Efficacy and Safety of Topical Dexpanthenol-Containing Spray and Cream in the Recovery of the Skin Integrity Compared with Petroleum Jelly after Dermatologic Aesthetic Procedures
Abstract
:1. Introduction
2. Methods
2.1. Study Design and Participants
2.2. Primary Outcomes
2.3. Secondary Outcomes
2.3.1. Clinical Efficacy—Dermatologists’ Assessments
2.3.2. Clinical Efficacy—Subjects’ Assessments
2.3.3. Safety and Tolerability
2.3.4. Product Acceptability
2.4. Statistical Analyses
3. Results
3.1. Primary Outcomes
3.1.1. Recovery of Skin Integrity
3.1.2. Reduction of Inflammation/Erythema
3.2. Secondary Outcomes
3.2.1. Clinical Efficacy
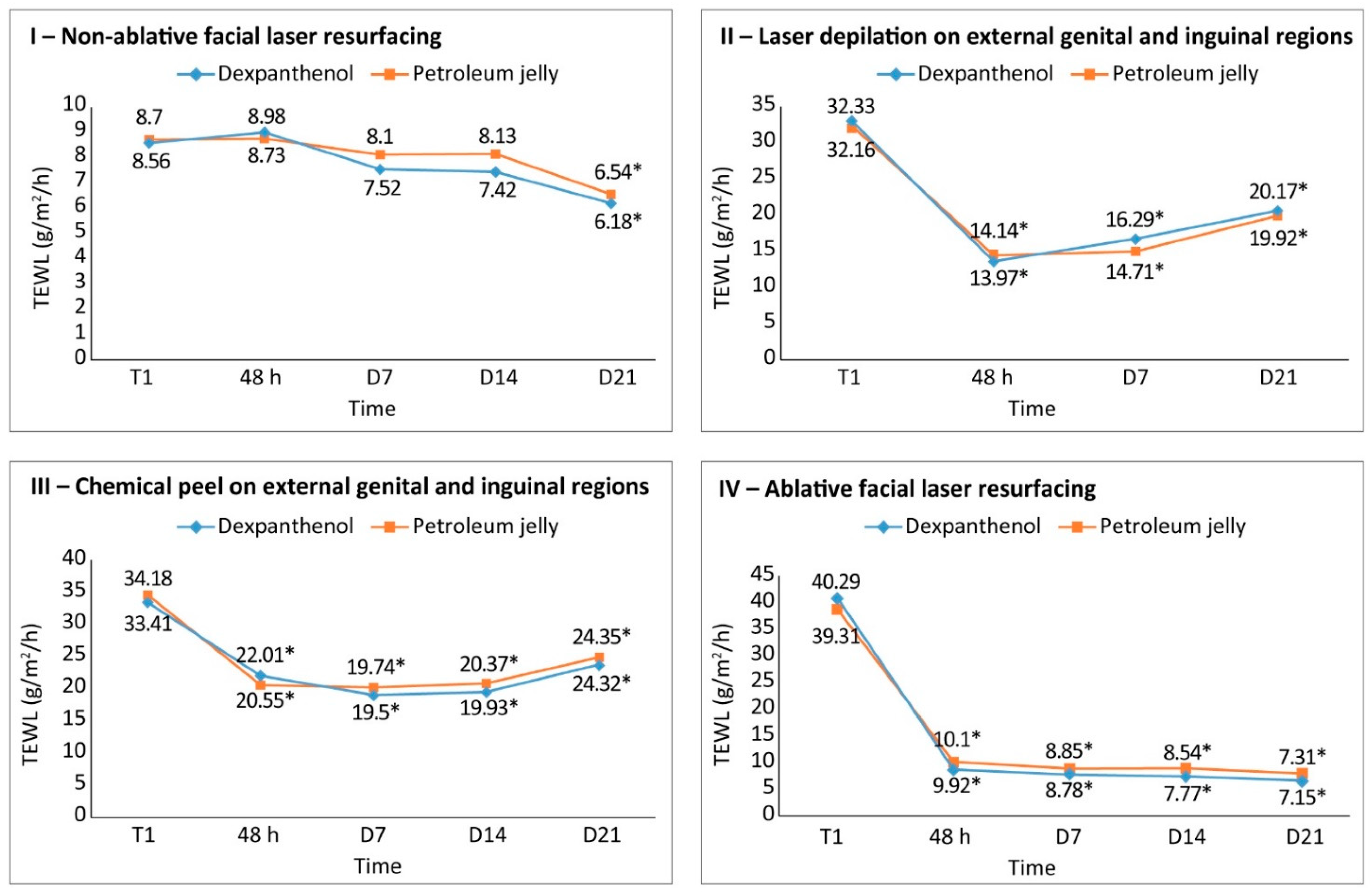
Study 1. Non-Ablative Facial Laser
Study 2: Laser Depilation around the External Genital Region (Skin Intimate Area)
Study 3: Chemical Peel in the Area around External Genitalia and Inguinal Area
Study 4: Dexpanthenol-Containing Emollient Cream after Facial Ablative Laser
3.2.2. Safety and Tolerability
3.3. Acceptability
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ong, M.W.S.; Bashir, S. Fractional laser resurfacing for acne scars: A review. Br. J. Dermatol. 2012, 166, 1160–1169. [Google Scholar] [CrossRef]
- Kaushik, S.B.; Alexis, A.F. Nonablative Fractional Laser Resurfacing in Skin of Color: Evidence-based Review. J. Clin. Aesthet. Dermatol. 2017, 10, 51. [Google Scholar]
- Narins, R.; Narins, D. Nonablative skin resurfacing. Aesthet. Surg. J. 2004, 24, 281–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manstein, D.; Herron, G.; Sink, R.; Tanner, H.; Anderson, R. Fractional photothermolysis: A new concept for cutaneous remodeling using microscopic patterns of thermal injury. Lasers Surg. Med. 2004, 34, 426–438. [Google Scholar] [CrossRef] [PubMed]
- Devgan, L.; Singh, P.; Durairaj, K. Minimally Invasive Facial Cosmetic Procedures. Otolaryngol. Clin. N. Am. 2019, 52, 443–459. [Google Scholar] [CrossRef]
- Rendon, M.I.; Berson, D.S.; Cohen, J.L.; Roberts, W.E.; Starker, I.; Wang, B. Evidence and considerations in the application of chemical peels in skin disorders and aesthetic resurfacing. J. Clin. Aesthet. Dermatol. 2010, 3, 32–43. [Google Scholar] [PubMed]
- Samargandy, S.; Raggio, B.S. Skin Resurfacing Chemical Peels. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, January 2021; [Updated 25 July 2021]. Available online: https://www.ncbi.nlm.nih.gov/books/NBK547752 (accessed on 27 August 2021).
- Garg, V.; Sinha, S.; Sarkar, R. Glycolic acid peels versus salicylic-mandelic acid peels in active acne vulgaris and post-acne scarring and hyperpigmentation: A comparative study. Dermatol. Surg. 2009, 35, 59–65. [Google Scholar] [CrossRef]
- Yokomizo, V.; Benemond, V.; Chisaki, C.; Benemond, P. Chemical peels: Review and practical applications. Surg. Cosmet. Dermatol. 2013, 5, 56–68. [Google Scholar]
- Castillo, D.E.; Keri, J.E. Chemical Peels in the Treatment of Acne: Patient Selection and Perspectives. Clin. Cosmet. Investig. Dermatol. 2018, 11, 365–372. [Google Scholar] [CrossRef] [Green Version]
- Sarkar, R.; Ghunawat, S.; Garg, V.K. Comparative Study of 35% Glycolic Acid, 20% Salicylic–10% Mandelic Acid, and Phytic Acid Combination Peels in the Treatment of Active Acne and Postacne Pigmentation. J. Cutan. Aesthet. Surg. 2019, 12, 158. [Google Scholar] [CrossRef]
- Gorski, J.; Proksch, E.; Baron, J.M.; Schmid, D.; Zhang, L. Dexpanthenol in wound healing after medical and cosmetic interventions (Postprocedure wound healing). Pharmaceuticals 2020, 13, 138. [Google Scholar] [CrossRef] [PubMed]
- Morales-Burgos, A.; Loosemore, M.P.; Goldberg, L.H. Postoperative wound care after dermatologic procedures: A comparison of 2 commonly used petrolatum-based ointments. J. Drugs Dermatol. 2013, 12, 163–164. [Google Scholar] [PubMed]
- Ebner, F.; Heller, A.; Rippke, F.; Tausch, I. Topical use of dexpanthenol in skin disorders. Am. J. Clin. Dermatol. 2002, 3, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Proksch, E.; de Bony, R.; Trapp, S.; Boudon, S. Topical use of dexpanthenol: A 70th anniversary article. J. Dermatol. Treat. 2017, 28, 766–773. [Google Scholar] [CrossRef] [PubMed]
- Slyshenkov, V.S.; Rakowska, M.; Moiseenok, A.G.; Wojtczak, L. Pantothenic acid and its derivatives protect ehrlich ascites tumor cells against lipid peroxidation. Free Radic. Biol. Med. 1995, 19, 767–772. [Google Scholar] [CrossRef]
- Heise, R.; Schmitt, L.; Huth, L.; Krings, L.; Kluwig, D.; Katsoulari, K.V.; Steiner, T.; Hölzle, F.; Baron, J.M.; Huth, S. Accelerated wound healing with a dexpanthenol-containing ointment after fractional ablative CO2 laser resurfacing of photo-damaged skin in a randomized prospective clinical trial. Cutan. Ocul. Toxicol. 2019, 38, 274–278. [Google Scholar] [CrossRef]
- Czarnowicki, T.; Malajian, D.; Khattri, S.; da Rosa, J.C.; Dutt, R.; Finney, R.; Dhingra, N.; Xiangyu, P.; Xu, H.; Estrada, Y.D.; et al. Petrolatum: Barrier repair and antimicrobial responses underlying this “inert” moisturizer. J. Allergy Clin. Immunol. 2016, 137, 1091–1102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bennani, I.; Lopez, R.; Bonnet, D.; Prevot, G.; Constantin, A.; Chauveau, D.; Paul, C.; Livideanu, C.B. Improvement of Microstomia in Scleroderma after Carbon Dioxide Laser Treatment. Case Rep. Dermatol. 2016, 8, 142–150. [Google Scholar] [CrossRef]
- Wall, T. Current Concepts: Laser Treatment of Adult Vascular Lesions. Semin. Plast. Surg. 2007, 21, 147–158. [Google Scholar] [CrossRef] [Green Version]
- Ghadially, R.; Halkier-Sorensen, L.; Elias, P.M. Effects of petrolatum on stratum corneum structure and function. J. Am. Acad. Dermatol. 1992, 26, 387–396. [Google Scholar] [CrossRef]
- Fluhr, J.W.; Feingold, K.R.; Elias, P.M. Transepidermal water loss reflects permeability barrier status: Validation in human and rodent in vivo and ex vivo models. Exp. Dermatol. 2006, 15, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Plataforma Brasil. Available online: https://plataformabrasil.saude.gov.br/login.jsf (accessed on 1 March 2021).
- Stettler, H.; Crowther, J.M.; Brandt, M.; Lu, B.; Boxshall, A.; de Salvo, R.; Laing, S.; Hennighausen, N.; Bielfeldt, S.; Blenkiron, P. Targeted dry skin treatment using a multifunctional topical moisturizer. Int. J. Cosmet. Sci. 2020, 43, 191–200. [Google Scholar] [CrossRef]
- Frankel, E.B. Acne Secondary to White Petrolatum Use. Arch. Dermatol. 1985, 121, 589–590. [Google Scholar] [CrossRef]
- Gehring, W.; Gloor, M. Effect of topically applied dexpanthenol on epidermal barrier function and stratum corneum hydration: Results of a human in vivo. Arzneim.-Forsch./Drug Res. Ed. 2000, 50, 659–663. [Google Scholar] [CrossRef]
- Proksch, E.; Nissen, H.P. Dexpanthenol enhances skin barrier repair and reduces inflammation after sodium lauryl sulphate-induced irritation. J. Dermatol. Treat. 2002, 13, 173–178. [Google Scholar] [CrossRef] [PubMed]

| Study | Procedure | Body Region | Test Products | Primary Outcome | Secondary Outcomes | Time of Assessment |
|---|---|---|---|---|---|---|
| 1 | Non-ablative laser resurfacing | Face | Dexpanthenol-containing spray vs. Petroleum jelly | TEWL; Reduction of erythema * | Clinical efficacy assessed by dermatologists, Efficacy perceived by subjects, Product safety, tolerability, and acceptability | T0, T1, T48, D7, D14, and D21 |
| 2 | Laser depilation | External genital and inguinal areas | Dexpanthenol-containing spray vs. Petroleum jelly | TEWL | Clinical efficacy assessed by dermatologists, Efficacy perceived by subjects, Product safety, tolerability, and acceptability | T0 T1, T48, D7, and D21 |
| 3 | Chemical peel with mandelic acid 20% | External genital and inguinal areas | Dexpanthenol-containing spray vs. Petroleum jelly | TEWL | Clinical efficacy assessed by dermatologists Efficacy perceived by subjects Product safety, tolerability, and acceptability | T0, T1, T48, D7, D14, and D21 |
| 4 | Ablative laser resurfacing | Face | Dexpanthenol-containing emollient cream vs. Petroleum jelly | TEWL; Reduction of erythema * | Clinical efficacy assessed by dermatologists Efficacy perceived by subjects Product safety, tolerability, and acceptability | T0, T1, T48, D7, D14, and D21 |
| Study | Number | Age, Years | Fitzpatrick Phototype | Gender | |
|---|---|---|---|---|---|
| Screened | Included | Mean (Range) | (I, II, III, IV) | Female/Male | |
| 1 | 35 | 33 | 49 (33–59) | I or II | Female |
| 2 | 37 | 33 | 46 (30–60) | I or II | Female |
| 3 | 38 | 33 | 41 (23–60) | I or II | Female |
| 4 | 65 | 33 | 46 (30–60) | I or II | Female |
| Treatment | Timepoint | Change from Baseline (%) | p Value |
|---|---|---|---|
| Dexpanthenol a | 48 h | −0.007% | 0.877 |
| Day 7 | −0.51% | 0.198 | |
| Day 14 | −0.92% | 0.068 | |
| Day 21 | −1.24% | 0.010 | |
| Petroleum jelly (control) | 48 h | −0.08% | 0.858 |
| Day 7 | −0.53% | 0.205 | |
| Day 14 | −0.95% | 0.058 | |
| Day 21 | −1.32% | 0.008 |
| Treatment | Timepoint | Change from Baseline (%) | p Value |
|---|---|---|---|
| Dexpanthenol a | 48 h | −1.57% | 0.005 |
| Day 7 | −1.98% | 0.003 | |
| Day 14 b | −1.67% | 0.002 b | |
| Day 21 | −1.58% | 0.001 | |
| Petroleum jelly (control) | 48 h | −1.09% | 0.072 |
| Day 7 | −1.58% | 0.025 | |
| Day 14 | −1.21% | 0.114 | |
| Day 21 | 1.13% | 0.053 |
| Study | 1—Non-Ablative Laser Resurfacing (n = 31) | 2—Laser Depilation on External Genital/Inguinal Region (n = 32) | 3—Chemical Peel on External Genital/Inguinal Region (n = 31) | 4—Ablative Facial Laser Resurfacing (n = 31) |
|---|---|---|---|---|
| Dexpanthenol-Containing Formulation | Spray | Spray | Spray | Cream |
| Attribute | ||||
| Smell (% excellent and good) | 54.84% | 50% | 38.71% * | 55.17% |
| Application (%very easy and easy) | 87.09% | 68.76% | 38.71% * | 82.76% |
| Spreadability (%very easy and easy) | 87.1% | 75% | 83.87% | 82.76% |
| Stickiness (% without stickiness and lightly sticky) | 67.74% | 75% | 74.19% | 72.42% |
| Absorption (% very easy/pleasant and easy) | 83.87% | 78.13% | 83.87% | 75.86% |
| Velvety touch (%Very velvety and velvety) | NA | NA | NA | 90.32% |
| Purchase intention (% certainly and probable) | 74.2% | 81.25% | 74.19% | 89.65% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Addor, F.A.S.; de Souza, M.C.; Trapp, S.; Peltier, E.; Canosa, J.M. Efficacy and Safety of Topical Dexpanthenol-Containing Spray and Cream in the Recovery of the Skin Integrity Compared with Petroleum Jelly after Dermatologic Aesthetic Procedures. Cosmetics 2021, 8, 87. https://doi.org/10.3390/cosmetics8030087
Addor FAS, de Souza MC, Trapp S, Peltier E, Canosa JM. Efficacy and Safety of Topical Dexpanthenol-Containing Spray and Cream in the Recovery of the Skin Integrity Compared with Petroleum Jelly after Dermatologic Aesthetic Procedures. Cosmetics. 2021; 8(3):87. https://doi.org/10.3390/cosmetics8030087
Chicago/Turabian StyleAddor, Flávia Alvim Sant’Anna, Maurício Cândido de Souza, Sonja Trapp, Erwan Peltier, and Juliana Machado Canosa. 2021. "Efficacy and Safety of Topical Dexpanthenol-Containing Spray and Cream in the Recovery of the Skin Integrity Compared with Petroleum Jelly after Dermatologic Aesthetic Procedures" Cosmetics 8, no. 3: 87. https://doi.org/10.3390/cosmetics8030087
APA StyleAddor, F. A. S., de Souza, M. C., Trapp, S., Peltier, E., & Canosa, J. M. (2021). Efficacy and Safety of Topical Dexpanthenol-Containing Spray and Cream in the Recovery of the Skin Integrity Compared with Petroleum Jelly after Dermatologic Aesthetic Procedures. Cosmetics, 8(3), 87. https://doi.org/10.3390/cosmetics8030087






