Abstract
Marine natural products are a good source of antioxidants due to the presence of a wide range of bioactive compounds. Accumulating evidence proves the potential use of seaweed-derived ingredients in skincare products. This study aims to evaluate the ultraviolet (UV) protective activity of the ethanol and water extracts of Padina australis. As the preliminary attempt for this discovery, the total phenolic content (TPC) and total flavonoid content (TFC) were measured, followed by the in vitro antioxidant activity using 2,2-diphenyl-1-picrylhydrazyl (DPPH) and reducing the power to shed light on its bioactivity. The UVB protective activity was examined on HaCaT human keratinocyte cells. The findings of this study reveal that the P. australis ethanol extract serves as a promising source of antioxidants, as it exhibits stronger antioxidant activities compared with the water extract in DPPH and the reducing power assays. The P. australis ethanol extract also demonstrated a higher level of total phenolic (76 mg GAE/g) and flavonoid contents (50 mg QE/g). Meanwhile, both the ethanol (400 µg/mL) and water extracts (400 µg/mL) protected the HaCaT cells from UVB-induced cell damage via promoting cell viability. Following that, LCMS analysis reveals that the P. australis ethanol extract consists of sugar alcohol, polysaccharide, carotenoid, terpenoid and fatty acid, whereas the water extract contains compounds from phenol, terpenoid, fatty acid, fatty alcohol and fatty acid amide. In summary, biometabolites derived from P. australis have diverse functional properties, and they could be applied to the developments of cosmeceutical and pharmaceutical products.
1. Introduction
Skin is one of the most complex and largest organs, serving as a protective barrier against internal and external stress. Skin aging is one of the factors which bring concern to humans, as they do not want to lose their youthful apperance. Skin aging can be catergorized into intrinsic and extrinsic aging, where extrinsic aging is mostly caused by ultraviolet (UV) radiation. Chronic exposure to ultraviolet (UV) radiation will cause damage to the intracellular biomolecules (proteins, lipids, polysaccharides and nucleic acids) and result in skin inflammation, photoaging, hyperpigmentation and skin cancer [1,2,3]. UV radiation, a ubiquitous environmental carcinogen, can be categorized into UV-A (320–400 nm), UV-B (280–320 nm) and UV-C (200–280 nm) [4]. Among these three types of UV rays, UVB radiation causes a deleterious effect on human skin by inducing genomic lesions in the nuclear and mitochondrial DNA or the production of reactive oxygen species (ROS) [5]. Thus, the usage of antioxidants is known to be effective against UV-induced photobiologic damages. However, commonly used synthetic antioxidants such as butylated hydroxyanisole (BHA), butylated hydroxytoluene (BHT), propyl gallate and tert-Butylhydroquinone (TBHQ) are suspected to cause liver damage and cancer [6,7,8]. This could be highly correlated with the side effects of synthetic antioxidants. Therefore, efforts are being oriented toward focusing on natural ingredients with potent antioxidant activity, such as marine algae.
Marine algae are a great resource for in-demand active compounds with a wide range of cosmeceutical applications [9]. They are rich in bioactive compounds such as unsaturated fatty acids, polysaccharides, vitamins and essential amino acids [10,11], which have therapeutic effects including anti-cancer, anti-inflammation, antioxidant, anti-microbial, antiaging, antiallergic and antiviral activities [12,13,14,15]. Marine algae such as Cladosiphon okamuranus, Sargassum fulvellum, Padina tetrastromatic (Ochrophyta, Phaeophyceae), Corallina pilulifera (Rhodophyta), Bryopsis plumose (Chlorophyta) and the microalgae Dunaliella salina (Chlorophyta), have been reported to possess antioxidant activity [16,17,18,19,20]. Notably, brown seaweeds are rich in biometabolites such as fucoidan, fucoxanthin, sulphated polysaccharide, polyphenol and fucosterol, which have been shown to possess anti-inflammatory [16], antioxidant [2], anti-cancer [21], antibacterial [22] and antiaging properties [23]. Therefore, tapping into algae as a source of the natural product may provide the impetus for the development of novel cosmeceuticals.
Malaysia is blessed with a long coastline, which is has sites for the flourishing growth of algae. The coastline is found to be an exceptionally harsh site for organisms to grow. As of yet, with these challenging conditions, algae species are thriving surprisingly well. It is postulated that the algae residing herein have developed several unique metabolic pathways that would allow them to tolerate harsh environmental conditions, such as higher levels of salinity and fluctuations in tidal gradients, temperature, pH, and UV exposure [8].
Padina australis Hauck is a brown alga (Ochrophyta, Phaeophyceae) which is distributed from the intertidal zone to the open ocean in Malaysian waters. P. australis has been reported to exhibit biological activities, including anti-angiogenic [24], antioxidant, anti-neuroinflammation [25,26], antimicrobial [27,28] antidiabetic, antihypertensive, antibacterial and anti-inflammatory activities [29]. The present study aims to evaluate the in vitro antioxidant and UVB protective potential of P. australis extracts in HaCaT human keratinocytes. Liquid chromatography–mass spectrometry (LC-MS) analysis was performed to profile the possible chemical constituents present in the P. australis extracts.
2. Materials and Methods
2.1. Cell Culture
The HaCaT human keratinocytes were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) GlutaMAX without HEPES (Gibco, Thermo Fisher, Waltham, MA, USA), supplemented with 1.0% of 100× antibiotic-antimycotic (100 U/mL penicillin, 100 µg/mL streptomycin and 25 µg/mL amphotericin B) (Gibco, Thermo Fisher, Waltham, MA, USA) and 10.0% fetal bovine serum (Gibco, Thermo Fisher, Waltham, MA, USA) [30].
2.2. Collection of P. australis and Preparation of Extracts
The fresh specimens were collected along the coast of Port Dickson, Negeri Sembilan, Malaysia, between March and May 2019. The specimens were identified based on morphology, and they were washed using saltwater to remove debris and then rinse with distilled water to be stored at −20 °C. The frozen samples were freeze dried (Labogene, Bjarkesvej, Germany) and grinded into powder using a grinder and stored at −20 °C. Seaweed powder was dissolved in two solvents—water or ethanol—at a ratio of 1:50 (w/v). We added 250 mL of the appropriate solvent to 5 g of seaweed powder, mixed in a shaker at 200 rpm for 48 h and then centrifuged at 4000 rpm at 4 °C for 20 min. The supernatant was sent for rotary evaporation (Fisher Scientific EYELA N-1200A Rotary Evaporator, Koishikawa Bunkyo, Tokyo) and further concentrated using a vacuum concentrator (ScanSpeed 40, Bio-Medical Science, Seoul, Korea) at 1000 rpm for 18–20 h. For the UVB irradiation assay, the ethanol extract was dissolved in DMSO, whereas the water extract was dissolved in distilled water. However, the final concentration of DMSO was maintained at 0.5% to prevent the toxic effect of the solvent.
2.3. In Vitro Antioxidant Activity
2.3.1. 2,2-Diphenyl-1-picrylhydrazyl (DPPH) Scavenging Activity Assay
The 2,2-Diphenyl-1-picrylhydrazyl (DPPH) scavenging activity was determined as described by Pang et al. [31]. A DPPH solution was used to determine the free radical scavenging activity, where 50 µL of each sample was dissolved in 1 mL of 0.1 mM DPPH and a negative control consisting of 50 µL of distilled water, whereas the positive control consisted of 50 µL of ascorbic acid (Sigma Aldrich, Tokyo, Japan). Both the controls and samples were incubated in the dark for 30 min at room temperature. The results were analyzed using a microplate reader at 518 nm using a UV-Vis spectrophotometer microplate reader (Infinite 200 Pro, Tecan, Männedorf, Switzerland).
The percentage of DPPH radical scavenging activity was calculated using the following formula (1):
2.3.2. Reducing Power Assay
The reducing power was determined according to the method of Pang et al. [31] with slight modifications. Ascorbic acid (Sigma-Aldrich, Darmstadt, Germany ) was the positive control, and 100 µL of algae extracts with different initial concentrations (0.05, 0.1, 0.2, 0.4, 0.8, 1.6 and 3.2 mg/mL) were utilized. The negative control consisted of 100 µL of distilled water for the water extract and 100 µL of ethanol for the ethanol extract, whereas the positive control consisted of 100 µL of ascorbic acid (Sigma-Aldrich, Tokyo, Japan) with different initial concentrations (4.625, 9.25, 18.75, 37.5, 75, 150 and 300 µg/mL). All the samples were dissolved with 250 μL of a 0.2 M phosphate buffer and 250 μL of 1% potassium ferricyanide and then incubated at 50 °C for 20 min. Then, 250 μL of 10% trichloroacetic acid was added into each mixture, and the mixture was centrifuged at 300× g for 10 min. Next, 250 µL of supernatant aliquots were mixed with 250 μL of 0.1% iron(III) chloride and 250 μL of distilled water. The absorbance was analyzed using a UV-Vis spectrophotometer microplate reader (Infinite 200 Pro, Tecan, Männedorf, Switzerland) at 700 nm.
2.4. Estimation of Total Phenolic Content (TPC) and Total Flavonoid Content (TFC)
2.4.1. Total Phenolic Content
The total phenolic content was determined as described in [32] using the Folin–Ciocalteu reagent. We dissolved 5 µL of each crude extract (6.4 mg/mL) in 25 µL of the Folin–Ciocalteu reagent (R & M Chemicals, Selangor, Malaysia) and 350 µL of distilled water. After 5 min, 75 µL of 20% of sodium carbonate was added to the mixture, and the volume was increased up to 500 µL with distilled water. The samples were incubated for 1 h at room temperature. The phenolic content was calculated as gallic acid equivalents (GAE/g) of a dry sample based on a standard curve of gallic acid (50, 100, 250, 500 and 1000 µg/mL). The concentration of the total phenolic content in the test samples was calculated from the calibration plot. All determinations were carried out in triplicate, and the results were analyzed at 750 nm with a UV-Vis spectrophotometric microplate reader (Infinite 200 Pro, Tecan, Männedorf, Switzerland).
2.4.2. Total Flavonoid Content
The total flavonoid content was determined according to the method of Pękal and Pyrzynska [33] with slight modification. The aluminum chloride colorimetric method was used for the determination of the total flavonoid content of the sample. For the total flavonoid determination, quercetin (Sigma-Aldrich, Bangalore, India) was used to make the standard calibration curve. The stock quercetin solution was prepared by dissolving 2.0 mg quercetin in 1.0 mL methanol, and then the standard solutions of quercetin were prepared by serial dilutions using distilled water (62.5–1000 μg/mL). An amount of 0.01 mL of diluted standard quercetin solutions or extracts (6.4 mg/mL) was separately mixed with 0.25 mL of 2% aluminum chloride (Sigma-Aldrich, Darmstadt, Germany), 0.25 mL of 1 M sodium acetate and 0.49 mL of distilled water. After mixing, the solution was incubated for 15 min at room temperature. The absorbance of the reaction mixtures was measured against a blank at 425 nm using a UV-Vis spectrophotometer microplate reader (Infinite 200 Pro, Tecan, Männedorf, Switzerland). The concentration of the total flavonoid content in the tested samples was calculated from the calibration plot and expressed as mg of quercetin equivalent ((QE)/g) of the dried sample. All the determinations were carried out in triplicate.
2.5. UVB Irradiation Assay
To determine the UVB protective properties of the algae extracts, the HaCaT cells were seeded at a cell density of 1 × 105 cells/mL before incubating for 24 h at 37 °C in a 5% CO2 atmosphere to allow adherence. The solvent extracts were diluted with 0.5% DMSO and PBS to test at the range of concentrations of 25–400 µg/mL for the ethanol and water extracts. Both the negative control and the unexposed control cells (covered with aluminum foil) were treated with only 0.5% DMSO and PBS. Rosmarinic acid was used as a positive control. After 24 h, the cells were treated with 50 μL of different concentrations of the extracts concurrently with UVB irradiation (50 mJ/cm2) [34]. The cells were irradiated with a Philip UVB Broadband TL 20 W/12 phototherapy lamp (Philip, Amsterdam, The Netherlands) with wavelength ranges of 290 and 315 nm, and the intensity was measured using a UV-340A UV light meter (Lutron, Taipei, Taiwan). After UVB irradiation, the PBS was discarded, and the cells were mounted with fresh growth medium (200 µL) and incubated in 5% CO2 at 37 °C for 24 h before proceeding with the MTT viability assay. Then, 20 µL of the MTT solution (5 mg/mL) was added to each well and incubated at 37 °C with 5% CO2 for 2 h [34]. The medium was discarded by gentle aspiration, and 100 µL of DMSO was added to dissolve the formazan crystals. The absorbance of each well was measured using a UV-Vis spectrophotometric microplate reader (Infinite 200 Pro, Tecan, Männedorf, Switzerland) at 570 nm (with 650 nm as a reference wavelength).
2.6. Liquid Chromatography–Mass Spectrometry Analysis
The P. australis ethanol and water extracts were analyzed using an LCMS on an Agilent 1290 infinity liquid chromatograph (Agilent Technologies, Wilmington, DE, USA) coupled with an Agilent 6520 Accurate-Mass Q-ToF mass spectrometer with a dual ESI source. Separation of the compound was achieved using an Agilent Zorbax Eclipse XDB-C18 column, Narrow-Bore 2.1 × 150 mm of a 3.5 µm particle size at 25 °C equilibrated with solvent A (0.1% formic acid in water) and solvent B (0.1% formic acid in acetonitrile). The flow rate was set at 0.5 mL per min. The total run time was 30 min, including a 25 min run time, and a 5 min post-run time. The ESI-TOF/MS conditions were optimized as follows: drying gas temperature, 300 °C; drying gas flow, 10 L/min; nebulizer gas pressure, 45 psi; capillary voltage, 4000 V for the positive ion mass spectra and 3500 V for the negative ion mass spectra; fragmentation voltage, 125 V; and skimmer, 65 V. The mass spectrum was scanned from m/z 100 to m/z 3200 in both the positive and negative ionization modes. The calibration reference solutions obtained from the Agilent were used to calibrate the mass spectrometer daily. The reference solution was used, and the two ions with m/z of 121.0508 and 922.0097 for the positive ion mass spectra and m/z of 119.03632 and 966.0097 for the negative ion mass spectra were selected for mass calibration to eliminate systematic errors.
2.7. Statistical Analysis
Statistical analysis was performed with the Statistical Package for Social Sciences (IBM SPSS Statistics 26, Chicago, IL, USA). All the tests were expressed as the means ± standard deviation (SD) of the three independent replicates. The significant difference between the treated and untreated groups was determined by one-way analysis of variance (ANOVA). A difference was considered statistically significant when p ≤ 0.05. The relationship between the phytochemical analysis and the antioxidant capacity of the extract was evaluated using Pearson’s correlation analysis.
3. Results
3.1. In Vitro Antioxidant Activity and Phytochemical Contents of the P. australis Extracts
The DPPH scavenging activity was evaluated using the one electron reduction principle method, which determined the free radical reducing capacity of the antioxidants present in each sample. There were many antioxidants assays available; however, the DPPH approach seemed to be rapid and accurate for evaluating the antioxidant property in algae. According to Gil-Izquierdo et al. [35], the results of the DPPH are highly reproducible compared with other assays, such as ABTS. Ascorbic acid was used as positive control. In the current study, the results showed a strong DPPH radical scavenging activity increase for the P. australis ethanol and water extracts in a dose-dependent manner (Figure 1). The highest DPPH radical scavenging activity was observed in the ethanol extract of P. australis (96.79 ± 4.63%) and the water extract of P. australis (81.09 ± 2.92%) when achieving the highest concentration at 152.38 µg/mL.
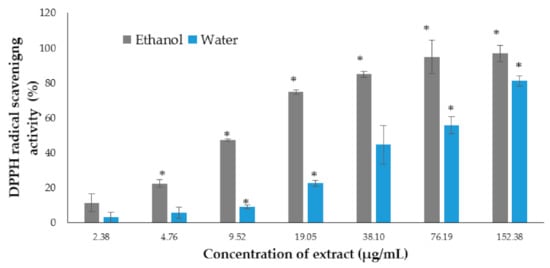
Figure 1.
DPPH radical scavenging activity of the ethanol and water extracts of P. australis. Data are expressed as the mean ± SD (n > 3). * p < 0.05 represents significant differences from the negative control.
The free radical reducing ability of the algae extracts was measured by the transformation of Fe3+ to Fe2+ in the presence of the extract. A high absorbance reading in the mixture indicated high reducing power activity. In our experimentation, the results showed the reducing power activity of both the ethanol and water extract, reporting that the absorbance reading increased in a dose-dependent manner, with the highest absorbance reading observed at the concentration of 66.67 µg/mL, which produced an absorbance reading of 0.60 and 0.37 for the ethanol and water, respectively (Figure 2).
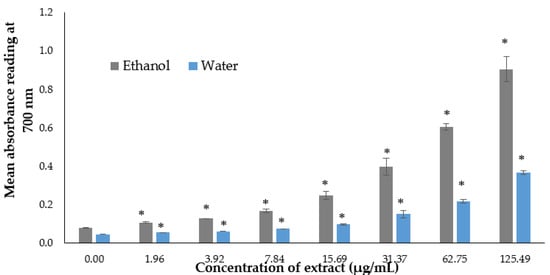
Figure 2.
Reducing power activity for the ethanol and water extracts of P. australis. Data are expressed as the mean ± SD (n > 3). * p < 0.05 represents significant differences from the negative control.
Chemical characterization analysis was carried out to determine the total phenolic and total flavonoid content in the crude extract. In the present study, the ethanol extracts of P. australis had a higher total phenolic content of 76.04 mg gallic acid equivalent ((GAE)/g), followed by the water extract with a total phenolic content of 27.01 mg GAE/g (Table 1). Flavonoids are secondary metabolites that are abundant in algae and belong to a large family of over 5000 hydroxylated polyphenolic compounds. The present study revealed that the ethanol extract of P. australis (50.07 mg QE/g) had a higher flavonoid content than the water extract (7.53 mg QE/g).

Table 1.
Phytochemical analysis of P. australis extracts.
Correlation analysis was carried out to investigate the relationship between the antioxidant assay, such as DPPH, and the reducing power activity and their phenolic and flavonoid contents. Table 2 shows that a strong and statistically significant correlation was observed when the analysis was being made between TPC and DPPH (r = 0.912), as well as reducing power activity (r = 0.994). Furthermore, the TFC was also found to exhibit a strong, statistically significant correlation with DPPH (r = 0.883) and the reducing power activity (r = 0.988). Previous studies have reported that the main contributor to the potential antioxidant activity in marine algae is the presence of phenolic compounds. Our data obtained from the study implied a similar positive correlation between the phytochemical analysis and antioxidant activity. Hence, based on the current findings, we could report that P. australis acts as a free radical scavenger. However, it should be noted that the structural diversity of the polyphenol, synergistic and antagonistic effects of this compound could also cause an effect on the antioxidant assay. Hence, there is a need to conduct chemical analysis to shed more light on this aspect.

Table 2.
Pearson’s correlation coefficients between the antioxidant assay and phytochemical analysis assay of P. australis extracts.
3.2. Effect of P. australis Extracts on the UVB-Induced Keratinocyte Death
The effect on favoring cell survival was evaluated in HaCaT keratinocytes to determine the potential of the extract as a photoprotective agent in the cosmetic field. HaCaT keratinocytes were exposed to UVB (50 mJ/cm2) in the presence of various concentrations of the ethanol extract and water extract, ranging between 25 µg/mL and 400 µg/mL, prior to the MTT assay. Based on the results, it was concluded that the ethanol extract showed a significant (p < 0.05) increase in cell viability at a concentration of 400 µg/mL (64%) (Figure 3a) and the water extract showed a significant increase in cell viability at the concentration of 400 µg/mL (60%) (Figure 3b). This suggests that both the ethanol and water extracts may have UVB protective properties.
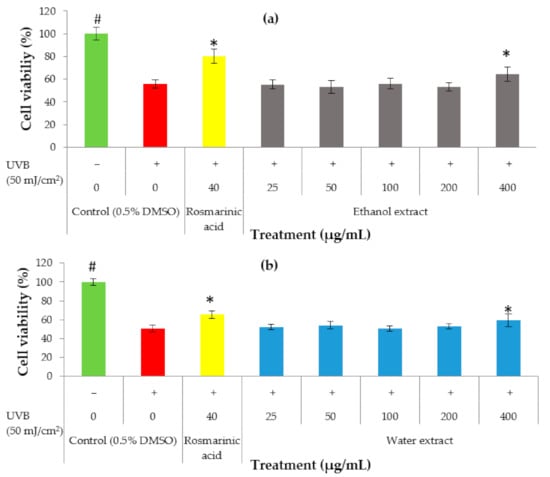
Figure 3.
Protective effect of P. australis ethanol (a) and water (b) extracts against UVB-induced cytotoxicity in HaCaT keratinocytes. The HaCaT cells were exposed to UVB (50 mJ/cm2) in the presence of algae extracts with various concentrations. The negative (0/+) and unexposed (0/−) controls were treated with 0.5% DMSO. Cell death was evaluated by an MTT test at 24 h in the HaCaT keratinocytes after UVB irradiation at a dose of 50 mJ/cm2 except for the unexposed cells, which were covered with aluminum foil. The results are expressed as a percentage of cell survival with respect to 0/− (untreated and unexposed). Data are expressed as the mean ± SD (n > 3). * p < 0.05 (samples) and # p < 0.05 (unexposed control) represent significant differences from the group exposed to UVB alone.
3.3. Chemical Profiling of P. australis Extracts
Using LCMS analysis, around 11 predicted compounds were present from the P. australis ethanol extract. These compounds were classified into polysaccharides, sugar alcohols, carotenoids, fatty acids and terpenoids. L-rhamnulose, a sulphated heteropolysaccharide compound, was detected at m/z 165.08. Dulcitol, a sugar alcohol compound, was detected at m/z 183.09. Fucoxanthin, (3S,4S,3′R)-4-hydroxyalloxanthin and 3,6-epoxy-5,5′,6,6′-tetrahydro-b,b-carotene-3′,5,5′,6′-tetrol were classified as carotenoids and detected at 681.41, 581.40, and 641.42, respectively. Fatty acid molecules such as 9Z,12Z,15E-octadecatrienoic acid, 2-hydroxyhexadecanoic acid, 9-keto palmitic acid, 9,16-dihydroxy-palmitic acid and 9,10-epoxy-18-hydroxystearate were detected at m/z of 279.23, 271.23, 269.21, 287.22 and 313.24, respectively, while 3α,12α-dihydroxy-5β-chol-8(14)-en-24-oic acid, a terpenoid compound, was detected at an m/z of 413.27 (Table 3).

Table 3.
Predicted compounds in the P. australis ethanol extract identified through LC-MS analysis.
For the P. australis water extract, five predicted compounds were determined through LCMS analysis, and these compounds were classified as fatty alcohols, fatty acids, fatty acid amides, terpenoids and phenol. In the experiments, 2,6-nonadien-1-ol, a fatty alcohol compound, was detected at an m/z of 158.15. Palmitic amide, a fatty acid amide compound, was detected at an m/z of 256.26. Emmotin A, a terpenoid, was detected at an m/z of 279.16. Docosanedioic acid is a fatty acid compound which was detected at an m/z of 393.30, and gingerol, a phenol compound, was detected at an m/z of 293.18 (Table 4).

Table 4.
Predicted compounds in the P. australis water extract identified through LC-MS analysis.
However, with our best efforts, a total of 51 detections from the ethanol extract and 16 detections in the water extract still fell under unknown compounds, even though the MFG scores achieved were above 90% and the DIFF MFG ppm value was between ±5. These findings were indicated as there was no matched identity of the compound when cross-checking through the available databases. This could mainly be due to the presence of a large family of novel or unusual biomolecules from the ethanol or water extracts that are yet to be uncovered in the routine studies conducted.
4. Discussion
UVB radiation in particular induces oxidative stress, and studies reported that the presence of natural products are effective against UV-induced skin damages [68]. The continuous search for natural products for cosmeceutical development is required due to the side effects of synthetic products such as benzoyl peroxide (antiseptic), which leads to skin irritation, and alpha hydroxy acid (exofoliant), which causes a burning sensation on the skin [69,70,71]. Great interest has been raised in the exploitation of marine algae-derived natural products, owing to their diversity and high abundance of secondary metabolites. In addition, they have been acknowledged to possess a wide range of biological activities which are well-suited for the development of natural cosmetic products with pharmaceutical and cosmeceutical benefits. Thus, cosmeceutical companies are focusing on marine algae and numerous algae species, as could be seen with the substantial number of innovative products that have been marketed as cosmetic products [72]. One of the best-selling products includes Elemis Pro-Collagen Marine Cream, using marine Padina pavonica extract, which was clinically proven to reduce the appearance of fine lines in the year 2015 [73]. To the best of our knowledge, the present study is the first to decipher the UVB radiation protective potential of Malaysian P. australis.
Human skin is vulnerable to free radical and oxidative damage, which are caused by overexposure to UV radiation. The exposure leads to the high generation of ROS, which is known to result in oxidative stress, a pathophysiological condition due to an imbalance between the oxidant and antioxidant levels. High production of ROS or a lack of an antioxidant defense system causes oxidative damage to be exerted on biomolecules, thus causing detrimental effects on the natural characteristics of biomolecules. These effects eventually cause an alteration in cellular functions [74]. The detrimental effects would then cascade through a series of events which ultimately lead to skin inflammation and eventually skin aging. Hence, antioxidants are crucial to neutralize ROS and mitigate oxidative damage. In this study, the antioxidant capacity of P. australis extract was analyzed through DPPH and reducing power activity, which are based on electron transfer reaction [75,76]. Our study revealed that the ethanol extract of P. australis demonstrated a higher antioxidant effect than the water extract, in accordance with the findings from Subermaniam et al. [77] and Akbary et al. [78]. Another Malaysian red alga, Gracilaria manilaensis, also demonstrated higher antioxidant activity in the ethanol extract compared with the hot water extract [31]. Hence, ethanol could be an ideal solvent for extracting antioxidant metabolites from algae.
Phenolic compounds consist of aromatic rings with one or more hydroxyl groups, whereas flavonoids have two benzene rings separated by a propane unit, and flavones and flavonols are the largest groups of phenolic compounds [79]. Polyphenolic compounds are able to scavenge free radicals such as superoxide and hydroxyl radicals, which can prevent oxidative disease. They can donate electrons [80] and stimulate the production of endogenous antioxidant molecules, which contribute to antioxidant activity. According to Singleton et al. [32], different phenolic compounds in algae extracts have different responses in the Folin–Ciocalteu method, depending on the number of phenolic groups they consist of. Our study showed that the ethanol extract had a higher total phenol content and flavonoid content compared with the water extract. Our findings are in accordance with a study described by Gunji et al. [81], which stated that the P.australis ethanol extract had a high phenolic content of 45 mg GAE/g and it had a significantly higher phenolic content compared with other seaweeds (Caulerpa sertularioides, Halimeda macroloba, Ulva reticulata, Sargassum polycystum and Turbinaria conoides). In addition, our results showed positive correlation between the phytochemical analysis and antioxidant activity, which suggest that the phenolic and flavonoid groups are highly responsible for antioxidant activity.
Interestingly, our findings showed that the P. australis ethanol and water extracts have the ability to attenuate the UVB-induced cytotoxicity in HaCaT cells. This is the first study to report that Malaysian P. australis has a promising UVB protectivity effect. Both the ethanol and water extracts of P. australis exhibit a UV protection effect, and a significant effect was observed at 400 µg/mL. The UV protective effect of the P. australis extract could be due to the presence of fucoxanthin, a carotenoid. A study by Matsui et al. [82] demonstrated that UV irradiation promotes sunburn and filaggrin downregulation. Fucoxanthin has played roles in exerting its protective effect against the damage caused by UV radiation through activation of the Cdx1-Flg axis. Another study by Heo and Jeon [83] revealed that fucoxanthin derived from Sargassum siliquastrum elevates the cell survival rate of human fibroblast cells when exposed to UVB radiation, and it significantly decreases the intracellular ROS production.
The gathered data demonstrated that the ethanol extract of P. australis could be a potent antioxidant agent for mitigating the oxidative damage caused by UVB radiation. The LCMS data revealed 11 compounds which belong to the chemical classes of carotenoids, sugar alcohols, polysaccharides, fatty acids and terpenoids. Carotenoids include fucoxanthin, which was present in both the positive and negative ion modes and has been reported to have antioxidant [47] and UVB protection activity [83]. The other carotenoids are (3S,4S,3′R)-4-hydroxyalloxanthin and 3,6-epoxy-5,5′,6,6′-tetrahydro-b,b-carotene-3′,5,5′,6′-tetrol. In addition, both dulcitol [40] and L-rhamnulose exhibited antioxidant activity. The LCMS results showed the presence of fatty acid molecules such as 9Z,12Z,15E-octadecatrienoic acid, 2-hydroxyhexadecanoic acid, 9-keto palmitic acid and 9,16-dihydroxy palmitic acid. However, studies showing the antioxidant activities of these compounds have not been found yet. Previous studies have revealed that fatty acids and lipids do exhibit antioxidant properties [84,85], and based on our LCMS results, 9,10 epoxy-18-hydroxystearate exhibits antioxidant activity. The LCMS showed that terpenoid compound such as 3α,12α-Dihydroxy-5β-chol-8(14)-en-24-oic acid was present in the ethanol extract. According to the study by Zhang et al. [19], terpenoids derived from algae exhibit antioxidant properties. Notably, the LCMS results did not reveal compounds belonging to the phenol classification. However, our results reported that the ethanol extract was rich in the phenolic and flavonoid contents; thus, most of the unknown compounds could belong to phenolic compounds and might have led to antioxidant and UVB protection activities, as was observed.
The water extract of P. australis was analyzed through LCMS, as per our effort to reveal the chemical constituents that might have contributed to their observed protective effect in the UVB-irradiated HaCaT keratinocyte experiment. The analysis revealed that the P. australis water extract consisted of compounds such as 2,6-nonadien-1-ol, palmitic amide, emmotin A, docosanedioic acid and gingerol. Out of the five proposed compounds, a study by Soib et al. [60] showed that the antioxidant property of Carica papaya extract could be due to palmitic amide. Additionally, 2,6-nonadien-1-ol [57], docosanedioic acid [63] and gingerol have been shown to have antioxidant activity [65]. Interestingly, gingerol is also reported to have a UVB protection effect [67]. One of the compounds belonged to the terpenoid classification, and studies have reported that algae species rich in terpenoids do possess antioxidant [86] and UV protection activities [87]. Notably, unknown compounds in the water extract could also be the reason for UVB protection activity.
5. Conclusions
Taken together, the present study revealed that Malaysian Padina australis ethanol and water extracts exhibited protective effects against UVB-induced cytotoxicity in HaCaT human keratinocytes. At the same time, the ethanol extract showed a higher antioxidant capacity and total phenolic and flavonoid contents compared with the water extract. The strong correlation between the antioxidant capacity and phytochemical contents suggests that the phenolic or flavonoid compounds could have contributed to the antioxidant activities observed in our experiments. The higher antioxidant effect of the ethanol extracts was further revealed by the LCMS results, which showed the presence of antioxidant molecules. The presence of bioactive constituents including fucoxanthin may have been responsible for the antioxidant and UVB-protective activities of the P. australis extracts. To summarize, this study demonstrated that P. australis possesses great antioxidative and UVB-protective effects. Nevertheless, P. australis may be a novel source of antioxidant and UVB protection compounds which has the potential to be used in cosmeceutical applications. However, further studies are required to identify the specific compounds which are responsible for these activities, namely by continuing with a deep study on their mechanism of action.
Author Contributions
Y.-Y.Y. and B.-H.G. conceived and designed the experiment. K.T. performed the experiments and data analysis and wrote the manuscript; Y.-Y.Y. and B.-H.G. validated the data and provided resources; Y.-Y.Y., B.-H.G., C.K.M. and L.T.G. reviewed and edited the manuscript; Y.-Y.Y. and B.-H.G. contributed to the key parts of the text associated with it. All authors have read and approved the final draft. All authors have read and agreed to the published version of the manuscript.
Funding
This investigation was supported by Sunway University.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors have declared that no competing interests exist.
References
- Hussein, M.R. Ultraviolet radiation and skin cancer: Molecular mechanisms. J. Cutan. Pathol. 2005, 32, 191–205. [Google Scholar] [CrossRef]
- Talero, E.; García-Mauriño, S.; Ávila-Román, J.; Rodríguez-Luna, A.; Alcaide, A.; Motilva, V. Bioactive compounds isolated from microalgae in chronic inflammation and cancer. Mar. Drugs 2015, 13, 6152–6209. [Google Scholar] [CrossRef] [PubMed]
- Berthon, J.Y.; Nachat-Kappes, R.; Bey, M.; Cadoret, J.P.; Renimel, I.; Filaire, E. Marine algae as attractive source to skin care. Free. Radic. Res. 2017, 51, 555–567. [Google Scholar] [CrossRef] [PubMed]
- D’Orazio, J.; Jarrett, S.; Amaro-Ortiz, A.; Scott, T. UV radiation and the skin. Int. J. Mol. Sci. 2013, 14, 12222–12248. [Google Scholar] [CrossRef]
- Nishigori, C.; Hattori, Y.; Toyokuni, S. Role of reactive oxygen species in skin carcinogenesis. Antioxid. Redox Signal. 2004, 6, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Lanigan, R.S.; Yamarik, T.A. Final report on the safety assessment of BHT (1). Int. J. Toxicol. 2002, 21, 19–94. [Google Scholar] [CrossRef]
- Vinayak, R.C.; Sabu, A.S.; Chatterji, A. Bio-prospecting of a few brown seaweeds for their cytotoxic and antioxidant activities. Evid. Based Complement. Altern. Med. 2011, 2011, 1–9. [Google Scholar] [CrossRef]
- Nursid, M.; Marasskuranto, E.; Atmojo, K.B.; Hartono, M.P.; Meinita, M.D.N.; Riyanti, R. Investigation on antioxidant compounds from marine algae extracts collected from binuangeun coast, banten, indonesia. Squalen. Bull. Mar. Fishs. Postharvest. Biotechnol. 2017, 11, 59–67. [Google Scholar] [CrossRef][Green Version]
- Thiyagarasaiyar, K.; Goh, B.H.; Jeon, Y.J.; Yow, Y.Y. Algae metabolites in cosmeceutical: An overview of current applications and challenges. Mar. Drugs 2020, 18, 323. [Google Scholar] [CrossRef]
- Thomas, N.V.; Kim, S.K. Beneficial effects of marine algal compounds in cosmeceuticals. Mar. Drugs 2013, 11, 146–164. [Google Scholar] [CrossRef] [PubMed]
- Fernando, I.S.; Nah, J.W.; Jeon, Y.J. Potential anti-inflammatory natural products from marine algae. Environ. Toxicol. Pharmacol. 2016, 48, 22–30. [Google Scholar] [CrossRef]
- Wang, H.M.D.; Chen, C.C.; Huynh, P.; Chang, J.S. Exploring the potential of using algae in cosmetics. Bioresour. Technol. 2015, 184, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Ariede, M.B.; Candido, T.M.; Jacome, A.L.M.; Velasco, M.V.R.; de Carvalho, J.C.M.; Baby, A.R. Cosmetic attributes of algae-A review. Algal Res. 2017, 25, 483–487. [Google Scholar] [CrossRef]
- Guillerme, J.B.; Couteau, C.; Coiffard, L. Applications for marine resources in cosmetics. Cosmetics 2017, 4, 35. [Google Scholar] [CrossRef]
- Florez, N.; Gonzalez-Munoz, M.J.; Ribeiro, D.; Fernandes, E.; Dominguez, H.; Freitas, M. Algae polysaccharides’ chemical characterization and their role in the inflammatory process. Curr. Med. Chem. 2017, 24, 149–175. [Google Scholar] [CrossRef]
- Peng, J.; Yuan, J.-P.; Wu, C.-F.; Wang, J.-H. Fucoxanthin, a marine carotenoid present in brown seaweeds and diatoms: Metabolism and bioactivities relevant to human health. Mar. Drugs 2011, 9, 1806–1828. [Google Scholar] [CrossRef] [PubMed]
- Antony, T.; Chakraborty, K. Xenicanes attenuate pro-inflammatory 5-lipoxygenase: Prospective natural anti-inflammatory leads from intertidal brown seaweed Padina tetrastromatica. Med. Chem. Res. 2019, 28, 591–607. [Google Scholar] [CrossRef]
- Ryu, B.; Qian, Z.J.; Kim, M.M.; Nam, K.W.; Kim, S.K. Anti-photoaging activity and inhibition of matrix metalloproteinase (MMP) by marine red alga, Corallina pilulifera methanol extract. Radiat. Phys. Chem. 2009, 78, 98–105. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, F.; Wang, X.; Liu, X.; Hou, Y.; Zhang, Q. Extraction of the polysaccharides from five algae and their potential antioxidant activity in vitro. Carbohydr. Polym. 2010, 82, 118–121. [Google Scholar] [CrossRef]
- Murthy, K.; Vanitha, A.; Rajesha, J.; Swamy, M.; Sowmya, P.; Ravishankar, G. In vivo antioxidant activity of carotenoids from Dunaliella salina—A green microalga. Life Sci. 2005, 76, 1381–1390. [Google Scholar] [CrossRef]
- Wang, H.M.D.; Li, X.C.; Lee, D.J.; Chang, J.S. Potential biomedical applications of marine algae. Bioresour. Technol. 2017, 244, 1407–1415. [Google Scholar] [CrossRef] [PubMed]
- Saidani, K.; Bedjou, F.; Benabdesselam, F.; Touati, N. Antifungal activity of methanolic extracts of four algerian marine algae species. Afr. J. Biotechnol. 2012, 11, 9496–9500. [Google Scholar] [CrossRef]
- Hwang, E.; Park, S.Y.; Sun, Z.W.; Shin, H.S.; Lee, D.G.; Yi, T.H. The protective effects of fucosterol against skin damage in UVB-irradiated human dermal fibroblasts. Mar. Biotechnol. 2014, 16, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Canoy, J.L.; Bitacura, J.G. Cytotoxicity and antiangiogenic activity of turbinaria ornata agardh and Padina australis hauck ethanolic extracts. Anal. Cell. Pathol. 2018, 2018. [Google Scholar] [CrossRef]
- Gany, S.A.; Tan, S.C.; Gan, S.Y. Antioxidative, anticholinesterase and anti-neuroinflammatory properties of Malaysian brown and green seaweeds. World. Acad. Sci. Eng. Technol. 2015, 8, 1269–1275. [Google Scholar] [CrossRef]
- Murugan, A.C.; Vallal, D.; Karim, M.R.; Govindan, N.; Yusoff, M.; Rahman, M.M. In vitro antiradical and neuroprotective activity of polyphenolic extract from marine algae Padina australis H. J. Chem. Pharm. Res 2015, 7, 355–362. [Google Scholar]
- Akbari, V.; Safaiee, F.; Yegdaneh, A. Bioassay-guided fractionation and antimicrobial activities of Padina australis extracts. Jundishapur J. Nat. Pharm. Prod. 2020, 15, e68304. [Google Scholar] [CrossRef]
- Salosso, Y.; Aisiah, S.; Toruan, L.N.L.; Pasaribu, W. Nutrient content, active compound and antibacterial activity of Padina australis against Aeromonas hydropilla. Pharmacogn. J. 2020, 12, 771–776. [Google Scholar] [CrossRef]
- Chellappan, D.K.; Chellian, J.; Leong, J.Q.; Liaw, Y.Y.; Gupta, G.; Dua, K.; Kunnath, A.P.; Palaniveloo, K. Biological and therapeutic potential of the edible brown marine seaweed Padina australis and their pharmacological mechanisms. J. Trop. Biol. Conserv. 2020, 17, 251–271. [Google Scholar]
- Tan, L.T.H.; Mahendra, C.K.; Yow, Y.Y.; Chan, K.G.; Khan, T.M.; Lee, L.H.; Goh, B.H. Streptomyces sp. MUM273b: A mangrove-derived potential source for antioxidant and UVB radiation protectants. BMC Microbiol. 2019, 8, 859. [Google Scholar] [CrossRef]
- Pang, J.R.; Goh, V.M.J.; Tan, C.Y.; Phang, S.M.; Wong, K.H.; Yow, Y.Y. Neuritogenic and in vitro antioxidant activities of Malaysian Gracilaria manilaensis Yamamoto & Trono. J. Appl. Phycol. 2018, 30, 3253–3260. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Pękal, A.; Pyrzynska, K. Evaluation of aluminium complexation reaction for flavonoid content assay. Food Anal. Methods 2014, 7, 1776–1782. [Google Scholar] [CrossRef]
- Mahendra, C.K.; Tan, L.T.H.; Yap, W.H.; Chan, C.K.; Pusparajah, P.; Goh, B.H. An optimized cosmetic screening assay for ultraviolet B (UVB) protective property of natural products. Prog. Drug Discov. Biomed. Sci. 2019, 2. [Google Scholar] [CrossRef]
- Gil-Izquierdo, A.; Gil, M.I.; Ferreres, F.; Tomás-Barberán, F.A. In vitro availability of flavonoids and other phenolics in orange juice. J. Agric. Food Chem. 2001, 49, 1035–1041. [Google Scholar] [CrossRef]
- Rao, N.P.; Sastry, K.V.; Rao, E.V. Carbohydrates of Padina tetrastromatica. Phytochemistry 1984, 23, 2531–2533. [Google Scholar] [CrossRef]
- Chen, L.; Huang, G.; Hu, J. Preparation, deproteinization, characterisation, and antioxidant activity of polysaccharide from cucumber (Cucumis saticus L.). Int. J. Biol. Macromol. 2018, 108, 408–411. [Google Scholar] [CrossRef]
- Cosmetics.specialchem.com. Rhamnosoft® HP ST-Solabia-Datasheet. Available online: https://cosmetics.specialchem.com/product/i-solabia-rhamnosoft-hp-st (accessed on 27 December 2020).
- Parameswaran, P.S.; Naik, C.G.; Das, B.; Kamat, S.Y.; Bose, A.K.; Nair, M.S.R. Constituents of the brown alga Padina tetrastromatica (Hauck)-II. Indian J. Chem. Sect. B 1996, 35, 463–467. [Google Scholar]
- Erukainure, O.L.; Chukwuma, C.I.; Islam, M.S. Raffia palm (Raphia hookeri) wine: Qualitative sugar profile, functional chemistry, and antidiabetic properties. Food Biosci. 2019, 30, 100423. [Google Scholar] [CrossRef]
- Agatonovic-Kustrin, S.; Morton, D.W. High-performance thin-layer chromatography-direct bioautography as a method of choice for alpha-amylase and antioxidant activity evaluation in marine algae. J. Chromatogr. A 2017, 1530, 197–203. [Google Scholar] [CrossRef]
- Raguraman, V.; MubarakAli, D.; Narendrakumar, G.; Thirugnanasambandam, R.; Kirubagaran, R.; Thajuddin, N. Unraveling rapid extraction of fucoxanthin from Padina tetrastromatica: Purification, characterization and biomedical application. Process Biochem. 2018, 73, 211–219. [Google Scholar] [CrossRef]
- Karkhaneh Yousefi, M.; Seyed Hashtroudi, M.; Mashinchian Moradi, A.; Ghasempour, A. Seasonal variation of fucoxanthin content in four species of brown seaweeds from Qeshm Island, Persian Gulf and evaluation of their antibacterial and antioxidant activities. Iran. J. Fish. Sci. 2020, 19, 2394–2408. [Google Scholar]
- Namjooyan, F.; Farasat, M.; Alishahi, M.; Jahangiri, A.; Mousavi, H. The anti-melanogenesis activities of some selected brown macroalgae from northern coasts of the Persian Gulf. Braz. Arch. Biol. Technol. 2019, 62, e1918019. [Google Scholar] [CrossRef]
- Jaswir, I.; Noviendri, D.; Salleh, H.M.; Taher, M.; Miyashita, K. Isolation of fucoxanthin and fatty acids analysis of Padina australis and cytotoxic effect of fucoxanthin on human lung cancer (H1299) cell lines. Afr. J. Biotechnol. 2011, 10, 18855–18862. [Google Scholar] [CrossRef]
- Karkhane Yousefi, M.; Seyed Hashtroudi, M.; Mashinchian Moradi, A.; Ghasempour, A.R. In vitro investigating of anticancer activity of focuxanthin from marine brown seaweed species. Glob. J. Environ. Sci. Manag. 2018, 4, 81–90. [Google Scholar] [CrossRef]
- Dang, T.T.; Bowyer, M.C.; Van Altena, I.A.; Scarlett, C.J. Comparison of chemical profile and antioxidant properties of the brown algae. Int. J. Food Sci. Technol. 2018, 53, 174–181. [Google Scholar] [CrossRef]
- Yap, W.F.; Tay, V.; Tan, S.H.; Yow, Y.Y.; Chew, J. Decoding antioxidant and antibacterial potentials of Malaysian green seaweeds: Caulerpa racemosa and Caulerpa lentillifera. Antibiotics 2019, 8, 152. [Google Scholar] [CrossRef]
- Foo, S.C.; Khong, N.M.; Yusoff, F.M. Physicochemical, microstructure and antioxidant properties of microalgae-derived fucoxanthin rich microcapsules. Algal Res. 2020, 51, 102061. [Google Scholar] [CrossRef]
- Arshad, A.; Rehman, T.; Saleem, H.; Khan, S.; Saleem, M.; Tousif, M.I.; Ahemad, S.; Ahemad, N.; Abdallah, H.H.; Mahomoodally, F.M. In vitro enzyme inhibition, antibacterial, UHPLC-MS chemical profiling and in silico studies of Indigofera argentea Burm. f. for potential biopharmaceutical application. S. Afr. J. Bot. 2020. [Google Scholar] [CrossRef]
- Azizan, A.; Ahamad Bustamam, M.S.; Maulidiani, M.; Shaari, K.; Ismail, I.S.; Nagao, N.; Abas, F. Metabolite profiling of the microalgal diatom Chaetoceros calcitrans and correlation with antioxidant and nitric oxide inhibitory activities via 1H NMR-based metabolomics. Mar. Drugs 2018, 16, 154. [Google Scholar] [CrossRef]
- Bakar, K.; Mohamad, H.; Latip, J.; Tan, H.S.; Herng, G.M. Fatty acids compositions of Sargassum granuliferum and Dictyota dichotoma and their anti-fouling activities. J. Sustain. Sci. Manag. 2017, 12, 8–16. [Google Scholar]
- Harper, D.R.; Gilbert, R.L.; O’Connor, T.J.; Kinchington, D.; Mahmood, N.; Mcllhinney, R.A.J.; Jeffries, D.J. Antiviral activity of 2-hydroxy fatty acids. Antivir. Chem. Chemother. 1996, 7, 138–141. [Google Scholar] [CrossRef][Green Version]
- Kajiwara, T.; Kodama, K.; Hatanaka, A.; Matsui, K. Biogeneration of volatile compounds via oxylipins in edible seaweeds. In Biotechnology for Improved Food and Flavors American Chemical Society Symposium Series 637; Takeoka, G.R., Teranishi, R., Williams, P.J., Kobayashi, A., Eds.; American Cancer Society: Washington, DC, USA, 1996; pp. 146–166. [Google Scholar]
- Espelie, K.E.; Dean, B.B.; Kolattukudy, P.E. Composition of lipid-derived polymers from different anatomical regions of several plant species. Plant Physiol. 1979, 64, 1089–1093. [Google Scholar] [CrossRef] [PubMed]
- Arshad, A.; Ahemad, S.; Saleem, H.; Saleem, M.; Zengin, G.; Abdallah, H.H.; Tousif, M.I.; Ahemad, N.; Fawzi Mahomoodally, M. RP-UHPLC-MS chemical profiling, biological and in silico docking studies to unravel the therapeutic potential of Heliotropium crispum Desf. as a Novel Source of Neuroprotective Bioactive Compounds. Biomolecules 2021, 11, 53. [Google Scholar] [CrossRef] [PubMed]
- Elkhateeb, W.; ELDien, A.N.; Fadl, E.; Elhagrasi, A.; Fayad, W.; Wen, T.C. Therapeutic potentials of n-hexane extracts of the three medicinal mushrooms regarding their anti-colon cancer, antioxidant, and hypocholesterolemic capabilities. Biodiversitas J. Biol. Divers. 2020, 21. [Google Scholar] [CrossRef]
- Ferreira, L.D.S.; Turatti, I.C.C.; Lopes, N.P.; Guaratini, T.; Colepicolo, P.; Oliveira Filho, E.C.; Garla, R.C. Apolar compounds in seaweeds from Fernando de Noronha archipelago (northeastern coast of Brazil). Int. J. Anal. Chem. 2012, 2012. [Google Scholar] [CrossRef]
- Wu, B.; Xiao, X.; Li, S.; Zuo, G. Transcriptomics and metabonomics of the anti-aging properties of total flavones of Epimedium in relation to lipid metabolism. J. Ethnopharmacol. 2019, 229, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Soib, H.H.; Ismail, H.F.; Husin, F.; Abu Bakar, M.H.; Yaakob, H.; Sarmidi, M.R. Bioassay-guided different extraction techniques of Carica papaya (Linn.) leaves on in vitro wound-healing activities. Molecules 2020, 25, 517. [Google Scholar] [CrossRef]
- Chia, Y.Y.; Kanthimathi, M.S.; Khoo, K.S.; Rajarajeswaran, J.; Cheng, H.M.; Yap, W.S. Antioxidant and cytotoxic activities of three species of tropical seaweeds. BMC Complement. Altern. Med. 2015, 15, 339. [Google Scholar] [CrossRef]
- Saleem, H.; Sarfraz, M.; Khan, K.M.; Anwar, M.A.; Zengin, G.; Ahmad, I.; Khan, S.U.; Mahomoodally, M.F.; Ahemad, N. UHPLC-MS phytochemical profiling, biological propensities and in-silico studies of Alhagi maurorum roots: A medicinal herb with multifunctional properties. Drug. Dev. Ind. Pharm. 2020, 46, 861–868. [Google Scholar] [CrossRef]
- Udaya Prakash, N.K.; Sripriya, N.S.; Raj, D.D.; Deepa, S.; Bhuvaneswari, S. Antioxidant potency and GC-MS composition of Origanum majorana Linn. Pak. J. Pharm. Sci. 2019, 32, 2117–2122. [Google Scholar]
- Xie, X.; Chen, C.; Fu, X. Screening α-glucosidase inhibitors from four edible brown seaweed extracts by ultra-filtration and molecular docking. LWT 2021, 138, 110654. [Google Scholar] [CrossRef]
- Dugasani, S.; Pichika, M.R.; Nadarajah, V.D.; Balijepalli, M.K.; Tandra, S.; Korlakunta, J.N. Comparative antioxidant and anti-inflammatory effects of [6]-gingerol,[8]-gingerol,[10]-gingerol and [6]-shogaol. J. Ethnopharmacol. 2010, 127, 515–520. [Google Scholar] [CrossRef]
- Park, M.; Bae, J.; Lee, D.S. Antibacterial activity of [10]-gingerol and [12]-gingerol isolated from ginger rhizome against periodontal bacteria. Phytother. Res. 2008, 22, 1446–1449. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Kim, Y.; Na, K.M.; Surh, Y.J.; Kim, T.Y. [6]-Gingerol prevents UVB-induced ROS production and COX-2 expression in vitro and in vivo. Free Radic. Res. 2007, 41, 603–614. [Google Scholar] [CrossRef] [PubMed]
- Ichihashi, M.; Ahmed, N.U.; Budiyanto, A.; Wu, A.; Bito, T.; Ueda, M.; Osawa, T. Preventive effect of antioxidant on ultraviolet-induced skin cancer in mice. J. Dermatol. Sci. 2000, 23, S45–S50. [Google Scholar] [CrossRef]
- Lauriola, M.M.; Sena, P.; De Bitonto, A.; Corazza, M. Irritant contact dermatitis after a curious therapeutic use of oregano essential oil. Contact Dermatitis 2020, 83, 129–130. [Google Scholar] [CrossRef]
- Mazzarello, V.; Gavini, E.; Rassu, G.; Donadu, M.G.; Usai, D.; Piu, G.; Pomponi, V.; Sucato, F.; Zanetti, S.; Montesu, M.A. Clinical assessment of new topical cream containing two essential oils combined with tretinoin in the treatment of acne. Clin. Cosmet. Investig. Dermatol. 2020, 13, 233. [Google Scholar] [CrossRef]
- Orchard, A.; van Vuuren, S. Commercial essential oils as potential antimicrobials to treat skin diseases. Evid. Based Complement. Altern. Med. 2017, 2017. [Google Scholar] [CrossRef]
- Joshi, S.; Kumari, R.; Upasani, V.N. Applications of algae in cosmetics: An overview. Int. J. Innov. Res. Sci. Eng. Technol. 2018, 7, 1269. [Google Scholar] [CrossRef]
- Uk.elemis.com. Available online: https://uk.elemis.com/pro-collagen-marine-cream.html (accessed on 17 April 2021).
- Babusikova, E.; Jurecekova, J.; Evinova, A.; Jesenak, M.; Dobrota, D. Oxidative damage and bronchial asthma. In Respiratory Diseases; Ghanei, M., Ed.; InTech: Rijeka, Croatia, 2012; pp. 151–176. [Google Scholar]
- Cerretani, L.; Bendini, A. Chapter 67—Rapid assays to evaluate the antioxidant capacity of phenols in virgin olive oil. In Olives and Olive Oil in Health and Disease Prevention; Preedy, V.R., Watson, R.R., Eds.; Academic Press: San Diego, CA, USA, 2010; pp. 625–635. [Google Scholar]
- Prieto, M.A.; Curran, T.P.; Gowen, A.; Vázquez, J.A. An efficient methodology for quantification of synergy and antagonism in single electron transfer antioxidant assays. Food Res. Int. 2015, 67, 284–298. [Google Scholar] [CrossRef]
- Subermaniam, K.; Yow, Y.Y.; Lim, S.H.; Koh, O.H.; Wong, K.H. Malaysian macroalga Padina australis Hauck attenuates high dose corticosterone-mediated oxidative damage in PC12 cells mimicking the effects of depression. Saudi J. Biol. Sci. 2020, 27, 1435. [Google Scholar] [CrossRef] [PubMed]
- Akbary, P.; Aminikhoei, Z.; Hobbi, M.; Kuchaksaraei, B.S.; Tavabe, K.R. Antioxidant properties and total phenolic contents of extracts from three macroalgae collected from Chabahar coasts. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2021, 91, 327–334. [Google Scholar] [CrossRef]
- Kaufman, P.B.; Cseke, L.J.; Warber, C.S.; James, A.S.; Brielmann, H.L. Natural Products from Plants; CRC Press: London, UK, 1999. [Google Scholar]
- Jayaprakasha, G.K.; Patil, B.S. In vitro evaluation of the antioxidant activities in fruit extracts from citron and blood orange. Food Chem. 2007, 101, 410–418. [Google Scholar] [CrossRef]
- Gunji, S.; Santoso, J.; Yoshie-Stark, Y.; Suzuki, T. Effects of extracts from tropical seaweeds on DPPH radicals and Caco-2 cells treated with hydrogen peroxide. Food Sci. Technol. Res. 2007, 13, 275–279. [Google Scholar] [CrossRef][Green Version]
- Matsui, M.; Tanaka, K.; Higashiguchi, N.; Okawa, H.; Yamada, Y.; Tanaka, K.; Taira, S.; Aoyama, T.; Takanishi, M.; Natsume, C.; et al. Protective and therapeutic effects of fucoxanthin against sunburn caused by UV irradiation. J. Pharmacol. Sci. 2016, 132, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Heo, S.J.; Jeon, Y.J. Protective effect of fucoxanthin isolated from Sargassum siliquastrum on UV-B induced cell damage. J. Photochem. Photobiol. B 2009, 95, 101–107. [Google Scholar] [CrossRef]
- Guermouche, B.; Soulimane-Mokhtari, N.A.; Bouanane, S.; Merzouk, H.; Merzouk, S.; Narce, M. Effect of dietary polyunsaturated fatty acids on oxidant/antioxidant status in macrosomic offspring of diabetic rats. BioMed Res. Int. 2014, 2014, 368107. [Google Scholar] [CrossRef] [PubMed]
- Luzia, D.M.; Jorge, N. Bioactive substance contents and antioxidant capacity of the lipid fraction of Annona crassiflora Mart. seeds. Ind. Crops Prod. 2013, 42, 231–235. [Google Scholar] [CrossRef]
- Grassmann, J. Terpenoids as plant antioxidants. Vitam. Horm. 2005, 72, 505–535. [Google Scholar] [CrossRef]
- Xuan, S.H.; Lee, N.H.; Park, S.N. Atractyligenin, a terpenoid isolated from coffee silverskin, inhibits cutaneous photoaging. J. Photochem. Photobiol. B 2019, 194, 166–173. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).