1. Introduction
Phytocosmetics, i.e., those cosmetics with added raw materials of plant origin, are notably drawing interest from the general population, who are increasingly concerned with safety of use and environmental issues. Acting in synergy with the components of the formulation, plant extracts with their complex composition can promote various beneficial actions to the skin and to the organism. Flavonoids, tannins, phenolics, amino acids, and vitamins in cosmetics can influence the biological functions of the skin [
1,
2] and contribute to the treatment of several skin disorders [
3].
The role of antioxidant agents has been widely discussed in reducing the damage from the production of reactive oxygen species (ROS) in the body [
4]. Polyphenols are associated with antioxidant activity in a wide range of plant species, and structurally they can have one or more hydroxyl groups and aromatic rings in free or glycosylated form. The effect of electron resonance demonstrates how conjugated double bonds give the substituents of the phenol molecule a greater power to stabilize the free radicals produced, thus minimizing their damage [
5].
The genus
Morus belongs to the Moraceae family, which presents about 24 species widely distributed throughout the world, being one of the most abundant families in tropical forests [
6].
Morus nigra (
Figure 1), known as black mulberry, is popularly used as a laxative, sedative, expectorant, emollient, diuretic, antiseptic, antioxidant, and anthelmintic with refreshing, soothing, anti-inflammatory, and hypoglycemic properties, and it is used in the treatment of eczema and oral inflammation [
7]. Its use is due to a high presence of flavonoids, tannins, coumarins, triterpenoids, and steroids [
8].
Studies have evaluated the antioxidant, anti-inflammatory, antibacterial, antiviral, and antinociceptive activities [
6,
9,
10], tyrosinase inhibitory activity [
11], and pre-clinical toxicity of the decoction [
12], as well as its antihyperglycemic and anti-obesity activities [
13,
14], in addition to various other activities, such as anticancer and organ protection activities, along with its effects on the female reproductive system [
15].
In this context, the pharmaceutical industry has been offering more and more space to natural products, recognizing the importance of phytotherapy and the popular use of medicinal plants, which historically have been a rich source for successful drugs. The scientific interest in plant-derived, natural-product-based drug discovery is evident, which results in new approaches for the identification, characterization, and resupply of natural products [
16].
Cosmetic alternatives have come to add value to traditional products. Hence, this work aims to develop a topical, antioxidant emulsion containing a hydroalcoholic extract of the leaves of Morus nigra, as well as to evaluate its stability and antioxidant efficacy via physicochemical and microbiological means.
2. Materials and Methods
2.1. Plant Material and Crude Hydroalcoholic Extract Preparation
Leaves of Morus nigra L. (Moraceae) were collected in the municipality of Petrolina-PE, northeastern Brazil, in November 2018 (coordinates: 9°23′23.8″ S 40°29′45.0″ W). A voucher specimen of the plant (1764) was deposited in the Herbarium Vale do São Francisco (HVASF) of the Federal University of Vale do São Francisco (UNIVASF). The plant material was dried in an oven with air circulation at 45 °C and pulverized in a knife mill (Solab, São Paulo, Brazil) to obtain an amount of 1756 g of powder, which was submitted to maceration with hydroalcoholic solution 70% (v/v) (ethanol 70%, Synth, São Paulo, Brazil). The final solution was placed in an air-circulating oven (Marconi, São Paulo, Brazil), which led the crude hydroalcoholic extract of Morus nigra (MnCE) to a yield of 16.6% (w/w). All procedures for access to genetic patrimony and associated traditional knowledge were carried out, and the project was registered in SisGen (Register #AC34CFC).
2.2. Analysis by HPLC-DAD
A Shimadzu
® (Kyoto, Japan) high-performance liquid chromatograph (HPLC) (LC-20 AT) equipped with an automatic sampler (SIL-20 A) and an LC-Solution
® 1.0 software (Shimadzu, Kyoto, Japan)-controlled diode-array detector (DAD) (SPD-M20A) was used. The plant extract, at the concentration of 1 mg/mL, was analyzed in triplicate, considering the following chromatographic conditions: stationary phase: C18 column with dimensions of 250 mm × 4.6 mm and particle size of 5 μm (Thermo Fisher Scientific
® Hypersil, Adelaide, Australia); mobile phase: solution A: water + 0.01% (
v/v) trifluoroacetic acid (TFA), and solution B: acetonitrile (ACN), considering the elution gradient shown in
Table 1. The temperature was kept constant at 30 °C, and the injection volume was 1 mg/mL for the extract and 200 μg/mL for standard solutions, at a flowrate of 0.8 mL/min. Before being analyzed in the chromatograph, the solutions were degassed and filtered through a 0.22 μm filter membrane (Chromafil
® Xtra, Bethlehem, PA, USA). Analyses were performed at the wavelength of 340 nm [
17]. All reagents for the chromatographic analyses were of HPLC-grade and were purchased from Tedia Brazil (Rio de Janeiro, Brazil).
2.3. Development of Topical Formulations
An anionic-type oil/water (O/W) emulsion was prepared according to the emulsification process, and the choice of the formulation took into account a previous study of compatibility between the components and the plant extract (unpublished data). The components, according to the International Nomenclature of Cosmetic Ingredients (INCI), were (percentages in w/w): cetearyl alcohol (and) sodium cetearyl sulfate 12% (Phase 1), propylparaben 0.10% (Phase 1), isopropyl myristate 6% (Phase 1), BHT 0.05% (Phase 1), methylparaben 0.20% (Phase 2), imidazolidinyl urea 0.30% (Phase 4), glycerin 6% (Phase 2), propylene glycol 4% (Phase 2), disodium EDTA 0.05% (Phase 2), cyclomethicone (and) dimethicone crosspolymer 2% (Phase 3), cyclopentasiloxane 3% (Phase 3), cyclopentasiloxane dimethiconol 2% (Phase 4) and water q.s.p. (quantity sufficient for preparation). The emulsion was prepared by heating phases 1 and 2 separately to 75 ± 1 °C. Then, phase 1 was mixed with phase 2 slowly and under constant agitation (1000 rpm). After formation of the emulsion, phases 3 and 4 were added at 40 °C, maintaining the system under agitation (500 rpm) until complete homogenization. After 24 h of maturation, the base (F1) was subjected to the preliminary stability tests, and the MnCE was then incorporated in the proportion of 10% (F2) and 20% (F3).
2.4. Stability Studies
Physical stability was performed by centrifugation (24 h after preparation of the formulations at 3000 rpm for 30 min at room temperature) and ice/thaw cycles, followed by accelerated stability for 90 days, and involved the following conditions: oven (Marconi, São Paulo, Brazil) at 37 ± 2 °C, refrigerator (Electrolux, São Paulo, Brazil) at 4 ± 2 °C, and room temperature of 20 ± 2 °C, under indirect light. Organoleptic characteristics (color, odor, and appearance) were evaluated with an aliquot of each sample placed in clock glass on a beige background [
18]. Samples were classified as: normal, without alteration; slightly modified; modified; and intensively modified.
The pH was measured using the potentiometric method with a digital pH meter (ITPH-2300, São Paulo, Brazil), previously calibrated, and the samples were diluted to 10% (
w/v) in distilled water. Viscosity determinations were obtained using a Brookfield viscometer (Quimis, model Q860 M21, São Paulo, Brazil) at 25 ± 2 °C. For analysis of spreadability, 0.3 g of sample was placed between glass plates measuring 10 cm × 18 cm and 0.5 cm thick, the first plate being laid on graph paper supported on a wooden plate. The following plates were added for reading the formed diameter, expressed in cm. The data were plotted on a spreadsheet, in which the spreadability was calculated according to Equation (1). Both tests were performed in triplicate.
where
Ei = spreadability of the sample for a given weight
i (mm
2);
d = mean diameter (mm) of the sample after plaque overlap.
2.5. Antioxidant Activity—DPPH Radical Scavenging Method
The formulations were evaluated on days 0, 30, 60, and 90 according to the free radical sequestration capacity by the DPPH assay [
19]. Solutions at concentrations of 243, 81, 27, 9, 3, and 1 µg/mL were prepared from the formulations and standards (ascorbic acid, butylated hydroxyanisole (BHA), and butylated hydroxytoluene (BHT)) and diluted in absolute ethanol. The results were expressed as the half-maximal inhibitory concentration (IC
50) and expressed in μg/mL.
2.6. Antibacterial Activity
The plant extract and formulations (F1, F2, and F3) were evaluated according to the microdilution technique of minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC). The extract of
M. nigra and the formulations were diluted in distilled water, yielding a 25 mg/mL stock solution. The microdilution, based on M07-A9 [
20], consisted of the distribution of 100 μL of Mueller–Hinton (MH) broth (Sigma-Aldrich, St. Louis, MO, USA) in microtiter plates. Afterwards, 100 μL of the stock solution were added to the first well and, after homogenization, transferred to the second well, and so on.
In the preparation of the inoculum, bacterial cultures of Staphylococcus aureus (ATCC 25923), Escherichia coli (ATCC 25922), Pseudomonas aeruginosa (ATCC 27853), Klebsiella pneumoniae (ATCC 13883), Shigella flexineri (ATCC 12028), Enterococcus faecalis (ATCC 19433), Serratia marcescens (ATCC 13880), Staphylococcus aureus MRSA (33591), and Salmonella enterica (ATCC 10708) were used.
After incubation at 37 °C for 24 h in Mueller–Hinton culture medium (Sigma-Aldrich, St. Louis, MO, USA) under aerobic conditions, 10 μL of 1% 2,3,5-triphenyl-tetrazolium chloride (CTT) was added to each well, determining the minimum inhibitory concentration (MIC) as the lowest concentration of the extract which visibly inhibited the bacterial growth. From all wells, an aliquot of 10 μL was removed, spread on the surface of MH agar, and incubated at 37 °C for 24 h. The minimum bactericidal concentration (MBC) was defined as the lowest concentration of the extract in the study capable of causing the death of the bacterial inoculum. The assays were performed in triplicate.
2.7. Microbiological Stability of Formulations—Challenge Test
In order to determine the resistance of the formulations to the microbial contamination, a preservative system efficacy challenge test was performed. The testing microorganisms
Staphylococcus aureus (ATCC 25923),
Escherichia coli (ATCC 25922), and
Pseudomonas aeruginosa (ATCC 27853) were used, which were suspended in saline solution (NaCl 0.9%) (Cristália, São Paulo, Brazil) from a recent (24–48 h) viable culture on an agar surface. The suspension was adjusted to a viable biomass of 1.5 × 10
8 colony-forming units (CFU)/mL [
21].
After obtaining the inoculum, the formulation to be tested was contaminated with 1% (v/v) of the bacterial suspension, thus giving rise to a centesimal dilution (1/100) of the inoculum. From each formulation, 0.5 g was withdrawn and diluted into 4.5 mL of saline solution, thus giving rise to a 10−1 dilution, and 1 mL was withdrawn therefrom. A serial decimal dilution was performed up to 10−6. A one milliliter aliquot of each of these decimal dilutions was pipetted to a Petri dish, and the pour plate method was applied for the inoculation. Briefly, agar was added to the Petri dish with the inoculum and incubated at 37 °C for 24 h prior to the determination of the total viable colonies. The test was performed over 28 days, and the viable counts were performed at intervals of 0, 7, 14, and 28 days. The cell viable counts were performed in triplicate for each decimal dilution.
2.8. Statistical Analyses
The results were statistically evaluated by the analysis of pairwise variance using one-way ANOVA for non-parametric variables. The program used was GraphPad Prism version 6.0 (San Diego, CA, USA). The aim of the statistical analysis was to verify whether there was a significant difference between the averages of the results obtained. A 95% confidence interval was used, and values of p < 0.05 were considered to be statistically significant.
3. Results and Discussion
With the purpose of obtaining the crude hydroalcoholic extract, the leaf powder of Morus nigra was subjected to maceration with a 70% hydroalcoholic solution. The final solution, after being concentrated in a rotatory evaporator (Quimis, São Paulo, Brazil), was placed in an air-circulating oven. The crude extract was previously evaluated for antioxidant potential, according to the DDPH radical scavenging assay, with an IC50 of 63.1 ± 5.85 μg/mL.
The analysis, using high-performance liquid chromatography coupled to a diode-array detector (HPLC–DAD), of the plant extract of
Morus nigra allowed the identification and quantification of rutin (calibration curve: y = 19,754x − 101,075; R
2 = 0.9977) and isoquercetin (calibration curve: y = 12,675x + 9443.8; R
2 = 0.9982), two analytical markers previously reported in this plant species [
17].
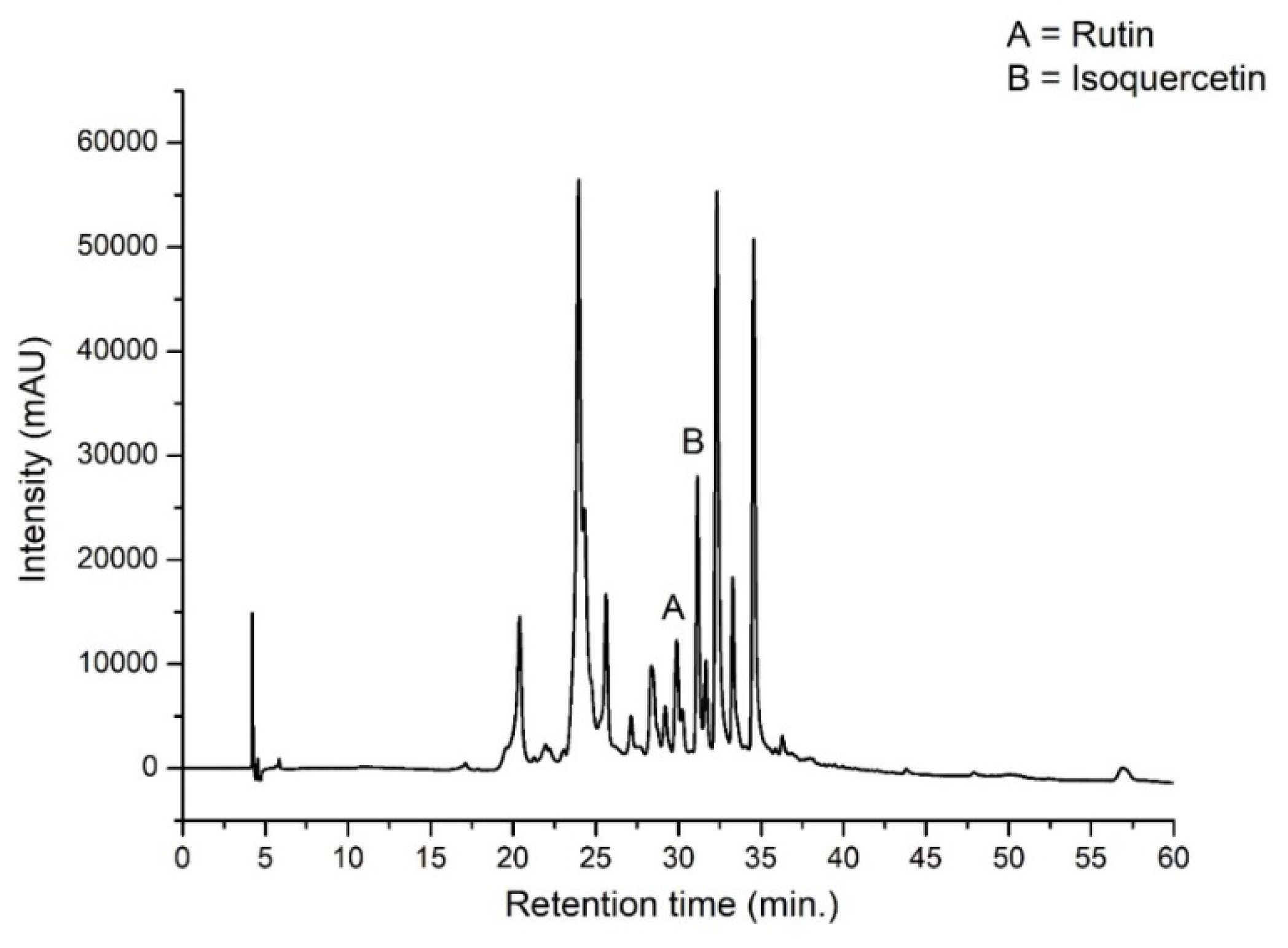
Figure 2 presents the chromatographic profile of the crude hydroalcoholic extract indicating the identified markers resolved by the line basis.
Resorting to the analysis of peak areas to quantify the contents of these polyphenols in the crude hydroalcoholic extract (MnCE), the amount of rutin and isoquercetin was 7.78 ± 0.08 and 2.59 ± 0.08 μg/mL, respectively. Numerous therapeutic properties of phenolic compounds are linked to their antioxidant and chelating capacities. Among these families of compounds, quercetin is known as one of the flavonoids with the highest antioxidative capacity [
22]. In its glycosylated form, called isoquercetin or quercetin-3-glucoside, the compound exhibits greater solubility in water [
23]. Rutin possesses a wide range of pharmacological activities (antioxidant, antiviral, anti-inflammatory, and vasoprotective, among others) [
24] and can be transformed into quercetin-3-glucoside, increasing its bioavailability.
Concerning the formulation of phytocosmetics, stability studies provide important parameters to ensure the safety of the product, as well as organoleptic, physicochemical, and microbiological aspects that are fundamental to the initial development of cosmetics. The choice of formulations for the current research work was based on a previous study (unpublished data) that analyzed the compatibility between different self-emulsifying systems and the efficacy between the use of hydroalcoholic fluid extract and the crude ethanolic extract of Morus nigra leaves. Accordingly, it was possible to observe the base formulation and the extract with greater compatibility, leading to the choice of the O/W emulsion of the anionic type incorporated into the 70% crude hydroalcoholic extract (MnCE).
The centrifugation of the formulations allowed us to verify the stability of the emulsified systems while preserving the homogeneous aspects. At time-zero, all the emulsions showed macroscopic stability, with a bright and homogeneous appearance. The aspect of the base (F1) was a white emulsion with a glossy color, while the emulsions added with the vegetal extract initially presented with a homogeneous appearance and the odor of the presence of extract. The formulation containing the extract at a concentration of 10% (FMn 10%—F2) presented with a moss-green color, and the extract at a concentration of 20% (FMn 20%—F3) showed higher viscosity and dark green coloration due to the greater amount of crude extract (
Figure 3). By the means of initial evaluations of antioxidant activity, it was verified that the FMn 10% emulsion presented with a very low antioxidant activity and was thus excluded from the study, yet the FMn 10% emulsion was maintained in some analyses for the sole purpose of comparison.
The emulsions remained unchanged during the study period at a temperature of 20 ± 2 °C, but the FMn F20% stored at 37 ± 2 °C showed signs of phase separation after 40 days, and the initial color and odor were preserved. At the end of the study, there was no intensification of this aspect, and the formulation still presented a homogeneous appearance, but without gloss.
Figure 4 displays the variations of the pH, viscosity, and spreadability of the emulsions (FMn 10% and FMn 20%) during the study period. The pH analysis was performed at 0, 30, 60, and 90 days (
Figure 4a) with no significant variations affecting the overall emulsion quality. The values observed were around 6.0, indicating biocompatibility with the skin, being potentially non-irritating, considering the pH gradient ranges from approximately 4 to 6 on the skin’s surface [
25]. The pH of the base ranged from 5.74 to 6.34, representing a 4.32% variation, whereas the formulation of 10% crude extract presented a pH between 5.77 and 6.12, with a coefficient of variation of 2.51%. Finally, the formulation with crude extract to 20% obtained pH values between 5.80 and 6.15, with a variation of 2.72% over the course of the study (90 days).
The viscosity analysis of the topical formulations (
Figure 4b), performed at the beginning and at the end of the study, did not show significant changes over the time. The tendency to return to the initial viscosity is the expected behavior for this type of fluid and, as expected, the introduction of the crude vegetable extract reduced the emulsion’s fluidity.
The evaluation of the spreadability of the formulations (
Figure 4c) showed differences between the storage conditions, but the time factor did not change significantly (
p < 0.05). Larger spreading was observed in the base, with the inclusion of the crude extract being a factor that significantly reduced this variable, as expected. Higher statistical differences were observed between samples stored at 4 °C (refrigerator) at the final time (90 days) in comparison with the topical base and oven conditions. The spreadability of the sample stored at room temperature did not present a significant statistical difference, an important aspect to be verified and corroborated so as to facilitate the storage and use of the product by the consumers.
The evaluation of the antioxidant activity was performed through the DPPH test, a free radical that, through interaction with antioxidant substances, can be ‘neutralized’ [
26]. Thus, after the incorporation of the plant extract, the formulations were monitored as a function of time (0, 30, 60, and 90 days) and storage conditions and compared to a positive control (a cream containing a high concentration of vitamin C). The results are presented in
Figure 5. It was observed that the temperature factor did not initially interfere with the antioxidant activity of the formulations. However, at the end of the experiment, corresponding to the 90th day, a significant decrease in the antioxidant activity of the sample stored in the refrigerator was observed.
The samples had an initial IC50 of 528.50 μg/mL, and at the end of the study (90 days), the samples presented a value of 618.31 μg/mL at room temperature (20 °C) and 627.40 μg/mL for the sample stored in the oven (37 °C), compared to the positive control that exhibited an IC50 of 73.60 μg/mL. Nonetheless, it is important to emphasize that the control adopted has a high content of ascorbic acid in the pure and soluble form in association with vitamin E, which potentiates the antioxidant action. It is also important to underline that, during the 90 days, no significant changes were observed in the antioxidant activity, except for the sample in the refrigerator at 90 days of storage.
It is important to highlight that the vehicle used (the base emulsion) did not present antioxidant activity, even though it presented a small amount of antioxidant in its formulation (0.05% BHT). Therefore, it is observed that the activity presented was due to the presence of the extract as well as its assets, thus showing a potential to be commercially explored, mainly considering the maintenance of the activity throughout the stability study, and noting that it was not affected by exposure to high temperatures. As a matter of fact, the use of extracts with higher levels of rutin and isoquercetin or in isolation to reduce the effects of excipient interaction, as well as the association of phenolic compounds with other vitamins, are alternatives for raising the antioxidant power of phytocosmetic formulations containing M. nigra leaf extract.
Regarding the evaluation of the antimicrobial activity, it is depicted in
Table 2 that the crude hydroalcoholic extract of
Morus nigra possesses activity against the strains of
Staphylococcus aureus, methicillin-resistant
Staphylococcus aureus (MRSA), and
Salmonella choleraesuis, and the extract presented better inhibitory activity against the three microorganisms. It is observed that the base emulsion also showed the ability to inhibit the growth of microorganisms, but only at higher amounts. Regarding the bactericidal concentration, the best activity of the extract, as well as that of the formulations, especially regarding the microorganisms MRSA and
Salmonella choleraesuis, is noticeable.
The findings of this study present some differences from research that also evaluated the antimicrobial activity of crude ethanolic extract and fractions of leaves of
Morus nigra. Bactericidal activity of the crude ethanol extract was observed against
B. cereus and
E. faecalis, as well as against
E. coli. Inhibitory activity against
Staphylococcus aureus and
Salmonella choleraesuis was achieved at higher concentrations (12.50 mg/mL for both microorganisms). In the same work, the hexane fraction was also effective against the growth of
B. cereus and
E. faecalis, further inhibiting the growth of
E. coli,
S. choleraesuis, and
S. marcescens at concentrations of 3.12 mg/mL [
10]. Tahir et al. [
27] reported antibacterial activities against four dental-caries-causing bacterial strains:
Streptococcus mutans, Escherichia coli, Staphylococcus aureus, and
Bacillus subtilis in the ethyl acetate fraction and against
Pseudomonas aeruginosa and
B. subtilis of the chloroform fraction of
M. nigra leaves.
Such differences in the findings may occur due to different variations in the performance of the tests, such as different sizes of bacterial inoculum, volume and type of broth or agar, well size, incubation period, among other factors. Thus, standardization through guidelines is the way to reduce the number of conflicts [
28].
Historically, flavonoids have been used in preparations for the treatment of several diseases, including infectious ones. In this sense, several studies have been developed to prove the antimicrobial activity of plant extracts, such as propolis, in addition to research with specific flavonoids, such as apigenin [
29], naringenin, quercetin, 3-
O-methylquercetin, and various quercetin glycosides [
30], for example.
The results of the challenge test of the formulations are presented in
Table 3, expressed in CFU/mL. It is observed that the high microbial load in the samples at time-zero is a fact that occurs due to intentional contamination and the short period of contact with the preservative of the formulation. From the seventh day on, there was a reduction/absence of viable colonies, a fact that was repeated in the tests at 14 and 28 days, thus indicating that the preservative system was able to inactivate the growth of all the microorganisms, obeying the established parameters for the test.