh tube, and the organic phase was collected for DNA isolation. RNA was precipitated by adding isopropanol (0.5 mL) to 1 mL of the TRIzol aqueous phase, and the sample was mixed, incubated for 10 min at 15–30 °C and centrifuged at 12,000×
g for 10 min at 2–8 °C. The supernatant was removed from each sample, and 1 mL of 75% ethanol per 1 mL TRIzol was added to wash the RNA pellet by vortexing and centrifugation at 7500×
g for 5 min at 2–8 °C. Finally, RNA pellets were dried for 10 min at room temperature (RT). RNA was eluted with 30 μL of RNase-free water, incubated for 10 min at 55 °C and stored at −70 °C until use. Semi qRT–PCR were performed to detect the expression of
IL-6,
IL-10 and an endogenous control, glyceraldehyde 3-phosphate dehydrogenase (
GAPDH), according to the manufacturer’s instructions. OnePCR™ plus master mix (GeneDireX, Las Vegas, NV, USA), 0.4 pmol of the primer pair, 100–200 ng of DNA template and DNase/RNase-free water were added to each sample to a total volume of 25 μL. The PCR conditions were predenaturation at 94 °C for 5 min; 40 cycles of denaturation at 94 °C for 1 min, annealing at 56 °C for 1 min, and extension at 72 °C for 1 min; and a final extension at 72 °C for 5 min to amplify the
IL-6,
IL-10 and
GAPDH genes. PCR products of approximately 86, 193 and 117 bp for the
IL-6,
IL-10 and
GAPDH genes, respectively (
Table 1), were subjected to 2% agarose gel electrophoresis. Gel Quant NET version 1.7.8 free software was used to analyze the nucleic acid band intensity in triplicate samples.
2.8. Enzyme-Linked Immunosorbent Assay (ELISA)
Three treatment samples (Phusang waterfall water, Phusang hot spring water and Phusang hot spring powder), a control (PBS) and an untreated control were available for ELISA. ELISAs were performed to detect IL-6 and IL-10 levels. Each plate was coated with mouse monoclonal anti-human IL-6 or IL-10 antibody (BD Biosciences, Becton, Dickinson, UK) and blocked with 10% fetal bovine serum in PBS according to the manufacturer’s instructions (BD Biosciences). Standard IL-6 or IL-10 solutions and the corresponding supernatant from cultured PBMCs (50 μL) were added, and samples were incubated at RT for 2 h. The cells were washed with PBS containing 0.5% Tween 20 for 3 times. Biotinylated anti-human IL-6 or IL-10 antibodies and avidin–horseradish peroxidase-conjugated reagent (50 μL) were added, and samples were incubated at RT for 1 h. The cells were washed with PBS containing 0.5% Tween 20 for 5 times. Tetramethylbenzidine (TMB) substrate solution (Invitrogen, 50 μL) was added to each sample, and the samples were incubated in the dark for 30 min. Each reaction was stopped with 25 μL of 2 N H2SO4. Optical density (OD) values for IL-6 and IL-10 were detected at 450 nm and compared with the standard curves to determine the concentrations.
2.9. The Effects (RNA Intensity, DNA Intensity, Optical Density, and CFUs/mL) of Different Water Samples on E. coli and LAB Growth
The effect of water as a prebiotic for E. coli and LAB growth must be confirmed for drinking water applications. Eight samples (Phusang hot spring water, Phusang waterfall water, Phusang hot spring powder, Phusang waterfall powder, traditional mineral water, distilled water [DW], control [PBS] and deionized water [DI]) were used to prepare the media. Nutrient broth and de Man–Rogosa–Sharpe (MRS) broth were used for E. coli and Lactobacillus spp. growth, respectively. RNA and DNA were extracted. RNA and DNA intensity were evaluated by semi qRT-PCR analyzing the area under the curve (band intensity). Optical densities (OD) were measured at 600 nm. Nutrient agar and MRS agar were used for E. coli and Lactobacillus spp. cultures, respectively. Colony forming units per milliliter (CFUs/mL) were counted.
2.10. Measurement of Antimicrobial Effects Using the Agar Well Diffusion Assay
Staphylococcus aureus (S. aureus), Staphylococcus epidermidis (S. epidermidis), Bacillus subtilis (B. subtilis) and E. coli were cultured at 37 °C for 18–24 h on swab plates. McFarland standard 0.5 was adjusted to a prepared bacterial concentration of 105–107 CFUs/mL at OD 600. All bacterial cultures were swabbed on Mueller–Hinton agar (HiMedia Laboratories Pvt. Ltd., Mumbai, India) in triplicate. A cork borer with a size of 7 mm was used to punch wells in Mueller–Hinton agar. The sterile water samples were filtered (membrane pore size 0.45 μm) (Phusang hot spring water, Phusang waterfall water, Phusang hot spring powder, and Phusang waterfall powder) and were added at 40 μL/well and incubated at 37 °C for 24 h. Deionized water was used as a negative control. Ciprofloxacin (512 μg/mL) and chloramphenicol (512 μg/mL) were used as positive controls for Gram-positive and Gram-negative bacteria, respectively. Total colonies were counted as CFUs/mL.
2.11. Liposome Preparation
The methodology described in a previous study [
37] was used to prepare the liposome formulation. Liposome formulations of 10 mM phosphatidylcholine (PC), 1 mM cholesterol (CHOL), 1 mM CUR and various types of rehydration solvents (Phusang hot spring water [PHSW] and PBS, pH 7.4) were prepared. The various compositions of the liposome formulations are shown in
Table 2.
Phosphatidylcholine (PC) from soybean (90%) was generously supplied by Lipoid GmbH (Ludwigshafen, Germany). Cholesterol (Chol) was purchased from Wako Pure Chemical Industries (Osaka, Japan). CUR 65% was supplied by Sigma–Aldrich Production GmbH (Buchs, Switzerland). All other chemicals used were of reagent grade.
2.12. Measurements of the Vesicle Size, Size Distribution and Zeta Potential
The mean vesicle sizes, size distributions and zeta potentials of the liposome formulations were measured using photon correlation spectroscopy (PCS; Zetasizer Nano Series, Malvern Instruments, Malvern, UK). All samples were controlled and collected at an ambient temperature of 25 °C. Twenty microliters of the sample formulations were diluted with 1480 μL of DI. At least three independent samples were collected, and the vesicle sizes, size distributions and zeta potentials were measured at least three times.
2.13. 2,2-Diphenyl-1-Picrylhydrazyl (DPPH) Radical-Scavenging Activity
The DPPH radical-scavenging activity was determined using a slightly modified version of a published method [
38]. Briefly, individual liposome formulations (40 μL) were added to 160 μL of a 0.2 mM DPPH radical solution. After reacting for 30 min, absorbance values were measured at 517 nm. DPPH radical-scavenging ability was calculated using the following equation:
where A
control is the absorbance of the control (containing all reagents except the sample extract) and A
sample is the absorbance of the liposome formulation. Trolox (25 μg/mL) was used as a positive control, as described by Bumrungthai et al. (2020) [
1].
2.14. High-Performance Liquid Chromatograph (HPLC) Analysis
The concentration of CUR in each formulation was initially determined after vesicle disruption with Triton® X-100 (0.1% w/v) at a 1:1 volumetric ratio and appropriate dilution with PBS. The vesicles in the Triton® X-100 solution were centrifuged at 14,000× g at 4 °C for 15 min. Each supernatant was filtered through a 0.45 μm nylon syringe filter. The concentration of CUR in each sample was analyzed using an HPLC Thermo Scientific™ UltiMate 3000 UHPLC System. A C18 reverse-phase column (Symmetry®, VertiSepTM, Vertical, Thailand) with dimensions of 5 μm and 4.6 × 150 mm was utilized. A mixture of acetonitrile and 0.01% phosphoric acid (65:35) was used as the mobile phase for CUR. The UV–VIS detector was set to 425 nm for CUR detection at 40 °C. The flow rate was 1.5 mL/min, and the injection volume was 20 μL. The calibration curve for CUR was ranged from 20 to 200 μg/mL, with a correlation coefficient of 0.999.
2.15. Metagenomics for Bacterial DNA Detection
Shotgun metagenomic sequencing was used to analyze the viral biodiversity of the hot spring water samples. Phenol–chloroform was used to extract DNA from a 0.45 μM filter membrane through which 20 L of water were passed according to the manufacturer’s instructions, and DNA was eluted with TE buffer and stored at −20 °C, as described by S Bumrungthai et al. (2020) [
1].
2.16. Viral DNA Detection
Water samples were collected from the Phusang hot spring for the detection of HPV and EBV; 2 L of water were obtained under the surface of the pool near the water inlet and stored on ice. A viral pellet 0.025 μm filter membrane (MCE membrane, nitrocellulose membrane, MF-Millipore
TM, 47 mm, Merck Millipore Ltd. Tullagreen, Carrigtwohill, Co., IRL REV 03/19 EPA EST 041237-IRL-001, Cork, MA, USA) was used for total DNA extraction. The cell lysis solution (10 mM Tris-HCl, pH 7.8; 5 mM EDTA; 0.5% SDS; and lysozyme) and proteinase K (50 μL, 20 mg/mL stock) were added to each sample, and samples were incubated at 55 °C for 1 h. Potassium acetate (5 M, 400 μL) was used for protein precipitation and then centrifuged at 12,000×
g for 10 min at 4 °C. Each supernatant was collected, an equal volume of phenol:chloroform:isoamyl (25:24:1) was added, and the sample was centrifuged at 12,000×
g for 10 min at 4 °C. Isopropanol was used to precipitate DNA from the aqueous phase by centrifugation at 12,000×
g for 10 min at 4 °C, followed by washing with 70% ethanol. DNA was rehydrated in TE buffer (10 mM Tris [pH 7.8] and 1 mM EDTA) and stored at −20 °C. Agarose (0.7%) gel electrophoresis was performed to confirm successful DNA extraction. The DNA quality was checked by staining with Redsafe
™ (iNtRON Biotechnology, Inc., Burlington, MA, USA) and imaged under a gel documentation system (Bio-Rad, Hercules, CA, USA). DNA extracted from the membrane was subjected to PCR. The
L1,
EBNA1 and
LMP1 genes were amplified by PCR (
Table 1). Each reaction volume consisted of OnePCR
TM Plus master mix (GeneDireX, Las Vegas, NV, USA), 0.4 pmol of each primer pair, 100–200 ng of DNA template and DNase/RNase-free water to a total volume of 25 μL. The PCR conditions were predenaturation at 94 °C for 5 min; 40 cycles of denaturation at 94 °C for 1 min, annealing at 1 min and extension at 72 °C for 1 min; and a final extension at 72 °C for 5 min. The annealing temperatures were 42 °C for the HPV
L1 gene (154 bps) and 58 °C for the EBV
EBNA1 gene (213 bps) and EBV
LMP1 (106 bps) genes. The sizes of the PCR products detected after electrophoresis on 2% agarose gels are listed in
Table 1. DNA from the Caski cell line was used as an HPV
L1 positive control, and DNA from the B95 cell line was used as an EBV positive control.
2.17. Evaluation of Acute Cutaneous Tolerance of Adult Subjects to Phusang Hot Spring Using Single Patch Tests
A patch test with a single application was performed using a monocentric and simple blind study method to determine the acute irritating potential of Phusang hot spring. Individuals with every skin type were included in the study. Inclusion criteria were subjects who provided written informed consent with no previous experience of intolerance or allergic reactions to this type of product and phototypes I to IV. The exclusion criteria were pregnant or breast-feeding women or women planning to be pregnant during the study; cutaneous pathology in the study zone (psoriasis, eczema, vitiligo, pityriasis versicolor, acne, etc.); subjects with medical treatments that may interfere with the acute skin tolerance evaluation according to the investigator; exposure to the sun or to UV rays on the back during the previous month; subjects with very irritated skin; subjects presenting particular hairiness of the back, freckles, beauty spots or a tattoo on the back; subjects with serious or progressive disease; and excessive use of alcohol or tobacco. Phusang hot spring treated patch was applied to the scapular area of the back for 48 h (the patch type was Finn Chamber® 8 mm, 50 mm2, occlusive, dose 25 μL). The control was a patch without product. Readings: The macroscopic skin examinations were performed under uniform conditions, specifically the lighting (standardized light), 30 min after removal of the patch and 24 h later. The grading of the possible irritation reaction in each zone that received the studied product and in the control zone was performed using the following scale: 0 = absent, 0.5 = very slight, 1 = slight, 2 = obvious, and 3 = important. A change in skin structure (dryness [D], roughness [R], thickness [T], and reflectivity [Re]) that might be linked to the nature of the studied product or one of its components was clinically described, and its intensity was graded using the following scale: 0.5 = doubtful, 1 = slight, 2 = obvious, and 3 = important. The results were analyzed and interpreted based on the experimental conditions. The analyses were descriptive and completed by calculating the cumulative irritation index (CII) for each subject using the following formula: CII = Σ of the grade (erythema + edema)/number of readings. This index was then divided by the number of subjects to obtain the mean cumulative irritation index (MCII): (MCII = ΣCII/number of subjects). The obtained index (maximum of 6 points) allowed us to arbitrarily classify the studied product according to the following scale: MCII < 0.25, nonirritating (NI); 0.25 ≤ MCII < 0.50, very slightly irritating (VSI); 0.5 ≤ MCII < 1, slightly irritating (SI); 1 ≤ MCII < 2, moderately irritating (MI); and MCII ≥ 2, irritating (I).
2.18. Statistical Analysis
The results were analyzed with IBM SPSS software. A p value < 0.05 was considered statistically significant. Independent Student’s t test and one-way ANOVA were used to compare separate sets of means ± S.D.
3. Results
3.1. Dry Weight and SEM–EDS Analysis
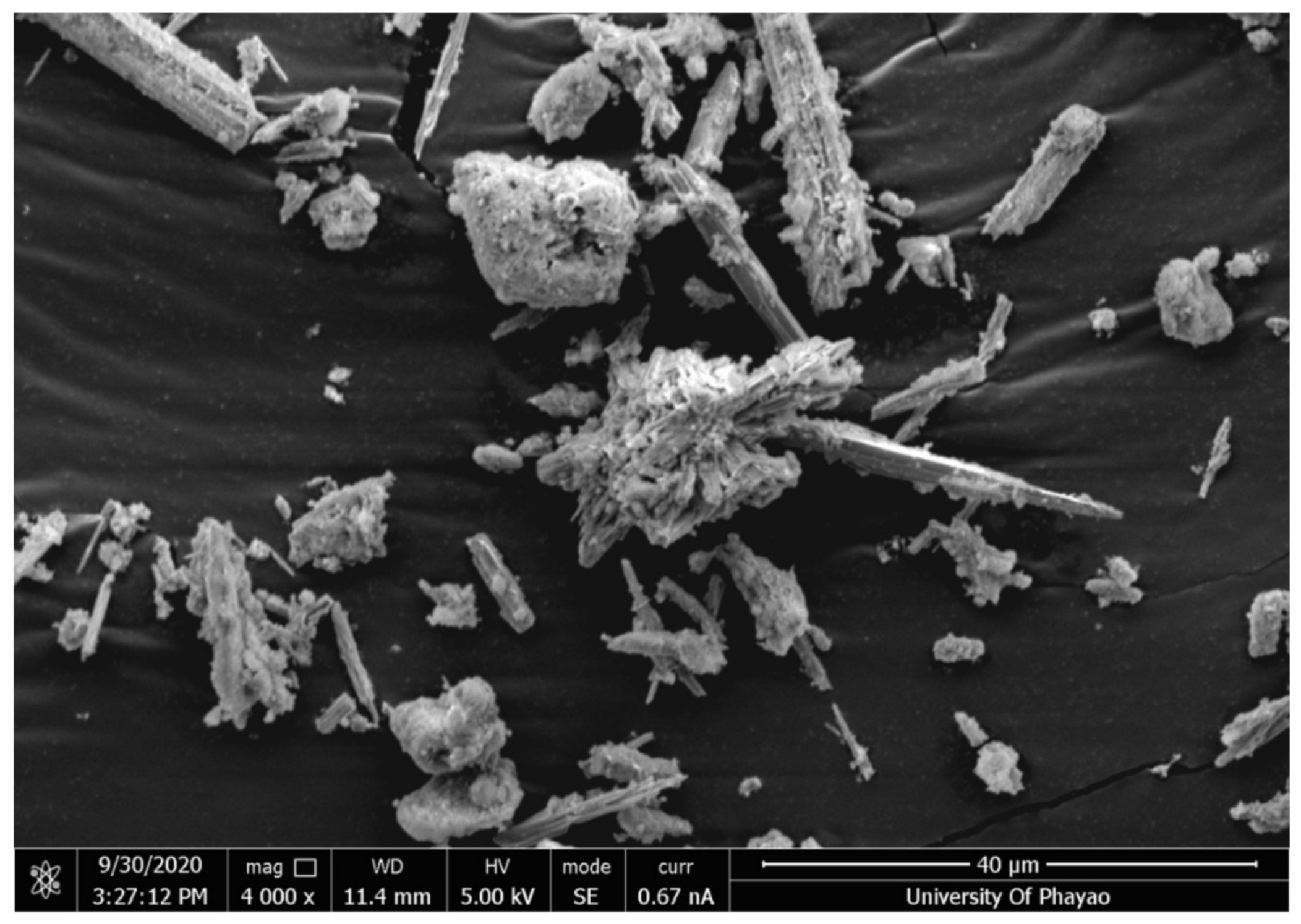
The dry weights of Phusang hot spring and Phusang waterfall water samples were 0.116 g/L and 0.145 g/L, respectively. SEM–EDS images are shown in
Figure 1,
Figure 2 and
Figure 3 and
Table 3. Phusang hot spring powder contained crystals that were slender and spiky. Phusang waterfall water powder contained other crystals that adhered together until the shape was not clearly visible. However, traditional mineral powder contained crystals that were slender, spiky and perfectly shaped. The results corresponded with the findings of a previous study [
1] that detected Ca and Na in Phusang hot spring water. Phusang hot spring powder consisted of oxygen (O), Ca and carbon (C), similar to traditional mineral powder. Magnesium (Mg) was detected in Phusang hot spring powder. Phusang hot spring powder did not contain aluminum (Al), aurum (Au), copper (Cu), or strontium (Sr), according to the SEM–EDS analysis.
3.2. Cytotoxicity toward L-929 Cells and HDFa
Three samples (Phusang hot spring water, Phusang waterfall water and Phusang hot spring powder), a control (PBS) and an untreated control were used to treat primary L-929 cells and HDFa cells and analyze their effects on cell viability using MTT assays.
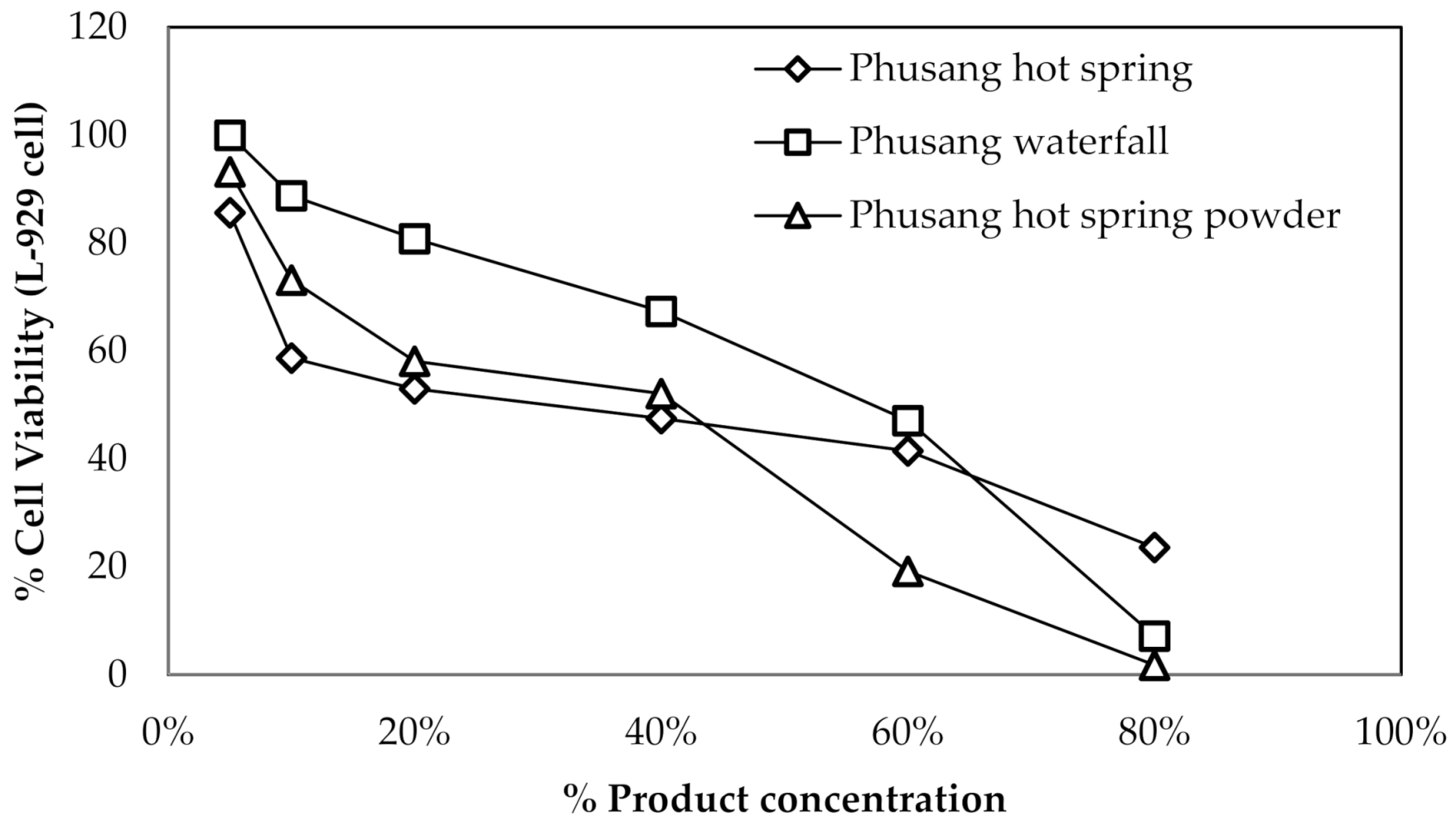
For L-929 cells, the IC50 values of Phusang hot spring water, Phusang waterfall water, and Phusang hot spring powder ranged from 40–60% (
Figure 4).
For HDFa cells, the IC50 values of Phusang hot spring water, Phusang waterfall water and Phusang hot spring powder were >25%. However, the IC50 of the DI water control was <6.25% (
Figure 5).
3.3. Semi qRT-PCR Analysis
Three treated samples (Phusang hot spring water, Phusang waterfall water and Phusang hot spring powder), a control (PBS) and an untreated control were available for the analysis of mRNA expression. The results of semiq qRT–PCR for
IL-6,
IL-10 and
GAPDH expression are shown in
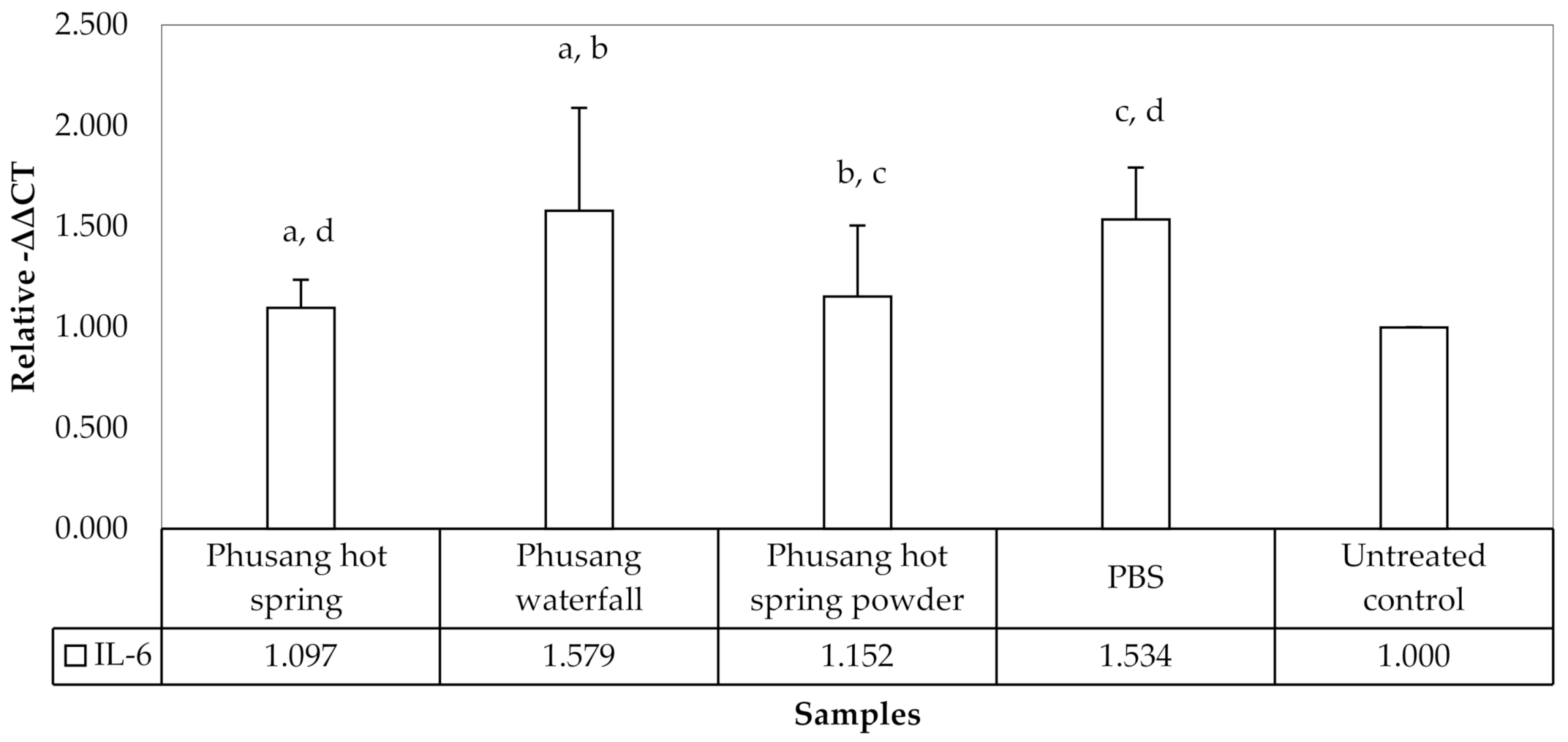
Figure 6.
Phusang hot spring water and Phusang hot spring powder significantly downregulated the expression of IL-6 (p values = 0.026 and 0.048, respectively) compared with PBS. Only Phusang waterfall water significantly upregulated IL-6 expression compared with the untreated control, Phusang hot spring water and Phusang hot spring powder groups (p values = 0.005, 0.016, and 0.029, respectively). However, PBS showed a statistically significant difference compared to the untreated control group (p value = 0.009). The levels of IL-6 among the Phusang hot spring water, Phusang hot spring powder and untreated control groups were not different (p value > 0.05).
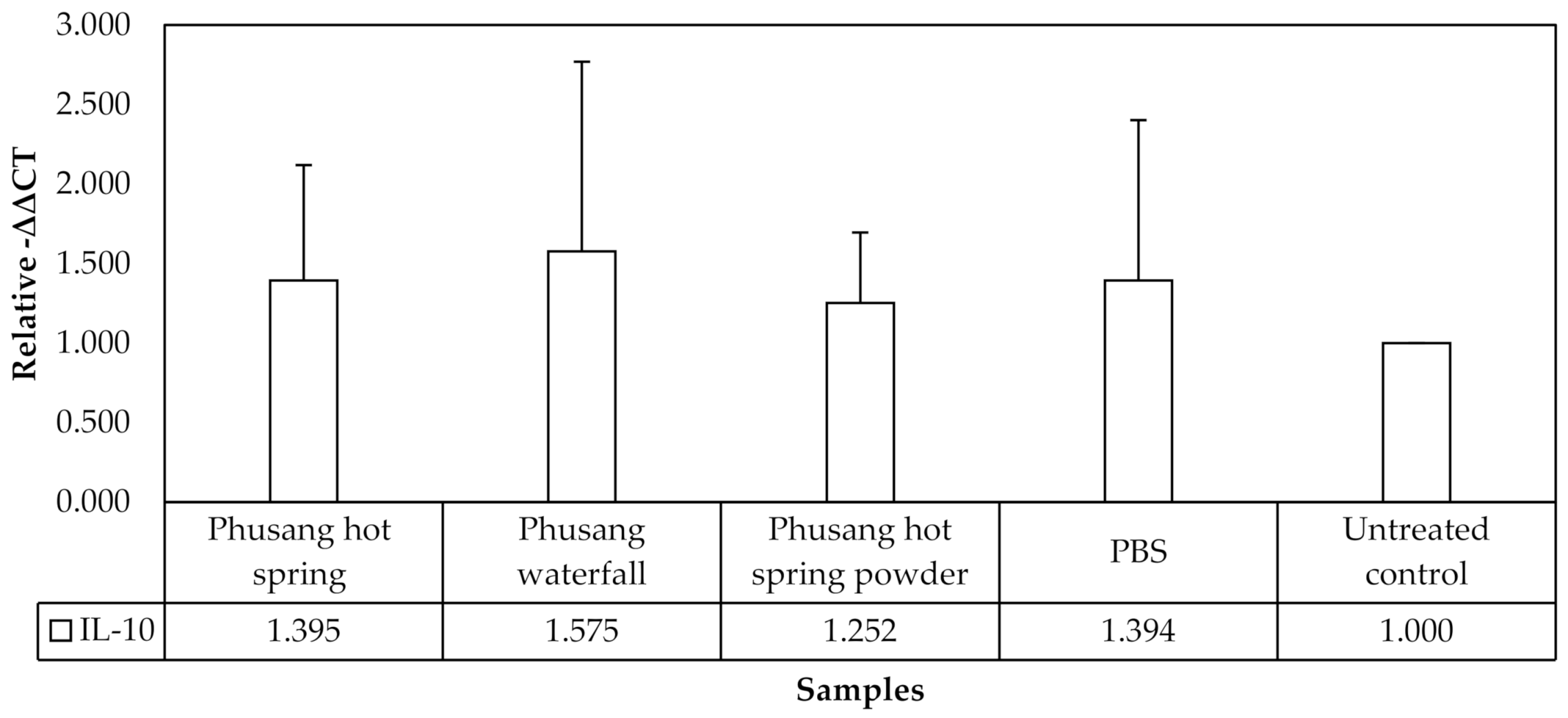
No statistically significant differences in
IL-10 expression were observed in this study (
Figure 7).
Therefore, Phusang hot spring water downregulated the expression of the IL-6 mRNA more strongly than Phusang waterfall water and PBS.
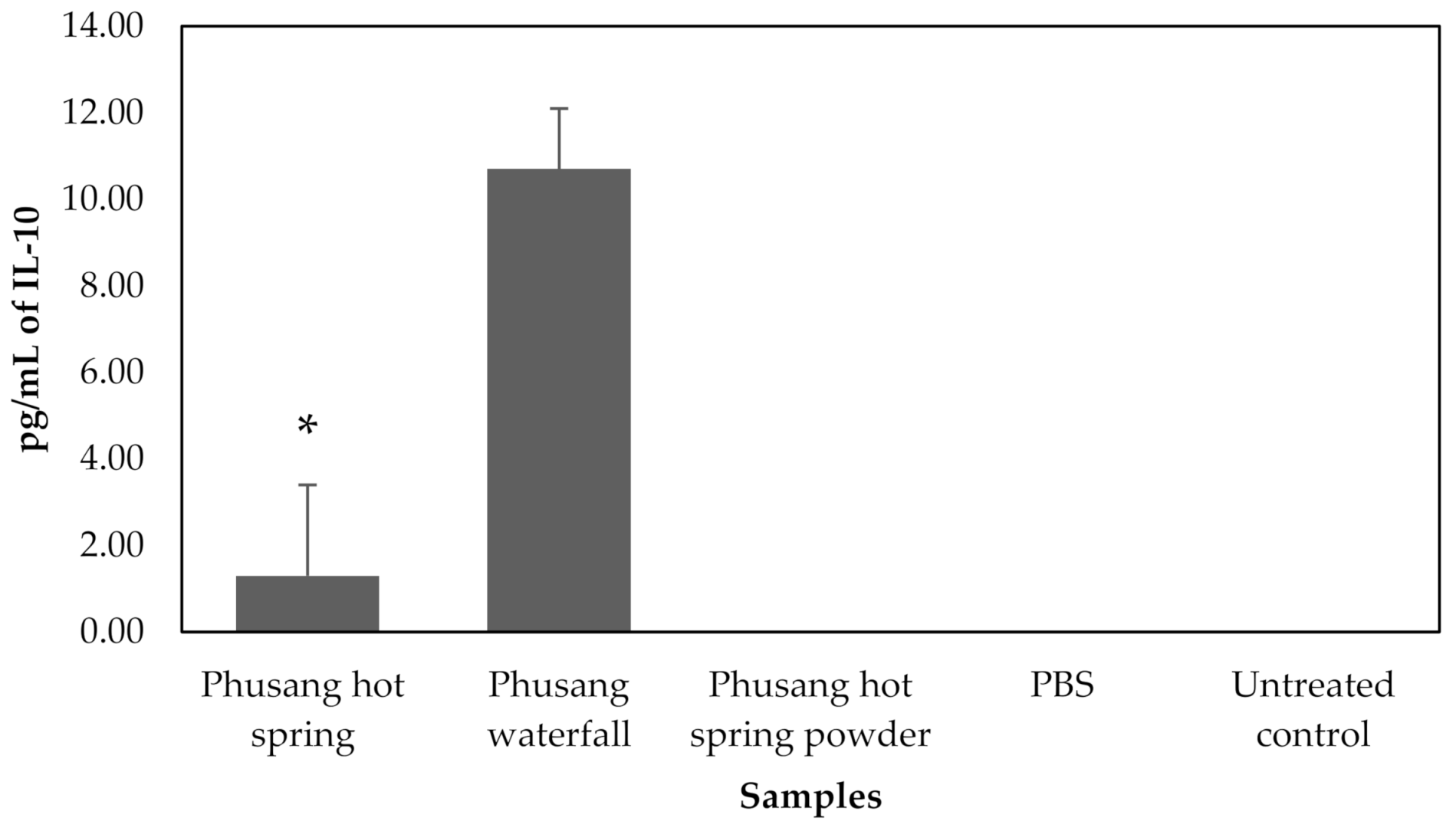
3.4. ELISA
Three treated samples (Phusang hot spring water, Phusang waterfall water and Phusang hot spring powder), a control (PBS) and an untreated control were available for ELISA. ELISA results for IL-6 and IL-10 are shown in
Figure 8 and
Figure 9.
Phusang hot spring water significantly reduced IL-6 protein levels (p value < 0.05) compared with the Phusang waterfall water, Phusang hot spring powder, PBS and untreated control groups.
Phusang waterfall significantly increased IL-10 levels (
p value = 0.000) compared to the Phusang hot spring water, Phusang hot spring powder, PBS and untreated control groups. Phusang hot spring water significantly increased IL-10 levels (
p value < 0.001) compared to the Phusang hot spring powder, PBS and untreated control groups (
Figure 9). IL-10 was not detected in the Phusang hot spring powder, PBS or untreated control groups in this study.
Therefore, Phusang hot spring water decreased IL-6 levels to a greater extent than Phusang waterfall water, PBS and the untreated control. Phusang hot spring water substantially increased the level of IL-10 compared with the PBS and untreated control groups.
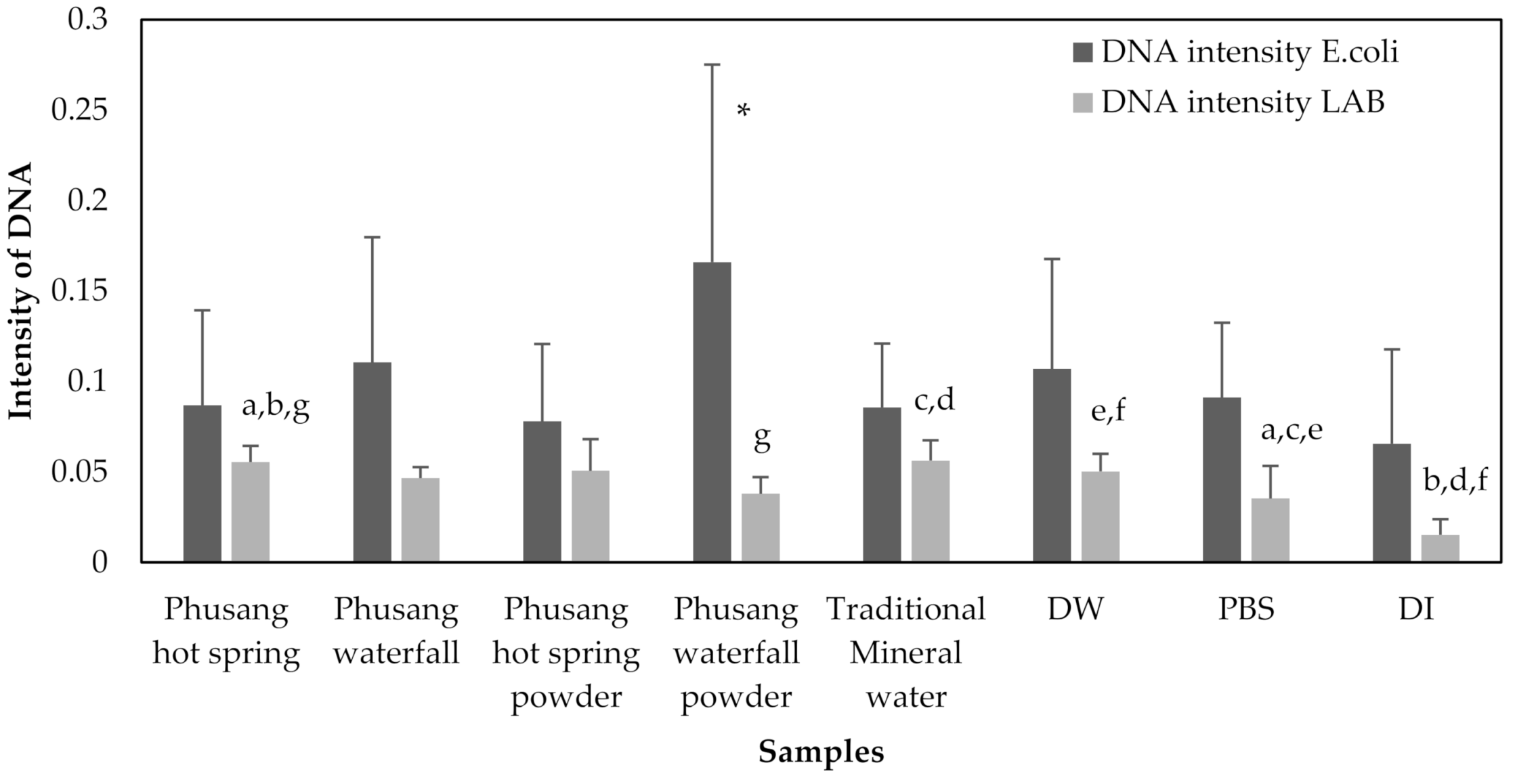
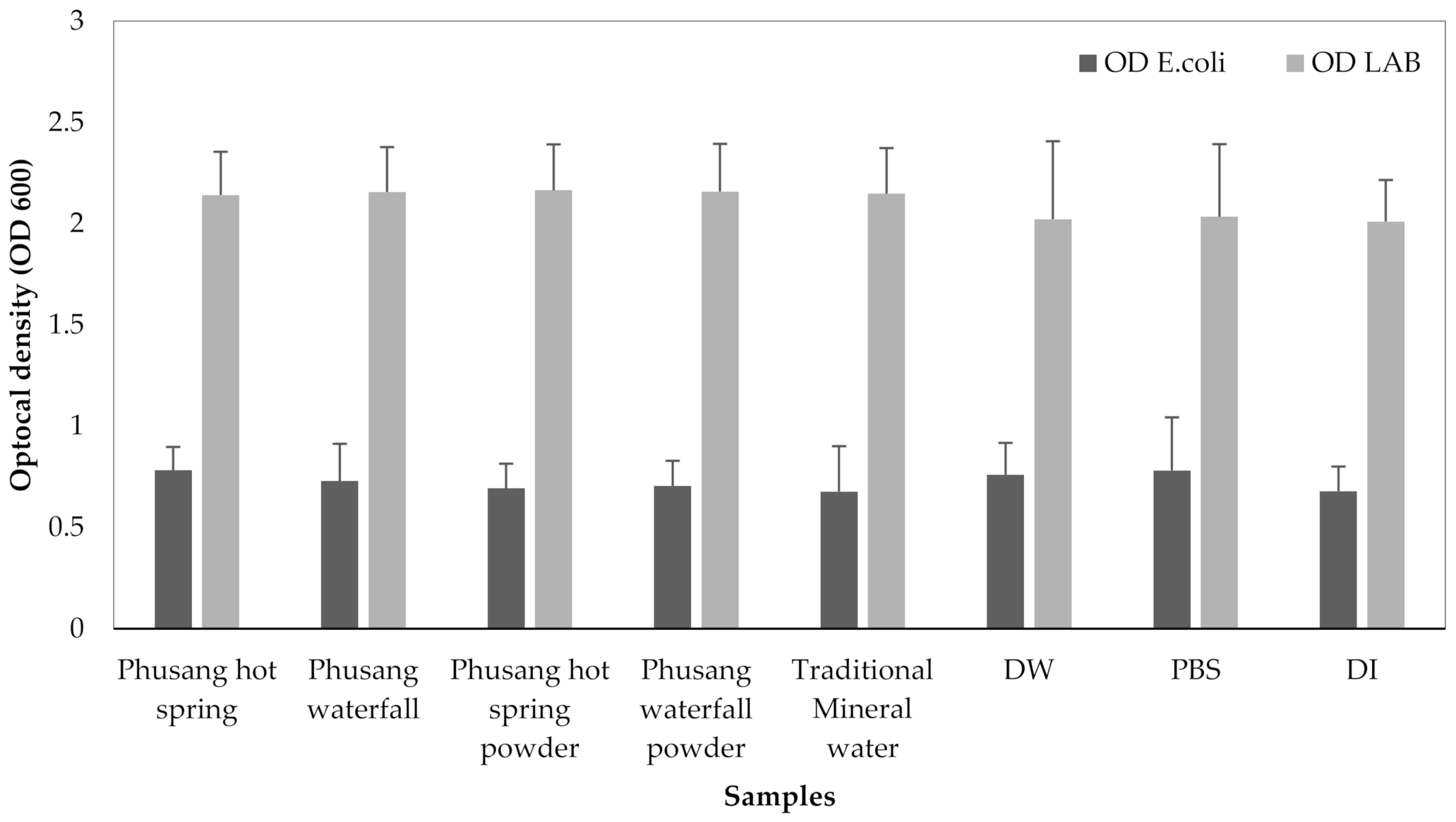
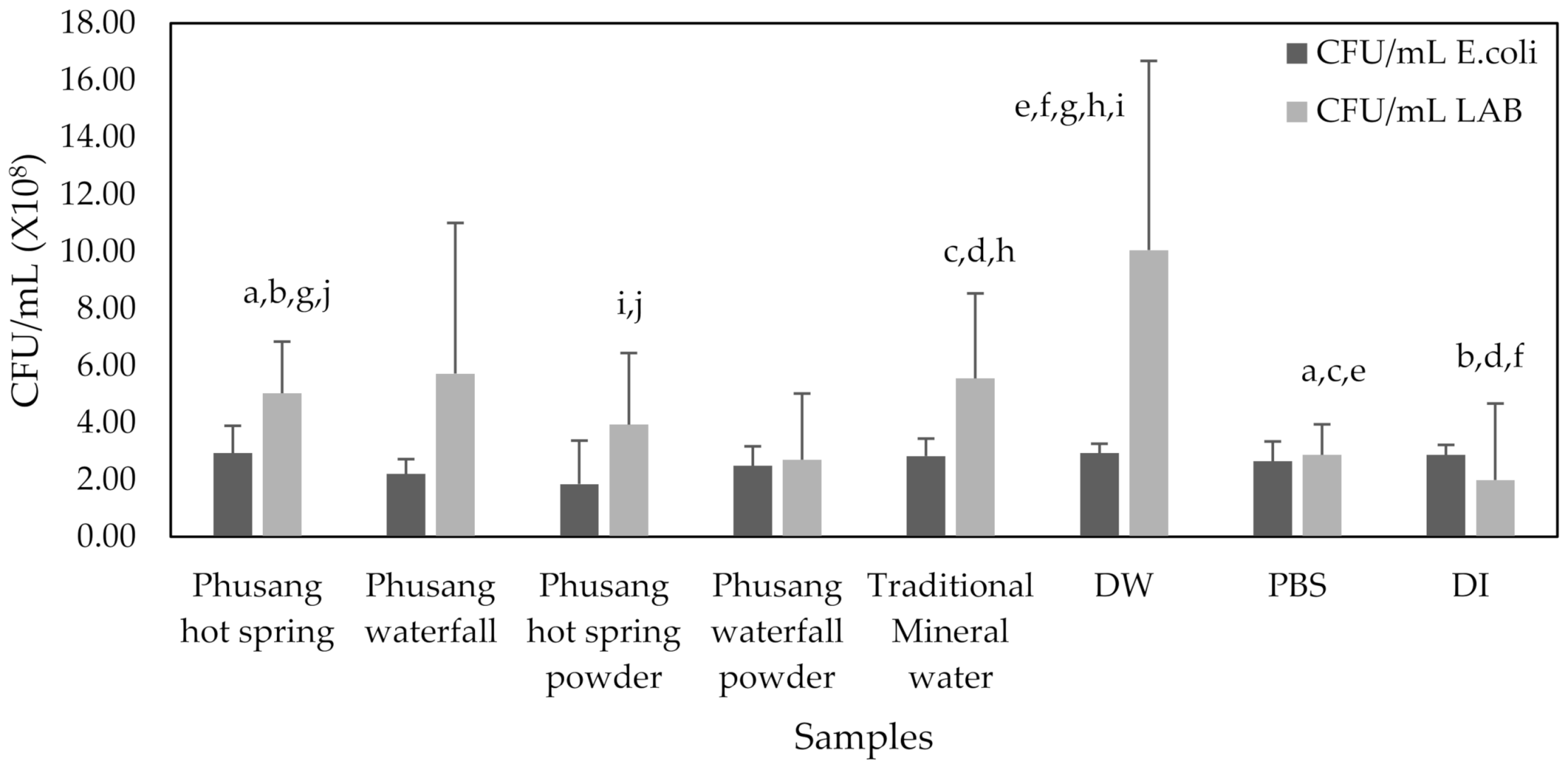
3.5. The Effects (RNA Intensity, DNA Intensity, Optical Density, and CFUs/mL) of Water Samples on E. coli and LAB Growth
Samples exposed to 8 treatments (Phusang hot spring water, Phusang waterfall water, Phusang hot spring powder, Phusang waterfall powder, traditional mineral water, DW, a control [PBS] and DI) were available for the detection of RNA intensity by performing a semi qRT-PCR analysis of the area under the curve (band intensity) for E. coli and LAB.
RNA extracted from
E. coli treated with Phusang hot spring water showed a significantly higher intensity than that from Phusang hot spring powder, traditional mineral water and DW (
p values = 0.036, 0.001, and 0.000, respectively). RNA extracted from
E. coli treated with Phusang waterfall water showed a significantly higher intensity than that from samples treated with Phusang hot spring powder, waterfall powder, traditional mineral water, DW and DI water (
p values = 0.011, 0.017, 0.000, 0.000, and 0.023, respectively) (
Figure 10).
The LAB RNA intensity in the samples was not significantly different (
Figure 10).
E. coli DNA extracted from the Phusang waterfall water group showed a significantly higher intensity than that extracted from the DI water group (
p value = 0.049).
E. coli DNA extracted from the Phusang waterfall powder group showed a significantly higher intensity than that from the Phusang hot spring water, Phusang waterfall water, Phusang hot spring powder, traditional mineral water, DW, PBS and DI water groups (
p values = 0.001, 0.016, 0.000, 0.001, 0.010, 0.001, and 0.000, respectively) (
Figure 11).
LAB DNA extracted from the Phusang hot spring water group showed a significantly higher intensity than that extracted from the Phusang waterfall powder, PBS and DI water groups (p values = 0.014, 0.005, and 0.000, respectively). The LAB DNA extracted from the Phusang hot spring powder group had a significantly higher intensity than that extracted from the PBS and DI water groups (p values = 0.031 and 0.000, respectively). LAB DNA extracted from the Phusang waterfall powder group showed a significantly lower intensity than that extracted from the traditional mineral water group (p value = 0.010). LAB DNA extracted from the traditional mineral water group had a significantly higher intensity than that extracted from the PBS and DI water groups (p values = 0.004 and 0.000, respectively). LAB DNA extracted from the DW group presented a significantly higher intensity than that extracted from the PBS and DI water groups (p values = 0.034 and 0.000, respectively).
The OD of
E. coli and LAB were not significantly different (
p values = 0.313 and 0.541, respectively, one-way ANOVA,
Figure 12).
E. coli CFUs/mL were not significantly different.
LAB treated with Phusang hot spring water generated significantly higher CFUs/mL than those treated with Phusang hot spring powder (
p value = 0.024). DW generated significantly higher CFUs/mL than Phusang hot spring water, Phusang hot spring powder, PBS and DI water (
p values = 0.031, 0.003, 0.005 and 0.001, respectively,
Figure 13).
Therefore, water from Phusang hot spring water and Phusang waterfall water showed a greater effect on E. coli RNA (but not DNA, CFUs/mL or OD) than traditional mineral water and DW. Phusang hot spring water, traditional mineral water and DW exerted greater effects on LAB DNA and CFUs/mL than PBS and DI water. DW produced a greater effect on LAB CFUs/mL than Phusang hot spring water, traditional mineral water, PBS and DI.
3.6. Antimicrobial Effects Analyzed Using the Agar Well Diffusion Assay
Phusang hot spring water, Phusang waterfall water, Phusang hot spring powder and Phusang waterfall powder did not exert antimicrobial effects on S. aureus, S. epidermidis, B. subtilis and E. coli in the agar well diffusion assay in the present study.
3.7. Physicochemical Characterization: Vesicle Size, Size Distribution, Zeta Potential and CUR Content
The physicochemical characteristics, such as vesicle size, size distribution, zeta potential and CUR content, in the formulations are shown in
Table 4. F1 and F2 were defined as the controls for F3 and F4, respectively. The vesicle size was smaller than 200 nm for all liposome formulations (
Table 4), with a size distribution of less than 0.4. The vesicle sizes for F3 and F4 were significantly smaller than those of their corresponding control formulations. Verma and coworkers reported that nanovesicles less than 120 nm in size can be delivered through the skin layer [
39]. We agreed that the F3 and F4 vesicles were an important first criterion to enable CUR to permeate the skin. The zeta potentials of all formulations were negative. The net surface charge of each liposome formulation was defined by the intrinsic properties of its composition. In the environment used for the experiment, the pH of the formulations (pH 7.4) was higher than the isoelectric point (IP) of PC (IP = 6). Therefore, PC carried a negative charge at this pH. Moreover, the addition of CHOL to a phospholipid membrane may decrease the surface charge of the bilayer [
40]. On the other hand, the pKa of CUR may be a factor affecting the net charges of liposome formulations. CUR has three pKa values of pKa1 = 7.8, pKa2 = 8.5 and pKa3 = 9. The CUR-loaded liposome formulation also carried a positive charge, as the pH was slightly lower than its pKa [
41]. Therefore, the net charge was defined by the total intrinsic properties of the formulation components. The zeta potential also plays an important role in the stability of the vesicle and requires careful consideration. Zeta potential ranges of –5 to +5 and –20 to +20 indicate fast aggregation and short-term stability, respectively [
42]. CUR is a water-insoluble compound. However, CUR can be added to the liposome formulation. The CUR content in F3 was significantly higher than that in F4. Liposome formulations deliver not only the entrapped compound but also the nonentrapped compound into the deep skin region [
43]. We concluded that the compositions were the major factors affecting the vesicle size, surface charge and CUR content in the formulations.
3.8. DPPH Radical-Scavenging Activity
The antioxidant activities of various liposome formulations with and without CUR are shown in
Table 4. The percent inhibition of F3 and F4 was significantly higher than that of Trolox (25 μg/mL) and the control. This result suggested that CUR-loaded liposome formulations prepared in PBS and PHSW can be promoted as antiaging cosmetic products.
One way to ensure the successful development of liposome formulations as antiaging cosmetics was to understand the factors affecting the safety, efficacy and stability of products. Thus, this study focused on the physicochemical characteristics and antioxidant activity.
3.9. Metagenomics for Bacteriophage Detection
Shotgun metagenomic sequencing was performed to analyze bacteriophage biodiversity in the hot spring water samples and revealed a 0.02% viral content (
Table 5,
Figure 14). In this study, 74% of the viruses were classified in order
Caudovirales 74 (families
Myoviridae, 70%;
Siphoviridae, 19%; and
Podoviridae; 1%), with 26% unclassified in Phusang hot spring water.
3.10. Viral DNA Detection
DNA was extracted and confirmed by agarose gel electrophoresis. The HPV L1, BENA1 and EBV LMP1 genes were not detected in 2 L of Phusang hot spring water.
3.11. Evaluation of the Acute Cutaneous Tolerance of Adult Subjects to Phusang Hot Spring Using Single Patch Tests
The main inclusion criteria for the studied population were an age > 18 years and phototypes I to IV (phototype III, 3 subjects; prototype IV, 18 subjects); the average age was 44 ± 3 years (23–60 years). The subjects analyzed included 21 females and 19 males. The readings obtained 30 min after the Phusang hot spring patch removal indicated that 6 subjects experienced very slight irritation (0.5, fairly detectable, discreet pinkness of one portion of the tested area), and 2 control subjects showed effects. The readings obtained 24 h after the Phusang hot spring occlusive patch removal indicated that 6 subjects exhibited very slight irritation (0.5, fairly detectable, discreet pinkness of one portion of the tested area), and 3 control subjects showed effects. The readings at 30 min and 24 h after occlusive patch removal yielded the MCII value for a nonirritating product at 48 h (MCII value: 0.14). This procedure was performed by a dermatologist.
4. Discussion
Similar to the results obtained with Avène thermal spring water, Phusang hot spring water (low mineral-content water) decreased the IL-6 level at 24 h compared with PBS and the untreated control. It may inhibit (anti-inflammatory properties) through IL-12 and IL-23 production [
8]. This study detected the expression of IL-10 at 24 h after treatment with Phusang hot spring water. Interestingly, IL-10 (protein) expression was upregulated 24 h after treatment with Phusang waterfall water, possibly because the expansion of CD4+ T cells was promoted. DCs decreased their ability to induce IL-4 production. Phusang waterfall water may modulate and change the DC capability to activate naïve CD4+ T cells by decreasing their potential to induce the production of proinflammatory Th1 and Th17 cells, as also observed with Avène thermal spring water [
8]. Phusang hot spring water might be used for balneotherapy, anti-inflammatory and immunomodulatory effects. However, further study is needed to evaluate the effects of Phusang hot spring water on cell signaling pathways. Likewise, serum IgE levels are significantly decreased in a diluted high-concentration mineral spring water group compared to the levels in negative control hairless mice. Similarly, the serum levels of inflammatory cytokines, such as IL-1β, IL-13 and tumor necrosis factor-α, are significantly reduced in groups treated with high concentrations of pure mineral spring water compared to the levels in negative control hairless mice [
3]. Mageumsan hot spring water also suppresses inflammation in subjects with atopic dermatitis (regulation of monocyte chemoattractant protein [MCP]-1 activation, and normal T cells expressed and secreted a cutaneous T-cell-attracting chemokine, eotaxin, macrophage inflammatory protein-1α, etc.) [
6]. In contrast, differentiation to T
reg cells is promoted by mineral water bathing. Balneotherapy not only is characterized by anti-inflammatory activity but also exerts positive effects on cutaneous barrier homeostasis [
7].
Viral infectious diseases are more common today, such as HPV, EBV, herpes, hepatitis, and viral diarrhea [
9]. However, this study did not detect oncogenic DNA viruses (HPV and EBV) that contact epithelial cells in Phusang hot spring water that might be used for balneotherapy. Moreover, the human pathogenic viruses
Caliciviridae,
Adenoviridae,
Hepeviridae,
Picornaviridae,
Reoviridae [
9] and enterovirus 71 (EV 71) [
10] are further considered when planning treatment procedures to reduce unexpected infections in individuals unprotected from viral infection [
12,
13]. Virus survival is higher in sterile water [
14]. Consequently, we must consider these findings before using hot spring water to ensure safety from viral infections. Therefore, the water may be sterilized before use for the greatest benefit.
This study found that the natural balance regulator is bacteriophages, according to findings from Manikaran hot springs (India) [
20,
21,
22]. Bacteriophages help control pathogens, including multidrug-resistant (MDR) bacterial populations [
23,
24]. A dynamic predator–prey mechanism called “kill the winner” suggests the elimination of most active bacterial populations by bacteriophages.
Myoviridae (30%) and
Podoviridae (23%) have been detected in fresh water, and
Rudiviridae (9%),
Globuloviridae (8%), and
Lipothrixviridae (1%) were exclusively observed in a hot water spring [
23]. An analysis of the microbial composition in circumneutral thermal springs in Chignahuapan (Mexico) revealed bacteriophages in the order
Caudovirales (
Siphoviridae,
Myoviridae, and
Podoviridae), but the family of
Herelleviridae was the most abundant in Chignahuapan samples [
25]. According to the present study,
Myoviridae,
Siphoviridae and
Podoviridae were present in Phusang hot spring water.
A novel thermophilic bacteriophage AP45 (family
Siphoviridae) and its host strain
Aeribacillus sp. CEMTC656 were isolated from the Valley of Geysers (Russia) [
26]. His1-like viruses detected in a South African hot spring (Brandvlei hot spring; 60 °C, pH 5.7) through a metavirome analysis have allowed researchers to expand the current clade of salterproviruses using a polymerase B gene phylogeny [
27]. Interest in the ability of bacteriophages to control bacterial populations has extended from medical applications to agriculture, aquaculture, the food industry [
28] and wastewater treatment systems [
19]. MMPphg from the thermophilic
Meiothermus bacteriophage MMP17 is a potential antimicrobial agent against both Gram-negative and Gram-positive bacteria such as
Escherichia coli (E. coli O157:H7),
S. aureus and
Klebsiella pneumoniae. The gene products of bacteriophage MMP17 can be used to combat bacterial infections and provide insights into bacteriophage-based strategies to develop alternatives to conventional antibiotics for human and veterinary applications [
29]. Bacteriophages have been applied to treat water contaminated with
Shigella.
Shigella is one of the most important waterborne and foodborne pathogens worldwide, according to a previous study [
30] that identified bacteriophages of
E. coli and
Vibrio.
Burkholderia pseudomallei,
Burkholderia ubonensis,
Burkholderia cenocepacia,
Burkholderia vietnamiensis,
Burkholderia mallei,
Burkholderia ambifaria, and
Burkholderia gladioli were detected in this study. Burkholderiales bacterium PBB2, 3, 4, 6, Burkholderiales bacterium RIFOXCYC12, Burkholderiales bacterium 68-12, and Burkholderiales bacterium RIFCSPLOW O2 12 FULL 65 40 were detected. Accordingly, bacteriophages of Burkholderia were not identified in the present study. Additionally, bacteriophages of Staphylococcus were not detected in the present study, consistent with our previous study that found more Staphylococcus in Phusang hot spring water [
1].
Sterile hot spring water (without bacteriophages) produced by filtration (membrane pore size 0.45 μm) was used to study the antimicrobial effects. Therefore, the levels of trace elements or other substances were tested, and bacteriophages were not involved in this analysis. An antimicrobial effect of Phusang hot spring water on S. aureus, S. epidermidis, B. subtilis or E. coli was not observed. Therefore, trace elements or other substances might not function as natural balance regulators.
The presence of Ca and Sr in Phusang hot spring water might be advantageous. However, Phusang hot spring water was not found to contain Sr using SEM–EDS. Sr was detected in our previous study using inductively coupled plasma (ICP, Perkin Elmer, Akron, OH, USA) [
1]. The agreement might have been poorer for some trace element concentrations [
44]. Therefore, other methods and increasing the number of particles examined using SEM–EDS should improve the analysis [
44] to confirm the presence of Sr. The amount of Sr may be beneficial to the elderly but not to children. Sr might reduce osteoporotic fractures in elderly individuals [
1,
45,
46] but inhibits bone formation in children. The detection of minerals is important for safety in the production of mineral water for drinking.
Phusang hot spring water may affect (prebiotic) activated LAB (DNA and CFUs/mL) growth. Phusang hot spring water might affect activated E. coli (RNA) functioning. However, the results of the nucleic acid, OD and CFUs/mL assays were different; therefore, the mechanism of Phusang hot spring water as a prebiotic must be confirmed before its use as drinking water.
In the present study, Phusang hot spring water was nontoxic to L-929 cells and HDFa cells and nonirritating at 48 h to humans with phototypes I to IV. Therefore, water may be applied in cosmetic ingredients, such as Phusang hot spring water combined with curcumin-loaded liposomes, which can be used to develop antiaging cosmetic products.
Therefore, the significant findings from this study allowed us to conclude that Phusang hot spring water exerts anti-inflammatory effects via IL-6 and might be used as a nontoxic-nonirritating agent. Combination with curcumin-loaded liposomes might be useful to develop antiaging cosmetic products. Specific bacteriophages may also help control environmental pathogens in Phusang hot spring water, except for Burkholderia and Staphylococcus. Consequently, treatment with sterile Phusang hot spring water might be used for safety and benefits.