Managing Skin Ageing as a Modifiable Disorder—The Clinical Application of Nourella® Dual Approach Comprising a Nano-Encapsulated Retinoid, Retilex-A® and a Skin Proteoglycan Replacement Therapy, Vercilex®
Abstract
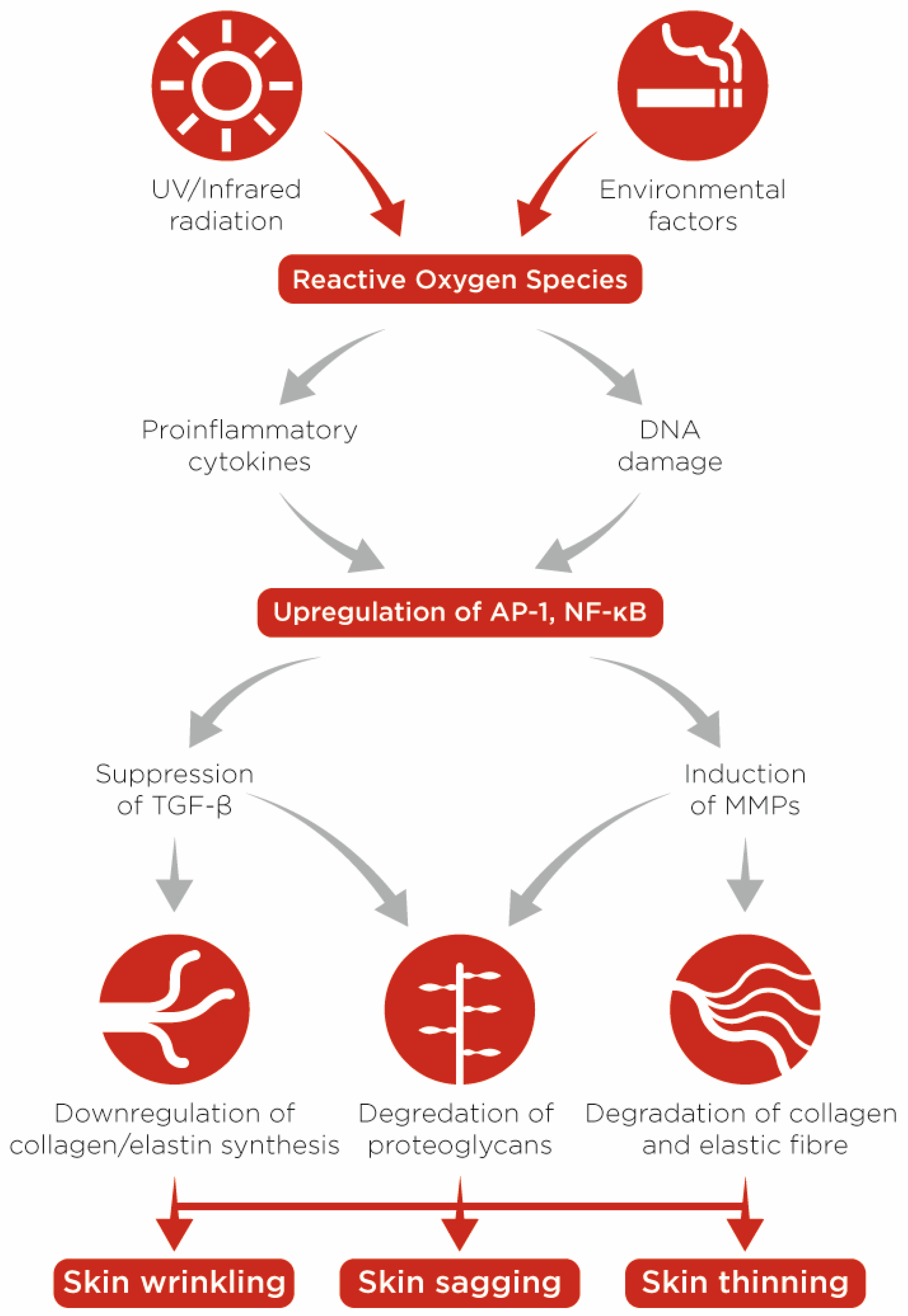
:1. Chronological (Intrinsic) and Accelerated (Extrinsic) Skin Ageing
2. Approaching Skin Ageing as a Modifiable Disorder
3. Natural Retinoids as the ‘Gold Standard’ Treatment of Skin Ageing
- A marked upturn in the proliferation rate of dermal stromal cells, particularly in the papillary dermis, compared to placebo-treated skin [22]
- Improvement of the dermoepidermal ECM microenvironment by two mechanisms. First, retinoids stimulate the activity of fibroblasts and boost the production of type-I and III collagen, fibronectin and tropoelastin in both chronologically aged and photoaged skin [21,22]; and second, these compounds can inhibit the expression of matrix metallopeptidases and thereby diminish the degradation of ECM components [26].
- Efficient anti-inflammatory effects through suppressing the release of proinflammatory cytokines and the activity of leukocytes [24]
3.1. Using Nano-Encapsulation to Enhance the Bioavailability and Safety of Retinoids
- Increasing drug availability at the skin surface. It is known that in aqueous solutions, lipophilic molecules, e.g., retinyl esters, compete for a space in carrier cavity, which forms a dynamic equilibrium between encapsulated and free drug molecules. At the surface, retinoid molecules that partition from the carrier cavity and penetrate the lipophilic skin barrier are replaced by newly partitioned active molecules (a buffering effect), such that a fresh pool of free, intact retinoid molecules is continuously available for absorption while the rest of the pool is protected from environmental factors inside the nano-capsules (a protective effect). The significant action of nano-encapsulation in producing a controlled, constant drug release profile has previously been documented [39].
- Augmenting retinoid net absorption ratio. Whether the result of improved availability or of an independent effect, nano-encapsulated retinyl ester exhibits augmented absorbability, as demonstrated by a comparative study on a model barrier system (Franz Diffusion Cell). The measurements demonstrated that retinoid molecules from Retilex-A® had a markedly higher penetration ratio into a skin model compared to a conventional, commercial formula in both water and isopropyl alcohol media [40].
- Reducing skin irritation side-effects. As explained earlier in this section, it is well established that specific forms of nano-encapsulation can alleviate the skin irritation caused by chemicals. Clinical studies with various skin irritants have verified this integral benefit of nano-encapsulation [36].
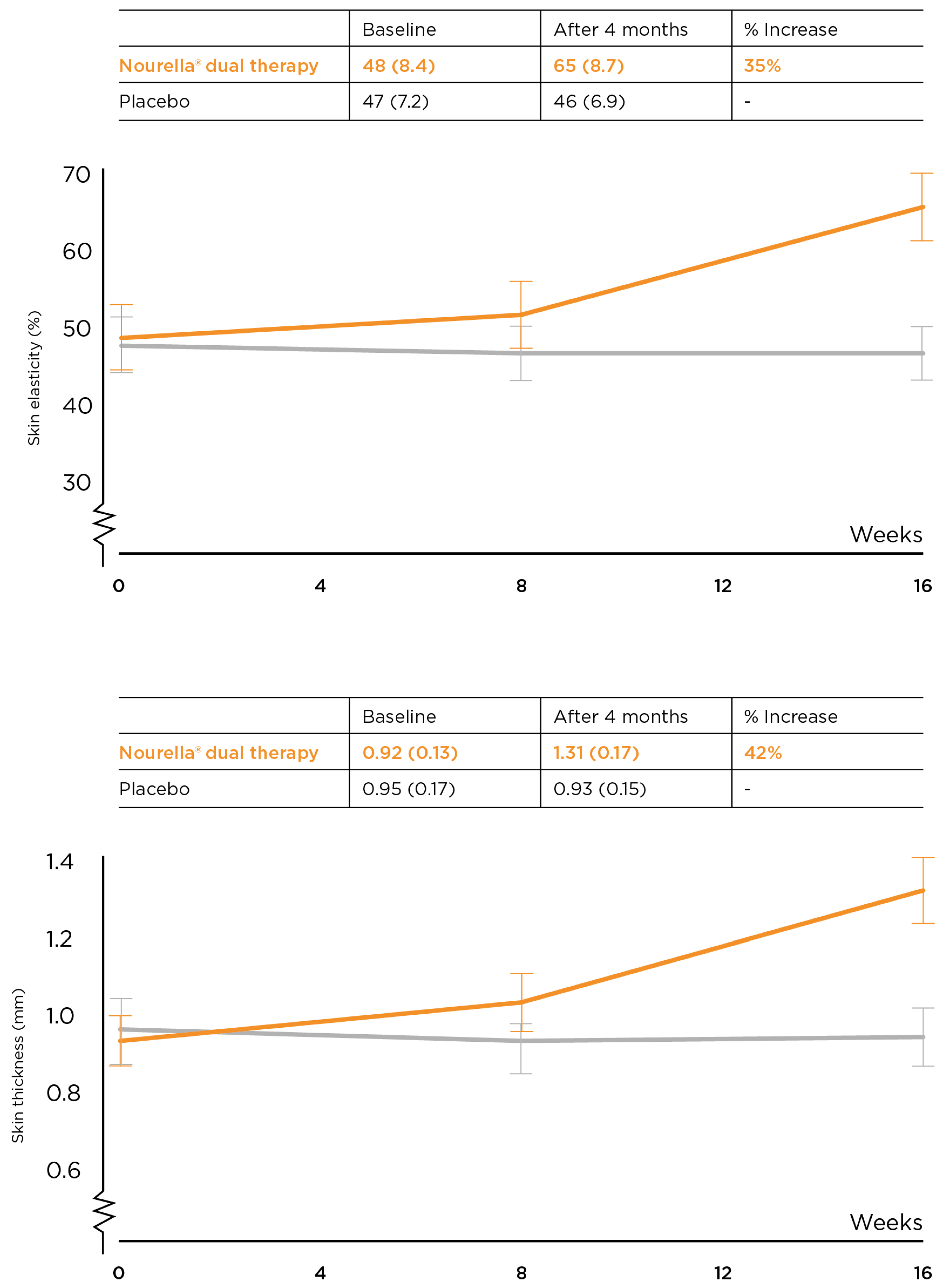
3.2. Supporting Evidence for the Anti-Ageing and Skin-Rejuvenating Efficacy of Retilex-A®
4. Proteoglycan Dysmetabolism in Ageing Skin
4.1. Proteoglycan Replacement Therapy (PRT) of Aged Skin Using Vercilex®
4.2. Potential Synergistic Anti-Aging Effects of Vercilex® with Retilex-A®
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Farage, M.A.; Miller, K.W.; Elsner, P.; Maibach, H.I. Structural Characteristics of the Aging Skin: A Review. Cutan. Ocul. Toxicol. 2007, 26, 343–357. [Google Scholar] [CrossRef] [PubMed]
- Green, A.C.; Hughes, M.C.; McBride, P.; Fourtanier, A. Factors Associated with Premature Skin Aging (Photoaging) before the Age of 55: A Population-Based Study. Dermatology 2011, 222, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Kubben, N.; Misteli, T. Shared molecular and cellular mechanisms of premature ageing and ageing-associated diseases. Nat. Rev. Mol. Cell Biol. 2017, 18, 595–609. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Duan, E. Fighting against Skin Aging: The Way from Bench to Bedside. Cell Transplant. 2018, 27, 729–738. [Google Scholar] [CrossRef]
- Kohl, E.; Steinbauer, J.; Landthaler, M.; Szeimies, R.M. Skin ageing. J. Eur. Acad. Dermatol. Venereol. 2011, 25, 873–884. [Google Scholar] [CrossRef]
- Bhawan, J.; Andersen, W.; Lee, J.; Labadie, R.; Solares, G. Photoaging versus intrinsic aging: A morphologic assessment of facial skin. J. Cutan. Pathol. 1995, 22, 154–159. [Google Scholar] [CrossRef]
- Langton, A.K.; Graham, H.K.; Griffiths, C.E.M.; Watson, R.E.B. Ageing significantly impacts the biomechanical function and structural composition of skin. Exp. Dermatol. 2019, 28, 981–984. [Google Scholar] [CrossRef] [Green Version]
- Bulterijs, S.; Hull, R.S.; Björk, V.C.E.; Roy, A.G. It is time to classify biological aging as a disease. Front. Genet. 2015, 6, 205. [Google Scholar] [CrossRef] [Green Version]
- Tsatsou, F.; Trakatelli, M.; Patsatsi, A.; Kalokasidis, K.; Sotiriadis, D. Extrinsic aging: UV-mediated skin carcinogenesis. Dermato-Endocrinology 2012, 4, 285–297. [Google Scholar] [CrossRef] [Green Version]
- Kaya, G.; Saurat, J.-H. Dermatoporosis: A Chronic Cutaneous Insufficiency/Fragility Syndrome. Clinicopathological features, mechanisms, prevention and potential treatments. Dermatology 2007, 215, 284–294. [Google Scholar] [CrossRef]
- Farage, M.A.; Miller, K.W.; Elsner, P.; Maibach, H.I. Functional and physiological characteristics of the aging skin. Aging Clin. Exp. Res. 2008, 20, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.A.; Gilchrest, B.A. Psychosocial Aspects Of Aging Skin. Dermatol. Clin. 2005, 23, 643–648. [Google Scholar] [CrossRef] [PubMed]
- Asakura, K.; Nishiwaki, Y.; Milojevic, A.; Michikawa, T.; Kikuchi, Y.; Nakano, M.; Iwasawa, S.; Hillebrand, G.; Miyamoto, K.; Ono, M.; et al. Lifestyle Factors and Visible Skin Aging in a Population of Japanese Elders. J. Epidemiol. 2009, 19, 251–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, J.-W.; Kwon, S.-H.; Choi, J.-Y.; Na, J.-I.; Huh, C.-H.; Choi, H.-R.; Park, K.-C. Molecular Mechanisms of Dermal Aging and Antiaging Approaches. Int. J. Mol. Sci. 2019, 20, 2126. [Google Scholar] [CrossRef] [Green Version]
- Baumann, L. How to Use Oral and Topical Cosmeceuticals to Prevent and Treat Skin Aging. Facial Plast. Surg. Clin. N. Am. 2018, 26, 407–413. [Google Scholar] [CrossRef]
- Baumann, L. Skin ageing and its treatment. J. Pathol. 2007, 211, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Ganceviciene, R.; Liakou, A.I.; Theodoridis, A.; Makrantonaki, E.; Zouboulis, C.C. Skin anti-aging strategies. Derm-Endocrinology 2012, 4, 308–319. [Google Scholar] [CrossRef] [Green Version]
- Kligman, L.H.; Duo, C.H.; Kligman, A.M. Topical Retinoic Acid Enhances the Repair of Ultraviolet Damaged Dermal Connective Tissue. Connect. Tissue Res. 1984, 12, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Gilman, R.H.; Buchanan, P.J.; Gilman, R.H. Retinoids: Literature review and suggested algorithm for use prior to facial resurfacing procedures. J. Cutan. Aesthetic Surg. 2016, 9, 139–144. [Google Scholar] [CrossRef]
- Lee, D.-D.; Stojadinovic, O.; Krzyzanowska, A.; Vouthounis, C.; Blumenberg, M.; Tomic-Canic, M. Retinoid-responsive transcriptional changes in epidermal keratinocytes. J. Cell. Physiol. 2009, 220, 427–439. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, S.; Date, A.; Patravale, V.; Korting, H.C.; Roeder, A.; Weindl, G. Retinoids in the treatment of skin aging: An overview of clinical efficacy and safety. Clin. Interv. Aging 2006, 1, 327–348. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; He, T.; Fisher, G.J.; Voorhees, J.J.; Quan, T. Molecular basis of retinol anti-ageing properties in naturally aged human skin in vivo. Int. J. Cosmet. Sci. 2016, 39, 56–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, Y.; Komi, Y.; Ashino, H.; Yamashita, J.; Inoue, J.; Yoshiki, A.; Eichmann, A.; Amanuma, H.; Kojima, S. Retinoic acid controls blood vessel formation by modulating endothelial and mural cell interaction via suppression of Tie2 signaling in vascular progenitor cells. Blood 2004, 104, 166–169. [Google Scholar] [CrossRef] [PubMed]
- Wolf, J.E., Jr. Potential anti-inflammatory effects of topical retinoids and retinoid analogues. Adv. Ther. 2002, 19, 109–118. [Google Scholar] [CrossRef]
- Duell, E.A.; Kang, S.; Elder, J.T.; Voorhees, J.J.; Derguini, F. Extraction of Human Epidermis Treated with Retinol Yields Retro-Retinoids in Addition to Free Retinol and Retinyl Esters. J. Investig. Dermatol. 1996, 107, 178–182. [Google Scholar] [CrossRef] [Green Version]
- Quan, T.; Qin, Z.; Shao, Y.; Xu, Y.; Voorhees, J.J.; Fisher, G.J. Retinoids suppress cysteine-rich protein 61 (CCN1), a negative regulator of collagen homeostasis, in skin equivalent cultures and aged human skin in vivo. Exp. Dermatol. 2011, 20, 572–576. [Google Scholar] [CrossRef] [Green Version]
- Lever, L.; Kumar, P.; Marks, R. Topical retinoic acid for treatment of solar damage. Br. J. Dermatol. 1990, 122, 91–98. [Google Scholar] [CrossRef]
- Weiss, J.S.; Ellis, C.N.; Headington, J.T.; Tincoff, T.; Hamilton, A.T.; Voorhees, J.J. Topical tretinoin improves photoaged skin. A double-blind vehicle-controlled study. JAMA 1988, 259, 527–532. [Google Scholar] [CrossRef]
- Ellis, C.N.; Weiss, J.S.; Hamilton, T.A.; Headington, J.T.; Zelickson, A.S.; Voorhees, J.J. Sustained improvement with prolonged topical tretinoin (retinoic acid) for photoaged skin. J. Am. Acad. Dermatol. 1990, 23 Pt 1, 629–637. [Google Scholar] [CrossRef]
- Kong, R.; Cui, Y.; Fisher, G.J.; Wang, X.; Chen, Y.; Schneider, L.M.; Majmudar, G. A comparative study of the effects of retinol and retinoic acid on histological, molecular, and clinical properties of human skin. J. Cosmet. Dermatol. 2016, 15, 49–57. [Google Scholar] [CrossRef] [Green Version]
- Thom, E.A. Comparative double-blind within subject study of the efficacy and tolerability of two different derivatives of vitamin a on skin thickness and elasticity: Retinoic acid and conjugated retinyl palmitate. J. Appl. Cosmetol. 1997, 15, 133–138. [Google Scholar]
- Roos, T.C.; Jugert, F.K.; Merk, H.F.; Bickers, D.R. Retinoid metabolism in the skin. Pharmacol. Rev. 1998, 50, 315–333. [Google Scholar]
- Lehman, P.A.; Slattery, J.T.; Franz, T.J. Percutaneous Absorption of Retinoids: Influence of Vehicle, Light Exposure, and Dose. J. Investig. Dermatol. 1988, 91, 56–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tolleson, W.H.; Cherng, S.-H.; Xia, Q.; Boudreau, M.; Yin, J.J.; Wamer, W.G.; Howard, P.C.; Yu, H.; Fu, P.P. Photodecomposition and Phototoxicity of Natural Retinoids. Int. J. Environ. Res. Public Health 2005, 2, 147–155. [Google Scholar] [CrossRef] [Green Version]
- Han, H.-S.; Kwon, Y.-J.; Park, M.-S.; Park, S.-H.; Cho, S.-M.K.; Rho, Y.-S.; Kim, J.-W.; Sin, H.-S.; Um, S.-J. Efficacy validation of synthesized retinol derivatives In vitro: Stability, toxicity, and activity. Bioorg. Med. Chem. 2003, 11, 3839–3845. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, M.; Fang, L. Biomaterials as novel penetration enhancers for transdermal and dermal drug delivery systems. Drug Deliv. 2013, 20, 199–209. [Google Scholar] [CrossRef] [Green Version]
- Kim, B.H.; Lee, Y.S.; Kang, K.S. The mechanism of retinol-induced irritation and its application to anti-irritant development. Toxicol. Lett. 2003, 146, 65–73. [Google Scholar] [CrossRef]
- Date, A.; Naik, B.; Nagarsenker, M. Novel Drug Delivery Systems: Potential in Improving Topical Delivery of Antiacne Agents. Ski. Pharmacol. Physiol. 2005, 19, 2–16. [Google Scholar] [CrossRef]
- Orienti, I.; Zecchi, V.; Bertasi, V.; Fini, A. Release of ketoprofen from dermal bases in presence of cyclodextrins: Effect of the affinity constant determined in semisolid vehicles. Arch. Der Pharm. 1991, 324, 943–947. [Google Scholar] [CrossRef]
- Wadstein, J. Penetration of different retinoid formulations in a model barrier system (Franz diffusion cell). In Proceedings of the Nordic Symposium on Focus on the Anti-Ageing Treatment of the Skin, Oslo, Norway, 24 March 1993. [Google Scholar]
- Thom, E. Skin treatment with two different galenical formulations of retinyl palmitate in humans. J. Appl. Cosmetol. 1993, 11, 71–76. [Google Scholar]
- Thom, E. Long-term effects after topical application of Active Retinyl Palmitate. J. Appl. Cosmetol. 1994, 12, 25–30. [Google Scholar]
- Wadstein, J.T.; Thom, E. A randomized, placebo-controlled doubleblind parallel group study in the Treatment of Aging Symptoms of the skin using Topical and Oral Treatments. J. Appl. Cosmetol. 2013, 31, 31–40. [Google Scholar]
- Lassus, A.E.T. Sincera in the treatment of solar elastosis in females—A pilot study. 1990; Unpublished. [Google Scholar]
- Iozzo, R.V. Matrix proteoglycans: From Molecular Design to Cellular Function. Annu. Rev. Biochem. 1998, 67, 609–652. [Google Scholar] [CrossRef] [Green Version]
- Couchman, J.R.; Pataki, C.A. An introduction to proteoglycans and their localization. J. Histochem. Cytochem. 2012, 60, 885–897. [Google Scholar] [CrossRef]
- Naba, A.; Hoersch, S.; Hynes, R.O. Towards definition of an ECM parts list: An advance on GO categories. Matrix Biol. 2012, 31, 371–372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, M.M.; Melrose, J. Proteoglycans in Normal and Healing Skin. Adv. Wound Care 2015, 4, 152–173. [Google Scholar] [CrossRef] [Green Version]
- Nomura, Y. Structural Change in Decorin with Skin Aging. Connect. Tissue Res. 2006, 47, 249–255. [Google Scholar] [CrossRef]
- Lee, D.H.; Oh, J.-H.; Chung, J.H. Glycosaminoglycan and proteoglycan in skin aging. J. Dermatol. Sci. 2016, 83, 174–181. [Google Scholar] [CrossRef]
- Corsi, A.; Xu, T.; Chen, X.-D.; Boyde, A.; Liang, J.; Mankani, M.; Sommer, B.; Iozzo, R.; Eichstetter, I.; Robey, P.; et al. Phenotypic Effects of Biglycan Deficiency Are Linked to Collagen Fibril Abnormalities, Are Synergized by Decorin Deficiency, and Mimic Ehlers-Danlos-Like Changes in Bone and Other Connective Tissues. J. Bone Miner. Res. 2002, 17, 1180–1189. [Google Scholar] [CrossRef]
- Danielson, K.G.; Baribault, H.; Holmes, D.F.; Graham, H.; Kadler, K.E.; Iozzo, R.V. Targeted Disruption of Decorin Leads to Abnormal Collagen Fibril Morphology and Skin Fragility. J. Cell Biol. 1997, 136, 729–743. [Google Scholar] [CrossRef] [Green Version]
- Tzellos, T.G.; Sinopidis, X.; Kyrgidis, A.; Vahtsevanos, K.; Triaridis, S.; Printza, A.; Klagas, I.; Karakiulakis, G.; Papakonstantinou, E. Differential hyaluronan homeostasis and expression of proteoglycans in juvenile and adult human skin. J. Dermatol. Sci. 2011, 61, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Carrino, D.A.; Sorrell, J.; Caplan, A. Age-related Changes in the Proteoglycans of Human Skin. Arch. Biochem. Biophys. 2000, 373, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, E.F.; Underhill, C.B.; Hahn, P.J.; Brown, D.B.; Uitto, J. Chronic sun exposure alters both the content and distribution of dermal glycosaminoglycans. Br. J. Dermatol. 1996, 135, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, E.F.; Fisher, L.W.; Li, K.; LeBaron, R.; Tan, E.M.; Uitto, J. Differential expression of the versican and decorin genes in photoaged and sun-protected skin. Comparison by immunohistochemical and northern analyses. Lab. Investig. 1995, 72, 662–669. [Google Scholar]
- Wadstein, J.; Thom, E.; Gadzhigoroeva, A. Integral Roles of Specific Proteoglycans in Hair Growth and Hair Loss: Mechanisms behind the Bioactivity of Proteoglycan Replacement Therapy with Nourkrin(R) with Marilex(R) in Pattern Hair Loss and Telogen Effluvium. Dermatol. Res. Pract. 2020, 2020, 8125081. [Google Scholar] [CrossRef]
- Tomonaga, A.; Takahashi, T.; Tanaka, Y.T.; Tsuboi, M.; Akihito, T.; Nagaoka, I. Evaluation of the effect of salmon nasal proteoglycan on biomarkers for cartilage metabolism in individuals with knee joint discomfort: A randomized double-blind placebo-controlled clinical study. Exp. Ther. Med. 2017, 14, 115–126. [Google Scholar] [CrossRef] [Green Version]
- Vergés, J.; Montell, E.; Herrero, M.; Perna, C.; Cuevas, J.; Dalmau, J.; Pérez, M.; Möller, I. Clinical and histopathological improvement of psoriasis with oral chondroitin sulfate: A serendipitous finding. Dermatol. Online J. 2005, 11. [Google Scholar] [CrossRef]
- Barthe, L.; Woodley, J.; Lavit, M.; Przybylski, C.; Philibert, C.; Houin, G. In vitro Intestinal Degradation and Absorption of Chondroitin Sulfate, a Glycosaminoglycan Drug. Arzneimittelforschung 2004, 54, 286–292. [Google Scholar] [CrossRef]
- Balogh, L.; Polyak, A.; Mathe, D.; Kiraly, R.; Thuroczy, J.; Terez, M.; Janoki, G.; Ting, Y.; Bucci, L.R.; Schauss, A.G. Absorption, Uptake and Tissue Affinity of High-Molecular-Weight Hyaluronan after Oral Administration in Rats and Dogs. J. Agric. Food Chem. 2008, 56, 10582–10593. [Google Scholar] [CrossRef]
- Tsuchiya, Y.; Tomita, M.; Tsuboi, M.; Takahashi, T.; Yonezuka, M.; Kikuchi, S.; Nagasawa, S.; Kumazawa, A.; Kubota, J. Absorption of proteoglycan via clathrin-mediated endocytosis in the small intestine of rats. Biosci. Biotechnol. Biochem. 2013, 77, 654–656. [Google Scholar] [CrossRef]
- Sano, M.; Shang, Y.; Nakane, A.; Saito, T. Salmon nasal cartilage proteoglycan enhances growth of normal human dermal fibroblast through Erk1/2 phosphorylation. Biosci. Biotechnol. Biochem. 2017, 81, 1379–1385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ito, G.; Kobayashi, T.; Takeda, Y.; Sokabe, M. Proteoglycan from salmon nasal cartridge [corrected] promotes in vitro wound healing of fibroblast monolayers via the CD44 receptor. Biochem. Biophys. Res. Commun. 2015, 456, 792–798. [Google Scholar] [CrossRef] [PubMed]
- Egea, J.; Garcia, A.G.; Verges, J.; Montell, E.; Lopez, M.G. Antioxidant, antiinflammatory and neuroprotective actions of chondroitin sulfate and proteoglycans. Osteoarthr. Cartil. 2010, 18 (Suppl. 1), S24–S27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poljšak, B.; Dahmane, R.G.; Godić, A. Intrinsic skin aging: The role of oxidative stress. Acta Dermatovenerol. Alp. Panon. Adriat. 2012, 21, 33–36. [Google Scholar]
- Poljšak, B.; Dahmane, R. Free Radicals and Extrinsic Skin Aging. Dermatol. Res. Pr. 2012, 2012, 135206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katagata, Y.; Goto, M.; Ito, S.; Kato, Y.; Yamazaki, S.; Yamamoto, K. Anti-aging effects of extracts prepared from salmon nasal cartilage in hairless mice. Mol. Med. Rep. 2011, 4, 779–784. [Google Scholar] [CrossRef]
- Takahashi, T.M.J.; Wakamatsu, K.; Tanaka, Y.T.; Masutani, T.; Yonezuka, M.; Kenichi Ito, I.; Tsuji-Takayama, K.; Tsuboi, M. Ingestion of Salmon Nasal Cartilage-Derived Proteoglycan Improves Skin Condition: A Randomized, Double-Blind, Controlled Study. Immun. Endo. Metabol. Agen. Medicin. Chem. 2015, 15, 120773. [Google Scholar] [CrossRef]
- Lassus, A.; Jeskanen, L.; Happonen, H.; Santalahti, J. Imedeen® for the Treatment of Degenerated Skin in Females. J. Int. Med. Res. 1991, 19, 147–152. [Google Scholar] [CrossRef]
- Majass, M.P.O. A double-blind, placebo-controlled study of Nourella for treatment of sun-damaged skin in females. Euro. J. Clin. Res. 1997, 9, 123–127. [Google Scholar]
- Thom, E. A randomized, double-blind, placebo-controlled study on the clinical efficacy of oral treatment with DermaVite on ageing symptoms of the skin. J. Int. Med. Res. 2005, 33, 267–272. [Google Scholar] [CrossRef]
- Asano, K.; Yoshimura, S.; Nakane, A. Alteration of Intestinal Microbiota in Mice Orally Administered with Salmon Cartilage Proteoglycan, a Prophylactic Agent. PLoS ONE 2013, 8, e75008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirose, S.; Asano, K.; Nakane, A. Attenuation of obesity-induced inflammation in mice orally administered with salmon cartilage proteoglycan, a prophylactic agent. Biochem. Biophys. Res. Commun. 2017, 484, 480–485. [Google Scholar] [CrossRef] [PubMed]
- Sashinami, H.; Takagaki, K.; Nakane, A. Salmon cartilage proteoglycan modulates cytokine responses to Escherichia coli in mouse macrophages. Biochem. Biophys. Res. Commun. 2006, 351, 1005–1010. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wadstein, J.; Alvarez, I.S.; López, L.B. Managing Skin Ageing as a Modifiable Disorder—The Clinical Application of Nourella® Dual Approach Comprising a Nano-Encapsulated Retinoid, Retilex-A® and a Skin Proteoglycan Replacement Therapy, Vercilex®. Cosmetics 2022, 9, 31. https://doi.org/10.3390/cosmetics9020031
Wadstein J, Alvarez IS, López LB. Managing Skin Ageing as a Modifiable Disorder—The Clinical Application of Nourella® Dual Approach Comprising a Nano-Encapsulated Retinoid, Retilex-A® and a Skin Proteoglycan Replacement Therapy, Vercilex®. Cosmetics. 2022; 9(2):31. https://doi.org/10.3390/cosmetics9020031
Chicago/Turabian StyleWadstein, Jan, Israel Sánchez Alvarez, and Lidia Bernal López. 2022. "Managing Skin Ageing as a Modifiable Disorder—The Clinical Application of Nourella® Dual Approach Comprising a Nano-Encapsulated Retinoid, Retilex-A® and a Skin Proteoglycan Replacement Therapy, Vercilex®" Cosmetics 9, no. 2: 31. https://doi.org/10.3390/cosmetics9020031
APA StyleWadstein, J., Alvarez, I. S., & López, L. B. (2022). Managing Skin Ageing as a Modifiable Disorder—The Clinical Application of Nourella® Dual Approach Comprising a Nano-Encapsulated Retinoid, Retilex-A® and a Skin Proteoglycan Replacement Therapy, Vercilex®. Cosmetics, 9(2), 31. https://doi.org/10.3390/cosmetics9020031




