UVB Radiation Protective Effect of Brown Alga Padina australis: A Potential Cosmeceutical Application of Malaysian Seaweed
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Collection of P. australis and Preparation of Extracts
2.3. In Vitro Antioxidant Activity
2.3.1. 2,2-Diphenyl-1-picrylhydrazyl (DPPH) Scavenging Activity Assay
2.3.2. Reducing Power Assay
2.4. Estimation of Total Phenolic Content (TPC) and Total Flavonoid Content (TFC)
2.4.1. Total Phenolic Content
2.4.2. Total Flavonoid Content
2.5. UVB Irradiation Assay
2.6. Liquid Chromatography–Mass Spectrometry Analysis
2.7. Statistical Analysis
3. Results
3.1. In Vitro Antioxidant Activity and Phytochemical Contents of the P. australis Extracts
3.2. Effect of P. australis Extracts on the UVB-Induced Keratinocyte Death
3.3. Chemical Profiling of P. australis Extracts
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hussein, M.R. Ultraviolet radiation and skin cancer: Molecular mechanisms. J. Cutan. Pathol. 2005, 32, 191–205. [Google Scholar] [CrossRef]
- Talero, E.; García-Mauriño, S.; Ávila-Román, J.; Rodríguez-Luna, A.; Alcaide, A.; Motilva, V. Bioactive compounds isolated from microalgae in chronic inflammation and cancer. Mar. Drugs 2015, 13, 6152–6209. [Google Scholar] [CrossRef] [PubMed]
- Berthon, J.Y.; Nachat-Kappes, R.; Bey, M.; Cadoret, J.P.; Renimel, I.; Filaire, E. Marine algae as attractive source to skin care. Free. Radic. Res. 2017, 51, 555–567. [Google Scholar] [CrossRef] [PubMed]
- D’Orazio, J.; Jarrett, S.; Amaro-Ortiz, A.; Scott, T. UV radiation and the skin. Int. J. Mol. Sci. 2013, 14, 12222–12248. [Google Scholar] [CrossRef]
- Nishigori, C.; Hattori, Y.; Toyokuni, S. Role of reactive oxygen species in skin carcinogenesis. Antioxid. Redox Signal. 2004, 6, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Lanigan, R.S.; Yamarik, T.A. Final report on the safety assessment of BHT (1). Int. J. Toxicol. 2002, 21, 19–94. [Google Scholar] [CrossRef]
- Vinayak, R.C.; Sabu, A.S.; Chatterji, A. Bio-prospecting of a few brown seaweeds for their cytotoxic and antioxidant activities. Evid. Based Complement. Altern. Med. 2011, 2011, 1–9. [Google Scholar] [CrossRef]
- Nursid, M.; Marasskuranto, E.; Atmojo, K.B.; Hartono, M.P.; Meinita, M.D.N.; Riyanti, R. Investigation on antioxidant compounds from marine algae extracts collected from binuangeun coast, banten, indonesia. Squalen. Bull. Mar. Fishs. Postharvest. Biotechnol. 2017, 11, 59–67. [Google Scholar] [CrossRef][Green Version]
- Thiyagarasaiyar, K.; Goh, B.H.; Jeon, Y.J.; Yow, Y.Y. Algae metabolites in cosmeceutical: An overview of current applications and challenges. Mar. Drugs 2020, 18, 323. [Google Scholar] [CrossRef]
- Thomas, N.V.; Kim, S.K. Beneficial effects of marine algal compounds in cosmeceuticals. Mar. Drugs 2013, 11, 146–164. [Google Scholar] [CrossRef] [PubMed]
- Fernando, I.S.; Nah, J.W.; Jeon, Y.J. Potential anti-inflammatory natural products from marine algae. Environ. Toxicol. Pharmacol. 2016, 48, 22–30. [Google Scholar] [CrossRef]
- Wang, H.M.D.; Chen, C.C.; Huynh, P.; Chang, J.S. Exploring the potential of using algae in cosmetics. Bioresour. Technol. 2015, 184, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Ariede, M.B.; Candido, T.M.; Jacome, A.L.M.; Velasco, M.V.R.; de Carvalho, J.C.M.; Baby, A.R. Cosmetic attributes of algae-A review. Algal Res. 2017, 25, 483–487. [Google Scholar] [CrossRef]
- Guillerme, J.B.; Couteau, C.; Coiffard, L. Applications for marine resources in cosmetics. Cosmetics 2017, 4, 35. [Google Scholar] [CrossRef]
- Florez, N.; Gonzalez-Munoz, M.J.; Ribeiro, D.; Fernandes, E.; Dominguez, H.; Freitas, M. Algae polysaccharides’ chemical characterization and their role in the inflammatory process. Curr. Med. Chem. 2017, 24, 149–175. [Google Scholar] [CrossRef]
- Peng, J.; Yuan, J.-P.; Wu, C.-F.; Wang, J.-H. Fucoxanthin, a marine carotenoid present in brown seaweeds and diatoms: Metabolism and bioactivities relevant to human health. Mar. Drugs 2011, 9, 1806–1828. [Google Scholar] [CrossRef] [PubMed]
- Antony, T.; Chakraborty, K. Xenicanes attenuate pro-inflammatory 5-lipoxygenase: Prospective natural anti-inflammatory leads from intertidal brown seaweed Padina tetrastromatica. Med. Chem. Res. 2019, 28, 591–607. [Google Scholar] [CrossRef]
- Ryu, B.; Qian, Z.J.; Kim, M.M.; Nam, K.W.; Kim, S.K. Anti-photoaging activity and inhibition of matrix metalloproteinase (MMP) by marine red alga, Corallina pilulifera methanol extract. Radiat. Phys. Chem. 2009, 78, 98–105. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, F.; Wang, X.; Liu, X.; Hou, Y.; Zhang, Q. Extraction of the polysaccharides from five algae and their potential antioxidant activity in vitro. Carbohydr. Polym. 2010, 82, 118–121. [Google Scholar] [CrossRef]
- Murthy, K.; Vanitha, A.; Rajesha, J.; Swamy, M.; Sowmya, P.; Ravishankar, G. In vivo antioxidant activity of carotenoids from Dunaliella salina—A green microalga. Life Sci. 2005, 76, 1381–1390. [Google Scholar] [CrossRef]
- Wang, H.M.D.; Li, X.C.; Lee, D.J.; Chang, J.S. Potential biomedical applications of marine algae. Bioresour. Technol. 2017, 244, 1407–1415. [Google Scholar] [CrossRef] [PubMed]
- Saidani, K.; Bedjou, F.; Benabdesselam, F.; Touati, N. Antifungal activity of methanolic extracts of four algerian marine algae species. Afr. J. Biotechnol. 2012, 11, 9496–9500. [Google Scholar] [CrossRef]
- Hwang, E.; Park, S.Y.; Sun, Z.W.; Shin, H.S.; Lee, D.G.; Yi, T.H. The protective effects of fucosterol against skin damage in UVB-irradiated human dermal fibroblasts. Mar. Biotechnol. 2014, 16, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Canoy, J.L.; Bitacura, J.G. Cytotoxicity and antiangiogenic activity of turbinaria ornata agardh and Padina australis hauck ethanolic extracts. Anal. Cell. Pathol. 2018, 2018. [Google Scholar] [CrossRef]
- Gany, S.A.; Tan, S.C.; Gan, S.Y. Antioxidative, anticholinesterase and anti-neuroinflammatory properties of Malaysian brown and green seaweeds. World. Acad. Sci. Eng. Technol. 2015, 8, 1269–1275. [Google Scholar] [CrossRef]
- Murugan, A.C.; Vallal, D.; Karim, M.R.; Govindan, N.; Yusoff, M.; Rahman, M.M. In vitro antiradical and neuroprotective activity of polyphenolic extract from marine algae Padina australis H. J. Chem. Pharm. Res 2015, 7, 355–362. [Google Scholar]
- Akbari, V.; Safaiee, F.; Yegdaneh, A. Bioassay-guided fractionation and antimicrobial activities of Padina australis extracts. Jundishapur J. Nat. Pharm. Prod. 2020, 15, e68304. [Google Scholar] [CrossRef]
- Salosso, Y.; Aisiah, S.; Toruan, L.N.L.; Pasaribu, W. Nutrient content, active compound and antibacterial activity of Padina australis against Aeromonas hydropilla. Pharmacogn. J. 2020, 12, 771–776. [Google Scholar] [CrossRef]
- Chellappan, D.K.; Chellian, J.; Leong, J.Q.; Liaw, Y.Y.; Gupta, G.; Dua, K.; Kunnath, A.P.; Palaniveloo, K. Biological and therapeutic potential of the edible brown marine seaweed Padina australis and their pharmacological mechanisms. J. Trop. Biol. Conserv. 2020, 17, 251–271. [Google Scholar]
- Tan, L.T.H.; Mahendra, C.K.; Yow, Y.Y.; Chan, K.G.; Khan, T.M.; Lee, L.H.; Goh, B.H. Streptomyces sp. MUM273b: A mangrove-derived potential source for antioxidant and UVB radiation protectants. BMC Microbiol. 2019, 8, 859. [Google Scholar] [CrossRef]
- Pang, J.R.; Goh, V.M.J.; Tan, C.Y.; Phang, S.M.; Wong, K.H.; Yow, Y.Y. Neuritogenic and in vitro antioxidant activities of Malaysian Gracilaria manilaensis Yamamoto & Trono. J. Appl. Phycol. 2018, 30, 3253–3260. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Pękal, A.; Pyrzynska, K. Evaluation of aluminium complexation reaction for flavonoid content assay. Food Anal. Methods 2014, 7, 1776–1782. [Google Scholar] [CrossRef]
- Mahendra, C.K.; Tan, L.T.H.; Yap, W.H.; Chan, C.K.; Pusparajah, P.; Goh, B.H. An optimized cosmetic screening assay for ultraviolet B (UVB) protective property of natural products. Prog. Drug Discov. Biomed. Sci. 2019, 2. [Google Scholar] [CrossRef]
- Gil-Izquierdo, A.; Gil, M.I.; Ferreres, F.; Tomás-Barberán, F.A. In vitro availability of flavonoids and other phenolics in orange juice. J. Agric. Food Chem. 2001, 49, 1035–1041. [Google Scholar] [CrossRef]
- Rao, N.P.; Sastry, K.V.; Rao, E.V. Carbohydrates of Padina tetrastromatica. Phytochemistry 1984, 23, 2531–2533. [Google Scholar] [CrossRef]
- Chen, L.; Huang, G.; Hu, J. Preparation, deproteinization, characterisation, and antioxidant activity of polysaccharide from cucumber (Cucumis saticus L.). Int. J. Biol. Macromol. 2018, 108, 408–411. [Google Scholar] [CrossRef]
- Cosmetics.specialchem.com. Rhamnosoft® HP ST-Solabia-Datasheet. Available online: https://cosmetics.specialchem.com/product/i-solabia-rhamnosoft-hp-st (accessed on 27 December 2020).
- Parameswaran, P.S.; Naik, C.G.; Das, B.; Kamat, S.Y.; Bose, A.K.; Nair, M.S.R. Constituents of the brown alga Padina tetrastromatica (Hauck)-II. Indian J. Chem. Sect. B 1996, 35, 463–467. [Google Scholar]
- Erukainure, O.L.; Chukwuma, C.I.; Islam, M.S. Raffia palm (Raphia hookeri) wine: Qualitative sugar profile, functional chemistry, and antidiabetic properties. Food Biosci. 2019, 30, 100423. [Google Scholar] [CrossRef]
- Agatonovic-Kustrin, S.; Morton, D.W. High-performance thin-layer chromatography-direct bioautography as a method of choice for alpha-amylase and antioxidant activity evaluation in marine algae. J. Chromatogr. A 2017, 1530, 197–203. [Google Scholar] [CrossRef]
- Raguraman, V.; MubarakAli, D.; Narendrakumar, G.; Thirugnanasambandam, R.; Kirubagaran, R.; Thajuddin, N. Unraveling rapid extraction of fucoxanthin from Padina tetrastromatica: Purification, characterization and biomedical application. Process Biochem. 2018, 73, 211–219. [Google Scholar] [CrossRef]
- Karkhaneh Yousefi, M.; Seyed Hashtroudi, M.; Mashinchian Moradi, A.; Ghasempour, A. Seasonal variation of fucoxanthin content in four species of brown seaweeds from Qeshm Island, Persian Gulf and evaluation of their antibacterial and antioxidant activities. Iran. J. Fish. Sci. 2020, 19, 2394–2408. [Google Scholar]
- Namjooyan, F.; Farasat, M.; Alishahi, M.; Jahangiri, A.; Mousavi, H. The anti-melanogenesis activities of some selected brown macroalgae from northern coasts of the Persian Gulf. Braz. Arch. Biol. Technol. 2019, 62, e1918019. [Google Scholar] [CrossRef]
- Jaswir, I.; Noviendri, D.; Salleh, H.M.; Taher, M.; Miyashita, K. Isolation of fucoxanthin and fatty acids analysis of Padina australis and cytotoxic effect of fucoxanthin on human lung cancer (H1299) cell lines. Afr. J. Biotechnol. 2011, 10, 18855–18862. [Google Scholar] [CrossRef]
- Karkhane Yousefi, M.; Seyed Hashtroudi, M.; Mashinchian Moradi, A.; Ghasempour, A.R. In vitro investigating of anticancer activity of focuxanthin from marine brown seaweed species. Glob. J. Environ. Sci. Manag. 2018, 4, 81–90. [Google Scholar] [CrossRef]
- Dang, T.T.; Bowyer, M.C.; Van Altena, I.A.; Scarlett, C.J. Comparison of chemical profile and antioxidant properties of the brown algae. Int. J. Food Sci. Technol. 2018, 53, 174–181. [Google Scholar] [CrossRef]
- Yap, W.F.; Tay, V.; Tan, S.H.; Yow, Y.Y.; Chew, J. Decoding antioxidant and antibacterial potentials of Malaysian green seaweeds: Caulerpa racemosa and Caulerpa lentillifera. Antibiotics 2019, 8, 152. [Google Scholar] [CrossRef]
- Foo, S.C.; Khong, N.M.; Yusoff, F.M. Physicochemical, microstructure and antioxidant properties of microalgae-derived fucoxanthin rich microcapsules. Algal Res. 2020, 51, 102061. [Google Scholar] [CrossRef]
- Arshad, A.; Rehman, T.; Saleem, H.; Khan, S.; Saleem, M.; Tousif, M.I.; Ahemad, S.; Ahemad, N.; Abdallah, H.H.; Mahomoodally, F.M. In vitro enzyme inhibition, antibacterial, UHPLC-MS chemical profiling and in silico studies of Indigofera argentea Burm. f. for potential biopharmaceutical application. S. Afr. J. Bot. 2020. [Google Scholar] [CrossRef]
- Azizan, A.; Ahamad Bustamam, M.S.; Maulidiani, M.; Shaari, K.; Ismail, I.S.; Nagao, N.; Abas, F. Metabolite profiling of the microalgal diatom Chaetoceros calcitrans and correlation with antioxidant and nitric oxide inhibitory activities via 1H NMR-based metabolomics. Mar. Drugs 2018, 16, 154. [Google Scholar] [CrossRef]
- Bakar, K.; Mohamad, H.; Latip, J.; Tan, H.S.; Herng, G.M. Fatty acids compositions of Sargassum granuliferum and Dictyota dichotoma and their anti-fouling activities. J. Sustain. Sci. Manag. 2017, 12, 8–16. [Google Scholar]
- Harper, D.R.; Gilbert, R.L.; O’Connor, T.J.; Kinchington, D.; Mahmood, N.; Mcllhinney, R.A.J.; Jeffries, D.J. Antiviral activity of 2-hydroxy fatty acids. Antivir. Chem. Chemother. 1996, 7, 138–141. [Google Scholar] [CrossRef][Green Version]
- Kajiwara, T.; Kodama, K.; Hatanaka, A.; Matsui, K. Biogeneration of volatile compounds via oxylipins in edible seaweeds. In Biotechnology for Improved Food and Flavors American Chemical Society Symposium Series 637; Takeoka, G.R., Teranishi, R., Williams, P.J., Kobayashi, A., Eds.; American Cancer Society: Washington, DC, USA, 1996; pp. 146–166. [Google Scholar]
- Espelie, K.E.; Dean, B.B.; Kolattukudy, P.E. Composition of lipid-derived polymers from different anatomical regions of several plant species. Plant Physiol. 1979, 64, 1089–1093. [Google Scholar] [CrossRef] [PubMed]
- Arshad, A.; Ahemad, S.; Saleem, H.; Saleem, M.; Zengin, G.; Abdallah, H.H.; Tousif, M.I.; Ahemad, N.; Fawzi Mahomoodally, M. RP-UHPLC-MS chemical profiling, biological and in silico docking studies to unravel the therapeutic potential of Heliotropium crispum Desf. as a Novel Source of Neuroprotective Bioactive Compounds. Biomolecules 2021, 11, 53. [Google Scholar] [CrossRef] [PubMed]
- Elkhateeb, W.; ELDien, A.N.; Fadl, E.; Elhagrasi, A.; Fayad, W.; Wen, T.C. Therapeutic potentials of n-hexane extracts of the three medicinal mushrooms regarding their anti-colon cancer, antioxidant, and hypocholesterolemic capabilities. Biodiversitas J. Biol. Divers. 2020, 21. [Google Scholar] [CrossRef]
- Ferreira, L.D.S.; Turatti, I.C.C.; Lopes, N.P.; Guaratini, T.; Colepicolo, P.; Oliveira Filho, E.C.; Garla, R.C. Apolar compounds in seaweeds from Fernando de Noronha archipelago (northeastern coast of Brazil). Int. J. Anal. Chem. 2012, 2012. [Google Scholar] [CrossRef]
- Wu, B.; Xiao, X.; Li, S.; Zuo, G. Transcriptomics and metabonomics of the anti-aging properties of total flavones of Epimedium in relation to lipid metabolism. J. Ethnopharmacol. 2019, 229, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Soib, H.H.; Ismail, H.F.; Husin, F.; Abu Bakar, M.H.; Yaakob, H.; Sarmidi, M.R. Bioassay-guided different extraction techniques of Carica papaya (Linn.) leaves on in vitro wound-healing activities. Molecules 2020, 25, 517. [Google Scholar] [CrossRef]
- Chia, Y.Y.; Kanthimathi, M.S.; Khoo, K.S.; Rajarajeswaran, J.; Cheng, H.M.; Yap, W.S. Antioxidant and cytotoxic activities of three species of tropical seaweeds. BMC Complement. Altern. Med. 2015, 15, 339. [Google Scholar] [CrossRef]
- Saleem, H.; Sarfraz, M.; Khan, K.M.; Anwar, M.A.; Zengin, G.; Ahmad, I.; Khan, S.U.; Mahomoodally, M.F.; Ahemad, N. UHPLC-MS phytochemical profiling, biological propensities and in-silico studies of Alhagi maurorum roots: A medicinal herb with multifunctional properties. Drug. Dev. Ind. Pharm. 2020, 46, 861–868. [Google Scholar] [CrossRef]
- Udaya Prakash, N.K.; Sripriya, N.S.; Raj, D.D.; Deepa, S.; Bhuvaneswari, S. Antioxidant potency and GC-MS composition of Origanum majorana Linn. Pak. J. Pharm. Sci. 2019, 32, 2117–2122. [Google Scholar]
- Xie, X.; Chen, C.; Fu, X. Screening α-glucosidase inhibitors from four edible brown seaweed extracts by ultra-filtration and molecular docking. LWT 2021, 138, 110654. [Google Scholar] [CrossRef]
- Dugasani, S.; Pichika, M.R.; Nadarajah, V.D.; Balijepalli, M.K.; Tandra, S.; Korlakunta, J.N. Comparative antioxidant and anti-inflammatory effects of [6]-gingerol,[8]-gingerol,[10]-gingerol and [6]-shogaol. J. Ethnopharmacol. 2010, 127, 515–520. [Google Scholar] [CrossRef]
- Park, M.; Bae, J.; Lee, D.S. Antibacterial activity of [10]-gingerol and [12]-gingerol isolated from ginger rhizome against periodontal bacteria. Phytother. Res. 2008, 22, 1446–1449. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Kim, Y.; Na, K.M.; Surh, Y.J.; Kim, T.Y. [6]-Gingerol prevents UVB-induced ROS production and COX-2 expression in vitro and in vivo. Free Radic. Res. 2007, 41, 603–614. [Google Scholar] [CrossRef] [PubMed]
- Ichihashi, M.; Ahmed, N.U.; Budiyanto, A.; Wu, A.; Bito, T.; Ueda, M.; Osawa, T. Preventive effect of antioxidant on ultraviolet-induced skin cancer in mice. J. Dermatol. Sci. 2000, 23, S45–S50. [Google Scholar] [CrossRef]
- Lauriola, M.M.; Sena, P.; De Bitonto, A.; Corazza, M. Irritant contact dermatitis after a curious therapeutic use of oregano essential oil. Contact Dermatitis 2020, 83, 129–130. [Google Scholar] [CrossRef]
- Mazzarello, V.; Gavini, E.; Rassu, G.; Donadu, M.G.; Usai, D.; Piu, G.; Pomponi, V.; Sucato, F.; Zanetti, S.; Montesu, M.A. Clinical assessment of new topical cream containing two essential oils combined with tretinoin in the treatment of acne. Clin. Cosmet. Investig. Dermatol. 2020, 13, 233. [Google Scholar] [CrossRef]
- Orchard, A.; van Vuuren, S. Commercial essential oils as potential antimicrobials to treat skin diseases. Evid. Based Complement. Altern. Med. 2017, 2017. [Google Scholar] [CrossRef]
- Joshi, S.; Kumari, R.; Upasani, V.N. Applications of algae in cosmetics: An overview. Int. J. Innov. Res. Sci. Eng. Technol. 2018, 7, 1269. [Google Scholar] [CrossRef]
- Uk.elemis.com. Available online: https://uk.elemis.com/pro-collagen-marine-cream.html (accessed on 17 April 2021).
- Babusikova, E.; Jurecekova, J.; Evinova, A.; Jesenak, M.; Dobrota, D. Oxidative damage and bronchial asthma. In Respiratory Diseases; Ghanei, M., Ed.; InTech: Rijeka, Croatia, 2012; pp. 151–176. [Google Scholar]
- Cerretani, L.; Bendini, A. Chapter 67—Rapid assays to evaluate the antioxidant capacity of phenols in virgin olive oil. In Olives and Olive Oil in Health and Disease Prevention; Preedy, V.R., Watson, R.R., Eds.; Academic Press: San Diego, CA, USA, 2010; pp. 625–635. [Google Scholar]
- Prieto, M.A.; Curran, T.P.; Gowen, A.; Vázquez, J.A. An efficient methodology for quantification of synergy and antagonism in single electron transfer antioxidant assays. Food Res. Int. 2015, 67, 284–298. [Google Scholar] [CrossRef]
- Subermaniam, K.; Yow, Y.Y.; Lim, S.H.; Koh, O.H.; Wong, K.H. Malaysian macroalga Padina australis Hauck attenuates high dose corticosterone-mediated oxidative damage in PC12 cells mimicking the effects of depression. Saudi J. Biol. Sci. 2020, 27, 1435. [Google Scholar] [CrossRef] [PubMed]
- Akbary, P.; Aminikhoei, Z.; Hobbi, M.; Kuchaksaraei, B.S.; Tavabe, K.R. Antioxidant properties and total phenolic contents of extracts from three macroalgae collected from Chabahar coasts. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2021, 91, 327–334. [Google Scholar] [CrossRef]
- Kaufman, P.B.; Cseke, L.J.; Warber, C.S.; James, A.S.; Brielmann, H.L. Natural Products from Plants; CRC Press: London, UK, 1999. [Google Scholar]
- Jayaprakasha, G.K.; Patil, B.S. In vitro evaluation of the antioxidant activities in fruit extracts from citron and blood orange. Food Chem. 2007, 101, 410–418. [Google Scholar] [CrossRef]
- Gunji, S.; Santoso, J.; Yoshie-Stark, Y.; Suzuki, T. Effects of extracts from tropical seaweeds on DPPH radicals and Caco-2 cells treated with hydrogen peroxide. Food Sci. Technol. Res. 2007, 13, 275–279. [Google Scholar] [CrossRef][Green Version]
- Matsui, M.; Tanaka, K.; Higashiguchi, N.; Okawa, H.; Yamada, Y.; Tanaka, K.; Taira, S.; Aoyama, T.; Takanishi, M.; Natsume, C.; et al. Protective and therapeutic effects of fucoxanthin against sunburn caused by UV irradiation. J. Pharmacol. Sci. 2016, 132, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Heo, S.J.; Jeon, Y.J. Protective effect of fucoxanthin isolated from Sargassum siliquastrum on UV-B induced cell damage. J. Photochem. Photobiol. B 2009, 95, 101–107. [Google Scholar] [CrossRef]
- Guermouche, B.; Soulimane-Mokhtari, N.A.; Bouanane, S.; Merzouk, H.; Merzouk, S.; Narce, M. Effect of dietary polyunsaturated fatty acids on oxidant/antioxidant status in macrosomic offspring of diabetic rats. BioMed Res. Int. 2014, 2014, 368107. [Google Scholar] [CrossRef] [PubMed]
- Luzia, D.M.; Jorge, N. Bioactive substance contents and antioxidant capacity of the lipid fraction of Annona crassiflora Mart. seeds. Ind. Crops Prod. 2013, 42, 231–235. [Google Scholar] [CrossRef]
- Grassmann, J. Terpenoids as plant antioxidants. Vitam. Horm. 2005, 72, 505–535. [Google Scholar] [CrossRef]
- Xuan, S.H.; Lee, N.H.; Park, S.N. Atractyligenin, a terpenoid isolated from coffee silverskin, inhibits cutaneous photoaging. J. Photochem. Photobiol. B 2019, 194, 166–173. [Google Scholar] [CrossRef] [PubMed]
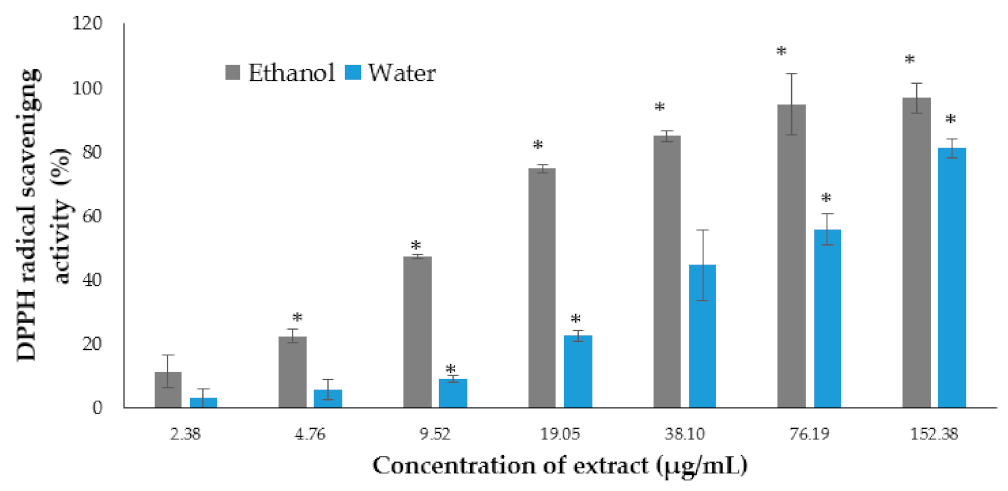
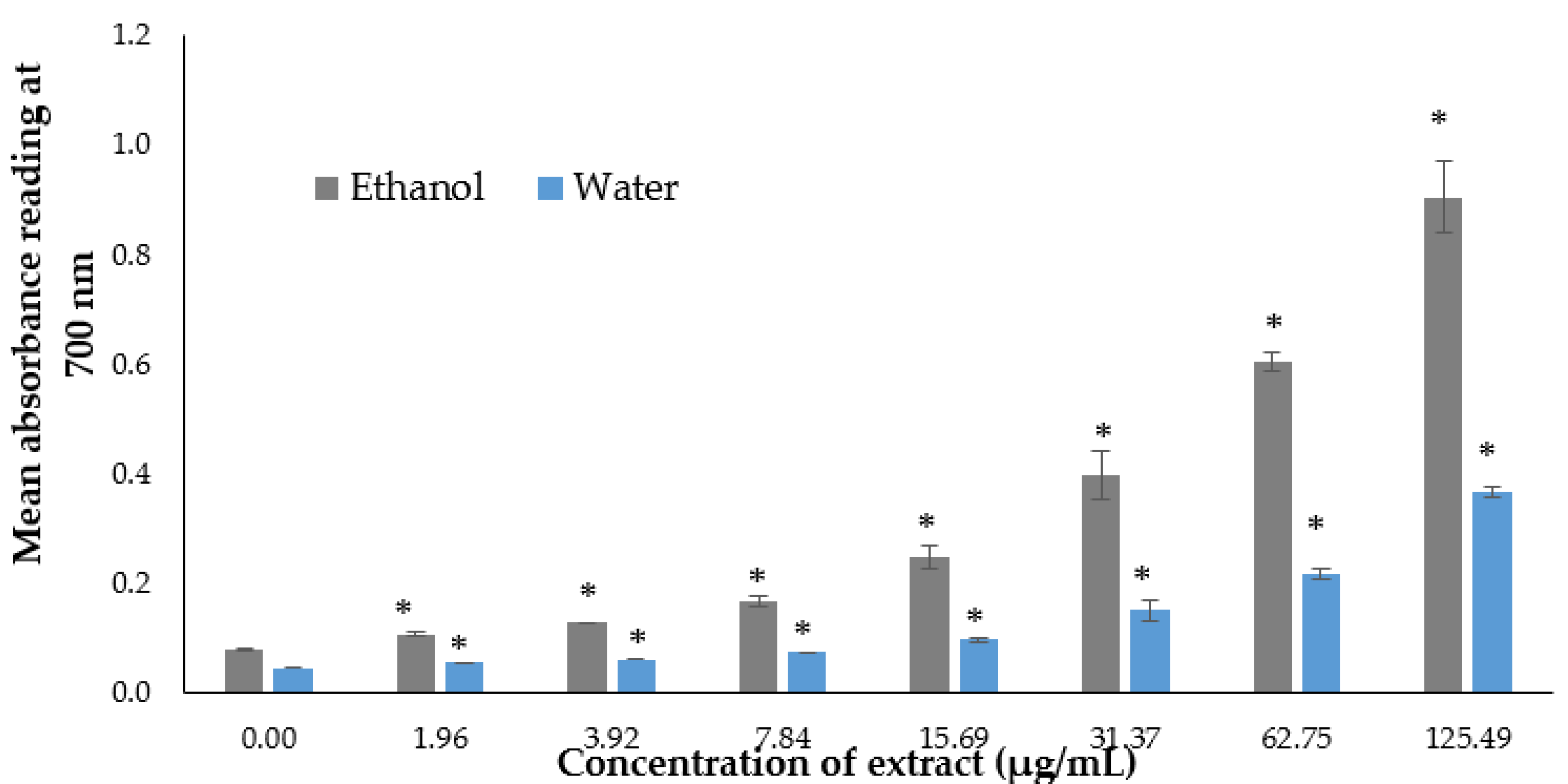

| Extract | Total Phenolic Content (mg GAE/g) | Total Flavonoid Content (mg QE/g) |
|---|---|---|
| Ethanol | 76.04 ± 7.35 | 50.07 ± 1.71 |
| Water | 27.01 ± 1.77 | 7.53 ± 0.38 |
| Antioxidant Activities | Phenolic Content | Flavonoid Content |
|---|---|---|
| DPPH radical scavenging activity | r = 0.912 * | r = 0.883 * |
| Reducing power activity | r = 0.994 * | r = 0.988 * |
| No | Compound Name | Formula | m/z | Mass | Polarity | Activity |
|---|---|---|---|---|---|---|
| 1 | L-rhamnulose | C6H12O5 | 165.08 | 164.07 | Positive | L-rhamnulose is a breakdown product of L-rhamnose, which is classified as a sulphated heteropolysaccharide. L-rhamnose is found in Padina tetrastromatica [36] and has antioxidant [37], anti-inflammation and anti-aging properties [38]. |
| 2 | Dulcitol | C6H14O6 | 183.09 | 182.08 | Positive | Galactitol or dulcitol is a sugar alcohol that is a metabolic breakdown product of galactose. The compound is found in Padina tetrastromatica [39]. Raffia palm wine sample contains dulcitol and exhibits antioxidant and antidiabetic properties [40]. |
| 3 | Fucoxanthin | C42H58O6 | 681.41 | 658.42 | Positive/Negative | This compound is found in Padina minor [41], Padina tetrastromatica [42], Padina boryana (formerly Padina tenuis) [43], Padina boergesenii [44] and P. australis [45]. The compound exhibits anti-cancer [46], anti-diabetic, anti-oxidative [47], neuroprotective and antimelanogenic activities [44]. |
| 4 | 9Z,12Z,15E-octadecatrienoic acid | C18H30O2 | 279.23 | 278.23 | Positive | 9Z,12Z,15E-octadecatrienoic acid is a fatty acid found in Caulerpa racemosa [48]. |
| 5 | 3α,12α-dihydroxy-5β-chol-8(14)-en-24-oic acid | C24H38O4 | 413.27 | 390.28 | Positive | This compound is classified as terpenoid and found in Chaetoceros calcitrans, a marine diatom [49]. This compound is also found in Indigofera argentea Burm. f., and the methanol crude extract exhibits antibacterial activity [50]. |
| 6 | (3S,4S,3′R)-4-hydroxyalloxanthin | C40H52O3 | 581.40 | 580.39 | Positive | This compound is found in Chaetoceros calcitrans and is classified as a carotenoid [51]. |
| 7 | 3,6-epoxy-5,5′,6,6′-tetrahydro-b,b-carotene-3′,5,5′,6′-tetrol | C40H58O5 | 641.42 | 618.43 | Positive | This compound is found in Chaetoceros calcitrans and is classified as a tetraterpenoid [49]. |
| 8 | 2-hydroxyhexadecanoic acid | C16H32O3 | 271.23 | 272.24 | Negative | The compound is categorized as a fatty acid and is found in Dictyota dichotoma. The compound has anti-fouling [52] and anti-viral activities [53]. |
| 9 | 9-keto palmitic acid | C16H30O3 | 269.21 | 270.22 | Negative | 9-keto palmitic acid is also known as 9-oxo palmitic acid. A study reported that 2-oxo palmitic acid is found in Ulva australis (formerly Ulva pertusa) [54]. |
| 10 | 9,16-dihydroxy-palmitic acid | C16H32O4 | 287.22 | 288.23 | Negative | The compound is presented in Vicia faba (vascular plant) [55]. |
| 11 | 9,10-epoxy-18-hydroxystearate | C18H34O4 | 313.24 | 314.25 | Negative | The compound is found in Heliotropium crispum (vascular plant) [56], and the methanol extract has antioxidant activity. |
| No | Compound Name | Formula | m/z | Mass | Polarity | Activity |
|---|---|---|---|---|---|---|
| 1 | 2,6-nonadien-1-ol | C9H16O | 158.15 | 140.12 | Positive | This compound is found in Phallus indusiatus (formerly Dictyophora indusiate) (Fungi) n-hexane extract and exhibits the highest antioxidant activity [57]. |
| 2 | Palmitic amide | C16H33NO | 256.26 | 255.26 | Positive | This compound belongs to fatty acid amides and is found in Padina gymnospora [58]. Epimedium (vascular plant) contains palmitic amide and exhibits anti-aging related to lipid metabolism [59]. This compound is found in Carica papaya (vascular plant), and the extract exhibits antioxidant and wound-healing properties [60]. |
| 3 | Emmotin A | C16H22O4 | 279.16 | 278.15 | Positive | Emmotin A is a terpenoid. Emmotin A is found in Padina tetrastromatica, and the extract has immunomodulatory activity [61]. Alhagi maurorum (vascular plant) roots contain emmotin A, and the extract has binding interaction with AChE followed by BChE, α-glucosidase, α-amylase and tyrosinase [62]. |
| 4 | Docosanedioic acid | C22H42O4 | 393.30 | 370.31 | Positive | This is a fatty acid molecule. Methanol extract of Origanum majorana (vascular plant) contains docosanedioic acid compound, and this extract exhibits significant antioxidant activity [63]. |
| 5 | Gingerol | C17H26O4 | 293.18 | 294.18 | Negative | This compound is found in Sargassum pallidum, Ecklonia cava subsp. kurome (formerly Ecklonia kurome) and Sargassum fusiforme (formerly Hizikia fusiforme) [64]. It exhibits antioxidant, anti-inflammation [65] and antibacterial activities against Porphyromonas gingivalis, Porphyromonas endodontalis and Prevotella intermedia [66] and possesses a UV protection effect [67]. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thiyagarasaiyar, K.; Mahendra, C.K.; Goh, B.-H.; Gew, L.T.; Yow, Y.-Y. UVB Radiation Protective Effect of Brown Alga Padina australis: A Potential Cosmeceutical Application of Malaysian Seaweed. Cosmetics 2021, 8, 58. https://doi.org/10.3390/cosmetics8030058
Thiyagarasaiyar K, Mahendra CK, Goh B-H, Gew LT, Yow Y-Y. UVB Radiation Protective Effect of Brown Alga Padina australis: A Potential Cosmeceutical Application of Malaysian Seaweed. Cosmetics. 2021; 8(3):58. https://doi.org/10.3390/cosmetics8030058
Chicago/Turabian StyleThiyagarasaiyar, Krishnapriya, Camille Keisha Mahendra, Bey-Hing Goh, Lai Ti Gew, and Yoon-Yen Yow. 2021. "UVB Radiation Protective Effect of Brown Alga Padina australis: A Potential Cosmeceutical Application of Malaysian Seaweed" Cosmetics 8, no. 3: 58. https://doi.org/10.3390/cosmetics8030058
APA StyleThiyagarasaiyar, K., Mahendra, C. K., Goh, B.-H., Gew, L. T., & Yow, Y.-Y. (2021). UVB Radiation Protective Effect of Brown Alga Padina australis: A Potential Cosmeceutical Application of Malaysian Seaweed. Cosmetics, 8(3), 58. https://doi.org/10.3390/cosmetics8030058









