Abstract
Para-phenylenediamine (PPD) is a chemical that is widely used in hair dyes. Multiple safety and regulatory agencies have categorized PPD as a potent sensitizer. In addition, PPD has carcinogenicity and genotoxicity attributes and, consequently, it is regulated at a maximal concentration of 2.0%. The aim of this study was to test whether the limit for PPD is surplus, and hence whether the consumer may be exposed to unnecessarily PPD levels. Experimentally, the analysis of PPD was performed using HPLC, where method validation and an inter-laboratory comparison test (ILC) were conducted to evaluate method performance. Thirty-three commercial products were analyzed, and five products were chosen to study the unconsumed PPD. Successfully, the implemented method confirmed its suitability and validity for the determination of PPD. For ILC results, PPD levels were 0.97 ± 0.04% and 0.92 ± 0.02%, quantified by our laboratory and an accredited laboratory, respectively. For all products, the initial concentration (T0) of PPD was lower than the regulatory limit. After 45 min, the content of PPD significantly reduced compared to T0. One product showed unconsumed PPD to be as high as 96% following the recommended dyeing time. In conclusion, the existence of high levels of unreacted PPD increases the likelihood of allergic events and elevates the risk of PPD-related chemicals. Collaborative efforts between industries, regulatory bodies, and health-related decision makers are deemed necessary to establish safe concentrations for PPD.
1. Introduction
Hair is a skin derivative representing definite characteristics of the human body. Structurally, the root and the shaft are the two main components of human body hair. Specifically, the shaft elements are organized into three layers: (1) the cuticle, which is the outermost protective part consisting of keratinized cells; (2) the cortex, which is the middle part forming the main bulk and containing the natural hair color pigments; (3) and medulla, which is the innermost layer [1,2]. Several factors can contribute to relative changes in hair texture or color, including diet, genes, environment, and hair dyes [3].
Hair dye products have become available in markets for various cosmetic purposes, such as covering gray hair, substituting hair color, and potentiating its natural color retention and longevity. These factors have accelerated a high demand for hair color products, concomitant with population aging [4,5]. As a result, the hair dye market has developed over the years into a multibillion-dollar industry, with permanent hair dyes representing the majority of its income (ca 70%). The popularity of permanent hair dyes arises from their desirable long-term effects and ease of application [5].
Hair dyes are divided into the following categories according to their origin: (1) vegetable hair dyes of which henna is the most widely used; (2) mineral hair dyes (silver nitrate or lead salts) requiring progressive usage and color treatments to reach the desired color lasting for weeks; and (3) synthetic hair dyes. Synthetic hair dyes are further classified, based on their permanence degree, into four types. (1) Temporary hair dyes, which last for a few days due to their high molecular weight and deposition on the cuticle surface. (2) Semi-permanent hair dyes that last for weeks due to their low molecular weight and shallow cortical penetration. (3) Permanent dyes, for which the coloring persists permanently due to their low molecular weight and deep cortical penetration. (4) Hair-bleaching dyes that oxidize melanin in the hair cortex in order to lighten the shade of the natural hair prior to hair coloring [6,7].
Mechanistically, permanent hair dyes normally constitute non-colored active ingredients that are subsequently oxidized to form the desired color. Accordingly, the term oxidative hair dye emerged [1,6]. There are two major components within oxidative hair dye formulations, i.e., the precursor part and coupler part. When mixed with hydrogen peroxide (developer), transient quinonediimine intermediates are formed. Consequently, these chemicals react with the coupler agent to generate the desired hair dye molecule. Of note, the entire dyeing procedure necessitates an oxidizing agent (frequently hydrogen peroxide) and an alkaline medium to ensure wide penetration of the staining constituents to the cuticle. Therefore, the oxidative dye products are originally packed into two separate bottles. One bottle contains the precursor and the couplers, whereas the other contains the developer [4,6].
During the past fifty years, p-phenylenediamine (PPD) has frequently been used as a primary precursor in the process of manufacturing oxidative hair dye products. PPD has the ability to provide a wide range of colors, hence gaining popularity among manufacturers [8]. Despite that, the safety of PPD is questionable as many health risks are associated with the use of PPD in both short and long-term applications. For example, there is accumulating evidence that PPD is associated with multiple cases of dermatitis and allergies [9,10,11,12,13,14]. In addition, PPD is considered a potent skin sensitizer, and thus a patch test is recommended before using any hair dyes containing PPD.
Furthermore, PPD may cause cross-allergic reactions to other chemicals [15]. Importantly, several experiments have shown a significant association between the use of products containing PPD and mutagenicity, according to the Scientific Committee on Consumer Products (SCCP) [10]. Owing to PPD risks, some countries such as Germany, France, and Sweden have banned the use of PPD during the twentieth century [11,12,13]. Alternatively, other regulatory agencies have set stringent levels for PPD in cosmetic products. For example, the European Union Cosmetic Directive Regulation has banned PPD in topical products that are intended for superficial purposes while allowing PPD inclusion in cosmetic products marketed as oxidizing coloring agents with a maximum concentration of 4% (free base). Specifically, the permitted concentration is 2% (free base) when mixing PPD with hydrogen peroxide following the preparation protocol of hair dyeing products [4,5,6]. On the contrary, several research studies have demonstrated violations of these limits. For example, a report showed that PPD was detected in 76 out of 115 products with a concentration range of 2.2 to 3.4%, rendering these products incompliant [11].
The well-documented evidence of PPD-related risk in cosmetics products raises alarm about the safety of this chemical. Recent studies have focused on designing new PPD alternatives to evade the health concerns associated with PPD usage [16,17]. A safe limit for PPD in hair dye products that can provide desirable coloring with minimum body exposure to PPD needs to be carefully defined. In this study, we aimed to test whether the current limit for PPD in hair dye products (i.e., 2%) is beyond a sufficient coloring need by measuring the PPD after mixing the ingredients of commercial color dyes. Using a validated HPLC method, we measured the unreacted amount of PPD at different time intervals to evaluate the potential of the current PPD limit to be overestimated. The output of the current study can potentially contribute to the regulatory science about the limits and regulations regarding PPD in cosmetics in general and, in particular, hair dyes.
2. Materials and Methods
2.1. Chemicals
Para-phenylenediamine (PPD) with CAS number (106-50-3), 99%, was purchased from Sigma-Aldrich (St. Louis, MO, USA). Potassium dihydrogen phosphate, sodium dihydrogen phosphate, sodium salt of 1-heptane sulfonic acid, sodium ascorbate, formic acid, acetonitrile (Lichrosolv, HPLC grade), and phosphoric acid were all obtained from Merck KGaA (Darmstadt, Germany).
2.2. Instrumentation and Chromatographic Conditions
An HPLC system (Shimadzu, Japan) equipped with dual LC-20AD pumps, a DGU-20A5R degasser, an SPD-M20A diode array detector, an SIL-20AC auto-sampler, and a CBM-20A system controller was used. Data were collected using LC-Solution software. The analytical method was adapted from previous work with minor modifications [18]. Briefly, the mobile phase was composed of A: 0.25 M phosphate buffer containing 0.1% sodium salt of 1-heptane sulfonic acid at pH of 6.0; and B: acetonitrile. Gradient elution run was carried out on a Supelco® (Bellefonte, PA, USA) with RP-amide C-16 column, 4.6 × 250 nm, particle size 5 mm or equivalent with a C-18 security-guard column at a flow rate of 0.8 mL/min. The gradient run was programmed as follows: 0.0–10.0 min linear gradient of 10% B; 10.0–20.0 min of 10 to 50% B; 20.0–30.0 min of 50 to 80% B; 30.0–55.0 min of 80 to 10% B for equilibration of the column with the initial conditions. The total run time was 55 min. The detection wavelength was set at 250 nm, and the injection volume was 20 μL.
2.3. Preparation of Standard Stock Solutions and Calibration Solutions
A stock solution of PPD was prepared by dissolving the appropriate amounts (50 mg) in 10 mL of 1% formic acid and 40 mL of 0.25 M phosphate buffer (pH 6.0) containing 0.1% 1-heptane sulfonic acid sodium salt and 0.05% sodium ascorbate/acetonitrile (90/10). Calibration curves were prepared by diluting stock solutions of PPD containing 20–200 µg/mL.
2.4. Sample Extraction
Approximately 0.5 g of color cream was mixed with about 0.5 g of peroxide developer with 10 mL of 1% formic acid and 40 mL of 0.25 M phosphate buffer (pH 6.0) containing 0.1% 1-heptane sulfonic acid sodium salt and 0.05% sodium ascorbate/acetonitrile (90/10), in a bottle with a screw cap. The mixture was shaken thoroughly and placed in an ultrasonic bath for 10 min. After that, the mixture was cooled to room temperature and then filtered through a 0.45 µm PTFE membrane filter. Finally, the filtrate (20 µL) was analyzed using HPLC.
2.5. Validation
The method was validated according to the Compendium of International Analysis of Methods-OIV Guide for validation and quality control [19,20]. This validation approach involves assessing the limit of detection (LOD), the limit of quantification (LOQ), linearity, selectivity, precision, and accuracy.
2.5.1. Linearity
Linearity was assessed by estimating several error values linked to the calibration during the experiment. A statistical test was then performed based on these results, making it possible to test the assumption of non-validity of the linear dynamic range using the Fischer–Snedecor test. The calculated experimental value Fobs was compared with the limit value, F1-α (n-2,np-n), extracted from the Snedecor law table. If Fobs ≥ F1-α, the assumption of the non-validity of the linear dynamic range was accepted. If Fobs < F1-α, the assumption of the non-validity of the linear dynamic range was rejected.
2.5.2. Selectivity
From linearity data, the parameters of the regression line were used to estimate selectivity. These parameters were standard deviation on the slope (Sb) and standard deviation on the intercept point (Sa). The purpose was to conclude the absence of any interference and acceptable specificity. In order to satisfy these requirements, two tests were carried out, namely, the test of the assumption that slope b of the overlap line was equal to 1.0, and the test of the assumption that intercept point a was equal to zero. These assumptions were tested using a Student’s t-test, generally associated with a risk of error of 1%.
2.5.3. Detection and Quantification Limit
LOD and LOQ were determined by calculating the standard deviation s’0 of ten replicate measurements of test samples with low concentrations of PPD [21].
2.5.4. Precision
From linearity data, the parameters for the regression line were used to estimate precision, and the repeatability was compared with the data reported by the reference method [18], using the Fischer–Snedecor test.
The standard deviation of the total variability (Sx) was calculated by the general formula:
where:
- Var (): variance of the mean of repeated replicas of all test materials.
- Var (repeata): variance of the repeatability of all the repetitions.
- k: repetitions.
The ratio was calculated using the following formula:
Here, S2ref is the precision for the reference method (5%).
Repeatability was calculated with one repetition on p test materials for the method (5 commercial products) and q test materials for the reference method (2 commercial products). The Fischer variable had a degree of freedom ν1 = p and ν2 = Q, i.e., F (p, Q, 1-α); therefore, the test could be interpreted as:
If Fobs > F1-α, then the repeatability value of the alternative method was significantly higher than that of the reference value, or:
If Fobs < F1-α, then we could not state that the repeatability value of the alternative method was significantly higher than that of the reference method.
In addition, the precision was further evaluated by participation in an inter-laboratory comparison test (ILC). The main criterion for selecting the comparable laboratory was its compliance with ISO/IEC 17025 in testing PPD within the same matrix. The participant laboratory was supplied with the analytical procedure, sample preparation, and the sequence of injections. We used the same test sample, and the PPD concentration was calculated as mean ± SD (n = 3).
2.5.5. Accuracy
The accuracy of the procedure was demonstrated by a recovery study which was carried out by measuring test samples, PPD-unspiked and PPD-spiked (10 replicates), at a concentration level corresponding to 100 µg/mL.
2.6. Method Performance Assessment Using Commercial Products
Commercially marketed samples (n = 33) labeled to contain PPD among their ingredients were used for this purpose. The products were marketed for personal use and contained different combinations of precursors and couplers. Products were chosen based on different intensities of color, reflecting a broad range of PPD concentrations.
2.7. Determination of Unconsumed PPD
Approximately 0.5 g of color gel was mixed with approximately 0.5 g of peroxide developer in a screw-capped bottle, and the mixture was incubated at 30 °C for 15, 30, and 45 min. Unincubated (T0) mixtures of 0.5 g of color gel and 0.5 g of the developer of each sample served as blanks (initial amount of PPD). After each specified incubation period, the oxidative reaction of precursor and coupler was quenched by acidifying the mixtures with 10 mL of 1% aqueous formic acid. Then, 40 mL of 0.25 M phosphate buffer (pH 6.0) containing 0.1% 1-heptane sulfonic acid sodium salt and 0.05% sodium ascorbate/acetonitrile (90/10) were added, followed by sonication for 10 min. The mixture was then filtered through a 0.45 µm filter. All experiments were performed in triplicate.
2.8. Statistical Analysis
Data for unconsumed PPD was analyzed using GraphPad Prism software (GraphPad Software Inc., San Diego CA, USA). One-way analysis of variance (ANOVA) followed by Tukey’s post hoc test was used to determine the significance between the different incubation times for each product. The difference was considered significant when p < 0.05.
3. Results
3.1. Method Optimization and Validation
3.1.1. Linearity
The linearity of PPD of the implemented method was statistically assessed by evaluating the generated results (Table 1). These errors are given as a standard deviation, resulting from the square root of the ratio between a sum of squares and a degree of freedom [19]. The linear regression analysis data are summarized in Table 2.

Table 1.
Calibration data in terms of PPD concentrations (µg/mL).

Table 2.
Linear regression parameters of PPD in standard solution.
The calculated experimental value Fobs is 0.557, less than the limit value F1-α (n-2,np-n) extracted from the Snedecor law table. The results showed excellent correlation, and the assumption of the non-validity of the linear dynamic range was rejected.
3.1.2. Selectivity
The selectivity of a method is expressed by its ability to only measure the PPD in oxidative hair dyes matrixes under testing (Figure 1). The selectivity data analysis is summarized in Table 3. The required conditions are that Tobs (0.18) should be lower than T critical and the slope of the regression line should be equivalent to 1. The other is that Tobs (0.50) should be lower than T critical and the intercept point of the regression line should be equivalent to zero. Both required conditions were met, and so the method was deemed to be specific [19].
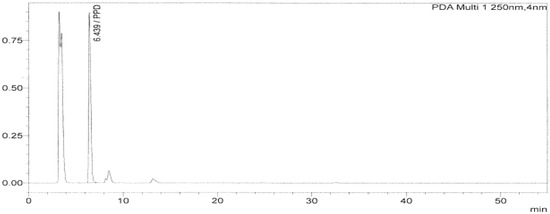
Figure 1.
HPLC chromatogram of a hair dye sample showing the peak of PPD at 6.4 min.

Table 3.
Summary for the selectivity data.
3.1.3. Detection and Quantification Limit
The limit of detection (LOD) is the lowest concentration of the measurement that can be detected at a specified level of confidence. The lowest concentration at which the performance of a method or measurement system is acceptable for a specified use is represented by the quantification limit (LOQ) [21]. For this study, the LOD and LOQ of PPD were 5.43 and 18.12 µg/mL, respectively.
3.1.4. Precision
The precision analysis data are summarized in Table 4. By using the Fischer–Snedecor test, the Fobs was less than F1-α, so we cannot state that the repeatability value of the PPD method was significantly higher than that of the reference method [19]. For the inter-laboratory comparison test (ILC), the results for PPD concentrations by our laboratory and the accredited laboratory were 0.97 % ± 0.04 and 0.92 % ± 0.02, respectively.

Table 4.
Summary for the precision data of PPD in hair dye samples.
3.1.5. Accuracy
The recoveries of PPD were assessed by analyzing a sample that was PPD-free, spiked at 100 µg/mL. Based on the investigated calibration range of the PPD, we found that the calculated recoveries ranged between 95.75 and 103.73% (Table 5).

Table 5.
Accuracy (recovery) data.
3.2. Method Performance
After successful application of the implemented validation parameters, the method was used to quantify PPD in real hair dyes samples. The results for PPD analysis are presented in Table 6. All samples were in accordance with the regulatory limit for PPD (2%).

Table 6.
Contents (% w/w) of PPD identified in the investigated hair dye products. ND: not detected.
3.3. Determination of Unconsumed PPD
The contents of PPD in five dark color hair dyes ranging from brown to black were analyzed using the procedure described in the experimental section. For all formulations, the concentrations of PPD at T0 were lower than the regulatory limit of 2% (Table 7). After 45 min from starting the oxidation reaction, the content of PPD significantly reduced compared to the initial amount at T0 (Figure 2). A minimum of 60% of the initial amount of PPD remained unreacted in the color formulations after the color development. Particularly, 76.92% and 74.30% of PPD remained unconsumed in the mixtures of sample numbers 29 and 32, respectively.

Table 7.
Contents of unreacted PPD % w/w in oxidative hair dye formulations.
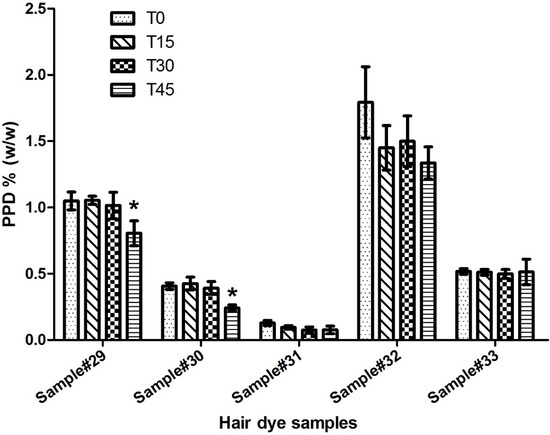
Figure 2.
Comparison of unreacted PPD in hair dye samples after different incubation times. Results are expressed as mean ± S.D. of three measurements. * p < 0.05 compared to T0.
4. Discussion
The risk associated with the use of PPD has motivated scientists to develop multiple analytical techniques to detect and quantify this chemical in cosmetic products to ensure their safety. Furthermore, because hair dyeing is based on oxidative chemical reactions, one can expect a variety of chemicals and intermediates with structural similarities, making their detection cumbersome. Consequently, several chromatographic methods have been reported for the analysis of the intermediates of oxidative hair dyes [18,22,23,24]. Some of these methods may be suitable only for checking the purity of the raw materials, while others may also be used for the analysis of intermediates of hair dyes in cosmetic formulations. ICP/MS, HPLC/MS, and GC-MS are reported analytical methods used to separate and determine the hair dye’s PPD intermediates [4]. However, these methods are costly, time-consuming, and some of them involve complicated procedures for sample preparation, such as extraction and chemical derivatization [22]. Because of that, a convenient and relatively easy-to-use technique is demanded in order to be used for routine analysis, and hence the advantage for the HPLC technique becomes visible. Practically, reported HPLC methods for the analysis of PPD in hair dye products suffer from major problems related to the quality of the chromatographic peak. Severe tailing, asymmetric chromatographic peak, and the inherent instability of PPD in hair dyes are common issues associated with HPLC analysis for any PPD-contained products. Fortunately, reversed-phase ion-pairing liquid chromatography has proven to counteract those issues with chromatographic separation [24]. In the current study, we used the ion-pairing technique in which we adapted the previous method with minor modifications. In order to improve peak shape, enhance detection sensitivity, and obtain a high response, a specified amount of ion-pair reagent was needed. We used 1-heptane sulfonic acid sodium salt as an ion-pair reagent to minimize the peak tailing. During method optimization, the resolution between the oxidizing hair dye components of commercial products was challenging. The original method used a flow rate of 1.0 mL/min, and authors achieved satisfactory resolution between some oxidizing components, but not for others [18]. In order to overcome this problem, authors recommended decreasing the flow rate from 1.0 to 0.8 mL/min, and this is the strategy we used in the current study.
Validation for the implemented method demonstrated suitability and validity for use in determining the PPD concentration in hair dyes. Calibration curves were prepared over 20–200 µg/mL, which was lower than the range in the earlier method (40–800) µg/mL (Table 1). This range was selected to avoid the point with a high degree of leverage, which can be problematic if one or two of the calibration points are far away from others along the x-axis. Consequently, these points will have a high degree of leverage even if they are not actual outliers. In other words, a relatively small error in the measured response will have a significant effect on the position of the regression line [25]. Additionally, the obtained LOD was 5.4 µg/mL, which was near the LOD of the original method (i.e., 5 µg/mL).
Recovery studies for PPD were evaluated by selecting a sample void of any of the target compounds, spiked at one concentration level (100 µg/mL). Successfully, we obtained an excellent recovery of 99.69% (Table 5), which was higher than the one reported by the earlier method (89.3% at 104 µg/mL). Of note, when the replicate preparations were injected at 25 °C with a run time of 55 min, the recovery percentage of the PPD decreased as the time increased (data not shown). An explanation for this observation is likely attributed to the continued oxidation of PPD by reacting with the couplers present in the replicate preparations. In order to counteract this process, the oxidative reaction was quenched by acidifying the mixtures with 10 mL of 1% aqueous formic acid, and this step effectively decreased the variation between results for the recovery study.
In order to assess the performance of the implemented method, marketed products were analyzed for PPD content. A total of 33 samples were randomly selected based on their inclusion of PPD among their ingredients manufactured by different companies. It is noteworthy that the consumer instructions for the time required for developing the hair colors varied between a minimum of 15 min and a maximum of 35 min. We chose a variety of levels for color intensity because it is recognized that intense colors imply higher PPD content compared to less intense colors. Table 6 shows that dark-brown and light-brown colors were tested for PPD, and the results were in accordance with the correlation between color intensity and PPD content. Specifically, sample #32, which is marketed as a dark dye, had a PPD level of 1.79%, while sample #21, which is marketed as dark blond, had a PPD level of 0.02%. Importantly, we participated in an inter-laboratory comparison test (ILC) with an accredited laboratory to evaluate the accuracy and the performance of our implemented method. Successfully, our laboratory detected PPD in a sample by quantifying 0.97% ± 0.04 PPD, while the accredited laboratory detected 0.92% ± 0.02 PPD, using the same sample and the same methodology, with RSD < 5%. This approach provides reliance and confidence in the implemented method and ensures its sensitivity and accuracy.
Within the hair dye products, the PPD is supposedly utilized in complex oxidation processes to yield the desired color. Unreacted PPD is said to be the main source of PPD-related allergies and/or sensitizations, and thus its estimation is of high importance. As far as we are concerned, there is only one study that has assessed the levels of unreacted PPD in dyeing products. Interestingly, that study showed that two PPD-containing hair dyes had 50% and 65% remaining PPD after incubating samples at 30 °C for 30 min [10,26]. In our study, we aimed to further investigate the levels of unconsumed PPD, utilizing more samples to better assess the regulatory control of PPD limits in hair dyes. We chose five samples for this analysis owing to their different levels of PPD (and, accordingly, different levels of color intensities). We calculated the remaining PPD by comparing the initial concentrations present in the hair dye formulations (at time 0) with the concentrations of PPD at different interval times (15, 30, and 45 min), and the results are shown in Table 7. Our results are in agreement with the previous study; hence, we found a minimum of about 60% remaining PPD in sample #30, while the maximum was found in sample #29 which contained about 77% remaining PPD after 45 min of incubation. There was an apparent increase in remaining PPD at T15, but that enhancement was not statically significant (Figure 2). Surprisingly, there was no statistically significant decrease in the unconsumed PPD between T0 and either T15 or T30 for any products (Figure 2). This finding is considered a concern, particularly when the average dying time for the tested samples is less than 30 min. Apparently, only a minute amount of PPD is likely utilized while the majority is washed after the recommended dyeing time. This note may aid in explaining some cases of the observed immediate hypersensitivities to PPD-containing dyes [27]. On the contrary, at T45, two products (samples 29 and 30) did show a statistical decrease in PPD levels compared to the initial PPD level in those samples (Figure 2). When taken together, these findings clearly raise concerns about the safety of hair dyes and the potential for consumers to be exposed to underestimated hazardous sensitizers.
A noticeable difference in the unconsumed concentration of PPD between hair dye formulations may reflect the different amounts and types of precursors, couplers, and other ingredients present in the formulation. Nevertheless, the existence of such a high level of PPD after dyeing the hair is alarming, keeping in mind that some consumers might leave the dye on the hair longer than recommended by the manufacturer in order to achieve intense color. This practice will likely expose consumers to unreacted PPD and/or other sensitizing intermediates, particularly during washing and conditioning the hair [10,26]. A retrospective study assessed the relationship between PPD-containing hair dye-related allergy and the calculated exposure time to the dye. Even though the exposure time was not significantly correlated with the number of allergy events represented by skin lesions, the dyeing frequency showed a positive correlation with allergy events on direct and indirect contact areas [28]. After 30 min of dyeing with product number 33, only about 4% of the PPD is consumed, while the consumer is exposed to about 96% of unnecessary PPD during a single dyeing event, which needs regulatory assessment. Moreover, frequent dyeing events using PPD-containing hair dyes and/or related products may extravagate the estimated risk. To that end, based on the chemistry of oxidative hair dyes, using a large excess of precursors and couplers greater than what is required to provide the desired color seems unjustifiable.
Mutagenic and carcinogenic outcomes, as well as hypersensitivity events, have been reported as consequences of hair dye practices [10,11,12,14]. Based on the chemistry of oxidative hair dyes, oxidative hair dye formation requires the production of quinonediimine (QDI) from the oxidation reaction between precursors and couplers (i.e., PPD being the precursor). Since these intermediates penetrate the hair fiber, all unreacted intermediates and/or chemicals are removed during the wash-off procedure [29,30]. However, the kinetics of color formation over 45 min revealed that the level of product (quinonediimine) increases with time, while the level of reactants (precursors and couplers) decrease. Nevertheless, the oxidation reaction of hair dyeing is inefficient, and it has been reported that as much as 20% to 80% of the applied amines/couplers remain unreacted at the end of a 30-minute dyeing application [9,31]. Importantly, the finding that product #32 contained both PPD and 2,4-toluenediamine (PTD) is interesting. PPD and PTD are considered “extreme” sensitizers by some agencies as they exhibit strong sensitization reactions [32]. Furthermore, the existence of surplus PPD and other couplers may enhance the chance of forming the teratogenic intermediate Bandrowski’s base, which is a trimer of PPD. Consequently, setting a minimum yet sufficient limit for PPD in color formation products is of great importance. Regulatory re-evaluation to amend PPD limits to control the unreacted PPD may be one way to prevent disorders such as allergy and dermatitis.
Despite the thorough analytical procedures performed in the current work, a few limitations should be considered. For the study of unreacted PPD, different commercial products with different ingredients were exposed to the same hydrogen peroxide quantity. The competition for oxidation reaction by hydrogen peroxide may vary between products depending on their ingredients. In addition, autoxidation and side reactions are common pathways in hair dye products, which may be reflected in the detected levels of the unreacted PPD. Nevertheless, the utilized analytical method demonstrated its accuracy in detecting PPD with a high level of certainty. Another limitation is the use of hair dye products instead of actual hair samples to measure the unconsumed PPD, which may not represent the actual dyeing behavior within the human scalp. Nevertheless, a collaborative work by five laboratories has estimated that a maximum of 32% and 30% of unconsumed PPD were observed within dye formulations and hair samples, respectively [10]. This relevant correlation clearly supports the fact that the methodology used in the current study is reasonable. To that end, as the data for unconsumed PPD is scarce in the literature, additional studies are favored to extrapolate the current findings.
5. Conclusions
A method for PPD quantification was successfully implemented and validated, circumventing the severe tailing and asymmetric chromatographic peak of PPD by using reversed-phase ion-pairing liquid chromatography. All tested samples complied with the current regulatory limits of PPD. The analysis of unreacted PPD revealed as much as 77% prevalence following extended dyeing time longer than what is recommended by the manufacturer. Eventually, higher PPD levels would likely be observed after strictly applying the manufacturers’ recommended dyeing times. Keeping in mind the health risks associated with PPD, as well as the carcinogenic potential of PPD-related chemicals, unconsumed PPD needs to be further explored and evaluated by regulatory bodies. Ideally, consumers are expected to be exposed to minimum levels of hazardous chemicals in hair dyes while obtaining the desired hair color. Collaborative efforts between industry, regulatory bodies, and health-related decision makers are deemed necessary to establish safe concentrations of sensitizing chemicals in hair dyes, particularly PPD.
Author Contributions
M.H.A.-E., conceptualization, formal analysis, methodology, validation, writing—original draft preparation; F.S.A., visualization, resources, supervision, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
We would like to thank the staff at the cosmetic section in the National Drug and Cosmetic Control Laboratory (NDCCL) of SFDA for their support in coordinating the work on the analytical instruments as well as granting access to other laboratory equipment.
Conflicts of Interest
The authors declare no conflict of interest.
Disclaimer
The views expressed in this paper are those of the authors and do not necessarily reflect those of the SFDA or its stakeholders. Guaranteeing the accuracy and the validity of data is a sole responsibility of research team.
References
- Guerra-Tapia, A.; Gonzalez-Guerra, E. Hair cosmetics: Dyes. Actas Dermosifiliogr. 2014, 105, 833–839. [Google Scholar] [CrossRef]
- Gavazzoni Dias, M.F. Hair cosmetics: An overview. Int. J. Trichol. 2015, 7, 2–15. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.B.; Shamim, H.; Nagaraju, U. Premature Graying of Hair: Review with Updates. Int. J. Trichol. 2018, 10, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Chisvert, A.; Miralles, P.; Salvador, A. Chapter 8—Hair Dyes in Cosmetics: Regulatory Aspects and Analytical Methods. In Analysis of Cosmetic Products, 2nd ed.; Salvador, A., Chisvert, A., Eds.; Elsevier: Boston, MA, USA, 2018; pp. 159–173. [Google Scholar]
- Morel, O.J.X.; Christie, R.M. Current Trends in the Chemistry of Permanent Hair Dyeing. Chem. Rev. 2011, 111, 2537–2561. [Google Scholar] [CrossRef] [PubMed]
- Da França, S.A.; Dario, M.F.; Esteves, V.B.; Baby, A.R.; Velasco, M.V.R. Types of Hair Dye and Their Mechanisms of Action. Cosmetics 2015, 2, 110–126. [Google Scholar] [CrossRef] [Green Version]
- Harrison, S.; Sinclair, R. Hair colouring, permanent styling and hair structure. J. Cosmet. Dermatol. 2003, 2, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Ezendam, J.; Salverda-Nijhof, J.G.W. Hair Dye Allergy in Consumers; RIVM Report; National Institute for Public Health and the Environment: Utrecht, The Netherlands, 2011. [Google Scholar]
- Scientific Committee on Consumer Products (SCCP). Skin Penetration of Oxidative Hair Dyes Formed by the Coupling of Precursors and Couplers under Simulated Conditions of Hair Dyeing; European Commission, Health & Consumer Protection Directorate-General: Luxembourg, 2006. [Google Scholar]
- Scientific Committee on Consumer Products (SCCP). Opinion on Exposure to Reactants and Reaction Products of Oxidative Hair Dye Formulations; European Commission, Health & Consumer Protection Directorate-General: Luxembourg, 2005. [Google Scholar]
- Chong, H.P.; Reena, K.; Ng, K.Y.; Koh, R.Y.; Ch, N.; Chye, S.M. Para-Phenylenediamine Containing Hair Dye: An Overview of Mutagenicity, Carcinogenicity and Toxicity. J. Environ. Anal. Toxicol. 2016, 6, 5. [Google Scholar]
- Shakoor, Z.; Al-Mutairi, A.S.; Al-Shenaifi, A.M.; Al-Abdulsalam, A.M.; Al-Shirah, B.Z.; Al-Harbi, S.A. Screening for skin-sensitizing allergens among patients with clinically suspected allergic contact dermatitis. Saudi Med. J. 2017, 38, 922–927. [Google Scholar] [CrossRef]
- Almogren, A.; Shakoor, Z.; Rab, M.O.G.; Adam, M.H. Pattern of patch test reactivity among patients with clinical diagnosis of contact dermatitis: A hospital-based study. Ann. Saudi Med. 2012, 32, 404–407. [Google Scholar] [CrossRef]
- Kim, K.-H.; Kabir, E.; Jahan, S.A. The use of personal hair dye and its implications for human health. Environ. Int. 2016, 89–90, 222–227. [Google Scholar] [CrossRef]
- Basketter, D.A.; English, J. Cross-reactions among hair dye allergens. Cutan. Ocul. Toxicol. 2009, 28, 104–106. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, G.; Dancik, Y.; Sinha, A.; Kyaw, H.M.; Srinivas, R.; Dawson, T.L., Jr.; Bigliardi, M.; Bigliardi, P.; Pastorin, G. Development of novel alternative hair dyes to hazardous para-phenylenediamine. J. Hazard. Mater. 2021, 402, 123712. [Google Scholar] [CrossRef] [PubMed]
- Goebel, C.; Troutman, J.; Hennen, J.; Rothe, H.; Schlatter, H.; Gerberick, G.F.; Blomeke, B. Introduction of a methoxymethyl side chain into p-phenylenediamine attenuates its sensitizing potency and reduces the risk of allergy induction. Toxicol. Appl. Pharmacol. 2014, 274, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, S.C. A method for the analysis of intermediates of oxidative hair dyes in cosmetic products. J. Sep. Sci. 2001, 24, 173–178. [Google Scholar] [CrossRef]
- Compendium of International Analysis of Methods—OIV. Practical Guide for the Validation, Quality Control, and Uncertainty Assessment of an Alternative Oenological Analysis Method; Resolution 10/2005; OIV: Paris, France, 2005.
- U.S. Pharmacopoeia. <1225> Validation of Compendial Procedures and <1210> Statistical Tools for Procedure Validation; U.S. Pharmacopeia National Formulary 2017: USP 40 NF 35; U.S. Pharmacopoeia: North Bethesda, MA, USA, 2017. [Google Scholar]
- Magnusson, B.; Örnemark, U. (Eds.) Eurachem Guide: The Fitness for Purpose of Analytical Methods—A Laboratory Guide to Method Validation and Related Topics, 2nd ed.; Eurachem: Windsor, UK, 2014. [Google Scholar]
- Ahmed, R.M.H.; Latif, F.; El-Dean, A.; El-Shaieb, K.; Vilanova, E.; Estevan, C. Different Analytical Methods of Para-Phenylenediamine Based Hair Dye. J. Cosmet. Dermatol. Sci. Appl. 2013, 3, 17–25. [Google Scholar] [CrossRef] [Green Version]
- Ko, H.Y.; Lin, Y.H.; Shih, C.J.; Chen, Y.L. Determination of phenylenediamines in hair colors derivatizated with 5-(4,6-dichlorotriazinyl)aminofluorescein via micellar electrokinetic chromatography. J. Food Drug Anal. 2019, 27, 825–831. [Google Scholar] [CrossRef] [Green Version]
- Santa Bárbara, M.C.; Yano, H.M.; Fernandes Farias, F.; Ortiz Gasparin, L.F.; Vieira, E.A.; Trujillo, L.M.; Pereira Martins, V.A.; Miyamaru, L.L.; Ortega Markman, B.E. Optimization and intralaboratorial validation of an analytical method by HPLC/UV for identification and quantification of p-phenylenediamine in henna dye hair and eyebrows. Vigil. Sanit. Debate 2019, 7, 62–68. [Google Scholar]
- Barwick, V. Preparation of Calibration Curves: A Guide to Best Practice; Report No. LGC/VAM/2003/032; LGC: Teddington, UK, 2003. [Google Scholar]
- Rastogi, S.C.; Sosted, H.; Johansen, J.D.; Menne, T.; Bossi, R. Unconsumed precursors and couplers after formation of oxidative hair dyes. Contact Dermat. 2006, 55, 95–100. [Google Scholar] [CrossRef]
- Wilkinson, M.; Solman, L.; Coenraads, P.J.; Goebel, C. Immediate hypersensitivity to p-phenylenediamine. Contact Dermat. 2019, 80, 177–178. [Google Scholar] [CrossRef]
- Lee, S.E.; Lee, S.H. Skin Barrier and Calcium. Ann. Dermatol. 2018, 30, 265–275. [Google Scholar] [CrossRef]
- Alinaghi, F.; Bennike, N.H.; Egeberg, A.; Thyssen, J.P.; Johansen, J.D. Prevalence of contact allergy in the general population: A systematic review and meta-analysis. Contact Dermat. 2019, 80, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Basketter, D.A.; Jefferies, D.; Safford, B.J.; Gilmour, N.J.; Jowsey, I.R.; McFadden, J.; Chansinghakul, W.; Duangdeeden, I.; Kullavanijaya, P. The impact of exposure variables on the induction of skin sensitization. Contact Dermat. 2006, 55, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Lewis, D.; Mama, J.; Hawkes, J. A Review of Aspects of Oxidative Hair Dye Chemistry with Special Reference to N-Nitrosamine Formation. Materials 2013, 6, 517–534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirchlechner, S.; Hubner, A.; Uter, W. Survey of sensitizing components of oxidative hair dyes (retail and professional products) in Germany. J. Dtsch. Dermatol. Ges. 2016, 14, 707–715. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).