Understanding the Sensitive Skin Subject to Achieve a More Holistic Diagnosis
Abstract
1. Introduction
2. A Global Phenomenon: Prevalence of Sensitive Skin around the World
2.1. Sensitive Skin Is a Whole Body Phenomenon
2.2. Signs, Symptoms and Underlying Causes
2.2.1. Altered Epidermal Barrier
2.2.2. Neurosensory Dysfunction
2.2.3. Altered Vasculature
3. Understanding Host Factors
3.1. Skin Type
3.2. Ethnicity
3.3. Gender
3.4. Menstrual Cycle
3.5. Age
3.6. Impact of Incontinence
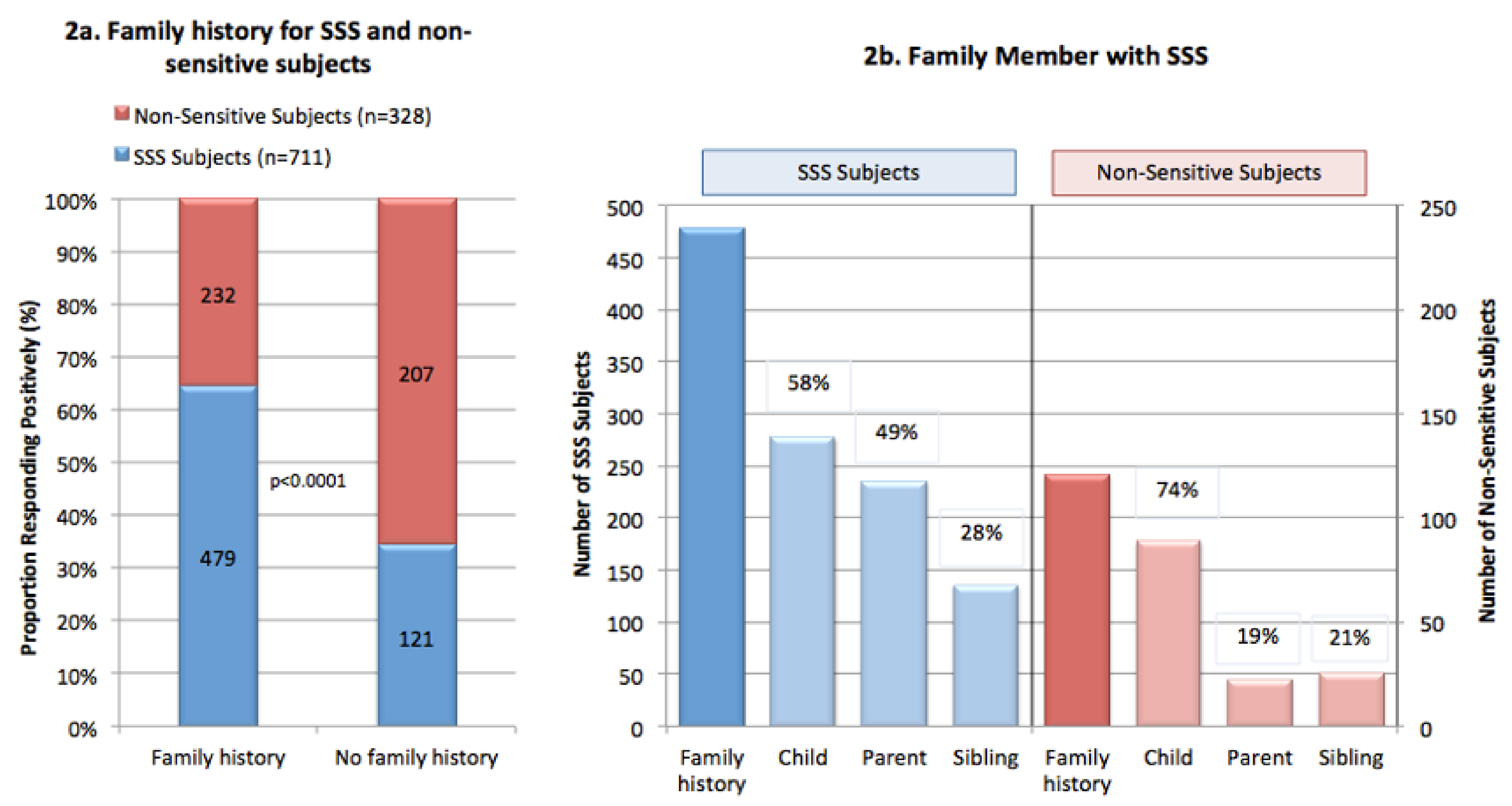
3.7. Familial and Genetic Links
4. Understanding the Triggering Factors for Sensitive Skin
4.1. Environmental, Lifestyle, Stress
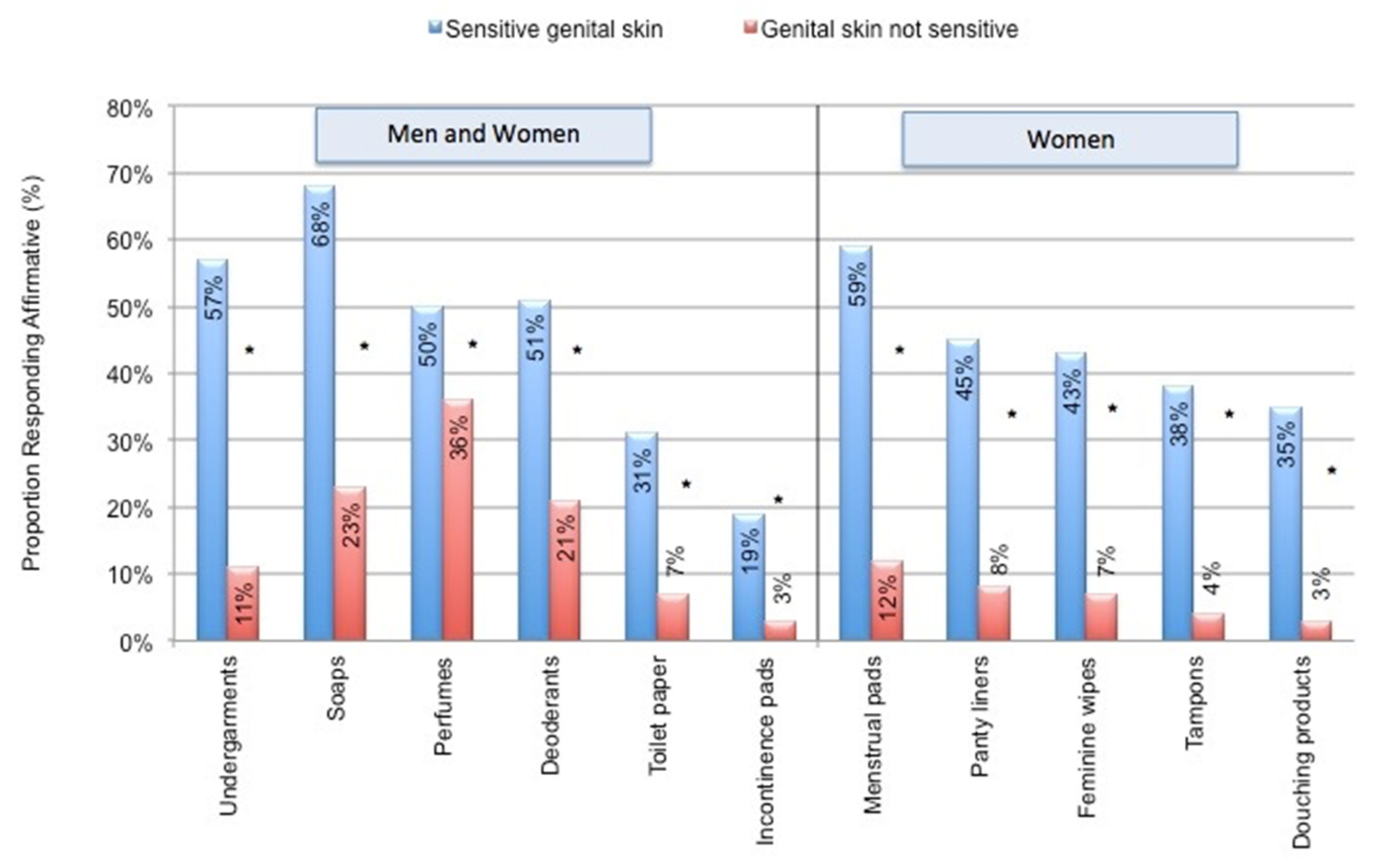
4.2. Impact of Everyday Products
5. Holistic Evaluation: Other Conditions Associated with Sensitive Skin
5.1. Dermatologic
5.2. Non-Dermatologic
5.2.1. Irritable Bowel Syndrome
5.2.2. Sleep Disorders
5.2.3. Stress
5.2.4. Menstrual Cycle
6. The Burden of SSS: How Does It Impact Daily Life?
6.1. Avoidance
6.2. Quality of Life
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Misery, L.; Ständer, S.; Szepietowski, J.C.; Reich, A.W.; Wallengren, J.; Evers, A.; Takamori, K.; Brenaut, E.; Gall-Ianotto, C.; Fluhr, J.; et al. Definition of Sensitive Skin: An Expert Position Paper from the Special Interest Group on Sensitive Skin of the International Forum for the Study of Itch. Acta Derm.-Venereol. 2017, 97, 4–6. [Google Scholar] [CrossRef]
- Berardesca, E.; Farage, M.; Maibach, H. Sensitive skin: An overview. Int. J. Cosmet. Sci. 2013, 35, 2–8. [Google Scholar] [CrossRef]
- Farage, M.A.; Maibach, H.I. Sensitive Skin: New Findings Yield New Insights. In Textbook of Cosmetic Dermatology; Informa Healthcare: New York, NY, USA, 2010; pp. 73–83. [Google Scholar]
- Do, L.H.D.; Azizi, N.; Maibach, H. Sensitive Skin Syndrome: An Update. Am. J. Clin. Dermatol. 2019, 21, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Farage, M.A. The Prevalence of Sensitive Skin. Front. Med. 2019, 6, 98. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Dai, R.; Li, L. The prevalence of self-declared sensitive skin: A systematic review and meta-analysis. J. Eur. Acad. Dermatol. Venereol. 2019, 34, 1779–1788. [Google Scholar] [CrossRef]
- Farage, M.A.; Mandl, C.P.; Berardesca, E.; Maibach, H.I. Sensitive Skin in China. J. Cosmet. Dermatol. Sci. Appl. 2012, 2, 184–195. [Google Scholar] [CrossRef][Green Version]
- Xu, F.; Yan, S.; Wu, M.; Li, F.; Sun, Q.; Lai, W.; Shen, X.; Rahhali, N.; Taieb, C.; Xu, J. Self-declared sensitive skin in China: A community-based study in three top metropolises. J. Eur. Acad. Dermatol. Venereol. 2013, 27, 370–375. [Google Scholar] [CrossRef]
- Wang, X.; Su, Y.; Zheng, B.; Wen, S.; Liu, D.; Ye, L.; Yan, Y.; Elias, P.M.; Yang, B.; Man, M. Gender-related characterization of sensitive skin in normal young Chinese. J. Cosmet. Dermatol. 2020, 19, 1137–1142. [Google Scholar] [CrossRef]
- Chew, A.; Maibach, H. Sensitive Skin. In Dry Skin and Moisturizers: Chemistry and Function; Loden, M., Miabach, H., Eds.; CRC Press: Boca Raton, FL, USA, 2000; pp. 429–440. [Google Scholar]
- Farage, M.A. Sensitive Skin in the Genital Area. Front. Med. 2019, 6, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Tagami, H. Functional characteristics of the stratum corneum in photoaged skin in comparison with those found in intrinsic aging. Arch. Dermatol. Res. 2008, 300, S1–S6. [Google Scholar] [CrossRef] [PubMed]
- Ya-Xian, Z.; Suetake, T.; Tagami, H. Number of cell layers of the stratum corneum in normal skin—relationship to the anatomical location on the body, age, sex and physical parameters. Arch. Dermatol. Res. 1999, 291, 555–559. [Google Scholar] [CrossRef] [PubMed]
- Farage, M.A. How do perceptions of sensitive skin differ at different anatomical sites? An epidemiological study. Clin. Exp. Dermatol. 2009, 34, e521–e530. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Guichard, A.; Humbert, P.; Zheng, S.; Tan, Y.; Yu, L.; Qin, O.; Wang, X. Evaluation of the severity and triggering factors of sensitive scalp in Chinese females. J. Cosmet. Dermatol. 2016, 15, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Willis, C.M.; Shaw, S.; De Lacharriere, O.; Baverel, M.; Reiche, L.; Jourdain, R.; Bastien, P.; Wilkinson, J. Sensitive skin: An epidemiological study. Br. J. Dermatol. 2001, 145, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Misery, L.; Rahhali, N.; Ambonati, M.; Black, D.; Saint-Martory, C.; Schmitt, A.-M.; Taieb, C. Evaluation of sensitive scalp severity and symptomatology by using a new score. J. Eur. Acad. Dermatol. Venereol. 2011, 25, 1295–1298. [Google Scholar] [CrossRef] [PubMed]
- Falcone, D.; Richters, R.J.; Uzunbajakava, N.E.; Van Erp, P.E.; Van De Kerkhof, P.C. Sensitive skin and the influence of female hormone fluctuations: Results from a cross-sectional digital survey in the Dutch population. Eur. J. Dermatol. 2017, 27, 42–48. [Google Scholar] [CrossRef]
- Saint-Martory, C.; Roguedas-Contios, A.; Sibaud, V.; Degouy, A.; Schmitt, A.; Misery, L. Sensitive skin is not limited to the face. Br. J. Dermatol. 2008, 158, 130–133. [Google Scholar] [CrossRef]
- Misery, L.; Cochener, B.; Brenaut, E.; Seite, S.; Taieb, C. Association of sensitive skin with sensitive corneas and sensitive eyelids. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 1358–1362. [Google Scholar] [CrossRef]
- Misery, L.; Morisset, S.; Seite, S.; Brenaut, E.; Ficheux, A.; Fluhr, J.W.; Delvigne, V.; Taieb, C. Relationship between sensitive skin and sleep disorders, fatigue, dust, sweating, food, tobacco consumption or female hormonal changes: Results from a worldwide survey of 10,743 individuals. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 1371–1376. [Google Scholar] [CrossRef]
- Pons-Guiraud, A. Sensitive skin: A complex and multifactorial syndrome. J. Cosmet. Dermatol. 2004, 3, 145–148. [Google Scholar] [CrossRef]
- Löffler, H.D.H.; Dickel, H.; Kuss, O.; Diepgen, T.L.; Effendy, I. Characteristics of Self-estimated Enhanced Skin Susceptibility. Acta Derm.-Venereol. 2001, 81, 343–346. [Google Scholar]
- Ständer, S.; Schneider, S.W.; Weishaupt, C.; Luger, T.A.; Misery, L. Putative neuronal mechanisms of sensitive skin. Exp. Dermatol. 2009, 18, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Diogo, L.; Papoila, A.L. Is it possible to characterize objectively sensitive skin? Ski. Res. Technol. 2010, 16, 30–37. [Google Scholar] [CrossRef]
- Löffler, H. Contact Allergy and Sensitive Skin. In Sensitive Skin Syndrome; Berardesca, E., Fluhr, J.W., Maibach, H.I., Eds.; Taylor & Francis: New York, NY, USA, 2006; pp. 225–235. [Google Scholar]
- Lee, C.H.; Maibach, H.I. The sodium lauryl sulfate model: An overview. Contact Dermat. 1995, 33, 1–7. [Google Scholar] [CrossRef]
- Seidenari, S.; Francomano, M.; Mantovani, L. Baseline biophysical parameters in subjects with sensitive skin. Contact Dermat. 1998, 38, 311–315. [Google Scholar] [CrossRef]
- Muizzuddin, N.; Marenus, K.D.; Maes, D.H. Factors defining sensitive skin and its treatment. Am. J. Contact Dermat. 1998, 9, 170–175. [Google Scholar] [PubMed]
- Draelos, Z.D. Sensitive skin: Perceptions, evaluation, and treatment. Am. J. Contact Dermat. 1997, 8, 67–78. [Google Scholar]
- Effendy, I.; Loeffler, H.; Maibach, H.I. Baseline transepidermal water loss in patients with acute and healed irritant contact dermatitis. Contact Dermat. 1995, 33, 371–374. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.J.; Chung, B.Y.; Lee, H.B.; Kim, H.O.; Park, C.W.; Lee, C.H. Quantitative study of stratum corneum ceramides contents in patients with sensitive skin. J. Dermatol. 2012, 39, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Buhé, V.; Vié, K.; Guéré, C.; Natalizio, A.; Lhéritier, C.; Gall-Ianotto, C.; Huet, F.; Talagas, M.; Lebonvallet, N.; Marcorelles, P.; et al. Pathophysiological Study of Sensitive Skin. Acta Derm.-Venereol. 2016, 96, 314–318. [Google Scholar] [CrossRef]
- Lampe, M.A.; Burlingame, A.L.; Whitney, J.; Williams, M.L.; Brown, B.E.; Roitman, E.; Elias, P.M. Human stratum corneum lipids: Characterization and regional variations. J. Lipid Res. 1983, 24, 120–130. [Google Scholar] [CrossRef]
- Roussaki-Schulze, A.V.; Zafiriou, E.; Nikoulis, D.; Klimi, E.; Rallis, E.; Zintzaras, E. Objective biophysical findings in patients with sensitive skin. Drugs Exp. Clin. Res. 2005, 31, 17–24. [Google Scholar]
- Cua, A.B.; Wilhelm, K.-P.; Maibach, H. Cutaneous sodium lauryl sulphate irritation potential: Age and regional variability. Br. J. Dermatol. 1990, 123, 607–613. [Google Scholar] [CrossRef]
- Chen, S.Y.; Yin, J.; Wang, X.M.; Liu, Y.Q.; Gao, Y.R.; Liu, X.P. A new discussion of the cutaneous vascular reactivity in sensitive skin: A sub-group of SS. Ski. Res. Technol. 2018, 24, 432–439. [Google Scholar] [CrossRef]
- Raj, N.; Voegeli, R.; Rawlings, A.V.; Doppler, S.; Imfeld, D.; Munday, M.R.; Lane, M.E. A fundamental investigation into aspects of the physiology and biochemistry of the stratum corneum in subjects with sensitive skin. Int. J. Cosmet. Sci. 2017, 39, 2–10. [Google Scholar] [CrossRef]
- Ehnis-Pérez, A.; Torres-Álvarez, B.; Cortés-García, D.; Hernández-Blanco, D.; Fuentes-Ahumada, C.; Castanedo-Cázares, J.P. Relationship between transient receptor potential vanilloid-1 expression and the intensity of sensitive skin symptoms. J. Cosmet. Dermatol. 2016, 15, 231–237. [Google Scholar] [CrossRef]
- Kueper, T.; Krohn, M.; Haustedt, L.O.; Hatt, H.; Schmaus, G.; Vielhaber, G. Inhibition of TRPV1 for the treatment of sensitive skin. Exp. Dermatol. 2010, 19, 980–986. [Google Scholar] [CrossRef]
- Sun, L.; Wang, X.; Zhang, Y.; Wang, T.; Li, X.; Ma, Y. The evaluation of neural and vascular hyper-reactivity for sensitive skin. Ski. Res. Technol. 2016, 22, 381–387. [Google Scholar] [CrossRef]
- Misery, L.; Weisshaar, E.; Brenaut, E.; Evers, A.W.M.; Huet, F.; Ständer, S.; Reich, A.; Berardesca, E.; Serra-Baldrich, E.; Wallengren, J.; et al. Pathophysiology and management of sensitive skin: Position paper from the special interest group on sensitive skin of the International Forum for the Study of Itch (IFSI). J. Eur. Acad. Dermatol. Venereol. 2020, 34, 222–229. [Google Scholar] [CrossRef]
- Querleux, B.; Dauchot, K.; Jourdain, R.; Bastien, P.; Bittoun, J.; Anton, J.-L.; Burnod, Y.; De Lacharrière, O. Neural basis of sensitive skin: An fMRI study. Ski. Res. Technol. 2008, 14, 454–461. [Google Scholar] [CrossRef]
- Jiang, W.C.; Zhang, H.; Xu, Y.; Jiang, C.; Xu, Y.; Liu, W.; Tan, Y. Cutaneous vessel features of sensitive skin and its underlying functions. Ski. Res. Technol. 2020, 26, 431–437. [Google Scholar] [CrossRef]
- Richters, R.; Falcone, D.; Uzunbajakava, N.; Verkruysse, W.; van Erp, P.; van de Kerkhof, P. What Is Sensitive Skin? A Systematic Literature Review of Objective Measurements. Ski. Pharm. Physiol. 2014, 28, 75–83. [Google Scholar] [CrossRef]
- Kim, Y.R.; Cheon, H.I.; Misery, L.; Taieb, C.; Lee, Y.W. Sensitive skin in Korean population: An epidemiological approach. Ski. Res. Technol. 2018, 24, 229–234. [Google Scholar] [CrossRef]
- Misery, L.; Ezzedine, K.; Corgibet, F.; Dupin, N.; Sei, J.; Philippe, C.; Joly, P.; Taieb, C.; Richard, M. Sex- and age-adjusted prevalence estimates of skin types and unpleasant skin sensations and their consequences on quality of life: Results of a study of a large representative sample of the French population. Br. J. Dermatol. 2019, 180, 1549–1550. [Google Scholar] [CrossRef]
- Berardesca, E.; Maibach, H.I. Racial differences in sodium lauryl sulphate induced cutaneous irritation: Black and white. Contact Dermat. 1988, 18, 65–70. [Google Scholar] [CrossRef]
- Löffler, H.; Pirker, C.; Aramaki, J.; Frosch, P.J.; Happle, R.; Effendy, I. Evaluation of skin susceptibility to irritancy by routine patch testing with sodium lauryl sulfate. Eur. J. Dermatol. 2001, 11, 416–419. [Google Scholar]
- Farage, M.A. Perceptions of Sensitive Skin with Age. In Textbook of Aging Skin; Farage, M.A., Miller, K.W., Maibach, H.I., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 1027–1046. [Google Scholar]
- Misery, L.; Jourdan, E.; Huet, F.; Brenaut, E.; Cadars, B.; Virassamynaïk, S.; Sayag, M.; Taieb, C. Sensitive skin in France: A study on prevalence, relationship with age and skin type and impact on quality of life. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 791–795. [Google Scholar] [CrossRef]
- Farage, M.A. Perceptions of Sensitive Skin with Age. In Textbook of Aging Skin, 2nd ed.; Farage, M.A., Miller, K.W., Maibach, H.I., Eds.; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Farage, M.A. Perceptions of sensitive skin: Women with urinary incontinence. Arch. Gynecol. Obstet. 2009, 280, 49–57. [Google Scholar] [CrossRef]
- Farage, M. Self-reported immunological and familial links in individuals who perceive they have sensitive skin. Br. J. Dermatol. 2008, 159, 237–238. [Google Scholar] [CrossRef]
- Farage, M.A.; Jiang, Y.; Tiesman, J.P.; Fontanillas, P.; Osborne, R. Genome-Wide Association Study Identifies Loci Associated with Sensitive Skin. Cosmetics 2020, 7, 49. [Google Scholar] [CrossRef]
- Bataille, A.; Le Gall-Ianotto, C.; Genin, E.; Misery, L. Sensitive Skin: Lessons from Transcriptomic Studies. Front. Med. 2019, 6, 115. [Google Scholar] [CrossRef]
- Misery, L.; Sibaud, V.; Merial-Kieny, C.; Taieb, C. Sensitive skin in the American population: Prevalence, clinical data, and role of the dermatologist. Int. J. Dermatol. 2011, 50, 961–967. [Google Scholar] [CrossRef]
- Jourdain, R.; De Lacharrière, O.; Bastien, P.; Maibach, H.I. Ethnic variations in self-perceived sensitive skin: Epidemiological survey. Contact Dermat. 2002, 46, 162–169. [Google Scholar] [CrossRef]
- Brenaut, E.; Misery, L.; Taieb, C. Sensitive Skin in the Indian Population: An Epidemiological Approach. Front. Med. 2019, 6, 29. [Google Scholar] [CrossRef]
- Taieb, C.; Auges, M.; Georgescu, V.; Cullell, N.P.; Miséry, L. Sensitive skin in Brazil and Russia: An epidemiological and comparative approach. Eur. J. Dermatol. 2014, 24, 372–376. [Google Scholar] [CrossRef]
- Farage, M.A.; Miller, K.W.; Zouboulis, C.C.; Piérard, G.E.; Maibach, H. Gender Differences in Skin Aging and the Changing Profile of the Sex Hormones with Age. J. Steroids Horm. Sci. 2012, 3, 109–124. [Google Scholar] [CrossRef]
- Farage, M.A.; Neill, S.; MacLean, A.B. Physiological changes associated with the menstrual cycle: A review. Obstet. Gynecol. Surv. 2009, 64, 58–72. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.G.; Maibach, H.I. Estrogen and skin. An overview. Am. J. Clin. Dermatol. 2001, 2, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Farage, M.A.; Miller, K.W.; Berardesca, E.; Maibach, H.I. Sex Hormones, the skin and the immune system: Interactions and implications for skin testing. Sex Hormones, the skin and the immune system: Interactions and Treatment Strategies. Dermatology 2011, 1, 62–70. [Google Scholar]
- Trowbridge, M.M.; Cheng, R.; Farage, M.A. The Association between Perception of Sensitive Skin and Objective and Subjective Measures in Women with Urinary Incontinence. Fam. Med. Med. Sci. Res. 2017, 6, 221. [Google Scholar] [CrossRef]
- Besné, I.; Descombes, C.; Breton, L. Effect of age and anatomical site on density of sensory innervation in human epidermis. Arch. Dermatol. 2002, 138, 1445–1450. [Google Scholar] [CrossRef]
- Xiao, X.; Qiao, L.; Ye, R.; Zuo, F. Nationwide Survey and Identification of Potential Stress Factor in Sensitive Skin of Chinese Women. Clin. Cosmet. Investig. Dermatol. 2020, 13, 867–874. [Google Scholar] [CrossRef]
- Misery, L.; Taïeb, C.; Brenaut, E.; Huet, F.; Abasq-Thomas, C.; Sayag, M.; Bodemer, C. Sensitive Skin in Children. Acta Derm.-Venereol. 2020, 100, adv00039. [Google Scholar] [CrossRef]
- Hägglund, D.; Olsson, H.; Leppert, J. Urinary incontinence: An unexpected large problem among young females. Results from a population-based study. Fam. Pract. 1999, 16, 506–509. [Google Scholar] [CrossRef]
- Jolleys, J.V. Reported prevalence of urinary incontinence in women in a general practice. Br. Med. J. Clin. Res. Ed. 1988, 296, 1300–1302. [Google Scholar] [CrossRef]
- Thomas, T.M.; Plymat, K.R.; Blannin, J.; Meade, T.W. Prevalence of urinary incontinence. Br. Med. J. 1980, 281, 1243–1245. [Google Scholar] [CrossRef]
- Kim, E.J.; Lee, D.H.; Kim, Y.K.; Kim, M.-K.; Kim, J.Y.; Lee, M.J.; Choi, W.W.; Eun, H.C.; Chung, J.H. Decreased ATP synthesis and lower pH may lead to abnormal muscle contraction and skin sensitivity in human skin. J. Dermatol. Sci. 2014, 76, 214–221. [Google Scholar] [CrossRef]
- Yang, L.; Lyu, L.; Wu, W.; Lei, D.; Tu, Y.; Xu, D.; Feng, J.; He, L. Genome-wide identification of long non-coding RNA and mRNA profiling using RNA sequencing in subjects with sensitive skin. Oncotarget 2017, 8, 114894–114910. [Google Scholar] [CrossRef] [PubMed]
- Szymanski, M.; Barciszewska, M.Z.; Erdmann, V.A.; Barciszewski, J. A new frontier for molecular medicine: Noncoding RNAs. Biochim. Biophys. Acta 2005, 1756, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Farage, M.A. Perceptions of sensitive skin: Changes in perceived severity and associations with environmental causes. Contact Dermat. 2008, 59, 226–232. [Google Scholar] [CrossRef]
- Brenaut, E.; Barnetche, T.; Le Gall-Ianotto, C.; Roudot, A.C.; Misery, L.; Ficheux, A. Triggering factors in sensitive skin from the worldwide patients’ point of view: A systematic literature review and meta-analysis. J. Eur. Acad. Dermatol. Venereol. 2019, 34, 230–238. [Google Scholar] [CrossRef]
- Xiong, X.-J.; Meng, X.-J.; Zheng, T.-L. Biosorption of C.I. Direct Blue 199 from aqueous solution by nonviable Aspergillus niger. J. Hazard. Mater. 2010, 175, 241–246. [Google Scholar] [CrossRef]
- Farage, M.A.; Miller, K.W.; Wippel, A.M.; Berardesca, E.; Misery, L. Sensitive Skin in the United States: Survey of Regional Differences. Fam. Med. Med. Sci. Res. 2013, 2, 1–8. [Google Scholar]
- Kluger, N.; Le Floc’h, C.; Niore, M.; Delvigne, V.; Le Dantec, G.; Taieb, C. Self-Reported Skin Sensation by People Who Have Experienced Containment During COVID-19 Pandemic. Clin. Cosmet. Investig. Dermatol. 2020, 13, 943–947. [Google Scholar] [CrossRef]
- Farage, M.A. Does sensitive skin differ between men and women? Cutan. Ocul. Toxicol. 2010, 29, 153–163. [Google Scholar] [CrossRef]
- Farage, M.A. Perceptions of Sensitive Skin of the Genital Area. In Topical Applications and the Mucosa; Surber, C., Elsner, P., Farage, M.A., Eds.; Karger: Basel, Switzerland, 2011; pp. 142–154. [Google Scholar]
- Farage, M.A.; Bowtell, P.; Katsarou, A. Self-diagnosed sensitive skin in women with clinically diagnosed atopic dermatitis. Clin. Med. Dermatol. 2008, 2, 21–28. [Google Scholar]
- Mikkelsen, C.S.; Holmgren, H.R.; Kjellman, P.; Heidenheim, M.; Karppinnen, A.; Bjerring, P.; Huldt-Nystrøm, T. Rosacea: A clinical review. Dermatol. Rep. 2016, 8, 6387. [Google Scholar] [CrossRef]
- Wang, X.Y.; Liu, Y.Y.; Liu, Y.X.; Ma, W.W.; Zhang, J.W.; Liu, Z.J.; Liu, J.; Zhou, B.R.; Xu, Y. A predictive model for differential diagnosis between rosacea and sensitive skin: A cross-sectional study. Chin. Med. J. Engl. 2020, 133, 2132–2134. [Google Scholar] [CrossRef] [PubMed]
- Misery, L.; Duboc, H.; Coffin, B.; Brenaut, E.; Huet, F.; Taieb, C. Association between two painful and poorly understood conditions: Irritable bowel and sensitive skin syndromes. Eur. J. Pain 2019, 23, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Halioua, B.; Misery, L.; Seite, S.; Delvigne, V.; Chelli, C.; Taieb, J.; Taieb, C. Influence of Skin Subjective Symptoms on Sleep Quality in Patients with Cutaneous Disorders: A Study of 2871 Subjects. Clin. Cosmet. Investig. Dermatol. 2021, 14, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.L.S.; Raber, I.; Xu, J.; Li, R.; Spitale, R.; Chen, J.; Kiefer, A.K.; Tian, C.; Eriksson, N.K.; Hinds, D.; et al. Assessment of the Genetic Basis of Rosacea by Genome-Wide Association Study. J. Investig. Dermatol. 2015, 135, 1548–1555. [Google Scholar] [CrossRef] [PubMed]
- Thompson, W.G.; Longstreth, G.F.; Drossman, D.A.; Heaton, K.W.; Irvine, E.J.; Muller-Lissner, S.A. Functional bowel disorders and functional abdominal pain. Gut 1999, 45, II43–II47. [Google Scholar] [CrossRef] [PubMed]
- Huet, F.; Misery, L. Sensitive skin is a neuropathic disorder. Exp. Dermatol. 2019, 28, 1470–1473. [Google Scholar] [CrossRef]
- Mostaghimi, L.; Hetzel, S. Insomnia and other sleep complaints in inflammatory versus noninflammatory skin disorders: An observational case-control study. Int. J. Dermatol. 2019, 58, 976–981. [Google Scholar] [CrossRef]
- Littlejohn, G.; Guymer, E. Neurogenic inflammation in fibromyalgia. Semin. Immunopathol. 2018, 40, 291–300. [Google Scholar] [CrossRef]
- Huynh, T.T. Burden of Disease: The Psychosocial Impact of Rosacea on a Patient’s Quality of Life. Am. Health Drug Benefits 2013, 6, 348–354. [Google Scholar]
- Reich, A.; Wójcik-Maciejewicz, A.; Slominski, A.T. Stress and the skin. G. Ital. Dermatol. Venereol. 2010, 145, 213–219. [Google Scholar] [PubMed]
- Blount, B.W.; Pelletier, A.L. Rosacea: A common, yet commonly overlooked, condition. Am. Fam. Physician 2002, 66, 435–440. [Google Scholar]
- Misery, L.; Myon, E.; Martin, N.; Consoli, S.; Boussetta, S.; Nocera, T.; Taieb, C. Sensitive skin: Psychological effects and seasonal changes. J. Eur. Acad. Dermatol. Venereol. 2007, 21, 620–628. [Google Scholar] [CrossRef] [PubMed]
- Misery, L.; Jean-Decoster, C.; Mery, S.; Georgescu, V.; Sibaud, V. A New Ten-Item Questionnaire for Assessing Sensitive Skin: The Sensitive Scale-10. Acta Derm.-Venereol. 2014, 94, 635–639. [Google Scholar] [CrossRef]
- Finlay, A.Y.; Khan, G. Dermatology Life Quality Index (DLQI)-a simple practical measure for routine clinical use. Clin. Exp. Dermatol. 1994, 19, 210–216. [Google Scholar] [CrossRef]
- Jenkinson, C.; Layte, R.; Jenkinson, D.; Lawrence, K.; Petersen, S.; Paice, C.; Stradling, J. A shorter form health survey: Can the SF-12 replicate results from the SF-36 in longitudinal studies. J. Public Health 1997, 19, 179–186. [Google Scholar] [CrossRef]
- Misery, L.; Jourdan, E.; Abadie, S.; Ezzedine, K.; Brenaut, E.; Huet, F.; Sayag, M.; Taieb, C. Development and validation of a new tool to assess the Burden of Sensitive Skin (BoSS). J. Eur. Acad. Dermatol. Venereol. 2018, 32, 2217–2223. [Google Scholar] [CrossRef] [PubMed]
- Misery, L.; Myon, E.; Martin, N.; Verriere, F.; Nocera, T.; Taieb, C. Sensitive skin in France: An epidemiological approach. Ann. Dermatol. Venereol. 2005, 132, 425–429. [Google Scholar] [CrossRef]
- Fauger, A.; Lhoste, A.; Chavagnac-Bonneville, M.; Sayag, M.; Jourdan, E.; Ardiet, N.; Perichaud, C.; Trompezinski, S.; Misery, L. Effects of a new topical combination on sensitive skin. J. Cosmet. Sci. 2015, 66, 79–86. [Google Scholar] [PubMed]
- Farage, M.A.; Nusair, T.L.; Hanseman, D.; Sherman, S.N.; Tsevat, J. The Farage Quality of Life Measure for Consumer Products: Development and Initial Implementation. Appl. Res. Qual. Life 2010, 5, 1–25. [Google Scholar] [CrossRef]
- Zhang, L.; Adique, A.; Sarkar, P.; Shenai, V.; Sampath, M.; Lai, R.; Qi, J.; Wang, M.; Farage, M.A. The Impact of Routine Skin Care on the Quality of Life. Cosmetics 2020, 7, 59. [Google Scholar] [CrossRef]




| Sensation | Ref. |
|---|---|
| Burning | [9,21,22] |
| Dryness | [23] |
| Itching | [9,21,22] |
| Pain | [9,21,24] |
| Prickling | [21,22] |
| Picking | [21] |
| Smarting | [25] |
| Stinging | [24] |
| Tickling | [26] |
| Tightening | [9,22] |
| Tingling | [9] |
| Factor | Ref. |
|---|---|
| Skin type | |
| Phototype I–IV | [21] |
| Fair skin, susceptible to sunburn | [5,16] |
| Susceptibility to blushing and/or flushing | [5,16] |
| Skin pigmentation | [5,48] |
| Dry skin | [16,21,46,47] |
| Ethnicity a | [14] |
| Gender | [5,9,16,21,49] |
| Menstrual cycle | [5,18,50] |
| Age | [5,51,52] |
| Incontinence | [53] |
| Familial and genetic links | [21,54,55,56] |
| General | Face | Body | Genital Area | |
|---|---|---|---|---|
| Caucasian (n = 805) | 68.1% | 78.4% | 59.8% | 54.2% |
| African-American (n = 128) | 72.7% | 73.4% | 67.2% | 66.4% |
| p = 0.15 | p = 0.24 | p = 0.13 | p = 0.012 |
| Total Number of Subjects | Women | Men | Significance | Ref | |
|---|---|---|---|---|---|
| England | 2316 | 51.4% | 38.2% | NR | [16] |
| 5 countries (France, China, USA, Brazil and Russia) | 10743 | 55.0% | 45.0% | p < 0.001 | [21] |
| India | 3012 | 36.7% | 27.9% | p < 0.001 | [59] |
| Brazil | 1022 | 45.7% | 22.3% | p < 0.001 | [60] |
| Russia | 1500 | 50.1% | 25.4% | p < 0.0001 | [60] |
| USA | 994 | 50.9% | 38.2% | p < 0.0001 | [57] |
| Germany | 420 | 53.9% | 36.4% | NR | [23] |
| Korea | 1000 | 59.2% | 54.4% | p = 0.187 | [46] |
| China | 9154 | 15.9% | 8.6% | p < 0.001 | [8] |
| China a | 954 | 30.0% | 20.0% | p = 0.0007 | [9] |
| Sensitive Skin in General | Sensitive Skin of Specific Sites | |||
|---|---|---|---|---|
| Face | Body | Genital Area | ||
| <30 (N = 291) | ||||
| Sensitive (any degree) | 67.4% | 76.6% | 57.4% | 53.3% d |
| Not sensitive | 32.6% | 23.4% | 42.6% | 46.7% |
| 31–39 (N = 491) | ||||
| Sensitive (any degree) | 69.5% | 78.4% b | 62.10% | 55.0% e |
| Not sensitive | 30.5% | 21.6% | 37.9% | 45.0% |
| 40–49 (N = 127) | ||||
| Sensitive (any degree) | 61.4% a | 69.3% c | 57.5% | 58.3% |
| Not sensitive | 38.6% | 30.7% | 42.5% | 41.7% |
| >50 (N = 101) | ||||
| Sensitive (any degree) | 74.3% | 83.2% | 64.4% | 66.3% |
| Not sensitive | 25.7% | 16.8% | 35.6% | 33.7% |
| Age vs. sensitive skin | p = 0.65 | p = 0.52 | p = 0.28 | p = 0.012 |
| SSS Subjects | Non-Sensitive Subjects | p Value | Ref | |
|---|---|---|---|---|
| Environmental | ||||
| Humid Weather a | 48% | 13% | <0.0005 | [75] |
| Dry weather a | 78% | 54% | <0.0005 | [75] |
| Hot weather a | 66% | 32% | <0.0005 | [75] |
| Cold weather a | 87% | 70% | <0.0005 | [75] |
| Sun a | 82% | 66% | <0.0005 | [75] |
| Wind a | 71% | 53% | <0.0005 | [75] |
| Air conditioning b | 13% | 5% | <0.001 | [57] |
| Temperature variation b | 47% | 19% | <0.001 | [57] |
| Water b | 15% | 6% | <0.001 | [57] |
| Pollution b | 63% | 33% | <0.001 | [21] |
| Dust b | 58% | 29% | <0.001 | [21] |
| Lifestyle and habits | ||||
| Rough fabrics a | 71% | 43% | <0.0005 | [75] |
| Cosmetics b | 58% | 22% | <0.001 | [57] |
| Fatigue/lack of sleep b | 65% | 34% | <0.001 | [21] |
| Sweating b | 54% | 27% | <0.001 | [21] |
| Tobacco smoke b | 40% | 20% | <0.001 | [21] |
| Food b | 44% | 17% | <0.001 | [21] |
| Psychological and physiological factors | ||||
| Stress a | 63% | 24% | <0.0005 | [75] |
| Emotion b | 53% | 47% | <0.001 | [57] |
| Menstrual cycle a,c | 60% | 33% | <0.0005 | [50] |
| Skin Conditions | References |
|---|---|
| Contact dermatitis | [55] |
| Atopic | [16,51,82] |
| Sensitivity of the corneas and eyelids | [20] |
| Rosacea | [59,83,84] |
| Acne | [46,59] |
| Atopic dermatitis (eczema) | [5,46,59] |
| Seborrheic dermatitis (dandruff) | [46,59] |
| Sensitive corneas and eyelids | [20] |
| Other associations with SSS | |
| Irritable Bowel | [85] |
| Sleep disorders | [21,86] |
| Stress | [2,4,19,78] |
| Menstrual cycle | [18,62] |
| Sensitive Skin in the Genital Area | Hot Weather | Cold Weather | Rough Fabric | Dry Weather | Stress | Humid Weather | Menstrual Cycle a | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All age groups | ||||||||||||||||||
| Total sensitive responders | 546 | 553 | 551 | 548 | 548 | 530 | 441 | |||||||||||
| Factor causes irritation (%) | 88% | b | 86% | 86% | c | 72% | 58% | 47% | 30% | d | ||||||||
| ≥50 | ||||||||||||||||||
| Total sensitive responders | 59 | 59 | 64 | 60 | 62 | 60 | 23 | |||||||||||
| Factor causes irritation (%) | 88% | b | 86% | 86% | c | 72% | 58% | 47% | 30% | d | ||||||||
| 40–49 | ||||||||||||||||||
| Total sensitive responders | 68 | 70 | 70 | 67 | 68 | 66 | 42 | |||||||||||
| Factor causes irritation (%) | 57% | 79% | 71% | 73% | 44% | e | 39% | 52% | ||||||||||
| 31–39 | ||||||||||||||||||
| Total sensitive responders | 256 | 261 | 255 | 257 | 256 | 245 | 229 | |||||||||||
| Factor causes irritation (%) | 64% | 86% | 71% | 79% | 62% | 44% | 62% | |||||||||||
| ≤30 | ||||||||||||||||||
| Total sensitive responders | 151 | 151 | 150 | 152 | 150 | 147 | 137 | |||||||||||
| Factor causes irritation (%) | 59% | 82% | 75% | 76% | 65% | 45% | 65% | |||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farage, M.A. Understanding the Sensitive Skin Subject to Achieve a More Holistic Diagnosis. Cosmetics 2021, 8, 81. https://doi.org/10.3390/cosmetics8030081
Farage MA. Understanding the Sensitive Skin Subject to Achieve a More Holistic Diagnosis. Cosmetics. 2021; 8(3):81. https://doi.org/10.3390/cosmetics8030081
Chicago/Turabian StyleFarage, Miranda A. 2021. "Understanding the Sensitive Skin Subject to Achieve a More Holistic Diagnosis" Cosmetics 8, no. 3: 81. https://doi.org/10.3390/cosmetics8030081
APA StyleFarage, M. A. (2021). Understanding the Sensitive Skin Subject to Achieve a More Holistic Diagnosis. Cosmetics, 8(3), 81. https://doi.org/10.3390/cosmetics8030081






