Abstract
The degradation and reduction in number of extracellular matrix (ECM) proteins are representative biological changes associated with decreased elasticity resulting in various skin problems. Elastin is an ECM protein that plays an important role in maintaining the skin’s structure. It is highly elastic and helps the tissue regain its shape after stretching or contracting. We aimed to evaluate the efficacy of the product containing amino acids, copper, and hyaluronic acid on the improvement of skin aging. A small open single-center study involved four treatments performed on five subjects at 1-week intervals with Elastic Lab®. As a result, eye wrinkles, skin moisture, inner elasticity, thickness, and density were improved 1 week after the last treatment in all subjects compared to the baseline. Among all evaluation items, skin elasticity, thickness, and density showed significant increases. Therefore, by using a composition containing amino acids, minerals, and hyaluronic acid, the biosynthesis of elastin and collagen in the skin increases, restoring skin elasticity and improving various skin problems.
Keywords:
elastin; collagen; hyaluronic acid; skin elasticity; skin density; skin moisture; wrinkle; skin thickness; skin aging; ECM 1. Introduction
Skin aging is a complex biological phenomenon that is affected by a combination of intrinsic (chronological, hormonal, and genetic) and extrinsic factors. The extrinsic factors include sun exposure, pollution, dietary habits, and chemicals [1,2]. As skin ages, protein degrading factors such as metalloproteinases (MMPs) increase, resulting in an abnormal balance in the extracellular matrix (ECM), a noncellular component that provides essential physical scaffolding and initiates crucial biochemical activities in tissues [2].
Moreover, the increased MMP, plasminogen activator inhibitor, and reactive oxygen species levels destroy the integrity of elastin networks and modify collagen fiber networks with aging [2]. Such changes in ECM proteins lead to skin aging phenotypes, including deep wrinkles, laxity, dullness, and sagging [1]. Therefore, stimulating ECM protein synthesis is an essential approach to preventing skin aging.
The dermis is mainly composed of fibroblasts that produce the primary structural constituents of the skin, including various types of collagen, elastin, and fibronectin [2]. Optimal quantities and delicate interactions of these components are necessary to maintain normal physiological properties of the skin [3]. Collagen is the most abundant fibrous protein within the interstitial ECM and constitutes up to 30% of the total protein mass of a multicellular animal. It provides tensile strength, regulates cell adhesion, supports chemotaxis and migration, and directs tissue development [2]. There are almost 30 types of collagen that exist, although not all are isolated to ECM. Collagen is arranged into fibrils to provide the required structural integrity to the tissues. Specifically, this fibril formation is defined by collagen types I, II, III, V, and XI. Collagen type I is the dominant form found extensively in almost all tissues, particularly in tendons and the skin. Other forms of collagen occur in defined areas. Collagen type II is found in cartilage and the cornea, whereas collagen type III is primarily found within the walls of blood vessels. Collagen is not only produced by fibroblasts, but also by endothelial and epithelial cells [4].
Elastin is another major ECM fiber that plays an important role in maintaining cutaneous homeostasis [5]. It is highly elastic and provides tissues with the ability to recover from continuous stretching [4]. Elastic fibers are composed of a microfibrillar scaffold (composed of various types of glycoproteins) and a core (composed of cross-linked elastin). Elastin is secreted from cells in the form of a water-soluble precursor called tropoelastin and is converted into insoluble elastin through cross-linking [6]. Unlike collagen, elastin synthesis mainly occurs during early and late neonatal periods. Moreover, the synthesis rate of functional levels of tropoelastin or elastin decreases with age [7]. Although elastin synthesis rarely occurs in adult fibroblasts, damaged elastin fibers are not always properly repaired. The elastic fibers that have undergone this process cannot function normally and tissues that should be elastic become hard, resulting in heart, blood vessel, lung, and skin diseases [8]. Moreover, when elastin is implanted it calcifies, causing arteriosclerosis, heart valve disease, and bioartificial heart valve calcification. However, the mechanisms of elastin calcification are still unknown [9]. Thus, the proper stimulation of elastin synthesis became an innovative target to maintain skin homeostasis and reduce skin aging.
Elastin is also known to possess wound-healing properties. These properties are most effective during the last-gestational stages of fetal development. However, in newborns, elastin expression significantly decreases, making scarless wound repair impossible. In various animal models, elastin has successfully been used on adult wounds to enhance wound healing [10,11]. Elastin-based scaffolds can reduce wound contraction by regulating fibroblast differentiation and improving dermal regeneration and skin elasticity [12]. It was also confirmed that the application of elastin peptides was therapeutically effective in animal models. A mixture of elastin and laminin peptides augmented the re-epithelialization of an injured rabbit ear and a proteolytic digest of elastin increased the synthesis of elastic fibers in mouse dermal fibroblasts [13,14]. Further investigation of the effects of elastin on wound healing in adults and fetuses as well as the signaling mechanisms of elastin in the wound healing process will open up new approaches to treatments that will enable complete wound healing.
Collagen and elastin have unusual amino acid compositions compared to other proteins. The triple-helical structure of collagen arises from an unusual abundance of three amino acids: glycine, proline, and hydroxyproline [15]. Elastin also has a similar amino acid composition to that of collagen in terms of glycine and proline residues, but with about 75% hydrophobic residues (Gly, Val, Ala) [12]. Therefore, many studies have focused on attempts to increase ECM protein levels with various components of amino acids.
Previous studies have attempted to induce ECM components such as elastin and collagen. To confirm these effects, elastin and collagen were extracted from animals and hydrolyzed, and cell tests or oral administration studies were conducted [16,17,18]. In addition, the elastin and collagen-inducing effects of polyphenols were also studied [19].
Hyaluronic acid is well known for its ability to replenish moisture in the skin. It has water-holding ability, resulting in smooth, moisturized skin. The hydration of the skin can slow down the formation of wrinkles and improve skin texture. The nutricosmetic effects of hyaluronic acid promote cell regeneration and even stimulate the production of collagen. Hyaluronic acid is already used in a number of products, including dermal fillers and cosmetics [20].
Lysyl oxidase (LOX) is a cuproenzyme that is necessary for the stabilization of ECM, especially the enzymatic cross-linking of collagen and elastin. Therefore, activation of LOX is required to increase the stable synthesis of elastin and collagen. Copper increases the activity of LOX, which will positively affect the synthesis of elastin and collagen [21].
We are interested in the investigation of the elastin–collagen-inducing effects through the biofeedback mechanism of the combination of mineral and amino acids constituting elastin and collagen. Moreover, we are looking for additional combinations that induce elastin and collagen with amino acids. In previous studies, researchers confirmed the elastin–collagen-inducing effects of a mixture of amino acids and hyaluronic acid [22].
Recently, we conducted an in vitro study to confirm whether the mixture of amino acids, copper, and hyaluronic acid that we developed stimulates the expression of ECM proteins in dermal fibroblasts. The composition increased the gene and protein expression levels of collagen and elastin by over two- and three-fold, respectively. The test results indicated that this unique composition successively promotes ECM protein synthesis in dermal fibroblasts [23]. However, further studies are still needed to confirm the effect of improving elastin synthesis on skin aging. In this study, a clinical trial was conducted to confirm whether this particular composition has anti-aging effects including wrinkle reduction, skin moisture, elasticity, thickness, and density improvement.
2. Materials and Methods
2.1. Study Product
Elastic Lab® is a product containing a mixture of amino acids such as glycine, L-proline, L-alanine, L-valine, L-leucine, L-lysine HCL, copper, and hyaluronic acid for the improvement of skin aging.
Materials
A list of the materials employed for each evaluation item in the study is described in Table 1.

Table 1.
Materials employed in the study.
2.2. Study Design
A small open single-center study was conducted from October 21st to December 17th, 2020, at the Global Medical Research Center. A total of five subjects were treated with Elastic Lab® to evaluate its efficacy on skin anti-aging by observing changes in eye wrinkles, skin moisture, inner elasticity, thickness, and density. The subjects received treatment on the face four times at one-week intervals. Moreover, measurement and questionnaire evaluations were conducted before the treatment and one week after the last treatment. Dermatologists proceeded with the safety evaluation. The inclusion and exclusion criteria are presented in Table 2.

Table 2.
Inclusion and exclusion criteria.
2.3. Statistical Analysis
The data were analyzed using the Wilcoxon signed-rank test to determine the results in terms of skin improvements. Statistical significance was determined by the paired t-test method with p-values less than 0.05 considered a significant difference.
3. Results
This study was conducted to confirm the efficacy of Elastic Lab® in the improvement of skin wrinkles, moisture, inner elasticity, thickness, and density. The rate of change was calculated as a percentage by subtracting the pre-treatment value from the post-treatment value and dividing it by the pre-treatment measurement value.
3.1. Wrinkles around the Eyes
The depth (mm) of wrinkles around the eyes was measured using Antera 3D CS before and after the treatment. This showed that the depth of wrinkles around the eyes of all subjects decreased (average decreased from 0.067 mm to 0.061 mm, Figure 1) after the treatments, and the average rate of change was −8.955.
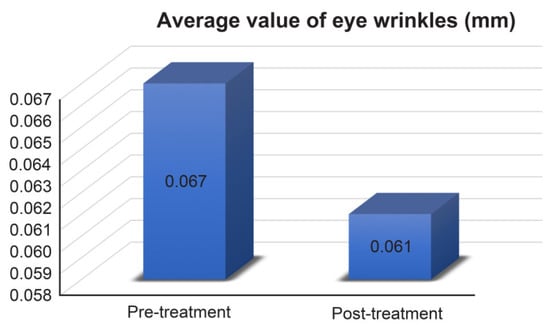
Figure 1.
Average values of eye wrinkles measurement results. Pre-treatment 0.067 ± 0.038 mm, post-treatment 0.061 ± 0.033 mm.
3.2. Skin Moisture
To compare skin hydration before and after product treatment, skin moisture was measured (PWC, %) with a Moisturemeter D Compact. The skin moisture content of all subjects showed improvement (average skin moisture increased from 48.900% to 55.893%, Figure 2), and the change rate also showed a large change of 14.301%.
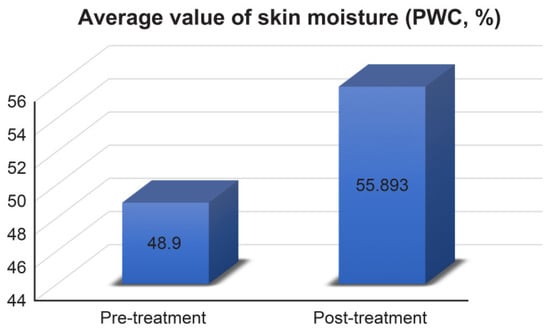
Figure 2.
Average values of skin moisture measurement results. Pre-treatment 48.9 ± 4.126%, post-treatment 55.893 ± 3.371%.
3.3. Skin Inner Elasticity
Using a dermal torque meter, the elasticity in the inner skin was measured (Ur/Ue) and the rate of change was evaluated. Similar to other results, skin elasticity was also improved in all subjects (average elasticity in the inner skin increased from 0.350 Ur/Ue to 0.432 Ur/Ue, Figure 3). Skin elasticity showed a 26.429% increase, which was the maximum improvement among other evaluation items.
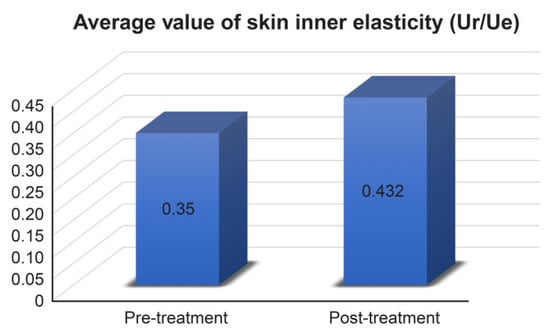
Figure 3.
Average values of skin inner elasticity measurement results. Pre-treatment 0.3 ± 0.029, post-treatment 0.432 ± 0.06.
3.4. Skin Thickness
Skin thickness was measured (μm) via imaging with an Ultrascan UC22. Skin thickness also increased in every subject (average skin thickness increased from 1652.800 μm to 1773.800 μm, Figure 4) and the change rate was 7.321%.
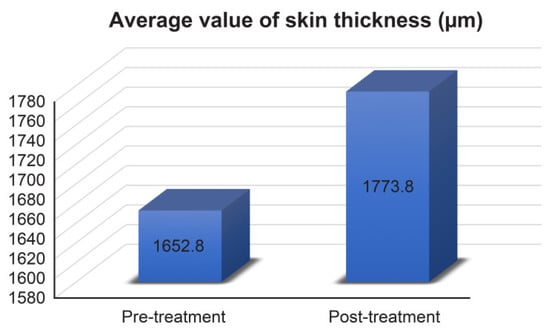
Figure 4.
Average values of skin thickness measurement results. Pre-treatment 1652.8 ± 302.064 μm, post-treatment 1773.8 ± 293.577 μm.
3.5. Skin Density
Skin density was also photographed and measured (μm) using an Ultrascan UC22. The principle of the device is as follows. A short electrical pulse is generated from the pulse generator and converted into an ultrasonic signal through an ultrasonic transducer. Ultrasonic signals are transmitted to the skin, creating inhomogeneous (cell-forming, vascular, etc.) reflections or “echoes” of the tissue. These echoes are received by the same transducer and converted into electrical signals, which are digitalized, stored, and processed in a computer to display an ultrasound image.
The skin density of subjects was improved (Figure 5) and the change rate was 12.509%. As shown in Figure 6 below, the skin density of subjects increased significantly.
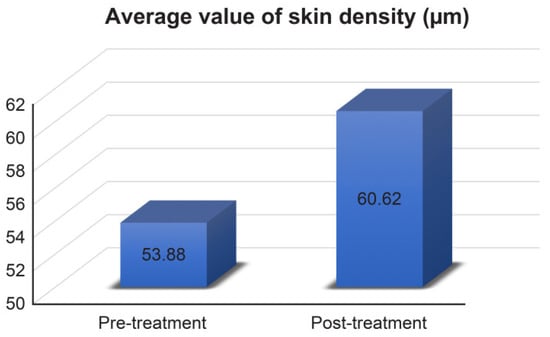
Figure 5.
Average values of skin density measurement results. Pre-treatment 53.88 ± 2.72 μm, post-treatment 60.62 ± 4.201 μm.
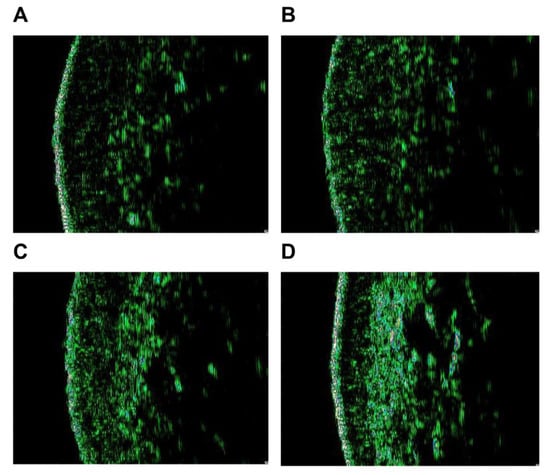
Figure 6.
Skin density photo results. Subject 2: Skin density before treatment (A) and 1 week after the last treatment (B). Subject 3: Skin density before treatment (C) and 1 week after the last treatment (D).
3.6. Safety Evaluation
The adverse reaction evaluation was performed by a dermatologist and test subjects, and there was no report of any specific adverse reaction during the clinical trial period.
3.7. Statistical Analysis
Table 3 presents the rate of change and the mean pre-treatment and post-treatment values for each measurement. The table also provides the estimates along with p-values. These p-values were provided from the results of the Wilcoxon signed-rank test. However, due to the small number of subjects, the statistical significance is relatively low.

Table 3.
Comparison of each evaluation item results pre and post-treatment.
4. Discussion
Skin aging is characterized by alterations in the extracellular matrix of the dermis through a combination of intrinsic and extrinsic factors. The most notable change is an imbalance between ECM synthesis and degradation, which promotes age-associated wrinkle formation [24]. In particular, collagen and elastin fibers, which are key to skin elasticity, are decomposed and reduced by proteolytic enzymes such as MMP [2]. In previous studies, a decrease in the distribution of elastin and collagen in the human dermal layer was observed through multiphoton microscopy [25,26].
In contrast to younger skin, aged skin showed fragmented and degraded collagen and elastic fibers. An increase in degradation and reduction in the biosynthesis of collagen and elastic fibers leads to a deficiency of collagen and elastin [27]. Collagen and elastin are not only involved in aging with time but also aging due to exposure to UV rays [28]. This process is called “solar elastosis”, and unlike endogenous aging, it is characterized by the accumulation of nonfunctional and disorganized elastic fibers throughout the dermis. If the skin is repeatedly exposed to UV rays, MMP, which is an enzyme that breaks down elastin and collagen, is activated and causes the degradation of collagen and elastic fibers, resulting in loss of skin elasticity [29].
This deficiency and degradation of collagen and elastic fibers results in a range of clinical changes such as wrinkles, loss of elasticity, and sagging of the skin [27,29,30]. The biomechanical properties of these clinical signs of aging showed common features. A decrease in collagen fiber density and fragmentation, atrophy of the elastin network, and reduction of skin thickness were observed [30].
Another study found that skin elasticity shows a rapid decrease from one’s 20s to the early 40s. Thereafter, wrinkle depth rapidly increases from the early 40s to the 60s [31]. These results indirectly indicate that skin aging starts from the loss of elasticity, meaning that skin aging care should be started from the care of elasticity. Thus, maintaining an adequate amount of ECM proteins is the most effective approach to delaying skin aging.
A representative approach for the maintenance of ECM proteins includes supplying adequate precursors of these proteins and creating proper conditions for cells to produce more. Supplementation of other components such as copper also showed positive effects on ECM protein synthesis by activating lysyl oxidase [32]. Recently, we developed a mixture composed of amino acids, copper, and hyaluronic acid, and demonstrated that it increased the expression of ECM proteins, collagen, and elastin in human skin fibroblasts by over two- and three-fold, respectively [23].
In this study, the results confirmed the anti-aging effects of Elastic Lab® products containing amino acids, copper, and hyaluronic acid. The product was administered to five subjects at one-week intervals for four sessions, and evaluations were performed a week later. The results showed improvements in all evaluation criteria for all subjects. On average, the wrinkle depth around the eyes decreased by 8.955%, skin moisture increased by 14.301%, skin elasticity increased by 23.429%, skin thickness increased by 7.321%, and skin density increased by 12.509%. Primarily, it is important to note the changes in skin elasticity, skin thickness, and skin density, which showed the greatest improvements.
Moreover, we measured skin elasticity using a dermal torque meter (DTM). A DTM is a device that applies a constant load to the skin by rotating a disk in a ring pattern to measure the angular rotation. The device records the rotation as an angular deformation and records the rotational recovery of the skin after the load is released [33]. According to a previous study, when a DTM, cutometer, and DPM (dermal phase meter) were compared with each other, the skin depths measured by DTM and cutometer were deeper than that by the DPM. In addition, the parameters and results for the DTM were found to be the most sensitive [34].
This result was consistent with the in vitro experiment previously conducted in which the mixture of amino acids stimulated ECM protein synthesis. We can conclude that skin elasticity, thickness, and density showed considerable improvements as amino acids and various components stimulated the skin fibroblasts to produce ECM proteins such as collagen and elastin. However, due to the small number of subjects, the statistical significance of this study is relatively low. Therefore, additional large-scale clinical trials are needed. Furthermore, studies to measure changes in elastin and collagen contents due to application of the product in tissues of various models using multiphoton microscopy are also needed.
5. Conclusions
As we age, ECM components degrade and elasticity decreases, resulting in deep wrinkles, laxity, dullness, and sagging of the skin. Using a composition containing amino acids, minerals, and hyaluronic acid, it is possible to induce an increase in the amount of biosynthesis of elastin and collagen in the fibroblasts of the skin dermis. This in turn restores skin elasticity and improves various skin problems, such as fine lines, redness, and skin pores. In addition to the effects of improving skin elasticity, elastin has properties that improve wound healing and regeneration. However, further studies will be needed to fully understand the effects and mechanisms of elastin. Additionally, since this study lacks statistical significance due to the small number of subjects studied, further studies such as large-scale clinical trials are needed to build up more scientific and statistical evidence.
Author Contributions
Conceptualization, M.-S.K.; methodology, M.-S.K.; software, M.-S.K.; validation, M.-S.K.; formal analysis, M.-S.K.; investigation, M.-S.K.; resources, M.-S.K.; data curation, M.-S.K.; writing-original draft, M.-S.K. and K.-E.C.; writing—review and editing, M.-S.K. and D.-K.L.; visualization, M.-S.K.; supervision, M.-S.K.; project administration, M.-S.K. and S.-H.S.; funding acquisition, M.-S.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Korea Institute of Startup and Entrepreneurship Development, grant number 10271672.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of Global Medical Research Center (No. GIRB-20O22-FT, 22 December 2020).
Informed Consent Statement
All of the patients gave their informed consent to participate in the study after the nature of the study had been fully explained.
Data Availability Statement
The data are available from the corresponding author upon reasonable request.
Acknowledgments
All authors are thankful to the Korea Institute of Startup and Entrepreneurship Development. We ensure that all authors have consented to the acknowledgment.
Conflicts of Interest
M.-S.K. is an employer of Elastic Lab Inc. K.-E.C. and D.-K.L. are employees of Elastic Lab Inc. All other authors declare no conflicts of interest.
References
- Zhang, S.; Duan, E. Fighting against skin aging: The way from bench to bedside. Cell Transplant. 2018, 27, 729–738. [Google Scholar] [CrossRef] [PubMed]
- Frantz, C.; Stewart, K.M.; Weaver, V.M. The extracellular matrix at a glance. J. Cell Sci. 2010, 123, 4195–4200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uitto, J.; Olsen, D.R.; Fazio, M.J. Extracellular matrix of the skin: 50 years of progress. J. Investig. Dermatol. 1989, 92, 61S–77S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kular, J.K.; Basu, S.; Sharma, R.I. The extracellular matrix: Structure, composition, age-related differences, tools for analysis and applications for tissue engineering. J. Tissue Eng. 2014, 5, 1–17. [Google Scholar] [CrossRef]
- Weihermann, A.C.; Lorencini, M.; Brohem, C.A.; de Carvalho, C.M. Elastin structure and its involvement in skin photoageing. Int. J. Cosmet. Sci. 2017, 39, 241–247. [Google Scholar] [CrossRef]
- Giro, M.G.; Oikarinen, A.I.; Oikarinen, H.; Sephel, G.; Uitto, J.; Davidson, J.M. Demonstration of elastin gene expression in human skin fibroblast cultures and reduced tropoelastin production by cells from a patient with atrophoderma. J. Clin. Investig. 1985, 75, 672–678. [Google Scholar] [CrossRef]
- Wen, Q.; Mithieux, S.M.; Weiss, A.S. Elastin biomaterials in dermal repair. Trends Biotechnol. 2020, 38, 280–291. [Google Scholar] [CrossRef]
- Baud, S.; Duca, L.; Bochicchio, B.; Brassart, B.; Belloy, N.; Pepe, A.; Dauchez, M.; Martiny, L.; Debelle, L. Elastin peptides in aging and pathological conditions. Biomol. Concepts 2013, 4, 65–76. [Google Scholar] [CrossRef]
- Truter, S.; Rosenbaum-Fiedler, J.; Sapadin, A.; Lebwohl, M. Calcification of elastic fibers in pseudoxanthoma elasticum. Mt. Sinai J. Med. 1996, 63, 210–215. [Google Scholar]
- Almine, J.F.; Wise, S.G.; Weiss, A.S. Elastin signaling in wound repair. Birth Defects Res. Part C 2012, 96, 248–257. [Google Scholar] [CrossRef]
- Rodríguez-Cabello, J.C.; de Torre, I.G.; Ibañez-Fonzeca, A.; Alonso, M. Bioactive scaffolds based on elastin-like materials for wound healing. Adv. Drug Deliv. Rev. 2018, 129, 118–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daamen, W.F.; Veerkamp, J.H.; van Hest, J.C.; van Kuppevelt, T.H. Elastin as a biomaterial for tissue engineering. Biomaterials 2007, 28, 4378–4398. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, T.; Suzuki, Y.; Tanihara, M.; Kakimaru, Y.; Suzuki, K. Development of alginate wound dressings linked with hybrid peptides derived from laminin and elastin. Biomaterials 2004, 25, 1407–1414. [Google Scholar] [CrossRef] [PubMed]
- Hinek, A.; Rabinovitch, M.; Keeley, F.; Okamura-Oho, Y.; Callahan, J. The 67-kD elastin/laminin-binding protein is related to an enzymatically inactive, alternatively spliced form of beta-galactosidase. J. Clin. Investig. 1993, 91, 1198–1205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lodish, H.; Berk, A.; Zipursky, S.L. Collagen: The fibrous proteins of the matrix. In Molecular Cell Biology, 4th ed.; W. H. Freeman: New York, NY, USA, 2000; pp. 145–154. [Google Scholar]
- Shiratsuchi, E.; Nakaba, M.; Yamada, M. Elastin hydrolysate derived from fish enhances proliferation of human skin fibroblasts and elastin synthesis in human skin fibroblasts and improves the skin conditions. J. Sci. Food Agric. 2015, 96, 1672–1677. [Google Scholar] [CrossRef] [PubMed]
- Barati, M.; Jabbari, M.; Navekar, R.; Farahmand, F.; Zeinalian, R.; Salehi-Sahlabadi, A.; Abbaszadeh, N.; Mokari-Yamchi, A.; Davoodi, S.H. Collagen supplementation for skin health: A mechanistic systematic review. J. Cosmet. Dermatol. 2020, 19, 2820–2829. [Google Scholar] [CrossRef]
- Bianchi, F.M.; Angelinetta, C.; Rizzi, G.; Pratico, A.; Villa, R. Evaluation of the Efficacy of a Hydrolyzed Collagen Supplement for Improving Skin Moisturization, Smoothness, and Wrinkles. J. Clin. Aesthet. Dermatol. 2022, 15, 48–52. [Google Scholar]
- Chowdhury, A.; Nosoudi, N.; Karamched, S.; Parasaram, V.; Vyavahare, N. Polyphenol treatments increase elastin and collagen deposition by human dermal fibroblasts; Implications to improve skin health. J. Dermatol. Sci. 2021, 102, 94–100. [Google Scholar] [CrossRef]
- Bukhari, S.N.A.; Roswandi, N.L.; Waqas, M.; Habib, H.; Hussain, F.; Khan, S.; Sohail, M.; Ramli, N.A.; Thu, H.E.; Hussain, Z. Hyaluronic acid, a promising skin rejuvenating biomedicine: A review of recent updates and pre-clinical and clinical investigations on cosmetic and nutricosmetic effects. Int. J. Biol. Macromol. 2018, 120 Pt B, 1682–1695. [Google Scholar] [CrossRef]
- Rucker, R.B.; Kosonen, T.; Clegg, M.S.; Mitchell, A.E.; Rucker, B.R.; Uriu-Hare, J.Y.; Keen, C.L. Copper, Lysyl oxidase, and extracellular matrix protein cross-linking. Am. J. Clin. Nutr. 1998, 67 (Suppl. 5), 996S–1002S. [Google Scholar] [CrossRef] [Green Version]
- de Servi, B.; Orlandini, A.; Caviola, E.; Meloni, M. Amino acid and hyaluronic acid mixtures differentially regulate extracellular matrix genes in cultured human fibroblasts. J. Biol. Regul. Homeost. Agents 2018, 32, 517–527. [Google Scholar] [PubMed]
- Chung, K.W.; Song, S.H.; Kim, M.S. Synergistic effect of copper and amino acid mixtures on the production of extracellular matrix proteins in skin fibroblasts. Mol. Biol Rep. 2021, 48, 3277–3284. [Google Scholar] [CrossRef] [PubMed]
- Panwar, P.; Butler, G.S.; Jamroz, A.; Azizi, P.; Overall, C.M.; Brömme, D. Aging-associated modifications of collagen affect its degradation by matrix metalloproteinases. Matrix Biol. 2018, 65, 30–44. [Google Scholar] [CrossRef] [PubMed]
- Pitte, J.C.; Freis, O.; Vazqueq-Duchene, M.D.; Perie, G.; Pauly, G. Evaluation of elastin/collagen content in human dermis in-vivo by multiphoton tomography variation with depth and correlation with aging. Cosmetics 2014, 1, 11–221. [Google Scholar]
- Wang, H.; Shyr, T.; Fevola, M.J.; Cula, G.O.; Stamatas, G.N. Age-related morphological changes of the dermal matrix in human skin documented in vivo by multiphoton microscopy. J. Biomed. Opt. 2018, 23, 030501. [Google Scholar] [CrossRef] [Green Version]
- Shin, J.-W.; Kwon, S.-H.; Choi, J.-Y.; Na, J.-I.; Huh, C.-H.; Choi, H.-R.; Park, K.-C. Molecular Mechanisms of Dermal Aging and Antiaging Approaches. Int. J. Mol. Sci. 2019, 20, 2126. [Google Scholar] [CrossRef] [Green Version]
- Baumann, L.; Bernstein, E.F.; Weiss, A.S.; Bates, D.; Humphrey, S.; Silberberg, M.; Daniels, R. Clinical Relevance of Elastin in the Structure and Function of Skin. Aesthet. Surg. J. Open Forum. 2021, 14, 1–8. [Google Scholar] [CrossRef]
- McCabe, M.C.; Hill, R.C.; Calderone, K.; Cui, Y.; Yan, Y.; Quan, T.; Fisher, G.J.; Hansen, K.C. Alterations in extracellular matrix composition during aging and photoaging of the skin. Matrix Biol. Plus 2020, 8, 100041. [Google Scholar] [CrossRef]
- Haydonta, V.; Bernardb, B.A.; Fortunel, N.O. Age-related evolutions of the dermis: Clinical signs, fibroblast and extracellular matrix dynamics. Mech. Ageing Dev. 2019, 177, 150–156. [Google Scholar] [CrossRef]
- Cho, C.; Cho, E.; Kim, N.; Shin, J.; Woo, S.; Lee, E.; Hwang, J.; Ha, J. Age-related biophysical changes of the epidermal and dermal skin in Korean women. Skin Res. Technol. 2019, 25, 504–511. [Google Scholar] [CrossRef]
- Hill, K.E.; Davidson, J.M. Induction of increased collagen and elastin biosynthesis in copper-deficient pig aorta. Arteriosclerosis 1986, 6, 98–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boyce, S.T.; Supp, A.P.; Wichkett, R.R.; Hoath, S.B.; Warden, G.D. Assessment with the Dermal Torque Meter of Skin Pliability After Treatment of Burns with Cultures Skin Substitutes. J. Bure Care Rehabil. 2000, 21, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Murray, B.C.; Wickett, R.R. Correlations between Dermal Torque Meter®, Cutometer®, and Dermal Phase Meter® measurements of human skin. Skin Res. Technol. 1997, 3, 101–106. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).