Clinical and Instrumental Exploration of Sensitive Skin in a Pediatric Population
Abstract
1. Introduction
2. Materials and Methods
2.1. Questionnaire for Identification of Sensitive Skin in Children
2.2. Clinical Evaluations
2.3. Instrumental Assessment
2.4. Quantification of Biological Markers in Skin Surface Samples
2.5. Statistical Analysis
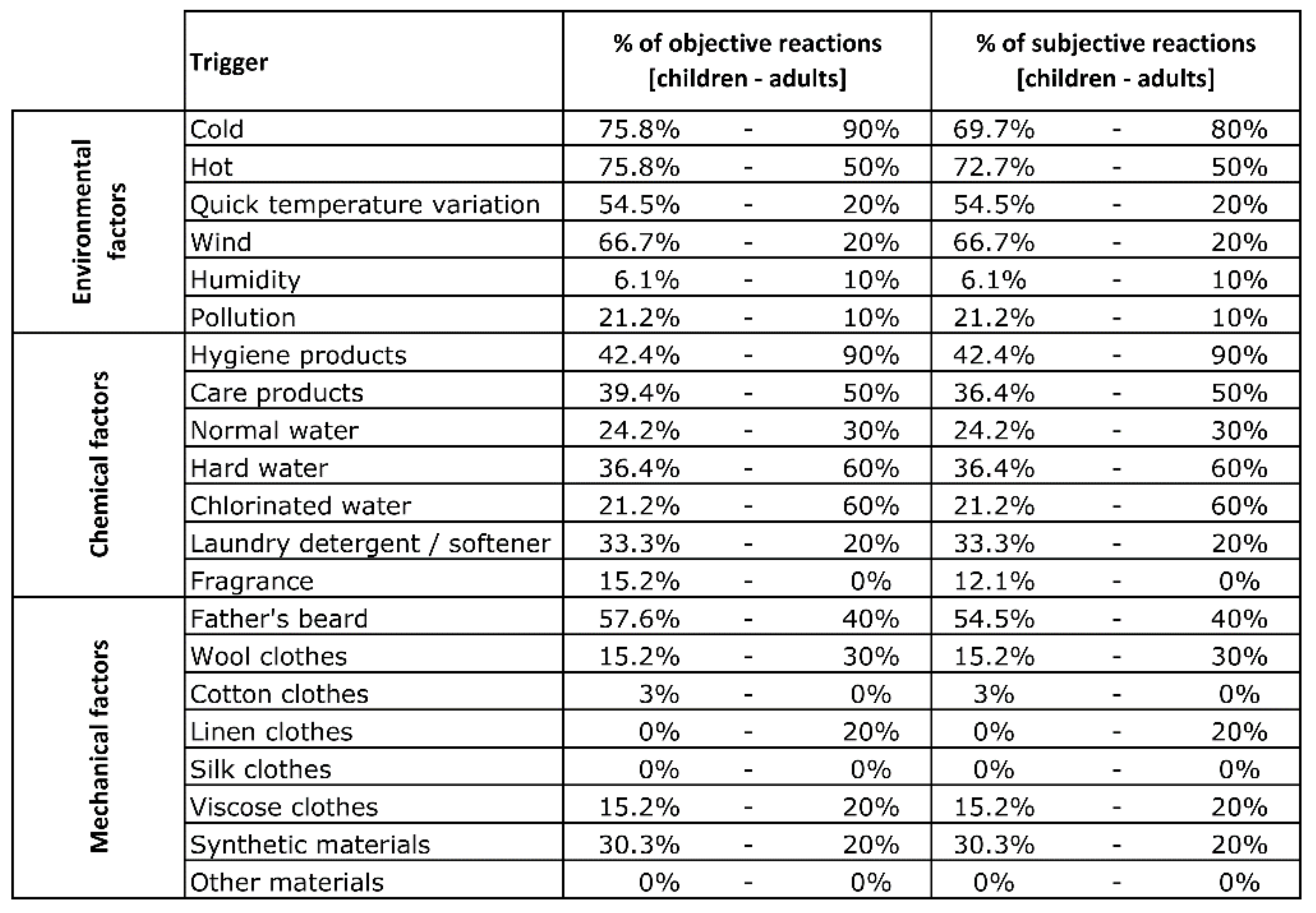
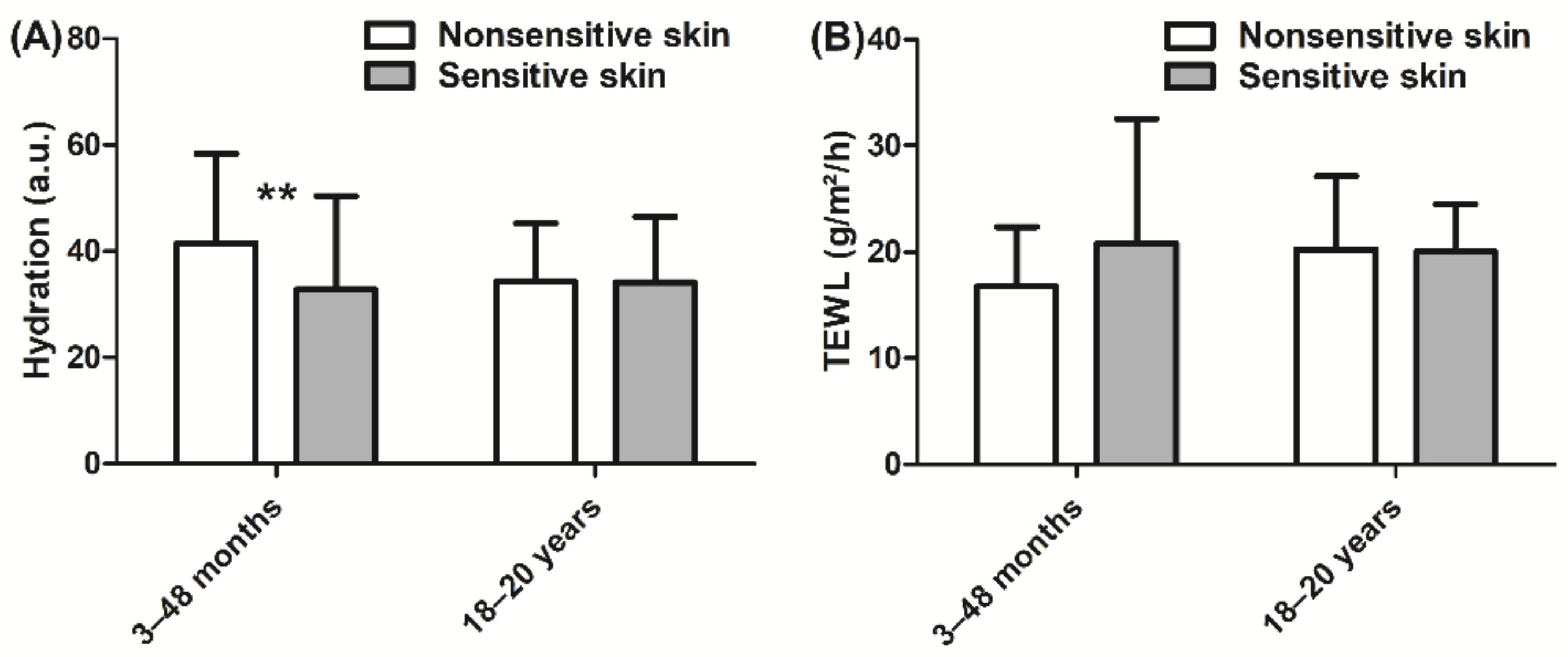
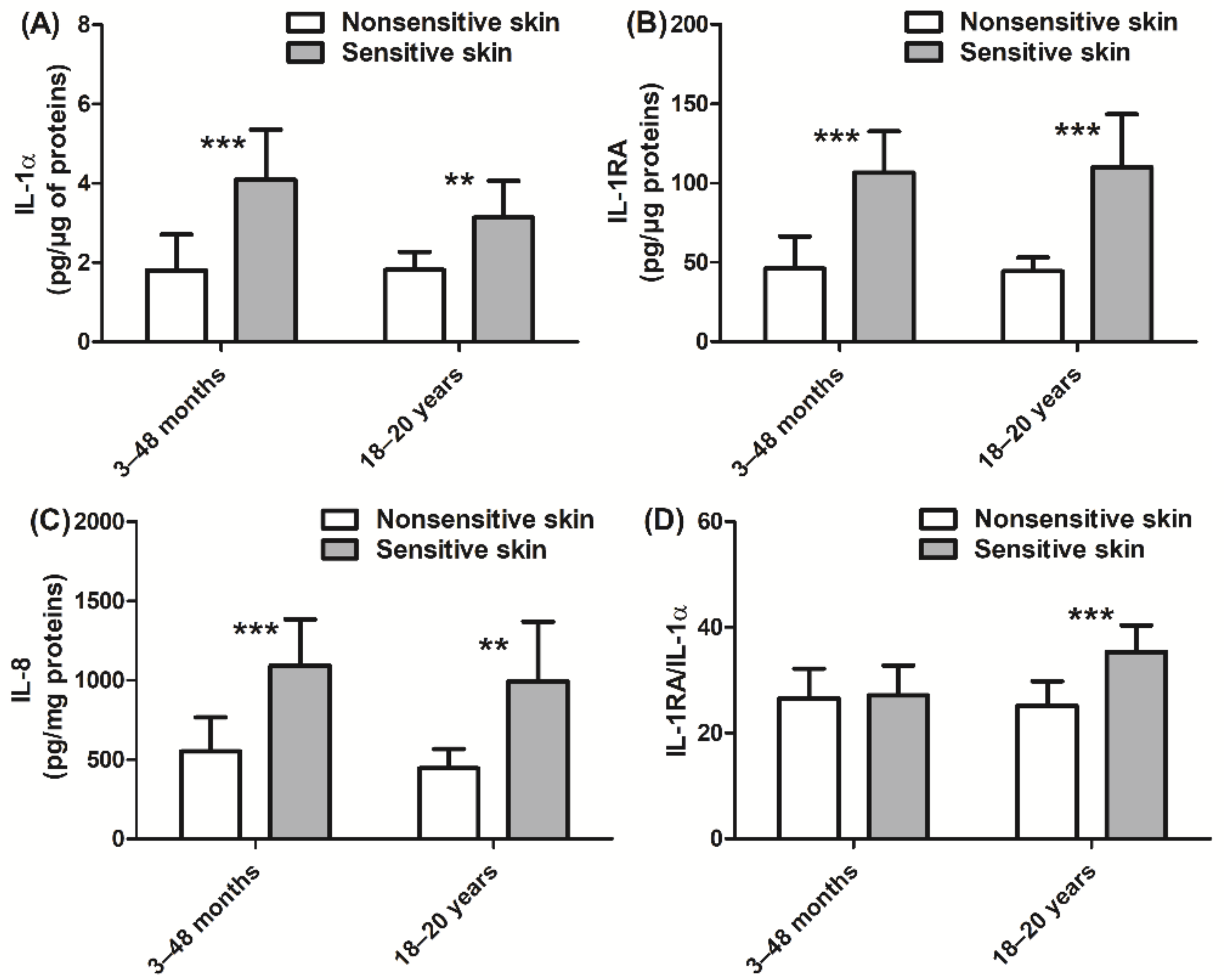
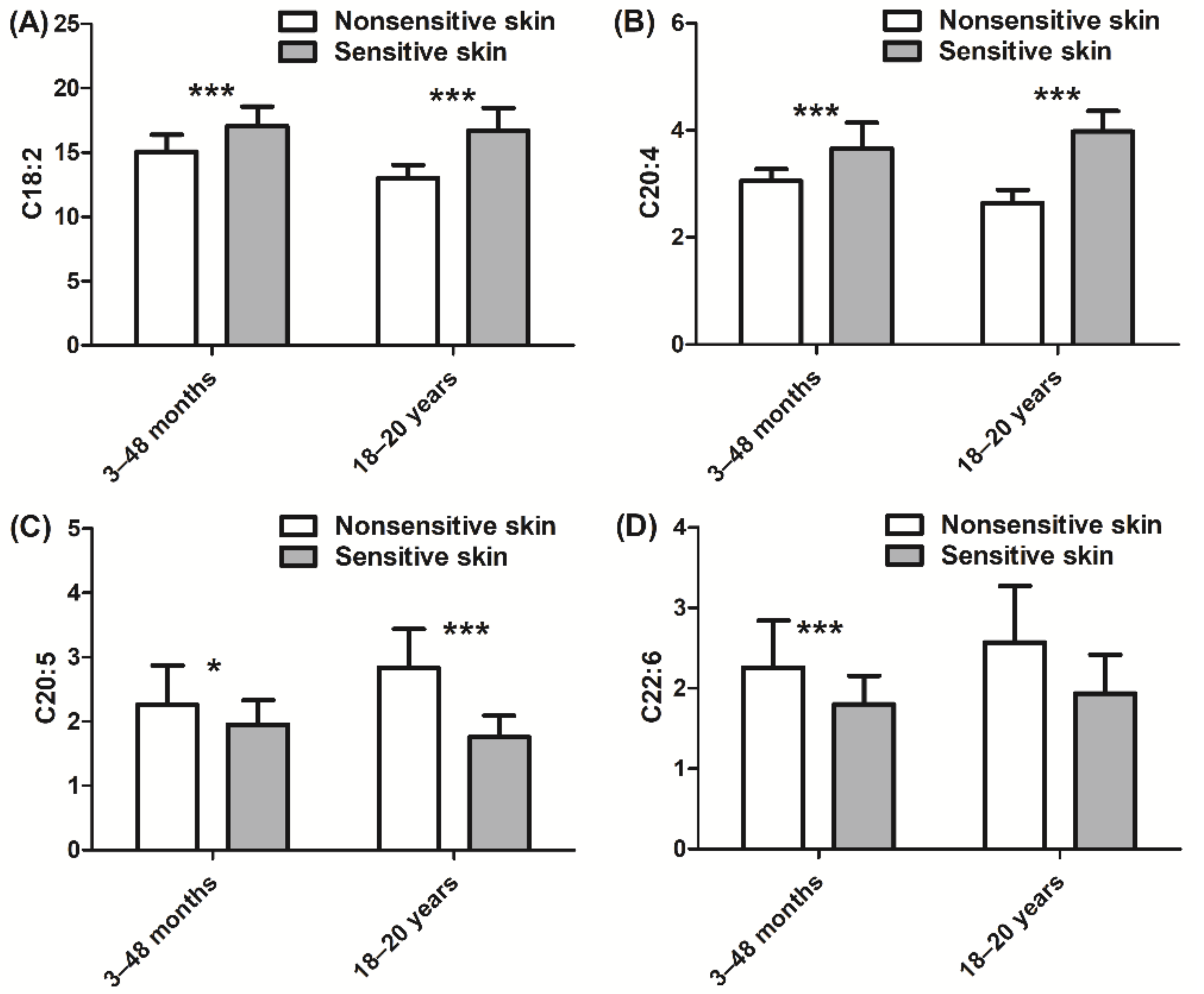
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Misery, L.; Ständer, S.; Szepietowski, J.; Reich, A.; Wallengren, J.; Evers, A.; Takamori, K.; Brenaut, E.; Gall-Ianotto, C.; Fluhr, J.; et al. Definition of Sensitive Skin: An Expert Position Paper from the Special Interest Group on Sensitive Skin of the International Forum for the Study of Itch. Acta Derm. Venereol. 2017, 97, 4–6. [Google Scholar] [CrossRef] [PubMed]
- Sparavigna, A.; Di Pietro, A.; Setaro, M. ‘Healthy skin’: Significance and results of an Italian study on healthy population with particular regard to ‘sensitive’ skin. Int. J. Cosmet. Sci. 2005, 27, 327–331. [Google Scholar] [CrossRef]
- Saint-Martory, C.; Roguedas-Contios, A.; Sibaud, V.; Degouy, A.; Schmitt, A.; Misery, L. Sensitive skin is not limited to the face. Br. J. Dermatol. 2007, 158, 130–133. [Google Scholar] [CrossRef]
- Brenaut, E.; Barnetche, T.; Ianotto, C.L.; Roudot, A.; Misery, L.; Ficheux, A. Triggering factors in sensitive skin from the worldwide patients’ point of view: A systematic literature review and meta-analysis. J. Eur. Acad. Dermatol. Venereol. 2019, 34, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Bowman, J.P.; Znaniecki, A.; Kligman, A.M.; Stoudemayer, T.; Mills, O.H. The use of chemical probes to assess the facial reactivity of women, comparing their self-perception and sensitive skin. J. Cosmet. Sci. 2000, 51, 267–273. [Google Scholar]
- Misery, L.; Jean-Decoster, C.; Mery, S.; Georgescu, V.; Sibaud, V. A New Ten-Item Questionnaire for Assessing Sensitive Skin: The Sensitive Scale-10. Acta Derm. Venereol. 2014, 94, 635–639. [Google Scholar] [CrossRef]
- Richters, R.; Uzunbajakava, N.; Hendriks, J.; Bikker, J.W.; Van Erp, P.; Van De Kerkhof, P. A model for perception-based identification of sensitive skin. J. Eur. Acad. Dermatol. Venereol. 2016, 31, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Distante, F.; Rigano, L.; D’Agostino, R.; Bonfigli, A.; Berardesca, E. Intra- and Inter-Individual Differences in Sensitive Skin. Cosmet. Toilet. 2002, 117, 39–46. [Google Scholar]
- Pinto, P.; Rosado, C.; Parreirão, C.; Rodrigues, L.M. Is there any barrier impairment in sensitive skin? A quantitative analysis of sensitive skin by mathematical modeling of transepidermal water loss desorption curves. Skin Res. Technol. 2011, 17, 181–185. [Google Scholar] [CrossRef]
- Löffler, H.D.H.; Dickel, H.; Kuss, O.; Diepgen, T.L.; Effendy, I. Characteristics of Self-estimated Enhanced Skin Susceptibility. Acta Derm. Venereol. 2001, 81, 343–346. [Google Scholar] [CrossRef] [PubMed]
- Diogo, L.; Papoila, A.L. Is it possible to characterize objectively sensitive skin? Skin Res. Technol. 2010, 16, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Roussaki-Schulze, A.V.; Afiriou, E.; Nikoulis, D.; Klimi, E.; Rallis, E.; Zintzaras, E. Objective biophysical findings in patients with sensitive skin. Drugs Exp. Clin. Res. 2005, 31, 17–24. [Google Scholar]
- Seidenari, S.; Francomano, M.; Mantovani, L. Baseline biophysical parameters in subjects with sensitive skin. Contact Dermat. 1998, 38, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Richters, R.J.; Falcone, D.; Uzunbajakava, N.E.; Varghese, B.; Caspers, P.J.; Puppels, G.J.; Van Erp, P.E.; Van De Kerkhof, P.C. Sensitive Skin: Assessment of the Skin Barrier Using Confocal Raman Microspectroscopy. Skin Pharmacol. Physiol. 2017, 30, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Buhé, V.; Vie, K.; Guéré, C.; Natalizio, A.; Lhéritier, C.; Gall-Ianotto, C.; Huet, F.; Talagas, M.; Lebonvallet, N.; Marcorelles, P.; et al. Pathophysiological Study of Sensitive Skin. Acta Derm. Venereol. 2016, 96, 314–318. [Google Scholar] [CrossRef]
- Ehnis-Pérez, A.; Torres-Alvarez, B.; Cortés-García, D.; Hernandez-Blanco, D.; Fuentes-Ahumada, C.; Castanedo-Cázares, J.P. Relationship between transient receptor potential vanilloid-1 expression and the intensity of sensitive skin symptoms. J. Cosmet. Dermatol. 2015, 15, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Kueper, T.; Krohn, M.; Haustedt, L.O.; Hatt, H.; Schmaus, G.; Vielhaber, G. Inhibition of TRPV1 for the treatment of sensitive skin. Exp. Dermatol. 2010, 19, 980–986. [Google Scholar] [CrossRef]
- Fernandes, E.S.; Fernandes, M.; E Keeble, J. The functions of TRPA1 and TRPV1: Moving away from sensory nerves. Br. J. Pharmacol. 2012, 166, 510–521. [Google Scholar] [CrossRef]
- Reilly, R.P.D.M. Inflammatory Mediators in Normal, Sensitive and Diseased Skin Types: Investigative Report. Acta Derm. Venereol. 2000, 80, 171–174. [Google Scholar] [CrossRef]
- Reinert, P.; Rybojad, M.; Humbert, P.; De Belilovsky, C.; Homsi, M. PRO investigation: PRevalence and Origins of Hyper-sensitive skin among more than 8000 children. J. Am. Acad. Dermatol. 2010, 62 (Suppl. 1), AB113. [Google Scholar]
- Yatagai, T.; Shimauchi, T.; Yamaguchi, H.; Sakabe, J.-I.; Aoshima, M.; Ikeya, S.; Tatsuno, K.; Fujiyama, T.; Ito, T.; Ojima, T.; et al. Sensitive skin is highly frequent in extrinsic atopic dermatitis and correlates with disease severity markers but not necessarily with skin barrier impairment. J. Dermatol. Sci. 2018, 89, 33–39. [Google Scholar] [CrossRef]
- Nettis, E.; Ortoncelli, M.; Pellacani, G.; Foti, C.; Di Leo, E.; Patruno, C.; Rongioletti, F.; Argenziano, G.; Ferrucci, S.M.; Macchia, L.; et al. A Multicenter Study on the Prevalence of Clinical Patterns and Clinical Phenotypes in Adult Atopic Dermatitis. J. Investig. Allergol. Clin. Immunol. 2020, 30, 448–450. [Google Scholar] [CrossRef]
- Serup, J. EEMCO guidance for the assessment of dry skin (xerosis) and ichthyosis: Clinical scoring systems. Skin Res. Technol. 1995, 1, 109–114. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R (Version 4.0.0); R Development Core Team: Vienna, Austria; Available online: http://www.R-project.org (accessed on 29 April 2020).
- Bianchi, M.E. DAMPs, PAMPs and alarmins: All we need to know about danger. J. Leukoc. Biol. 2007, 81, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Akdis, M.; Aab, A.; Altunbulakli, C.; Azkur, K.; Costa, R.A.; Crameri, R.; Duan, S.; Eiwegger, T.; Eljaszewicz, A.; Ferstl, R.; et al. Interleukins (from IL-1 to IL-38), interferons, transforming growth factor β, and TNF-α: Receptors, functions, and roles in diseases. J. Allergy Clin. Immunol. 2016, 138, 984–1010. [Google Scholar] [CrossRef] [PubMed]
- Bando, M.; Hiroshima, Y.; Kataoka, M.; Shinohara, Y.; Herzberg, M.C.; Ross, K.F.; Nagata, T.; Kido, J.-I. Interleukin-1 α regulates antimicrobial peptide expression in human keratinocytes. Immunol. Cell Biol. 2007, 85, 532–537. [Google Scholar] [CrossRef]
- Steude, J.; Kulke, R.; Christophers, E. Interleukin-1-stimulated Secretion of Interleukin-8 and Growth-related Oncogene-α Demonstrates Greatly Enhanced Keratinocyte Growth in Human Raft Cultured Epidermis. J. Investig. Dermatol. 2002, 119, 1254–1260. [Google Scholar] [CrossRef] [PubMed]
- Hirao, T.; Aoki, H.; Yoshida, T.; Sato, Y.; Kamoda, H. Elevation of Interleukin 1 Receptor Antagonist in the Stratum Corneum of Sun-exposed and Ultraviolet B-irradiated Human Skin. J. Investig. Dermatol. 1996, 106, 1102–1107. [Google Scholar] [CrossRef]
- Falcone, D.; Spee, P.; Kerkhof, P.; Erp, P.E.J.V.; Van De Kerkhof, P.C.M. Minimally-invasive Sampling of Interleukin-1? and Interleukin-1 Receptor Antagonist from the Skin: A Systematic Review of in Vivo Studies in Humans. Acta Derm. Venereol. 2017, 97, 1066–1073. [Google Scholar] [CrossRef]
- Terui, T.; Hirao, T.; Sato, Y.; Uesugi, T.; Honda, M.; Iguchi, M.; Matsumura, N.; Kudoh, K.; Aiba, S.; Tagami, H. An increased ratio of interleukin-1 receptor antagonist to interleukin-1? in inflammatory skin diseases. Exp. Dermatol. 1998, 7, 327–334. [Google Scholar] [CrossRef]
- Perkins, M.A.; Osterhues, M.A.; Farage, M.A.; Robinson, M.K. A noninvasive method to assess skin irritation and compromised skin conditions using simple tape adsorption of molecular markers of inflammation. Skin Res. Technol. 2001, 7, 227–237. [Google Scholar] [CrossRef]
- Tsuruta, J.; Sugisaki, K.; Dannenberg, A.M.; Yoshimura, T.; Abe, Y.; Mounts, P. The cytokines NAP-1 (IL-8), MCP-1, IL-1 beta, and GRO in rabbit inflammatory skin lesions produced by the chemical irritant sulfur mustard. Inflammation 1996, 20, 293–318. [Google Scholar] [CrossRef]
- Kondo, S.; Kono, T.; Sauder, D.N.; McKenzie, R.C. IL-8 Gene Expression and Production in Human Keratinocytes and Their Modulation by UVB. J. Investig. Dermatol. 1993, 101, 690–694. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, M.; Nishizawa, T.; Ohigashi, H.; Tanaka, T.; Hou, D.-X.; Colburn, N.H.; Murakami, A. Linoleic acid metabolite suppresses skin inflammation and tumor promotion in mice: Possible roles of programmed cell death 4 induction. Carcinogenesis 2009, 30, 1209–1216. [Google Scholar] [CrossRef] [PubMed]
- Fritsche, K.L. Too much linoleic acid promotes inflammation—Doesn’t it? Prostaglandins Leukot. Essent. Fat. Acids 2008, 79, 173–175. [Google Scholar] [CrossRef] [PubMed]
- Innes, J.K.; Calder, P.C. Omega-6 fatty acids and inflammation. Prostaglandins Leukot. Essent. Fat. Acids 2018, 132, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Johnson, G.H.; Fritsche, K. Effect of Dietary Linoleic Acid on Markers of Inflammation in Healthy Persons: A Systematic Review of Randomized Controlled Trials. J. Acad. Nutr. Diet. 2012, 112, 1029–1041.e15. [Google Scholar] [CrossRef] [PubMed]
- Khnykin, D.; Miner, J.H.; Jahnsen, F. Role of fatty acid transporters in epidermis. Derm.-Endocrinol. 2011, 3, 53–61. [Google Scholar] [CrossRef]
- Jang, H.-Y.; Koo, J.-H.; Lee, S.-M.; Park, B.-H. Atopic dermatitis-like skin lesions are suppressed in fat-1 transgenic mice through the inhibition of inflammasomes. Exp. Mol. Med. 2018, 50, 1–9. [Google Scholar] [CrossRef]
- Sala-Vila, A.; Miles, E.A.; Calder, P. Fatty acid composition abnormalities in atopic disease: Evidence explored and role in the disease process examined. Clin. Exp. Allergy 2008, 38, 1432–1450. [Google Scholar] [CrossRef]
- Johnson, M.; Bradford, C. Omega-3, Omega-6 and Omega-9 Fatty Acids: Implications for Cardiovascular and Other Diseases. J. Glycom. Lipidom. 2014, 4, 2153-0637. [Google Scholar] [CrossRef]
- Ziboh, V.A.; Miller, C.C.; Cho, Y. Metabolism of polyunsaturated fatty acids by skin epidermal enzymes: Generation of antiinflammatory and antiproliferative metabolites. Am. J. Clin. Nutr. 2000, 71, 361s–366s. [Google Scholar] [CrossRef] [PubMed]
- Patterson, E.; Wall, R.; Fitzgerald, G.F.; Ross, R.P.; Stanton, C. Health Implications of High Dietary Omega-6 Polyunsaturated Fatty Acids. J. Nutr. Metab. 2012, 2012, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Stamatas, G.; Morello, A.; Mays, D. Early Inflammatory Processes in the Skin. Curr. Mol. Med. 2013, 13, 1250–1269. [Google Scholar] [CrossRef]
- Fluhr, J.; Lachmann, N.; Baudouin, C.; Msika, P.; Darlenski, R.; De Belilovsky, C.; Bossert, J.; Colomb, E.; Burdin, B.; Haftek, M. Development and organization of human stratum corneum after birth: Electron microscopy isotropy score and immunocytochemical corneocyte labelling as epidermal maturation’s markers in infancy. Br. J. Dermatol. 2014, 171, 978–986. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boyer, G.; Belilovsky, C.D.; Brédif, S.; Baudouin, C.; Misery, L.; Bellemère, G. Clinical and Instrumental Exploration of Sensitive Skin in a Pediatric Population. Cosmetics 2021, 8, 43. https://doi.org/10.3390/cosmetics8020043
Boyer G, Belilovsky CD, Brédif S, Baudouin C, Misery L, Bellemère G. Clinical and Instrumental Exploration of Sensitive Skin in a Pediatric Population. Cosmetics. 2021; 8(2):43. https://doi.org/10.3390/cosmetics8020043
Chicago/Turabian StyleBoyer, Gaëtan, Clarence De Belilovsky, Stéphanie Brédif, Caroline Baudouin, Laurent Misery, and Gaëlle Bellemère. 2021. "Clinical and Instrumental Exploration of Sensitive Skin in a Pediatric Population" Cosmetics 8, no. 2: 43. https://doi.org/10.3390/cosmetics8020043
APA StyleBoyer, G., Belilovsky, C. D., Brédif, S., Baudouin, C., Misery, L., & Bellemère, G. (2021). Clinical and Instrumental Exploration of Sensitive Skin in a Pediatric Population. Cosmetics, 8(2), 43. https://doi.org/10.3390/cosmetics8020043






