Revealing the Correlation between Altered Skin Lipids Composition and Skin Disorders
Abstract
:1. Introduction
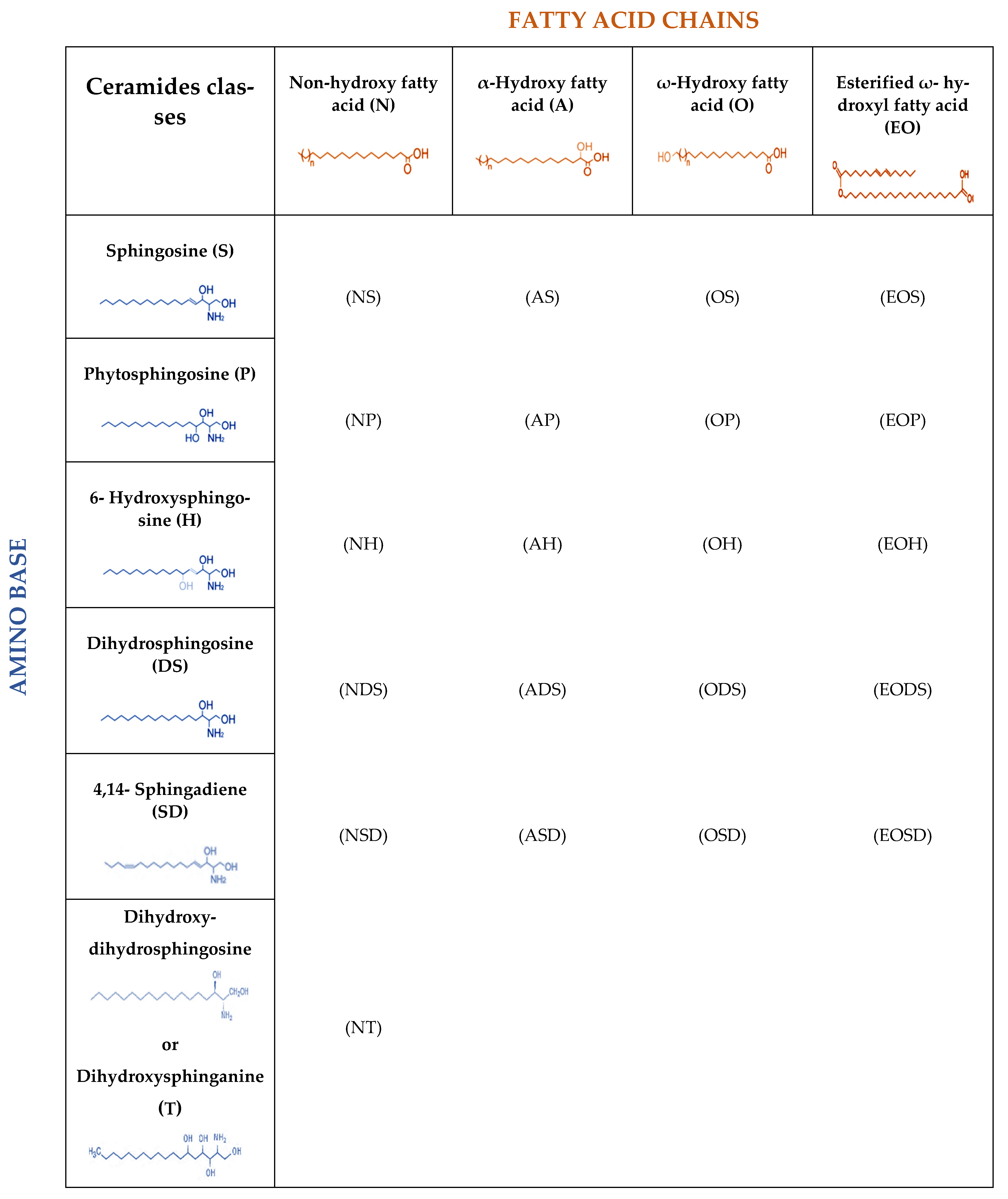
2. Lipids of the Skin
3. Lipids and Microbiome in Skin Diseases
3.1. Acne Vulgaris
3.2. Atopic Dermatitis
3.3. Rosacea
3.4. Psoriasis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Graham-Brown, R.A.C.; Bourke, J.F. Mosby’s Color Atlas and Text of Dermatology; Mosby Ltd.: St. Louis, MI, USA, 2006. [Google Scholar]
- Zhou, M.; Yang, M.; Zheng, Y.; Dong, K.; Song, L.; He, C.; Liu, W.; Wang, Y.; Jia, Y. Skin surface lipidomics revealed the correlation between lipidomic profile and grade in adolescent acne. J. Cosmet. Dermatol. 2020, 19, 3349–3356. [Google Scholar] [CrossRef] [PubMed]
- Ní Raghallaigh, S.; Bender, K.; Lacey, N.; Brennan, L.; Powell, F.C. The fatty acid profile of the skin surface lipid layer in papulopustular rosacea. Br. J. Dermatol. 2012, 166, 279–287. [Google Scholar] [CrossRef]
- Knox, S.; O’Boyle, N.M. Skin lipids in health and disease: A review. Chem. Phys. Lipids 2021, 236, 105055. [Google Scholar] [CrossRef]
- Kefala, V.; Biskanaki, F.; Rallis, E. The microbiome of human skin: Α key factor in managing problems in aesthetics and dermatology. Rev. Clin. Pharmacol. Pharmacokinet. 2020, 38, 151–160. [Google Scholar]
- McGrath, J.A.; Eady, R.A.J.; Pope, F.M. Anatomy and organization of human skin. In Rook’s Textbook of Dermatology; Burns, T., Breathnach, S., Cox, N., Christopher, G., Eds.; Blackwell Science Ltd.: Oxford, UK, 2004; pp. 3.1–3.84. [Google Scholar]
- Fore, J. A review of skin and the effects of aging on skin structure and function. Ostomy. Wound. Manage. 2006, 52, 24–35. [Google Scholar]
- Koszyczarek, M.M. An Investigation of Skin and Plasma Lipids in Psoriasis. Ph.D. Thesis, The University of Manchester, Manchester, UK, 2020. [Google Scholar]
- Geerligs, M. Skin Layer Mechanics; Universiteitsdrukkerij TU Eindhoven: Eindhoven, The Netherlands, 2010; ISBN 9789074445924. [Google Scholar]
- Coelho, M.; Oliveira, T.; Fernandes, R. Biochemistry of adipose tissue: An endocrine organ. Arch. Med. Sci. 2013, 9, 191–200. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Fang, H.; Dang, E.; Wang, G. The role of ceramides in skin homeostasis and inflammatory skin diseases. J. Dermatol. Sci. 2020, 97, 2–8. [Google Scholar] [CrossRef]
- Sahle, F.F.; Gebre-Mariam, T.; Dobner, B.; Wohlrab, J.; Neubert, R.H.H. Skin diseases associated with the depletion of stratum corneum lipids and stratum corneum lipid substitution therapy. Skin Pharmacol. Physiol. 2015, 28, 42–55. [Google Scholar] [CrossRef] [PubMed]
- Kwan, P.; Sills, G.J.; Brodie, M.J. Understanding the Role of Natural Moisturizing Factor in Skin Hydration. Pract. Dermatol. 2012, 36–40, 21–34. [Google Scholar]
- Jungersted, J.M.; Hellgren, L.I.; Jemec, G.B.E.; Agner, T. Lipids and skin barrier function—A clinical perspective. Contact Dermat. 2008, 58, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Kawana, M.; Miyamoto, M.; Ohno, Y.; Kihara, A. Comparative profiling and comprehensive quantification of stratum corneum ceramides in humans and mice by LC/MS/MS. J. Lipid Res. 2020, 61, 884–895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pappas, A. Epidermal surface lipids. Derm. -Endocrinol. 2009, 1, 72–76. [Google Scholar] [CrossRef] [Green Version]
- Nicollier, M.; Massengo, T.; Rémy-Martin, J.P.; Laurent, R.; Adessi, G.L. Free fatty acids and fatty acids of triacylglycerols in normal and hyperkeratotic human stratum corneum. J. Invest. Dermatol. 1986, 87, 68–71. [Google Scholar] [CrossRef] [Green Version]
- De Luca, C.; Valacchi, G. Surface lipids as multifunctional mediators of skin responses to environmental stimuli. Mediat. Inflamm. 2010, 2010, 321494. [Google Scholar] [CrossRef]
- Opálka, L.; Kováčik, A.; Pullmannová, P.; Maixner, J.; Vávrová, K. Effects of omega-O-acylceramide structures and concentrations in healthy and diseased skin barrier lipid membrane models. J. Lipid Res. 2020, 61, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Dreno, B.; Martin, R.; Moyal, D.; Henley, J.B.; Khammari, A.; Seité, S. Skin microbiome and acne vulgaris: Staphylococcus, a new actor in acne. Exp. Dermatol. 2017, 26, 798–803. [Google Scholar] [CrossRef] [Green Version]
- Moradi Tuchayi, S.; Makrantonaki, E.; Ganceviciene, R.; Dessinioti, C.; Feldman, S.R.; Zouboulis, C.C. Acne vulgaris. Nat. Rev. Dis. Prim. 2015, 1, 15029. [Google Scholar] [CrossRef] [PubMed]
- Mohiuddin, A. A Comprehensive Review of Acne Vulgaris. J. Clin. Res. Dermatol. 2019, 6, 1–34. [Google Scholar] [CrossRef]
- Camera, E.; Ludovici, M.; Tortorella, S.; Sinagra, J.L.; Capitanio, B.; Goracci, L.; Picardo, M. Use of lipidomics to investigate sebum dysfunction in juvenile acne. J. Lipid Res. 2016, 57, 1051–1058. [Google Scholar] [CrossRef] [Green Version]
- Zhou, M.; Gan, Y.; He, C.; Chen, Z.; Jia, Y. Lipidomics reveals skin surface lipid abnormity in acne in young men. Br. J. Dermatol. 2018, 179, 732–740. [Google Scholar] [CrossRef]
- Zhou, M.; Wang, H.; Yang, M.; He, C.; Yang, M.; Gao, Y.; Jia, Y. Lipidomic analysis of facial skin surface lipids reveals an altered lipid profile in infant acne. Br. J. Dermatol. 2020, 182, 817–818. [Google Scholar] [CrossRef]
- Okoro, E.; Adenle, A.; Ludovici, M.; Truglio, M.; Marini, F.; Camera, E. Lipidomics of Facial Sebum in the Comparison Between Acne and Non-Acne Adolescents with Dark Skin. Sci. Rep. 2021, 11, 16591. [Google Scholar] [CrossRef] [PubMed]
- Ikaraoha, C.I.; Taylor, G.O.L.; Anetor, J.I.; Onuegbu, J.A. Pattern of skin surface lipids in some south-western Nigerians with acne vulgaris. West Afr. J. Med. 2004, 24, 65–68. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Li, H. Acne, the Skin Microbiome, and Antibiotic Treatment. Am. J. Clin. Dermatol. 2019, 20, 335–344. [Google Scholar] [CrossRef]
- Rocha, M.A.; Bagatin, E. Skin barrier and microbiome in acne. Arch. Dermatol. Res. 2018, 310, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.N.; Braue, A.; Varigos, G.A.; Mann, N.J. The effect of a low glycemic load diet on acne vulgaris and the fatty acid composition of skin surface triglycerides. J. Dermatol. Sci. 2008, 50, 41–52. [Google Scholar] [CrossRef]
- Fitz-Gibbon, S.; Tomida, S.; Chiu, B.H.; Nguyen, L.; Du, C.; Liu, M.; Elashoff, D.; Erfe, M.C.; Loncaric, A.; Kim, J.; et al. Propionibacterium acnes strain populations in the human skin microbiome associated with acne. J. Invest. Dermatol. 2013, 133, 2152–2160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gruber, F.; Kremslehner, C.; Narzt, M.S. The impact of recent advances in lipidomics and redox lipidomics on dermatological research. Free Radic. Biol. Med. 2019, 144, 256–265. [Google Scholar] [CrossRef]
- Emmert, H.; Baurecht, H.; Thielking, F.; Stölzl, D.; Rodriguez, E.; Harder, I.; Proksch, E.; Weidinger, S. Stratum corneum lipidomics analysis reveals altered ceramide profile in atopic dermatitis patients across body sites with correlated changes in skin microbiome. Exp. Dermatol. 2020, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Schafer, L.; Kragballe, K. Abnormalities in epidermal lipid metabolism in patients with atopic dermatitis. J. Invest. Dermatol. 1991, 96, 10–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hara, J.; Higuchi, K.; Okamoto, R.; Kawashima, M.; Imokawa, G. High-expression of sphingomyelin deacylase is an important determinant of ceramide deficiency leading to barrier disruption in atopic dermatitis. J. Invest. Dermatol. 2000, 115, 406–413. [Google Scholar] [CrossRef] [Green Version]
- Proksch, E.; Jensen, J.M.; Elias, P.M. Skin lipids and epidermal differentiation in atopic dermatitis. Clin. Dermatol. 2003, 21, 134–144. [Google Scholar] [CrossRef]
- Wang, H.; Cui, L.; Jia, Y.; Gao, Y.; Zhang, G.; He, C. Application of lipidomics to reveal differences of facial skin surface lipids between atopic dermatitis and healthy infants. J. Cosmet. Dermatol. 2020, 19, 1528–1534. [Google Scholar] [CrossRef]
- Töröcsik, D.; Weise, C.; Gericke, J.; Szegedi, A.; Lucas, R.; Mihaly, J.; Worm, M.; Rühl, R. Transcriptomic and lipidomic profiling of eicosanoid/docosanoid signalling in affected and non-affected skin of human atopic dermatitis patients. Exp. Dermatol. 2019, 28, 177–189. [Google Scholar] [CrossRef] [Green Version]
- Jia, Y.; Gan, Y.; He, C.; Chen, Z.; Zhou, C. The mechanism of skin lipids influencing skin status. J. Dermatol. Sci. 2018, 89, 112–119. [Google Scholar] [CrossRef]
- Elias, P.M.; Schmuth, M. Abnormal skin barrier in the etiopathogenesis of atopic dermatitis. Curr. Opin. Allergy Clin. Immunol. 2009, 9, 437–446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howell, M.D.; Gallo, R.L.; Boguniewicz, M.; Jones, J.F.; Wong, C.; Streib, J.E.; Leung, D.Y.M. Cytokine milieu of atopic dermatitis skin subverts the innate immune response to vaccinia virus. Immunity 2006, 24, 341–348. [Google Scholar] [CrossRef] [Green Version]
- Nakatsuji, T.; Gallo, R.L. The role of the skin microbiome in atopic dermatitis. Ann. Allergy Asthma Immunol. 2019, 122, 263–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kong, H.H.; Oh, J.; Deming, C.; Conlan, S.; Grice, E.A.; Beatson, M.A.; Nomicos, E.; Polley, E.C.; Komarow, H.D.; Mullikin, J.; et al. Temporal shifts in the skin microbiome associated with disease flares and treatment in children with atopic dermatitis. Genome Res. 2012, 22, 850–859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lynde, C.W.; Andriessen, A.; Bertucci, V.; McCuaig, C.; Skotnicki, S.; Weinstein, M.; Wiseman, M.; Zip, C. The skin microbiome in atopic dermatitis and its relationship to emollients. J. Cutan. Med. Surg. 2016, 20, 21–28. [Google Scholar] [CrossRef]
- Pascolini, C.; Sinagra, J.; Pecetta, S.; Bordignon, V.; De Santis, A.; Cilli, L.; Cafiso, V.; Prignano, G.; Capitanio, B.; Passariello, C.; et al. Molecular and immunological characterization of Staphylococcus aureus in pediatric atopic dermatitis: Implications for prophylaxis and clinical management. Clin. Dev. Immunol. 2011, 2011, 718708. [Google Scholar] [CrossRef] [Green Version]
- Williams, M.R.; Gallo, R.L. The Role of the Skin Microbiome in Atopic Dermatitis. Curr. Allergy Asthma Rep. 2015, 15, 65. [Google Scholar] [CrossRef]
- Flores, G.E.; Seite, S.; Henley, J.B.; Martin, R.; Zelenkova, H.; Aguilar, L.; Fierer, N. Microbiome of affected and unaffected skin of patients with atopic dermatitis before and after emollient treatment. J. Drugs Dermatol. 2014, 13, 1365–1372. [Google Scholar]
- Kobayashi, T.; Glatz, M.; Horiuchi, K.; Kawasaki, H.; Akiyama, H.; Kaplan, D.H.; Kong, H.H.; Amagai, M.; Nagao, K. Dysbiosis and Staphyloccus aureus Colonization Drives Inflammation in Atopic Dermatitis. Immunity 2015, 42, 756–766. [Google Scholar] [CrossRef] [Green Version]
- Biskanaki, F.; Kefala, V. The role of Photodynamic Therapy in Rosacea Acne. Rev. Clin. Pharmacol. Pharmacokinet. 2020, 38, 109–117. [Google Scholar]
- Rebora, A. Rosacea. J. Invest. Dermatol. 1987, 88, 56–60. [Google Scholar] [CrossRef] [Green Version]
- Addor, F.A.S. Skin barrier in rosacea. Ann. Bras. Dermatol. 2016, 91, 59–63. [Google Scholar] [CrossRef]
- Pye, R.J.; Meyrick, G.; Burton, J.L. Skin surface lipid composition in rosacea. Br. J. Dermatol. 1976, 94, 161–164. [Google Scholar] [CrossRef] [PubMed]
- Reinholz, M.; Ruzicka, T.; Schauber, J. Cathelicidin LL-37: An antimicrobial peptide with a role in inflammatory skin disease. Ann. Dermatol. 2012, 24, 126–135. [Google Scholar] [CrossRef] [Green Version]
- Yamasaki, K.; Di Nardo, A.; Bardan, A.; Murakami, M.; Ohtake, T.; Coda, A.; Dorschner, R.A.; Bonnart, C.; Descargues, P.; Hovnanian, A.; et al. Increased serine protease activity and cathelicidin promotes skin inflammation in rosacea. Nat. Med. 2007, 13, 975–980. [Google Scholar] [CrossRef]
- Wang, R.; Farhat, M.; Na, J.; Li, R.; Wu, Y. Bacterial and fungal microbiome characterization in patients with rosacea and healthy controls. Br. J. Dermatol. 2020, 183, 1112–1114. [Google Scholar] [CrossRef]
- Hsu, D.K.; Fung, M.A.; Chen, H.-L. Role of skin and gut microbiota in the pathogenesis of psoriasis, an inflammatory skin disease. Med. Microecol. 2020, 4, 100016. [Google Scholar] [CrossRef]
- Parisi, R.; Symmons, D.P.M.; Griffiths, C.E.M.; Ashcroft, D.M. Global epidemiology of psoriasis: A systematic review of incidence and prevalence. J. Invest. Dermatol. 2013, 133, 377–385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Ganguli-Indra, G.; Indra, A.K. Lipidomic analysis of epidermal lipids: A tool to predict progression of inflammatory skin disease in humans. Expert Rev. Proteom. 2016, 13, 451–456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Łuczaj, W.; Wroński, A.; Domingues, P.; Rosário Domingues, M.; Skrzydlewska, E. Lipidomic analysis reveals specific differences between fibroblast and keratinocyte ceramide profile of patients with psoriasis vulgaris. Molecules 2020, 25, 630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathew, A.J.; Chandran, V. Lipidomics in psoriatic disease: The new kid on the omics block. Indian J. Rheumatol. 2019, 14, 261–262. [Google Scholar] [CrossRef]
- Chang, H.W.; Yan, D.; Singh, R.; Liu, J.; Lu, X.; Ucmak, D.; Lee, K.; Afifi, L.; Fadrosh, D.; Leech, J.; et al. Alteration of the cutaneous microbiome in psoriasis and potential role in Th17 polarization. Microbiome 2018, 6, 154. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.; Wen, B.; Hou, G.; Lei, L.; Mei, Z.; Jia, X.; Chen, X.; Zhu, W.; Li, J.; Kuang, Y.; et al. Lipidomics profiling reveals the role of glycerophospholipid metabolism in psoriasis. Gigascience 2017, 6, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Dei-Cas, I.; Giliberto, F.; Luce, L.; Dopazo, H.; Penas-Steinhardt, A. Metagenomic analysis of gut microbiota in non-treated plaque psoriasis patients stratified by disease severity: Development of a new Psoriasis-Microbiome Index. Sci. Rep. 2020, 10, 12754. [Google Scholar] [CrossRef]
- Langan, E.A.; Griffiths, C.E.M.; Solbach, W.; Knobloch, J.K.; Zillikens, D.; Thaçi, D. The role of the microbiome in psoriasis: Moving from disease description to treatment prediction? Br. J. Dermatol. 2018, 178, 1020–1027. [Google Scholar] [CrossRef]
- Fyhrquist, N.; Muirhead, G.; Prast-Nielsen, S.; Jeanmougin, M.; Olah, P.; Skoog, T.; Jules-Clement, G.; Feld, M.; Barrientos-Somarribas, M.; Sinkko, H.; et al. Microbe-host interplay in atopic dermatitis and psoriasis. Nat. Commun. 2019, 10, 4703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tett, A.; Pasolli, E.; Farina, S.; Truong, D.T.; Asnicar, F.; Zolfo, M.; Beghini, F.; Armanini, F.; Jousson, O.; De Sanctis, V.; et al. Unexplored diversity and strain-level structure of the skin microbiome associated with psoriasis. NPJ Biofilms Microbiomes 2017, 3, 14. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drakou, K.; Tsianni, A.; Vrani, F.; Kefala, V.; Rallis, E. Revealing the Correlation between Altered Skin Lipids Composition and Skin Disorders. Cosmetics 2021, 8, 88. https://doi.org/10.3390/cosmetics8030088
Drakou K, Tsianni A, Vrani F, Kefala V, Rallis E. Revealing the Correlation between Altered Skin Lipids Composition and Skin Disorders. Cosmetics. 2021; 8(3):88. https://doi.org/10.3390/cosmetics8030088
Chicago/Turabian StyleDrakou, Katerina, Andrea Tsianni, Faye Vrani, Valia Kefala, and Efstathios Rallis. 2021. "Revealing the Correlation between Altered Skin Lipids Composition and Skin Disorders" Cosmetics 8, no. 3: 88. https://doi.org/10.3390/cosmetics8030088
APA StyleDrakou, K., Tsianni, A., Vrani, F., Kefala, V., & Rallis, E. (2021). Revealing the Correlation between Altered Skin Lipids Composition and Skin Disorders. Cosmetics, 8(3), 88. https://doi.org/10.3390/cosmetics8030088





