Anti-Oxidant and Anti-Aging Activities of Callus Culture from Three Rice Varieties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Callus Induction
2.3. Rice Seed and Callus Extraction
2.4. Total Phenolic Content (TPC) Determination
2.5. Amino Acids Identification
2.6. Potassium Ferricyanide Reducing Power Assay (PFRAP)
2.7. DPPH Radical Scavenging Assay (DPPH)
2.8. Lipid Peroxidation Inhibition (LPO)
2.9. Superoxide Dismutase Activity (SOD)
2.10. Cytotoxicity
2.11. Promoting Keratinocyte Proliferation
2.12. Anti-Collagenase Activity
2.13. Anti-Inflammatory Activity
2.14. Anti-Tyrosinase Activity
2.15. Statistical Analysis
3. Results and Discussion
3.1. Callus Induction
3.2. Bioactive Compounds
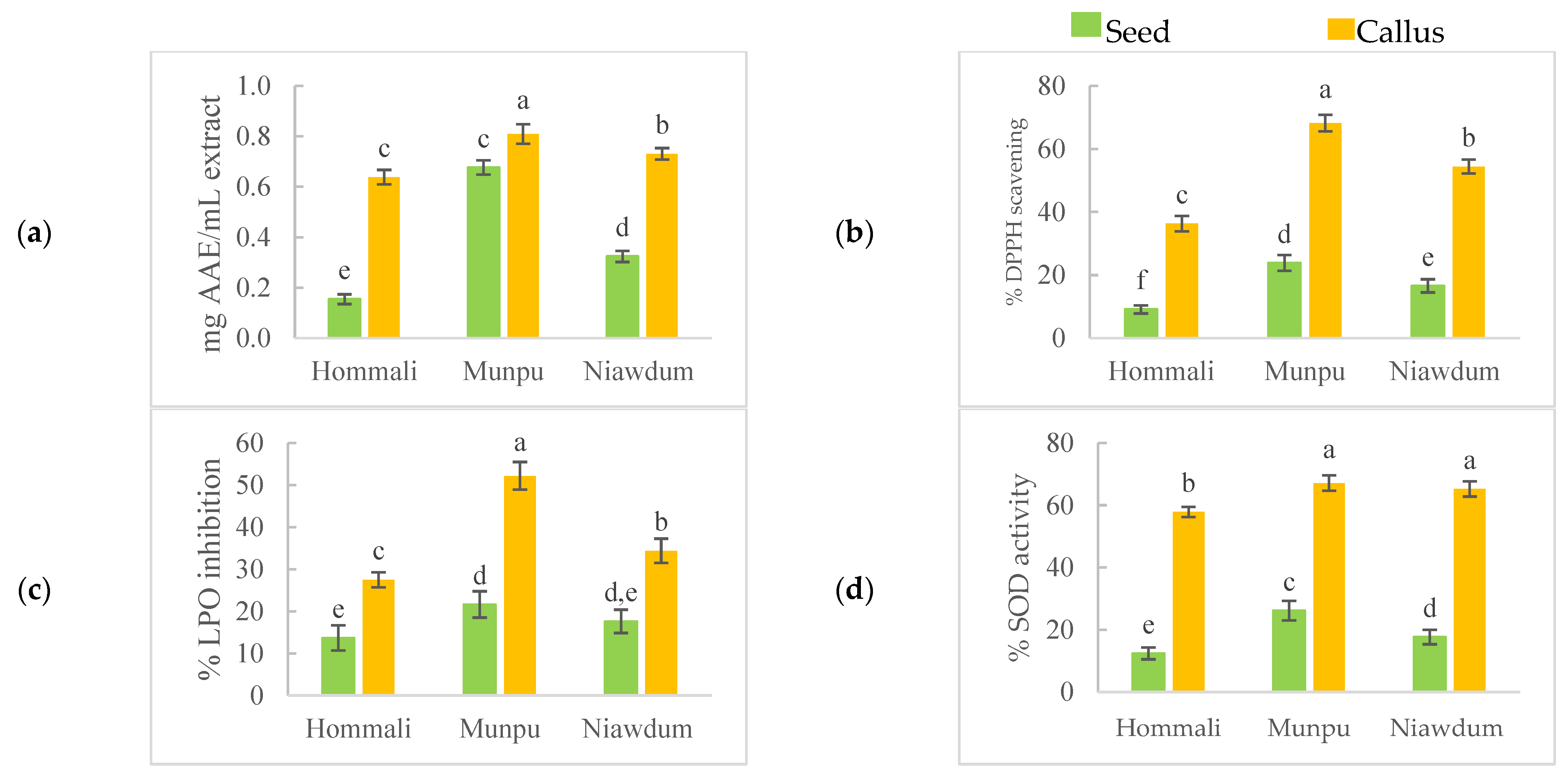
3.3. Anti-Oxidant Activities
3.4. Anti-Aging Activities
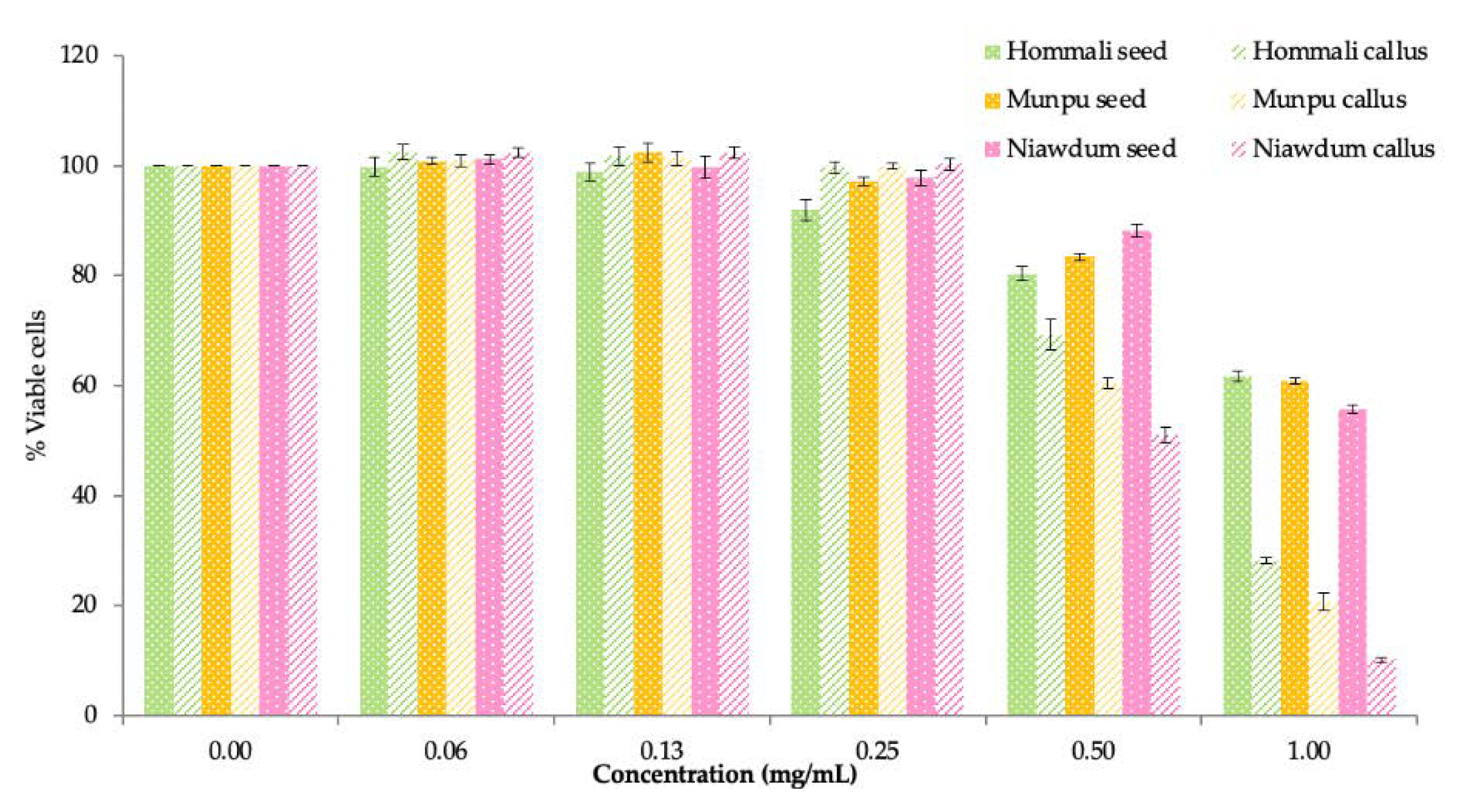
3.4.1. Cytotoxicity
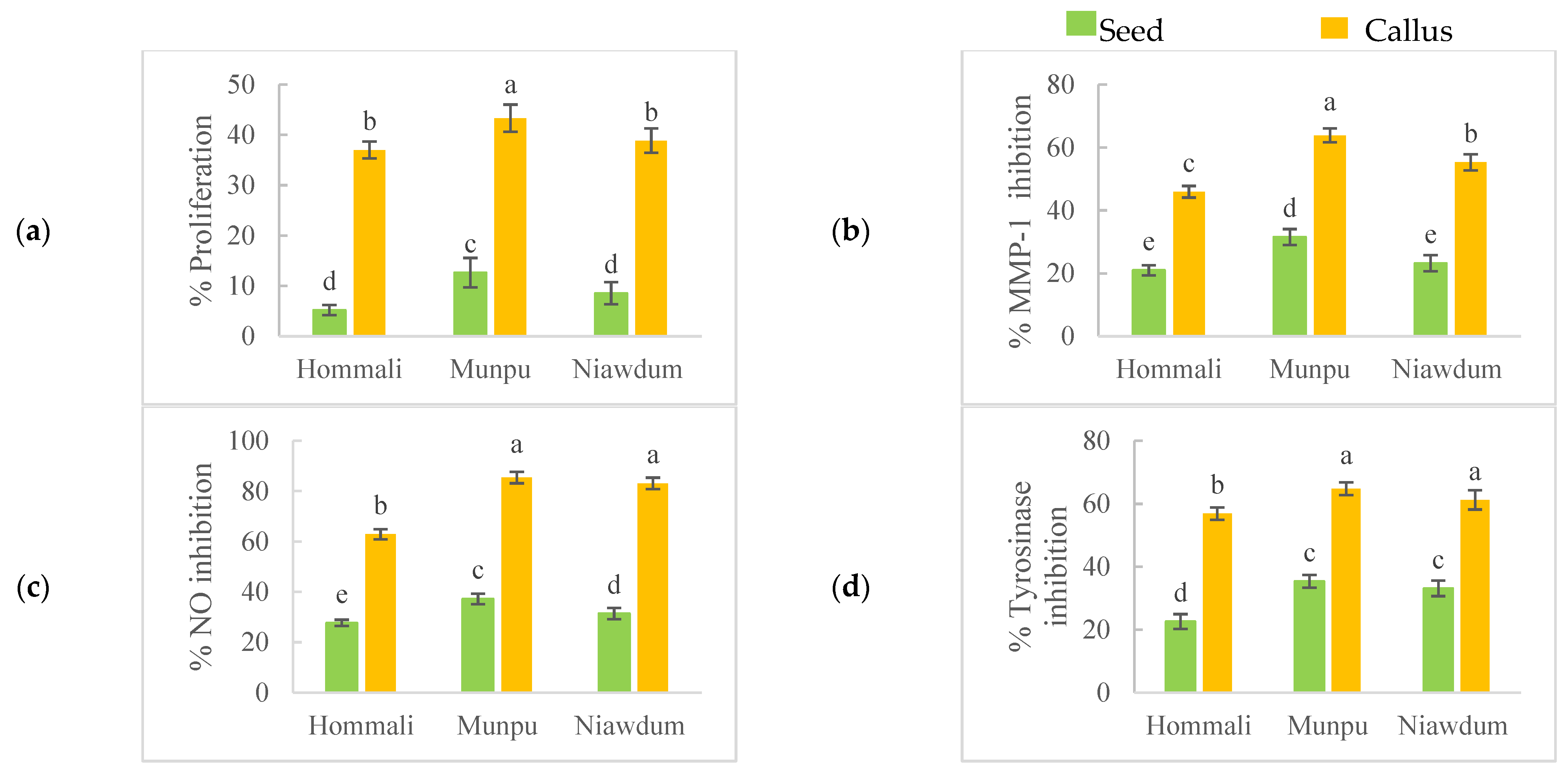
3.4.2. Promoting Keratinocytes’ Proliferation
3.4.3. Anti-Collagenase Activity
3.4.4. Anti-Inflammatory Activity
3.4.5. Anti-Tyrosinase Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Raschke, C.; Elsner, P. Skin Aging: A Brief Summary of Characteristic Changes. In Textbook of Aging Skin, 2nd ed.; Farage, M., Miller, K., Maibach, H., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 37–43. [Google Scholar]
- Chiu, P.C.; Chan, C.C.; Lin, H.M.; Chiu, H.C. The clinical anti-aging effects of topical kinetin and niacinamide in Asians: A randomized, double-blind, placebo-controlled, split-face comparative trial. J. Cosmet. Dermatol. 2007, 6, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Schürch, C.; Blum, P.; Zülli, F. Potential of plant cells in culture for cosmetic application. Phytochem. Rev. 2008, 7, 599–605. [Google Scholar] [CrossRef]
- Schmid, D.; Schürch, C.; Blum, P.; Belser, E.; Zülli, F. Plant stem cell extract for longevity of skin and hair. SOFW 2008, 134, 29–35. [Google Scholar]
- Ji, S.H.; Akter, M.; Ko, E.Y.; Choi, E.H.; Keum, Y.S.; Han, I. Enhancing antioxidant activities and anti-aging effect of rice stem cell extracts by plasma treatment. Appl. Sci. 2022, 12, 2903. [Google Scholar] [CrossRef]
- Draelos, Z.D. Plant stem cells and skin care. Cosmet. Dermatol. 2012, 25, 395–396. [Google Scholar]
- Schmid, D.; Zülli, F. Stimulating epidermal regeneration with plant-derived stem cells. C&T 2010, 125, 61–71. [Google Scholar]
- Barbulova, A.; Tito, A.; Carola, A.; Bimonte, M.; de Laurentis, F.; D’Ambrosio, P.; Apone, F.; Colucci, G.; Monoli, I.; Cucchiara, M. Raspberry stem cell extract to protect skin from inflammation and oxidative stress. C&T 2010, 125, 38–47. [Google Scholar]
- Tito, A.; Carola, A.; Bimonte, M.; Barbulova, A.; Arciello, S.; de Laurentiis, F.; Monoli, I.; Hill, J.; Gibertoni, S.; Colucci, G.; et al. A tomato stem cell extract, containing anti-oxidant compounds and metal chelating factors, protects skin cells from heavy metal-induced damages. Int. J. Cosmet. Sci. 2011, 33, 543–552. [Google Scholar] [CrossRef]
- Ludwig, P.; Bennett, S.; Gruber, J. Rice meristem stimulates epigenetic rejuvenation: New technologies. SA Pharm. Cosmet. Rev. 2011, 38, 34–37. [Google Scholar]
- Zawistowski, J.; Kopec, A.; Kitts, D.D. Effects of a black rice extract (Oryza sativa L. indica) on cholesterol levels and plasma lipid parameters in Wistar Kyoto rats. J. Funct. Foods 2009, 1, 50–56. [Google Scholar] [CrossRef]
- Chung, H.S.; Shin, J.C. Characterization of anti-oxidant alkaloids and phenolic acids from anthocyanin-pigmented rice (Oryza sativa cv. Heugjinjubyeo). Food Chem. 2007, 104, 1670–1677. [Google Scholar] [CrossRef]
- Yawadio, R.; Tanimori, S.; Morita, N. Identification of phenolic compounds isolated from pigmented rices and their aldose reductase inhibitory activities. Food Chem. 2007, 101, 1616–1625. [Google Scholar] [CrossRef]
- Finocchiaro, F.; Ferrari, B.; Gianinetti, A. A study of biodiversity of flavonoid content in the rice caryopsis evidencing simultaneous accumulation of anthocyanins and proanthocyanidins in a black-grained genotype. J. Cereal Sci. 2010, 51, 28–34. [Google Scholar] [CrossRef]
- Bhat, F.M.; Sommano, S.R.; Riar, C.S.; Seesuriyachan, P.; Chaiyaso, T.; Prom-u-Thai, C. Status of bioactive compounds from bran of pigmented traditional rice varieties and their scope in production of medicinal food with nutraceutical importance. Agronomy 2020, 10, 1817. [Google Scholar] [CrossRef]
- Guo, H.; Ling, W.; Wang, Q.; Liu, C.; Hu, Y.; Xia, M.; Feng, X.; Xia, X. Effect of anthocyanin-rich extract from black rice (Oryza sativa L. indica) on hyperlipidemia and insulin resistance in fructose-fed rats. Plant Foods Hum. Nutr. 2007, 62, 1–6. [Google Scholar] [CrossRef]
- Nam, S.H.; Choi, S.P.; Kang, M.Y.; Kozukue, N.; Friedman, M. Antioxidative, antimutagenic, and anticarcinogenic activities of rice bran extracts in chemical and cell assays. J. Agric. Food Chem. 2005, 53, 816–822. [Google Scholar] [CrossRef]
- Chulasiri, M. Anti-aging cosmeceuticals from pigmented rice. Asian J. Pharm. Sci. 2016, 11, 30. [Google Scholar] [CrossRef]
- Vargas, C.G.; da Silva Junior, J.D.; Rabelo, T.K.; Moreira, J.C.F.; Gelain, D.P.; Rodrigues, E.; Augusti, P.R.; Rios, A.d.O.; Flôres, S.H. Bioactive compounds and protective effect of red and black rice brans extracts in human neuron-like cells (SH-SY5Y). Food Res. Int. 2018, 113, 57–64. [Google Scholar] [CrossRef]
- Oki, T.; Masuda, M.; Kobayashi, M.; Nishiba, Y.; Furuta, S.; Suda, I.; Sato, T. Polymeric procyanidins as radical-scavenging components in red-hulled rice. J. Agric. Food Chem. 2002, 50, 7524–7529. [Google Scholar] [CrossRef]
- Vichit, W.; Saewan, N. Anti-oxidant activities and cytotoxicity of Thai pigmented rice. Int. J. Pharm. Pharm. 2015, 7, 329–334. [Google Scholar]
- Nam, S.H.; Choi, S.P.; Kang, M.Y.; Koh, H.J.; Kozukue, N.; Friedman, M. Antioxidative activities of bran extracts from twenty one pigmented rice cultivars. Food Chem. 2006, 94, 613–620. [Google Scholar] [CrossRef]
- Saewan, N.; Vichit, W.; Prinyarux, T. Anti-aging efficacy of thai red rice callus cosmetic product. J. Appl. Sci. 2018, 17, 63–72. [Google Scholar]
- Mousumee, K.; Soyeon, P.; Hyeon-Jin, K.; Kui-Jae, L.; Dea-Heon, K.; So-Hyeon, B.; Seong-Tshool, H. The Resveratrol Rice DJ526 Callus Significantly Increases the Lifespan of Drosophila (Resveratrol Rice DJ526 Callus for Longevity). Nutrients 2019, 11, 983. [Google Scholar]
- Gajula, D.; Verghese, M.; Boateng, J.; Walker, L.T.; Shackelford, T.; Mentreddy, S.R.; Cedric, S. Determination of total phenolics, flavonoids and anti-oxidant and chemopreventive potential of basil (Ocimum basilicum L. and Ocimum tenuiflorum L.). Int. J. Cancer Res. 2009, 5, 130–143. [Google Scholar] [CrossRef]
- Liming, W.; Jinhui, Z.; Xiaofeng, X.; Yi, L.; Jing, Z. Fast determination of 26 amino acids and their content changes in royal jelly during storage using ultra-performance liquid chromatography. J. Food Compost. Anal. 2009, 22, 242–249. [Google Scholar] [CrossRef]
- Kuda, T.; Yano, T. Changes of radical-scavenging capacity and ferrous reducing power in chub mackerel Scomber japonicus and Pacific saury Cololabis saira during 4 °C storage and retorting. LWT-Food Sci. Technol. 2009, 42, 1070–1075. [Google Scholar] [CrossRef]
- Vichit, W.; Saewan, N. Effect of germination on anti-oxidant, anti-inflammatory and keratinocyte proliferation of rice. Int. Food Res. J. 2016, 23, 2006–2015. [Google Scholar]
- Rangkadilok, N.; Sitthimonchai, S.; Worasuttayangkurn, L.; Mahidol, C.; Ruchirawat, M.; Satayavivad, J. Evaluation of free radical scavenging and antityrosinase activities of standardized longan fruit extract. Food Chem. Toxicol. 2007, 45, 328–336. [Google Scholar] [CrossRef]
- Miguel, M.G. Anti-oxidant and anti-inflammatory activities of essential oils: A short review. Molecules 2010, 15, 9252–9287. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.M.; Sato, N.; Li, X.Y.; Nakamura, N.; Hattori, M. Flavan-3-ol contents, anti-oxidative and α-glucosidase inhibitory activities of Cynomorium songaricum. Food Chem. 2010, 118, 116–119. [Google Scholar] [CrossRef]
- Takahashi, H.; Tsuji, H.; Hashimoto, Y.; Ishida-Yamamoto, A.; Iizuka, H. Cell proliferation and cytokine induction by TNF-alpha of psoriatic keratinocytes are not different from normal keratinocytes in vitro. Indian J. Dermatol. 2009, 54, 237–239. [Google Scholar] [CrossRef] [PubMed]
- Karim, A.A.; Azlan, A.; Ismail, A.; Hashim, P.; Abd Gani, S.S.; Zainudin, B.H.; Abdullah, N.A. Phenolic composition, anti-oxidant, anti-wrinkles and tyrosinase inhibitory activities of cocoa pod extract. BMC Complement. Altern. Med. 2014, 14, 381. [Google Scholar] [CrossRef]
- Laskin, J.D.; Heck, D.E.; Laskin, D.L. Multifunctional role of nitric oxide in inflammation. Trends Endocrinol. Metab. 1994, 5, 377–382. [Google Scholar] [CrossRef]
- Piktel, E.; Wnorowska, U.; Cieśluk, M.; Deptula, P.; Pogoda, K.; Misztalewska-Turkowicz, I.; Paprocka, P.; Niemirowicz-Laskowska, K.; Wilczewska, A.Z.; Janmey, P.A.; et al. Inhibition of inflammatory response in human keratinocytes by magnetic nanoparticles functionalized with PBP10 peptide derived from the PIP2-binding site of human plasma gelsolin. J. Nanobiotechnol. 2019, 17, 22. [Google Scholar] [CrossRef] [PubMed]
- Saewan, N.; Koysomboon, S.; Chantrapromma, K. Anti-tyrosinase and anti-cancer activities of flavonoids from Blumea balsamifera DC. J. Med. Plant Res. 2011, 5, 8. [Google Scholar]
- Tadhani, M.B.; Patel, V.H.; Subhash, R. In vitro anti-oxidant activities of Stevia rebaudiana leaves and callus. J. Food Compost. Anal. 2007, 20, 323–329. [Google Scholar] [CrossRef]
- Hakkim, F.L.; Shankar, C.G.; Girija, S. Chemical composition and anti-oxidant property of holy basil (Ocimum sanctum L.) leaves, stems, and inflorescence and their in vitro callus cultures. J. Agric. Food Chem. 2007, 55, 9109–9117. [Google Scholar] [CrossRef]
- Venkatramesh, M.; Wagner, D.R.; Lall, S.; Lejard, F.Y.; Yoo, S.H. Production and Extraction of Procyanidins from Plant Cell Cultures. U.S. Patent No. WO2010/114567A1, 7 October 2010. [Google Scholar]
- Zheng, H.-Z.; Wei, H.; Guo, S.-H.; Yang, X.; Feng, M.-X.; Jin, X.-Q.; Fang, Y.-L.; Zhang, Z.-W.; Xu, T.-F.; Meng, J.-F. Nitrogen and phosphorus co-starvation inhibits anthocyanin synthesis in the callus of grape berry skin. Plant Cell Tissue Organ Cult. 2020, 142, 313–325. [Google Scholar] [CrossRef]
- Ali, A.M.A.; El-Nour, M.E.M.; Yagi, S.M. Total phenolic and flavonoid contents and antioxidant activity of ginger (Zingiber officinale Rosc.) rhizome, callus and callus treated with some elicitors. J. Genet. Eng. Biotechnol. 2018, 16, 677–682. [Google Scholar] [CrossRef]
- Robinson, M.; Visscher, M.; Laruffa, A.; Wickett, R. Natural moisturizing factors (NMF) in the stratum corneum (SC). I. Effects of lipid extraction and soaking. J. Cosmet. Sci. 2010, 61, 13–22. [Google Scholar]
- Saini, R.; Badole, S.; Zanwar, A. Arginine derived nitric oxide: Key to healthy skin. In Bioactive Dietary Factors and Plant Extracts in Dermatology; Watson, R.R., Zibadi, S., Eds.; Nutrition and Health; Humana Press: Totowa, NJ, USA, 2013; pp. 73–82. [Google Scholar]
- Chessa, M.A.; Iorizzo, M.; Richert, B.; López-Estebaranz, J.L.; Rigopoulos, D.; Tosti, A.; Gupta, A.K.; Di Chiacchio, N.; Di Chiacchio, N.G.; Rubin, A.I.; et al. Pathogenesis, clinical signs and treatment recommendations in brittle nails: A review. Derm. Ther. 2020, 10, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Yatazawa, M.; Furuhashi, K.; Kurihara, N.; Ohnishi, Y. Amino acid composition of rice callus tissue grown with different kinds of nitrogen sources. Soil Sci. Plant Nutr. 1968, 14, 85–88. [Google Scholar] [CrossRef]
- Lee, K.K.; Furuhashi, K.; Yatazawa, M. Utilization of arginine dy rice callus tissue. Soil Sci. Plant Nutr. 1972, 18, 179–184. [Google Scholar] [CrossRef]
- Pulido, R.; Bravo, L.; Saura-Calixto, F. Anti-oxidant activity of dietary polyphenols as determined by a modified ferric reducing/anti-oxidant power assay. J. Agric. Food Chem. 2000, 48, 3396–3402. [Google Scholar] [CrossRef] [PubMed]
- Prabha, M.R.; Vasantha, K. Anti-oxidant, cytotoxicity and polyphenolic content of Calotropis procera (Ait.) R. Br. flowers. J. Appl. Pharm. Sci. 2011, 1, 136–140. [Google Scholar]
- Alam, M.N.; Bristi, N.J.; Rafiquzzaman, M. Review on in vivo and in vitro methods evaluation of anti-oxidant activity. Saudi Pharm. J. 2013, 21, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Pieme, C.A.; Penlap, V.N.; Ngogang, J.; Costache, M. In vitro cytotoxicity and anti-oxidant activities of five medicinal plants of Malvaceae family from Cameroon. Environ. Toxicol. Pharmacol. 2010, 29, 223–228. [Google Scholar] [CrossRef]
- Song, H.; Kumar, P.; Arivazhagan, G.; Lee, S.; Yoon, H.M.; Kim, I.H.; Kwon, H.J.; Kim, J.M.; Hakkim, F.L. Anti-oxidant property of leaves and calluses extracts of in-vitro grown 5 different Ocimum species Plant Biotechnol. J. 2012, 39, 146–153. [Google Scholar]
- Sunitha, K.; Chary, K.B.; Nimgulkar, C.C.; Kumar, B.D.; Rao, D.M. Identification, quantification and anti-oxidant activity of secondary metabolites in leaf and callus extracts of Coleus forskohlii. Int. J. Pharma Bio Sci. 2013, 4, 1139–1149. [Google Scholar]
- Ślesak, I.; Libik, M.; Miszalski, Z. Superoxide dismutase activity in callus from the C3-CAM intermediate plant Mesembryanthemum crystallinum. PCTOC 2003, 75, 49–55. [Google Scholar] [CrossRef]
- Adil, M.; Ren, X.; Jeong, B.R. Light elicited growth, antioxidant enzymes activities and production of medicinal compounds in callus culture of Cnidium officinale Makino. J. Photochem. Photobiol. B Biol. 2019, 196, 111509. [Google Scholar] [CrossRef] [PubMed]
- Furtana, G.B.; Sönmez, K.; Ellialtıoğlu, Ş.Ş.; Tıpırdamaz, R. The effects of hydrogen peroxide and nitric oxide on ion contents and lipid peroxidation levels of pepper callus tissues under salt stress. Acta Hortic. 2019, 1257, 123–130. [Google Scholar] [CrossRef]
- Wang, Y.; Graves, D.T. Keratinocyte function in normal and diabetic wounds and modulation by FOXO1. J. Diabet. Res. 2020, 2020, 3714704. [Google Scholar] [CrossRef]
- Diegelmann, R.F.; Evans, M.C. Wound healing: An overview of acute, fibrotic and delayed healing. Front. Biosci. 2004, 9, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Debnath, T.; Park, S.; Kim, D.; Jo, J.; Lim, B. Anti-oxidant and anti-inflammatory activities of Inonotus obliquus and germinated brown rice extracts. Molecules 2013, 18, 9293–9304. [Google Scholar] [CrossRef] [PubMed]
- Daniela, L.; Alla, P.; Maurelli, R.; Elena, D.; Giovanna, P.; Vladimir, K.; Roberto, D.T.; Chiara, D.L.; Saveria, P.; Liudmila, K. Anti-Inflammatory effects of concentrated ethanol extracts of edelweiss (Leontopodium alpinum Cass.) callus cultures towards human keratinocytes and endothelial cells. Mediat. Inflamm. 2012, 2012, 498373. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, S.; Ichiyama, T.; Sonaka, I.; Ohsaki, A.; Hirano, R.; Haneda, Y.; Fukano, R.; Hara, M.; Furukawa, S. Amino acids exhibit anti-inflammatory effects in human monocytic leukemia cell line, THP-1 cells. Inflamm. Res. 2011, 60, 1013–1019. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, S.; Ichiyama, T.; Sonaka, I.; Ohsaki, A.; Okada, S.; Wakiguchi, H.; Kudo, K.; Kittaka, S.; Hara, M.; Furukawa, S. Cysteine, histidine and glycine exhibit anti-inflammatory effects in human coronary arterial endothelial cells. Clin. Exp. Immunol. 2012, 167, 269–274. [Google Scholar] [CrossRef]
- Parvez, S.; Kang, M.; Chung, H.S.; Bae, H. Naturally occurring tyrosinase inhibitors: Mechanism and applications in skin health, cosmetics and agriculture industries. Phytother. Res. 2007, 21, 805–816. [Google Scholar] [CrossRef] [PubMed]
- Momtaz, S.; Mapunya, B.M.; Houghton, P.J.; Edgerly, C.; Hussein, A.; Naidoo, S.; Lall, N. Tyrosinase inhibition by extracts and constituents of Sideroxylon inerme L. stem bark, used in South Africa for skin lightening. J. Ethnopharmacol. 2008, 119, 507–512. [Google Scholar] [CrossRef]

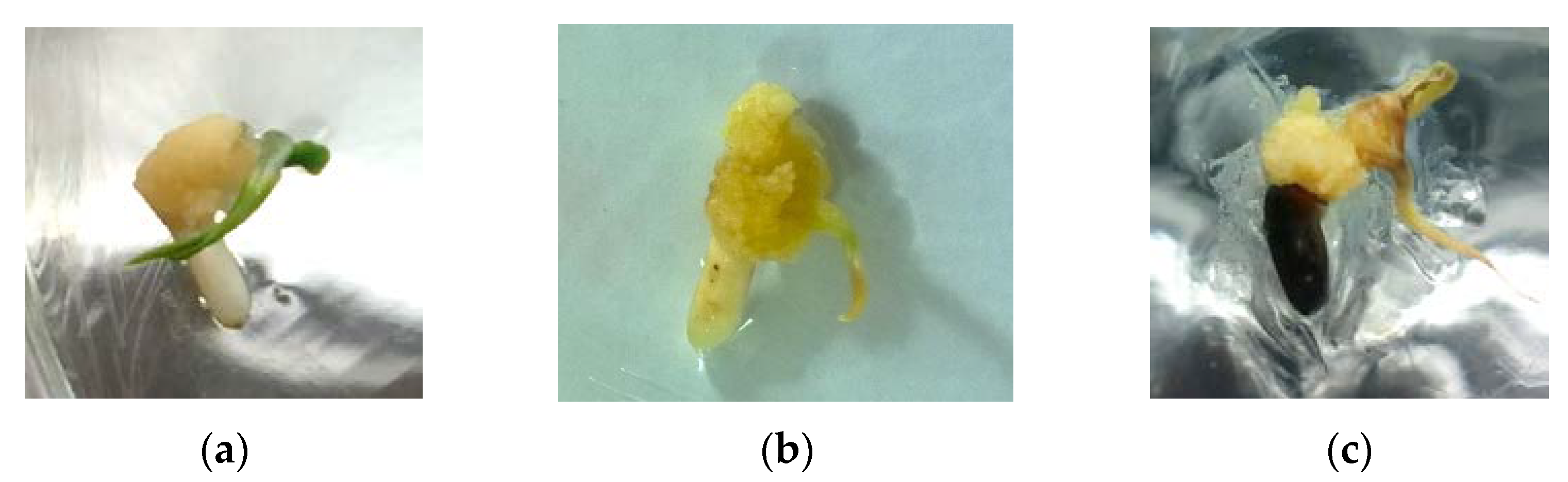
| Clorox Concentration (%) | ||||
|---|---|---|---|---|
| 10 | 20 | 30 | 40 | |
| Contamination (%) | 25.71 ± 4.04 c | 2.14 ± 1.01 b | 0.00 ± 0.00 a | 0.00 ± 0.00 a |
| Callus induction (%) | 73.57 ± 3.03 b | 95.71 ± 2.02 a | 97.86 ± 1.01 a | 95.71 ± 4.04 a |
| Clorox Incubation Time (min) | ||||
|---|---|---|---|---|
| 10 | 20 | 30 | 40 | |
| Contamination (%) | 33.57 ± 5.05 c | 4.29 ± 2.02 b | 0.00 ± 0.00 a | 0.00 ± 0.00 a |
| Callus induction (%) | 65.00 ± 5.05 c | 95.00 ± 1.01 a | 97.86 ± 1.01 a | 89.29 ± 3.03 b |
| Bioactive Compounds | Hommali | Munpu | Niawdum | |||
|---|---|---|---|---|---|---|
| Seed | Callus | Seed | Callus | Seed | Callus | |
| Total phenolic content (mg GAE/mL extract) | 0.09 ± 0.01 | 0.31 ± 0.01 * | 0.48 ± 0.02 | 0.59 ± 0.02 * | 0.30 ± 0.03 | 0.42 ± 0.01 * |
| Amino Acid | Hommali | Munpu | Niawdum | |||
|---|---|---|---|---|---|---|
| Seed | Callus | Seed | Callus | Seed | Callus | |
| HIS | n.d. | 0.07 ± 0.03 | n.d. | 0.06 ± 0.05 | n.d. | 0.02 ± 0.01 |
| SER | n.d. | 0.52 ± 0.11 | n.d. | 0.57 ± 0.04 | 0.45 ± 0.03 | 0.03 ± 0.01 |
| ARG | 0.00 ± 0.01 | 0.33 ± 0.07 | 0.00 ± 0.02 | 0.18 ± 0.02 | 0.25 ± 0.03 | 0.26 ± 0.03 |
| GLY | 0.06 ± 0.02 | 0.31 ± 0.06 | 0.38 ± 0.04 | 0.41 ± 0.03 | 0.39 ± 0.02 | 0.25 ± 0.04 |
| ASP | n.d. | 0.17 ± 0.06 | n.d. | 0.18 ± 0.15 | n.d. | 0.17 ± 0.03 |
| GLU | 0.21 ± 0.07 | 4.13 ± 0.57 | 0.17 ± 0.03 | 4.05 ± 0.18 | 0.13 ± 0.01 | 3.89 ± 0.07 |
| THR | n.d. | 0.23 ± 0.07 | n.d. | 0.32 ± 0.15 | n.d. | 0.23 ± 0.02 |
| ALA | 1.03 ± 0.20 | 2.79 ± 0.49 | 0.26 ± 0.01 | 2.60 ± 0.15 | 2.86 ± 0.03 | 2.49 ± 0.04 |
| PRO | 0.15 ± 0.01 | 0.94 ± 0.18 | 0.09 ± 0.01 | 0.22 ± 0.08 | 0.20 ± 0.03 | 0.13 ± 0.02 |
| Cys | n.d. | n.d. | n.d. | n.d. | 0.06 ± 0.03 | n.d. |
| LYS | 0.01 ± 0.03 | 0.19 ± 0.06 | n.d. | 0.06 ± 0.04 | n.d. | 0.04 ± 0.01 |
| TYR | n.d. | 0.75 ± 0.14 | n.d. | 0.81 ± 0.03 | n.d. | 0.04 ± 0.00 |
| MET | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| VAL | 0.36 ± 0.04 | 0.34 ± 0.07 | 0.31 ± 0.03 | 0.30 ± 0.06 | 0.45 ± 0.02 | 0.35 ± 0.04 |
| LLE | 0.13 ± 0.01 | 0.06 ± 0.02 | n.d. | 0.05 ± 0.01 | 0.12 ± 0.02 | 0.14 ± 0.02 |
| LEU | 0.27 ± 0.03 | 0.04 ± 0.02 | n.d. | 0.07 ± 0.01 | 0.43 ± 0.01 | 0.19 ± 0.02 |
| PHE | 0.05 ± 0.02 | 0.04 ± 0.01 | n.d. | 0.13 ± 0.01 | 0.12 ± 0.02 | 0.22 ± 0.04 |
| GABA | n.d. | 2.32 ± 0.35 | n.d. | 2.37 ± 0.04 | n.d. | 2.37 ± 0.04 |
| Total | 2.25 ± 0.30 | 13.20 ± 1.20 * | 1.20 ± 0.14 | 12.36 ± 1.17 * | 5.45 ± 0.80 | 10.81 ± 1.16 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vichit, W.; Saewan, N. Anti-Oxidant and Anti-Aging Activities of Callus Culture from Three Rice Varieties. Cosmetics 2022, 9, 79. https://doi.org/10.3390/cosmetics9040079
Vichit W, Saewan N. Anti-Oxidant and Anti-Aging Activities of Callus Culture from Three Rice Varieties. Cosmetics. 2022; 9(4):79. https://doi.org/10.3390/cosmetics9040079
Chicago/Turabian StyleVichit, Wannisa, and Nisakorn Saewan. 2022. "Anti-Oxidant and Anti-Aging Activities of Callus Culture from Three Rice Varieties" Cosmetics 9, no. 4: 79. https://doi.org/10.3390/cosmetics9040079
APA StyleVichit, W., & Saewan, N. (2022). Anti-Oxidant and Anti-Aging Activities of Callus Culture from Three Rice Varieties. Cosmetics, 9(4), 79. https://doi.org/10.3390/cosmetics9040079






