Abstract
Two novel body/face wash gels enriched with emollient ingredients (including dexpanthenol) were developed for the daily care of dry skin. Two similarly designed 2-week studies (N = 42 each) were conducted to assess the biophysical and cosmetic performance of each of the new wash gels in healthy adults with dry skin. Instrumental measurements quantified the effects of the wash gels on stratum corneum (SC) hydration and transepidermal water loss (TEWL) (with and without a previous sodium lauryl sulfate (SLS) challenge) after single and repeated usage. Following single and repeated applications of the face wash gel to facial skin, as well as to dry SLS-undamaged and SLS-damaged skin of the forearm, skin hydration significantly increased. Similarly, after single and repeated usage of the body wash gel to dry SLS-undamaged and SLS-damaged skin of the forearm, skin moisturization increased significantly from baseline; comparisons with control areas provided inconsistent results for SLS-undamaged skin. No effects on TEWL were observed for either product. Both wash gels were well tolerated and the cosmetic performances were appreciated by the subjects. The study results suggest that daily use of the new wash gels was associated with significant skin-moisturizing effects without adversely affecting skin barrier function and repair.
Keywords:
body wash; cleanser; dexpanthenol; dry skin; face wash; moisturization; skin barrier; study; wash gel 1. Introduction
Skin cleansers are available worldwide in different forms, such as bars, creams, liquids, or gels, to be applied together with water [1]. The most frequently used cleansing products are soap (ionic)-based [1]. However, soap-based products may cause damage to the stratum corneum (SC) and extensive dryness [2,3,4]. This is of particular relevance for dry skin sufferers because xerotic skin is associated with an already existing reduced skin hydration and damaged SC. The latter leads to deficient barrier function with increased transepidermal water loss (TEWL), thereby fostering the ‘dry skin cycle’ [5,6]. Other features of dry skin include an altered lipid organization/composition and content, as well as impaired corneocyte differentiation [7]. Gentle skin cleansing without further compromising the skin’s natural protective barrier and moisture level is therefore an important element of body/face wash products to be used on a regular basis by dry skin sufferers.
Against this background, two new dexpanthenol-containing wash gels for daily external use (face wash gel (Bepanthen® Derma Gentle Face Cleanser) and body wash gel (Bepanthen® Derma Gentle Body Cleanser)) were developed for dry skin conditions; dry skin (xerosis) represents a common dermatological disorder [8]. Apart from the amount of non-soap (non-ionic)-based surfactants ensuring cleansing and foaming, the key ingredients are the same in the two wash gels and comprise dexpanthenol, argan oil, glycerin, and niacinamide. The face wash gel contains a lower proportion of surfactants (e.g., disodium cocoyl glutamate, lauryl glucoside) than the body wash gel. Other ingredients in common for the two wash gels include 1,2-hexanediol, lysine, guar hydroxypropyltrimonium chloride, xanthan gum, and citric acid. The composition was largely motivated by the objective to provide simultaneously gentle effective cleansing at a skin-friendly pH (5.5) and skin-moisturizing effects with the same product, thereby complying with modern cleanser technology [9] and appropriately addressing the needs of dry skin sufferers. Maintenance of barrier function and good tolerability should equally be ensured. These objectives were to be met by the addition of ingredients that are typically used in emollients, without compromising the cleansing efficacy of the wash gels. Specifically, the new wash gels contain a non-physiological lipid (argan oil), a humectant (glycerin), an antipruritic/soothing agent (niacinamide), and a multifunctional ingredient, including the enhancement of epidermal differentiation (dexpanthenol) [10]. These ingredients belong to the key components of an ideal emollient for dry skin management [7]. The benefits of using a body/face wash enriched with emollient ingredients were demonstrated previously; less unfavorable effects on skin barrier integrity and reduced loss of SC hydration were observed compared with regular wash products, while still functioning as cleansers [3,9,11,12].
According to cosmetic regulations, claims for cosmetic products have to be supported by adequate and verifiable evidence [13]. Taking these recommendations into account, we explored the biophysical and cosmetic properties of each of the new wash gels in individual randomized 2-week studies (with similar designs) involving healthy adult subjects with dry skin. Instrumental measurements quantified the effects of the wash gels on SC hydration and TEWL (with and without a previous sodium lauryl sulfate (SLS) challenge) after single and repeated usage. The cutaneous tolerability and the subjective cosmetic performance of the wash gels were assessed as well.
2. Methods
The two studies were conducted in healthy adult subjects with a dry skin condition under the supervision of a dermatologist at Eurofins Evic Product Testing Romania SRL, Bucharest, Romania, between November 2019 and December 2019. The trials were performed according to the Declaration of Helsinki with all its amendments. Subjects gave written informed consent to participate after being informed about the study procedures. The new wash gels used in the trials were provided by Bayer Consumer Care AG, Basel, Switzerland. For both trials, ethics approval was obtained from an independent institutional ethics committee.
Given the exploratory nature of the two studies, no primary or secondary variables were defined. For the same reason, no formal sample size calculation was performed. Based on historical data from similar studies, it was expected that scientifically reliable results could be gathered with the selected sample size [10,14].
2.1. Study 1: Face Wash Gel
2.1.1. Study Design
Study 1 was a randomized, open-label, intraindividual comparison study in healthy adults with dry skin. Study participants were sequentially allocated into two groups. Group 1 had test skin areas on the face (cheeks) and forearm, and group 2 had test skin areas on the face only. Both groups visited the study center on day 0 (baseline) and study days 1, 2, 7, and 14. In the group 1 subjects, skin barrier dysfunction was experimentally induced at one skin area on each volar forearm via the application of 0.8% SLS (1 mL) under a semiocclusive patch (Tegaderm®, 3M Health Care, Neuss, Germany) for 24 h, as reported previously [10]. Subjects undergoing the SLS challenge had an additional study visit on day -1 to have the SLS and patch applied.
Study participants came to the study site without having administered the face wash gel on that day.
All subjects in both groups had test areas F1 and F2 on their upper cheeks. Areas on the face were randomly allocated to the right or left cheek. Subjects of group 1 had additional test areas marked on their volar forearms (A1, A2, A3, A4). Each of these test areas had a size of 16 cm2. Skin areas A1 and A3 were always on the same arm, while A2 and A4 were located on the contralateral arm. On areas A3 and A4, the SLS challenge was performed. The face wash gel was applied on skin areas F1 and F2, as well as on areas of one volar forearm (A1, A3). The areas of the contralateral arm (A2, A4) served as controls for A1 and A3, respectively, and were not treated with the face wash gel. The locations of A1 and A3 on the same forearm were chosen via randomization. Likewise, the allocation of forearms (left or right) to treatment with the face wash gel was carried out in a randomized fashion. Subjects in group 2 had only the face as the investigational site (skin areas F1 and F2).
During the study, the face wash gel was applied to the assigned test areas (F1, F2, A1, and A3 for group 1; F1 and F2 for group 2). For the face, subjects were instructed to wet the face, gently massage the face wash gel onto the entire face, and then rinse and gently wipe dry. For the forearms, they were instructed to wet the skin, make a foam in the palms of their hands and apply the product to the assigned forearm, then rinse and gently wipe dry. The first application was made with the support of a technician at the investigational site on day 0 after baseline assessments. No face wash gel was applied within the first 24 h after the initial application. Thereafter, the administration of the study product was performed by the subjects themselves at home on a twice-daily schedule (morning and evening) until day 14. In the group 1 subjects, SLS patches were removed 30 min before the first product administration (day 0, baseline). At that time, the SLS challenge was finalized. Compliance was assured by weighing each plastic bottle (200 mL) with face wash gel before and after the treatment period.
2.1.2. Subjects and Assessments
Generally, the inclusion and exclusion criteria were in line with a previous study that was recently conducted at the same study center using topical dexpanthenol-containing cosmetic body emollients [10].
Healthy male and female subjects, aged 18 to 70 years and having skin types II–IV on the Fitzpatrick scale [15] were eligible for study enrolment. Subjects were required to have very dry and flaky skin on the face, as judged clinically by the dermatologist involved in the study. For inclusion, females had to be nonpregnant and nonbreastfeeding. Female subjects of childbearing age were requested to use a reliable method of contraception during the study.
Subjects were excluded from study participation if they had: any skin condition at the target areas that would interfere with the interpretation of the study results (e.g., pigmentation disorders, many freckles or naevi, history of atopic skin, presence of acne or rosacea, excessive pilosity, irritated skin, scars); a condition requiring the use of drugs interfering with study assessments within 6 months (systemic retinoids), 3 months (other systemic antiacne medication), 2 months (topical retinoids), 1 month (other topical antiacne medication), or 2 weeks (antiacne cosmetic products, antibiotics, topical or systemic use of anti-inflammatory drugs or antihistamines) prior to or during the study; initiation/change of estrogen-progesterone contraception or hormonal treatment within 3 months prior to or during the trial; allergies to any ingredient of the study product; history of adverse reactions to cosmetic products; vaccination within 2 weeks before or during the study; desensitization treatment within 6 months prior to start of the study.
Study participants were not permitted to have had any noninvasive (e.g., skin cleansing, scrub) or invasive (e.g., peeling, laser treatment) cosmetic interventions on the face and forearms within 1 month and 2 months, respectively, before and during the study. Likewise, intensive exposure of face and forearms to ultraviolet light was not allowed within 1 month prior to and during the trial. Subjects were also not allowed to use topical preparations other than the study product on the face and forearms throughout the study course. On visit days at the study site, subjects had to come to the trial center without having applied any product to the target areas. Moreover, subjects were not permitted to use detergents on the face and forearms since the previous evening; the consumption of hot beverages (e.g., coffee or tea) within 2 h before instrumental assessments was not allowed either.
The SC moisturization status was measured using a corneometer (Corneometer® CM825, Courage & Khazaka, Cologne, Germany). This corneometer determines the electrical capacitance of the skin surface, which is a function of the SC water content [16]. Measurements took place on the face area F2 (groups 1 and 2) and on each of the forearm skin areas A1 and A2 (group 1) at baseline and at 1, 2, 5, and 24 h (day 1) after the first and single application of the face wash gel. Additional assessments took place on study days 7 and 14. For all subjects in group 1, SC hydration was also determined in skin areas A3 and A4 of the forearms. For these test areas, the assessment times were identical to those of areas F2, A1, and A2, except for an additional measurement on day -1 prior to the SLS application. Three measurements were performed in the individual test areas per assessment time. An increase in skin capacitance/corneometry values corresponds to improved skin hydration [17].
TEWL (MPA Tewameter® TM300, Courage & Khazaka, Cologne, Germany) measurements took place on the face area F1 (groups 1 and 2) and on each of the forearm skin areas A1 and A2 (group 1) according to the same schedule as the SC hydration measurements. For subjects in group 1, TEWL was also quantified in skin areas A3 and A4 on day -1 when the SLS application took place; subsequent TEWL assessment times in these test areas were identical to those of areas F1, A1, and A2. There was one measurement per allocated skin area and assessment time. By means of TEWL measurements, the SC barrier function can be reliably quantified in an easy and noninvasive fashion; an increase in TEWL reflects worsening barrier function [18,19].
Instrumental measurements (i.e., corneometry, TEWL) have been widely used to study the effects of cleansing products on the SC [9] and were conducted as reported previously [10]. In brief, prior to instrumental measurements, subjects remained in a climatized room (20 ± 2 °C, 45 ± 15% relative humidity) for 15–20 min. During the measurements, the ventilation system of the air conditioner was stopped to limit the movement of air. The skin target areas treated with the face wash gel were wiped dry with a paper towel before measurements and after the product was rinsed off. If various instrumental measurements were conducted in the same skin area at a given time point, different skin sites were to be used.
To determine the cosmetic performance of the face wash gel, the subjects had to complete a validated self-assessment questionnaire when they visited the study center on day 14. The questionnaire was designed based on the guidance provided by the American Society for Testing and Materials [20]. For the assessment of cosmetic performance, the subjects had to rate 45 statements on a numerical scale ranging from 0 to 6 (0 = strongly disagree, 1 = moderately disagree, 2 = slightly disagree, 3 = neither agree nor disagree, 4 = slightly agree, 5 = moderately agree, 6 = strongly agree). Cutaneous tolerability and adverse events (AEs) were monitored throughout the study period.
2.1.3. Statistical Evaluation
The statistical evaluations were conducted using SPSS (version 22, IBM, Armonk, NY, USA). For the corneometry and TEWL determinations, a global repeated measures analysis of variance (ANOVA) was performed to identify any differences between the mean values assessed at baseline and measurements thereafter. Depending on the data distribution, the paired t-test or Wilcoxon signed-rank test was applied to test whether there was any significant mean change in instrumental measurements between the baseline and each postbaseline determination. A paired t-test was used to detect differences in the corneometry and TEWL values between the skin areas that were treated and nontreated (control) with face wash gel. For all statistical analyses, the level of significance was set at 0.05. For the analysis of data from the corneometry and TEWL measurements (change from baseline), a Bonferroni correction was applied to control for multiple comparisons over time. In these cases, the p-value for significance was set at a lower level. AEs and results from the questionnaire were analyzed in a descriptive way. As prospectively defined, results from the instrumental measurements in skin areas F1 and F2 were pooled for groups 1 and 2.
2.2. Study 2: Body Wash Gel
Study 2 was a randomized, open-label, intraindividual comparison study in healthy adults with dry skin. Study participants were sequentially allocated into two study groups. Group 1 had the test areas A1, A2, A3, and A4 marked on their volar forearms; group 2 had the test areas A1 and A2 only. Each of the test areas had a size of 16 cm2. Skin areas A1 and A3 were always on the same arm, while A2 and A4 were located on the contralateral arm. In areas A3 and A4, an SLS challenge was performed as described for study 1. The body wash gel was applied to skin areas A1 and A3; the areas of the contralateral arm (A2, A4) served as controls and remained untreated. The locations of A1 and A3 on the same forearm and the forearm (left or right) to be treated with the body wash gel were chosen via randomization. The body wash gel was applied once daily on the assigned test areas (A1 and A3 for group 1; A1 for group 2). Subjects were instructed to wet the skin, make a foam in the palms of their hands, and apply the study product to the assigned forearm, then rinse and gently wipe dry. By using the same procedure, the rest of the body (except the forearm with control areas A2 and A4) was treated once daily with the body wash gel. The first application was made with the support of a technician at the investigational site on day 0 after baseline assessments. No body wash gel was applied within the first 24 h after the first application. Thereafter, the administration of the study product was performed by the subjects themselves at home using a once-daily schedule (evening) until day 14. Compliance was assured by weighing each plastic bottle (200 mL) with body wash gel before and after the treatment period.
The visit schedule, inclusion/exclusion criteria, assessments (including time points), and statistics related to study 2 were identical to study 1, with the exception that subjects were required to have very dry, flaky skin on the body (instead of the face), as judged clinically by the dermatologist involved in the study. In addition, in study 2, the results from the instrumental measurements in skin areas A1 and A2 were pooled for groups 1 and 2, as prospectively defined in the study protocol.
3. Results
3.1. Study 1: Face Wash Gel
In total, 42 healthy Caucasian subjects (35 females, 7 males) were enrolled and all subjects completed the study. The mean age was 59 years (range: 22–70 years). The first 22 subjects considered eligible for study participation were allocated into group 1 and the subsequent 20 subjects into group 2.
3.1.1. SC Hydration—Face (Pooled Data, Group 1 + Group 2)
Table 1 shows the corneometry results for the F2 skin area over the study course; mean absolute values and mean changes from the baseline skin surface capacitance are displayed. The single application of the face wash gel, as well as the following twice-daily treatment induced an increase in the SC moisturization status, as reflected by an enhanced electrical capacitance of the skin surface compared with the baseline (p < 0.001 for all comparisons with baseline). In addition, global ANOVA revealed a significant difference between the skin capacitance mean value determined at baseline and subsequent measurements (p < 0.05). After a single application, the skin-hydrating effect was rapidly achieved and was maintained over the entire study. On day 14, there was a 58.8% improvement in skin hydration compared to the baseline (+16.55 a.u.; p < 0.001).

Table 1.
Absolute skin capacitance measurement values (face, area F2) and changes from baseline after a single application of the face wash gel (first 24 h) followed by twice-daily applications for 13 days.
3.1.2. SC Hydration—Forearms (Group 1)
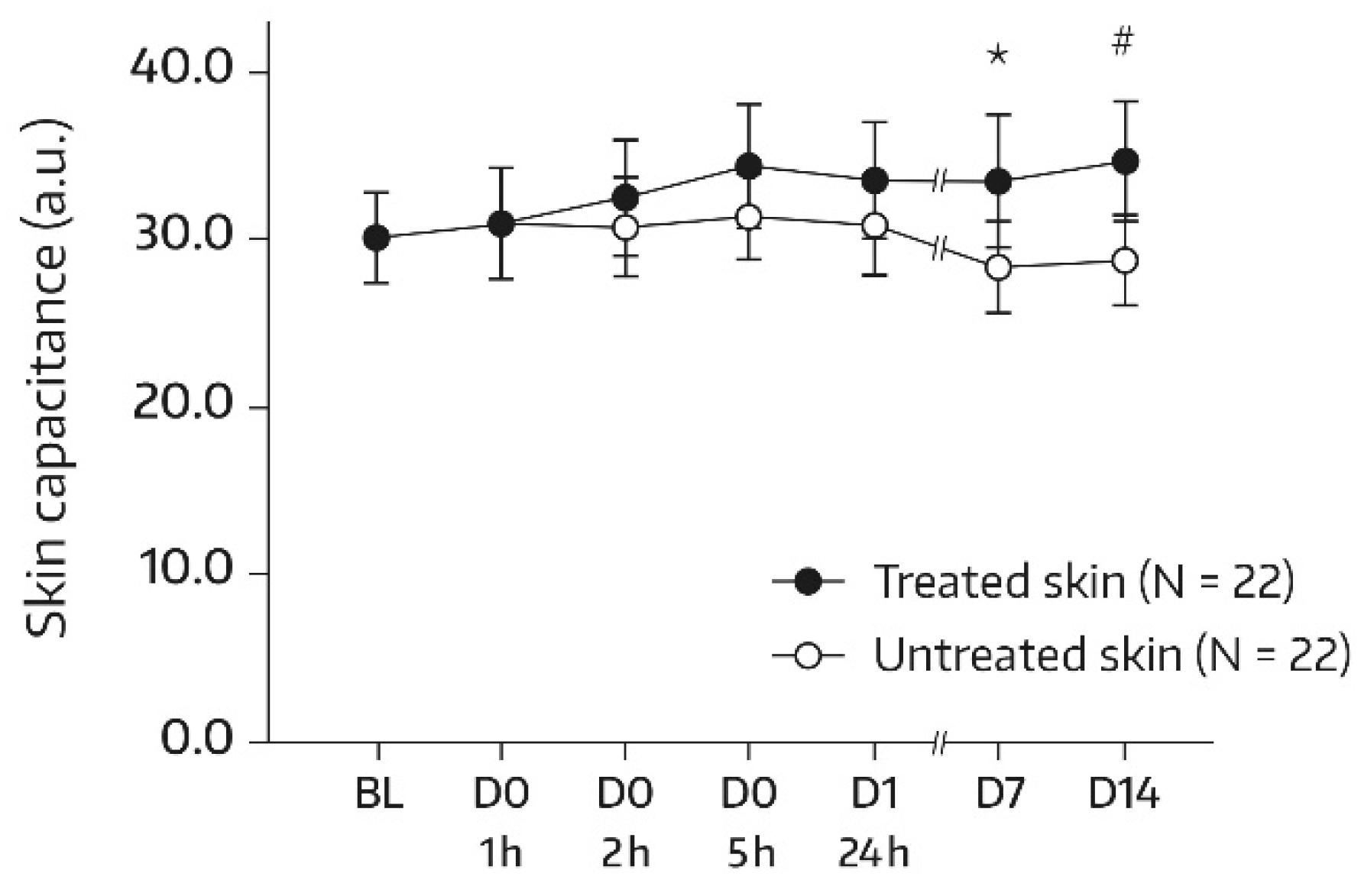
At baseline, the two forearm skin areas treated with the face wash gel (A1) or left untreated (A2) had comparable mean ± standard deviation (SD) values for dryness (30.07 ± 6.30 and 30.05 ± 6.11 arbitrary units (a.u.), respectively). Table 2 displays the corneometry results for the area treated with the face wash gel over the course of the study. A significant increase from the baseline in SC moisturization status was observed at 5 h after the first application and at all subsequent determinations, while a global ANOVA revealed no significant difference (p = 0.444) between the skin capacitance mean value determined at the baseline and subsequent measurements. On day 14, there was a 17.9% improvement in skin hydration compared to the baseline (+4.49 a.u., p = 0.019). In the untreated control area (A2), no change in SC hydration from the baseline was observed; the mean electrical capacitance remained unchanged at 28–31 a.u. throughout the study. Bilateral differences between the treated and untreated areas in terms of absolute mean values for skin capacitance were significant and in favor of the face wash gel on days 7 and 14. After the single administration, bilateral differences did not reach significance but the differences became more pronounced upon continued twice-daily use (Figure 1). On day 14, the mean bilateral difference amounted to 5.86 a.u. (p = 0.010).

Table 2.
Absolute skin capacitance measurement values (forearm, area A1) and changes from baseline after a single application of the face wash gel (first 24 h) followed by twice-daily applications for 13 days.
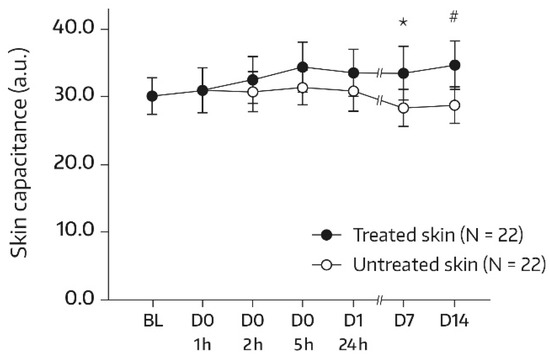
Figure 1.
Results from skin capacitance measurements (forearm) in untreated skin and skin undergoing a single application of the face wash gel (first 24 h) followed by twice-daily applications for 13 days. The values were determined using corneometry and are presented as mean ± SD. BL—baseline skin capacitance value before the first product application; D—day; h—hour. * p = 0.026 if compared to the untreated control, as measured using a paired t-test. # p = 0.010 if compared to the untreated control, as measured using a paired t-test.
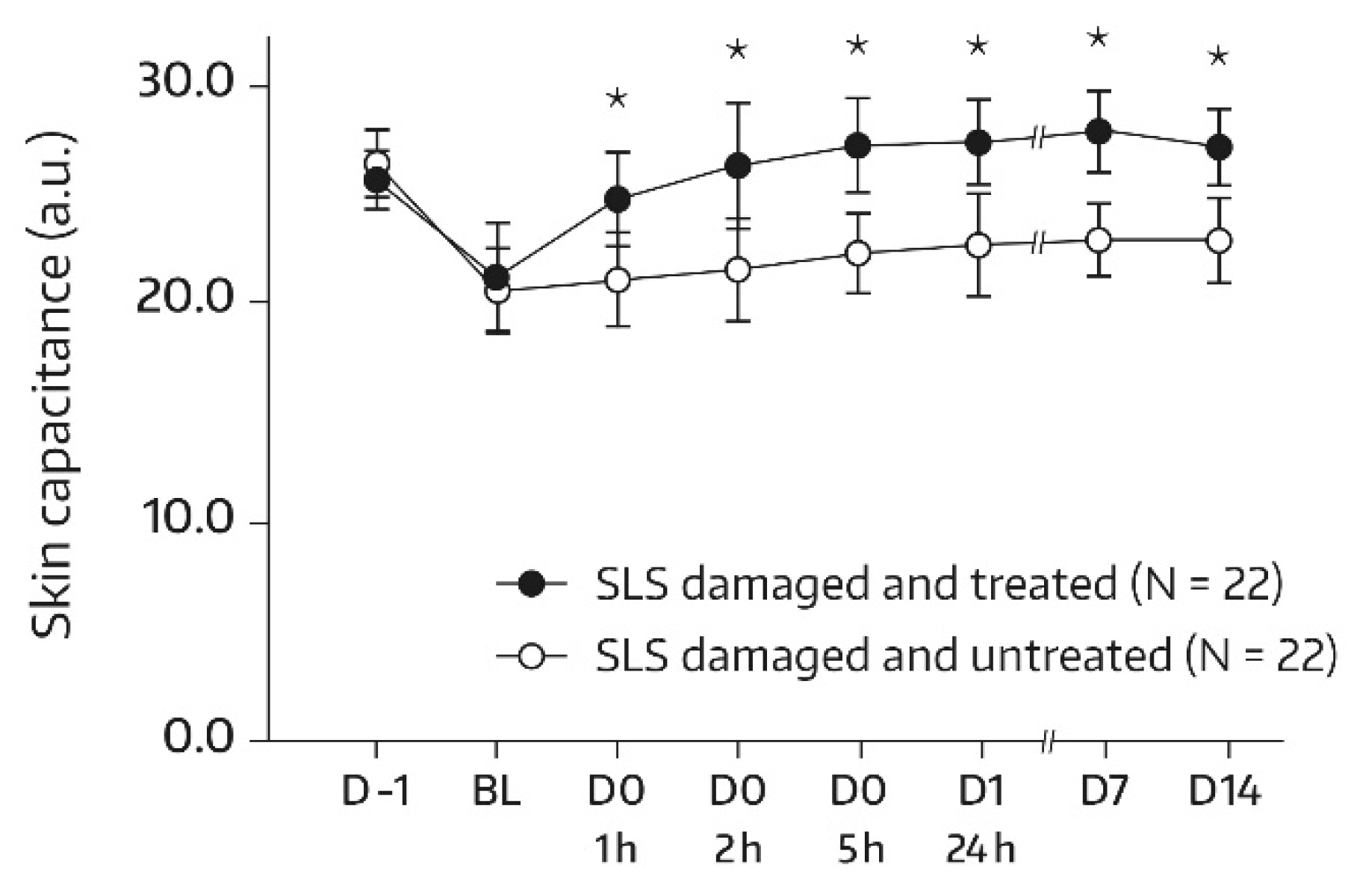
A similar pattern was observed in subjects undergoing an SLS challenge. Following the SLS challenge, skin hydration increased significantly in the SLS-damaged skin upon treatment with the face wash gel. There was a significant increase from baseline at all assessments (p ≤ 0.001 for all comparisons with baseline). In addition, a global ANOVA revealed a significant difference between the skin capacitance mean value that was determined at baseline and subsequent measurements (p < 0.05). On day 14, there was a 73.1% improvement in skin hydration compared with baseline (+34.86 a.u.; p < 0.001). The bilateral difference between the face-wash-gel-treated SLS-damaged skin area (A3) and the untreated SLS-damaged skin area (A4) in terms of absolute values for skin capacitance evolved over the study period and was significant and in favor of the face wash gel at the study’s end (Figure 2). Specifically, on day 14, the mean bilateral difference reached 6.43 a.u. (p = 0.039). The increase in skin hydration in the untreated skin area (A4) during the first 24 h following the SLS patch removal was in accordance with previous findings and was caused by transient inflammatory reactions [10,14].
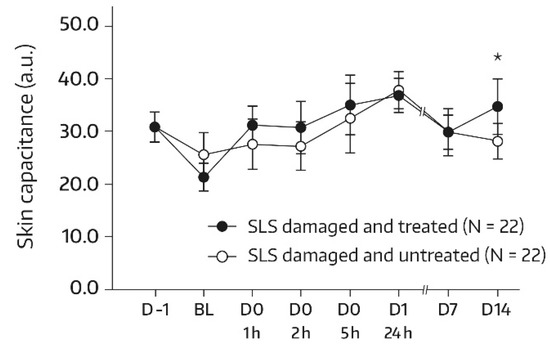
Figure 2.
Results from skin capacitance measurements (forearm) in untreated SLS-damaged skin and SLS-damaged skin undergoing a single application of the face wash gel (first 24 h) followed by twice-daily applications for 13 days. The values were determined using corneometry and are presented as mean ± SD. The SLS challenge started on day -1 and stopped 24 h later (30 min before the BL assessment). BL—baseline skin capacitance value before the first product application; D—day; h—hour; SLS—sodium lauryl sulfate. * p = 0.039 if compared to untreated control, as measured using a paired t-test.
3.1.3. TEWL—Face (Pooled Data, Group 1 + Group 2)
Table 3 summarizes the results from TEWL determinations in the face skin area F1; mean absolute values and mean changes from the baseline for the TEWL values are shown. The single application of the face wash gel, as well as the subsequent twice-daily treatments, had no adverse effect on skin barrier function. In fact, the mean TEWL remained stable at 12–13 g/m2·h throughout the study. Changes from the baseline were not significant at all postbaseline assessments. In addition, a global ANOVA revealed no significant difference between the mean TEWL value determined at baseline and subsequent measurements.

Table 3.
Absolute transepidermal water loss (TEWL) measurement values (face, area F1) and changes from the baseline after a single application of the face wash gel (first 24 h) followed by twice-daily applications for 13 days.
3.1.4. TEWL—Forearms (Group 1)
At baseline, the mean TEWL values were comparable between face-wash-gel-treated (A1) and untreated (A2) forearm skin areas (7.90 ± 1.97 and 8.21 ± 2.25 g/m2·h, respectively). Table 4 displays the TEWL results found for the area treated with the face wash gel over the course of the study.

Table 4.
Absolute transepidermal water loss (TEWL) measurement values (forearm, area A1) and changes from the baseline after a single application of the face wash gel (first 24 h) followed by twice-daily applications for 13 days.
Furthermore, in the target area of the forearm (A1), the single application of the face wash gel and the following twice-daily treatments had no adverse effect on skin barrier function. The mean TEWL showed only minor variations from the baseline and remained stable at 8–9 g/m2·h until the study’s end. In fact, all changes from the baseline were not significant for all postbaseline assessments. In addition, a global ANOVA revealed no significant difference between the mean TEWL value determined at the baseline and subsequent measurements. As expected, in the untreated control area (A2), the TEWL values remained essentially unchanged throughout the study, showing mean TEWL changes from the baseline between −1.14 and 1.62 g/m2·h (p > 0.05 for all comparisons with the baseline). Bilateral differences between the face-wash-gel-treated and untreated areas in terms of absolute mean values for TEWL were also not significant at all postbaseline assessments (data not shown), thereby underscoring that the face wash gel maintained skin barrier integrity upon single and repeated daily use.
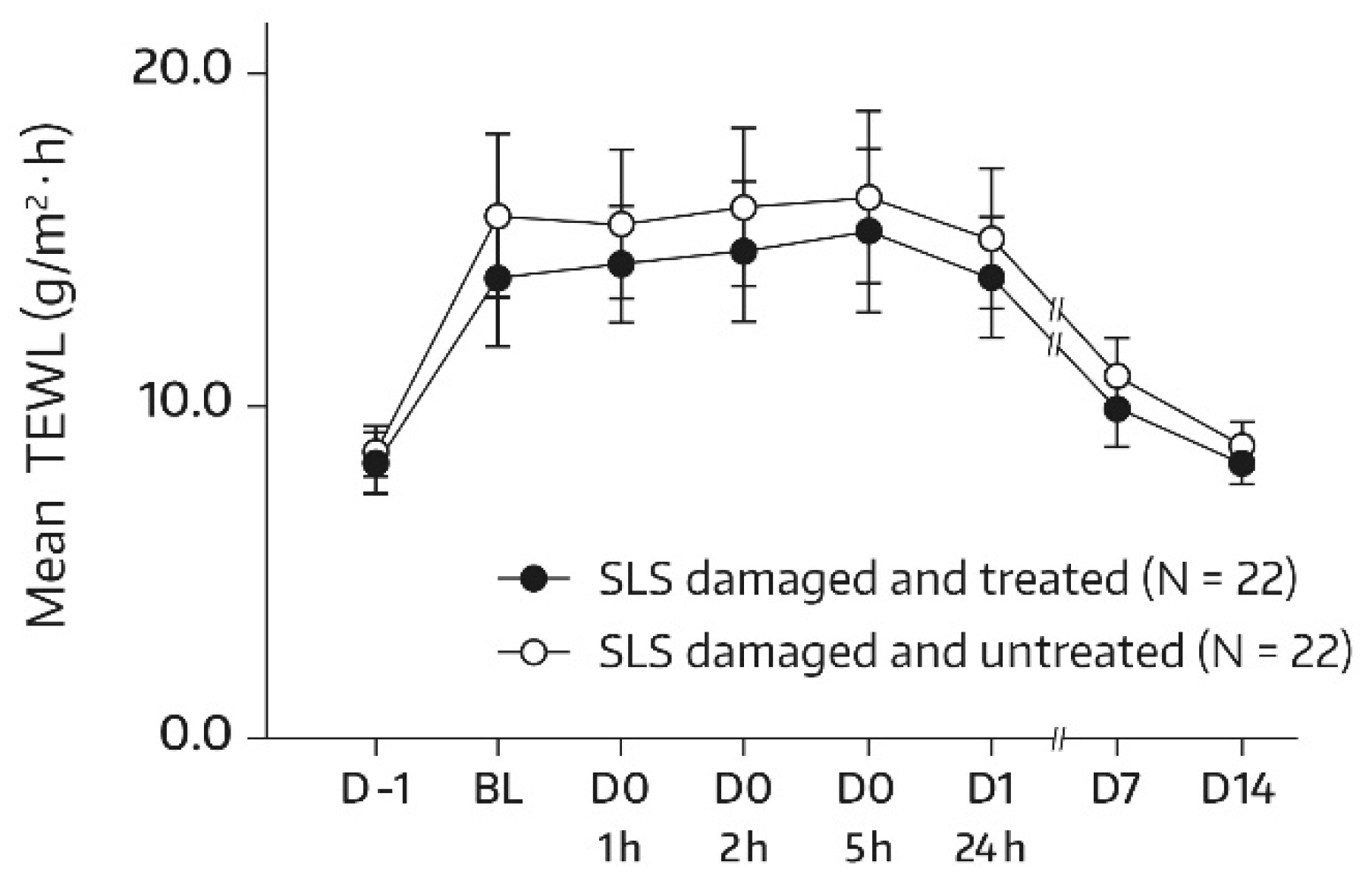
The SLS challenge caused skin barrier dysfunction as mirrored by an approximately twofold increase in TEWL at the baseline in comparison with the unchallenged test areas on day -1. By the study’s end (day 14), the mean TEWL had almost reached the prechallenge level, thereby suggesting the restoration of skin barrier function. This applied to both treated and untreated skin areas. Relevant bilateral differences between the face-wash-gel-treated SLS-damaged forearm skin area (A3) and the nontreated SLS-damaged forearm skin area (A4) were not observed, and absolute mean values for TEWL were not significantly different for all postbaseline assessments (Figure 3), which indicates that application of the face wash gel did not interfere with spontaneous barrier healing of 0.8% SLS-damaged skin. However, an accelerated TEWL reduction was not observed either.
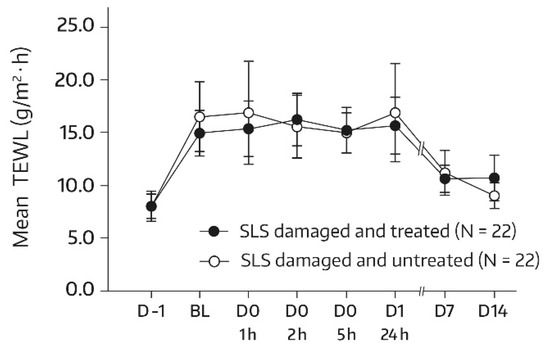
Figure 3.
Results from the TEWL measurements (forearm) in untreated SLS-damaged skin and SLS-damaged skin undergoing a single application of the face wash gel (first 24 h) followed by twice-daily applications for 13 days. The values are presented as mean ± SD. The SLS challenge started on day -1 and stopped 24 h later (30 min before the BL assessment). BL—baseline TEWL value before the first product application; D—day; h—hour; SLS—sodium lauryl sulfate.
3.1.5. Self-Assessment Questionnaire and Tolerability
The cosmetic performance of the face wash gel was appreciated by the overwhelming majority of study participants. In fact, all statements related to the cosmetic performance were rated as 6 (strongly agree), 5 (moderately agree), or 4 (slightly agree) by >70% of the subjects at the study’s end, thereby suggesting that the face wash gel performed favorably with regard to the convenience of application, cleansing effect, presence of unpleasant residues, and rinse-off capability, as well as soothing, smoothing, softening and moisturization features, and appearance of the facial skin. For instance, 98%, 98%, and 81% of the subjects provided a score of 4–6 for the statements that the face wash gel “effectively cleans without irritating dry skin,” “is perfect for daily cleansing of skin,” and “leaves the skin feeling moisturized,” respectively.
In terms of safety, applications of the face wash gel were well tolerated. None of the subjects experienced a systemic or local AE that was considered to be related to the study product according to the investigator. The SLS challenge caused some local reactions (e.g., erythema or burning), which improved as the study progressed and had resolved or were of minor severity by day 14. In the SLS-damaged skin area undergoing single and repeated usage of the face wash gel (A3), fewer AEs with shorter durations were recorded than in the untreated SLS-damaged skin area (A4). On day 1 (24 h after the first application of the study product), 5 AEs were reported for skin area A3 compared with 17 AEs for skin area A4.
3.2. Study 2: Body Wash Gel
In total, 42 healthy Caucasian subjects (40 females, 2 males) were enrolled and all subjects completed the study. The mean age was 58 years (range: 42–70 years). The first 22 subjects that were considered eligible for study participation were allocated into group 1 and the subsequent 20 subjects into group 2.
3.2.1. SC Hydration—Unchallenged Skin Areas (Pooled Data, Group 1 + Group 2)
At baseline, the two forearm skin areas that were treated with the body wash gel (A1) or left untreated (A2) had comparable mean ± SD values for dryness (24.83 ± 6.19 and 25.61 ± 5.35 a.u., respectively). Table 5 shows the corneometry results for the skin area A1 of the forearm over the study’s course; mean absolute values and mean changes from the baseline of skin surface capacitance are displayed.

Table 5.
Absolute skin capacitance measurement values (forearm, area A1) and changes from the baseline after a single application of the body wash gel (first 24 h) followed by once-daily applications for 13 days.
The single application of the body wash gel, as well as the following once-daily treatments, caused an increase in SC moisturization, as reflected by the enhanced electrical capacitance of the skin’s surface compared with the baseline (p < 0.001 for all comparisons with baseline). Global ANOVA did not detect a significant difference between the skin capacitance mean value determined at the baseline and subsequent measurements (p = 0.390). After a single application, the skin-hydrating effect was rapidly achieved and was maintained until the study’s end. On day 14, there was a 16.4% improvement in skin hydration compared with the baseline (+3.24 a.u., p < 0.001). In the untreated control area (A2), the electrical capacitance remained unchanged at approximately 25–26 a.u. during the study. Bilateral differences between the body-wash-gel-treated and untreated areas in terms of absolute mean values for skin capacitance were significant and in favor of the body wash gel at 5 h (3.01 a.u., p = 0.015) and on day 7 (2.86 a.u., p = 0.030) after the first application. At the other assessment times, the differences reached a statistical trend (p < 0.1). On day 14, the mean bilateral difference was 2.37 a.u. (p = 0.061).
3.2.2. SC Hydration—Challenged Skin Areas (Group 1)
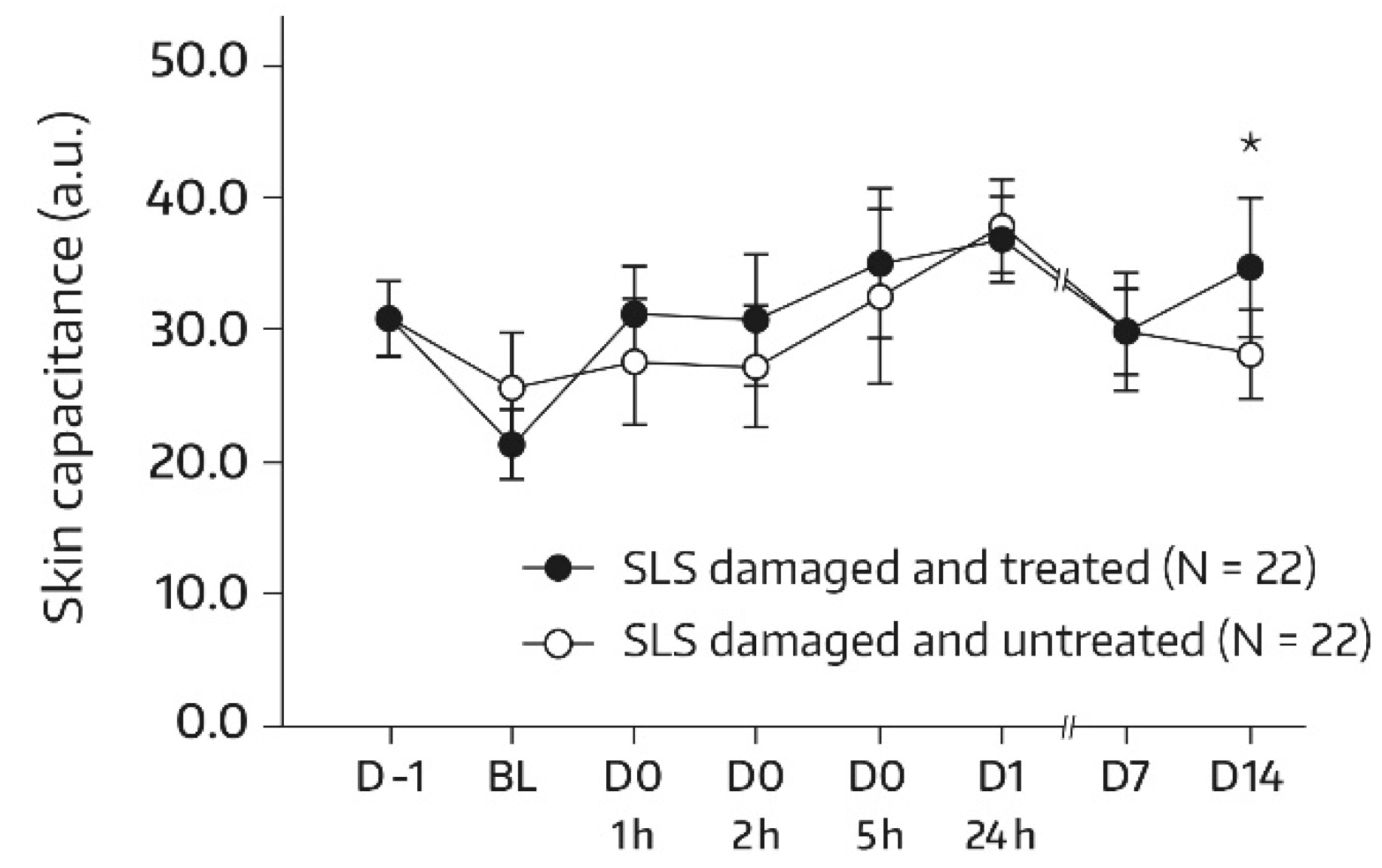
In subjects undergoing the SLS challenge, skin hydration increased significantly in the skin area (A3) that was damaged with SLS and subsequently treated with the body wash gel. With the exception of the first postbaseline measurement (1 h), there was a significant increase from the baseline at all assessments (p = 0.006 at 2 h and p < 0.001 for the remaining comparisons with baseline). In addition, global ANOVA revealed a significant difference between the skin capacitance mean value that was determined at the baseline and subsequent measurements (p < 0.05). On day 14, there was a 36.2% improvement in skin hydration compared with the baseline (+5.86 a.u., p < 0.001). Bilateral differences between the body-wash-gel-treated SLS-damaged skin area (A3) and the untreated SLS-damaged skin area (A4) in terms of absolute values for skin capacitance were significant and in favor of the body wash gel at all postbaseline assessments (Figure 4). On day 14, the mean bilateral difference amounted to 4.34 a.u. (p = 0.002).
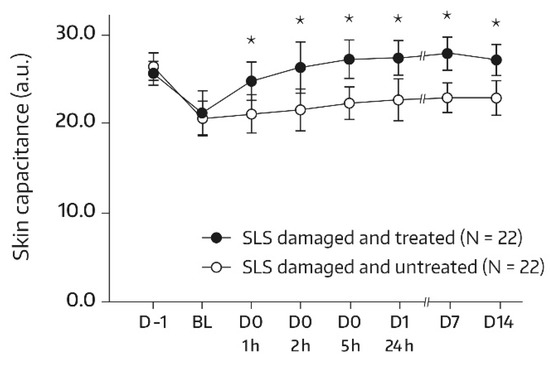
Figure 4.
Results from the skin capacitance measurements (forearm) of untreated SLS-damaged skin and SLS-damaged skin undergoing a single application of the body wash gel (first 24 h) followed by once-daily applications for 13 days. The values were determined using corneometry and are presented as mean ± SD. The SLS challenge started on day -1 and stopped 24 h later (30 min before the BL assessment). BL—baseline skin capacitance value before the first product application; D—day; h—hour; SLS—sodium lauryl sulfate. * p < 0.05 if compared to the untreated control, as measured using a paired t-test.
3.2.3. TEWL—Unchallenged Skin Areas (Pooled Data, Group 1 + Group 2)
The mean TEWL baseline values were comparable between the body-wash-gel-treated (A1) and untreated (A2) forearm skin areas (8.17 ± 1.30 and 7.78 ± 1.53 g/m2·h, respectively). Table 6 displays the TEWL results found for the body-wash-gel-treated area over the study’s course; mean absolute values and mean changes from the baseline for the TEWL values are shown.

Table 6.
Absolute transepidermal water loss (TEWL) measurement values (forearm, area A1) and changes from the baseline after a single application of the body wash gel (first 24 h) followed by once-daily applications for 13 days.
In the forearm target area A1, the single application of the body wash gel and the following once-daily treatments had no adverse effect on skin barrier function. The mean TEWL remained stable at approximately 8 g/m2·h throughout the study. In fact, all changes from the baseline were not significant for all postbaseline assessments. In addition, a global ANOVA revealed no significant difference between the mean TEWL value determined at the baseline and subsequent measurements. In the untreated control area (A2), the TEWL values also remained unchanged over the study’s course at approximately 8 g/m2·h (p > 0.05 for all comparisons with the baseline). Bilateral differences between the body-wash-gel-treated and untreated areas in terms of absolute mean values for TEWL were also not significant for all postbaseline assessments (data not shown), thereby providing further evidence that the body wash gel did not adversely affect skin barrier integrity upon single and repeated daily usage.
3.2.4. TEWL—Challenged Skin Areas (Group 1)
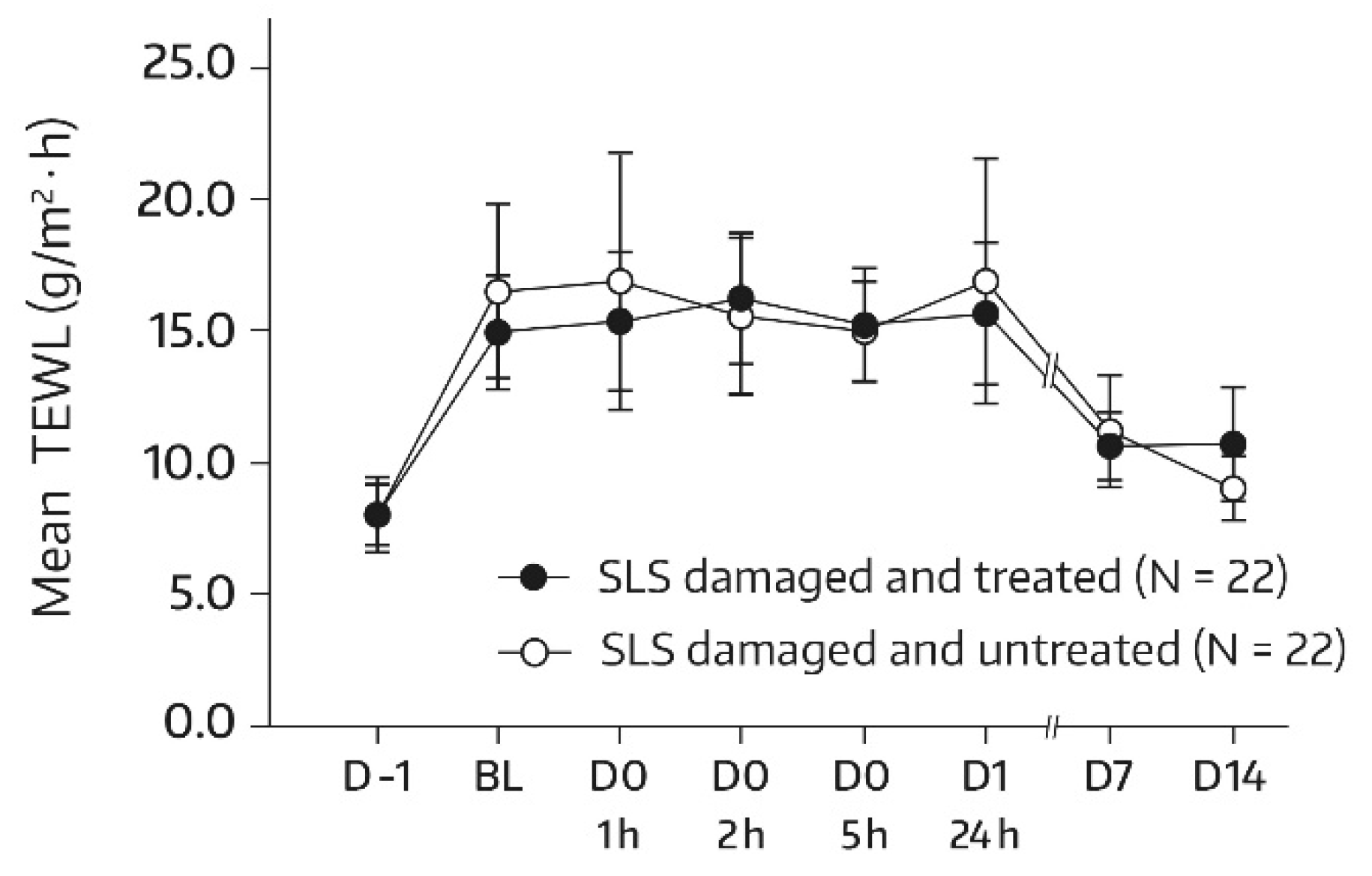
For subjects in group 1, the SLS challenge caused a dysfunction of the skin barrier, as seen by an approximately 1.8-fold increase in the TEWL at the baseline in comparison with the unchallenged test areas on day -1. By day 14, the mean TEWL had reached the prechallenge level, which suggests that the restoration of skin barrier function took place by the study’s end. This applied to both the treated and untreated target areas. Relevant bilateral differences between the body-wash-gel-treated SLS-damaged forearm skin area (A3) and the nontreated SLS-damaged forearm skin area (A4) were not observed. In fact, absolute mean values for TEWL were not significantly different for all postbaseline assessments (Figure 5), thereby indicating that regular use of the body wash gel did not interfere with the spontaneous barrier healing of 0.8% SLS-damaged skin. However, an accelerated TEWL reduction was not observed either.
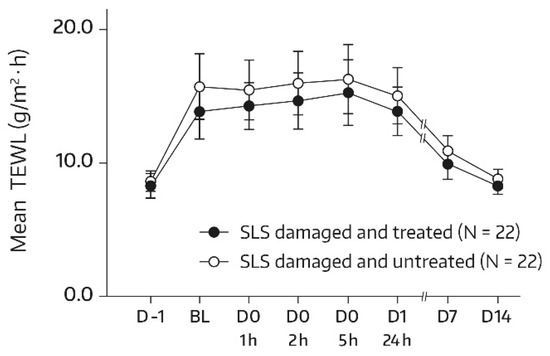
Figure 5.
Results from the TEWL measurements (forearm) in untreated SLS-damaged skin and SLS-damaged skin undergoing a single application of the body wash gel (first 24 h) followed by once-daily applications for 13 days. The values are presented as mean ± SD. The SLS challenge started on day -1 and stopped 24 h later (30 min before the BL assessment). BL—baseline TEWL value before the first product application; D—day; h—hour; SLS—sodium lauryl sulfate.
3.2.5. Self-Assessment Questionnaire and Tolerability
Generally, the cosmetic performance of the body wash gel was well received by the subjects. Specifically, 35 of the 45 statements related to cosmetic performance were rated as 6 (strongly agree), 5 (moderately agree), or 4 (slightly agree) by >70% of subjects at the study’s end. All statements related to cosmetic performance were scored 4–6 by >50% of the study participants at the end of the study. These results indicate that the body wash gel performed favorably overall with regard to the convenience of application, cleansing effect, presence of unpleasant residues, and rinse-off capability, as well as soothing, smoothing, softening and hydrating features, and appearance of the body’s skin. For instance, 83%, 83%, and 81% of subjects provided a score of 4–6 for the statements that the body wash gel “effectively cleans without irritating dry skin,” “is perfect for daily cleansing of skin,” and “leaves the skin feeling moisturized,” respectively.
Applications of the body wash gel were well tolerated. None of the subjects reported a systemic or local AE that was considered to be related to the studied product according to the investigator. The local reactions caused by the SLS challenge (e.g., erythema or itching) had resolved or were of minor severity by the end of the study. In the SLS-damaged skin area undergoing single and repeated daily usage of the body wash gel (A3), fewer AEs with shorter durations were recorded than in the untreated SLS-damaged skin area (A4). On day 1 (24 h after the first application of the studied product), 8 AEs were reported for skin area A3 compared with 22 AEs for skin area A4.
4. Discussion
Two novel wash gels for daily external use (face wash gel and body wash gel) were developed to address the needs of dry skin conditions. Both wash gels are enriched with emollient ingredients (e.g., dexpanthenol) and only differ in the content of non-ionic-based surfactants. The basic objective was to formulate wash products that simultaneously deliver gentle effective cleansing and skin-hydrating effects, without impairment of the SC barrier function. In this context, two studies explored for the first time the effects of the new wash gels on SC hydration and TEWL (with and without SLS challenge), as well as their cutaneous tolerability and cosmetic performance after single and continued applications in subjects with dry skin.
If compared with contralateral skin areas and/or baseline assessments, the study results can be summarized as follows: (1) following a single application of the face wash gel to dry skin of the face, skin capacitance was significantly increased for up to 24 h, indicating long-lasting facial skin hydration; (2) after twice-daily applications of the face wash gel to dry facial skin, as well as to dry SLS-undamaged and SLS-damaged skin of the forearm for approximately 2 weeks, skin capacitance was significantly increased at the final or both weekly assessments, suggesting long-term hydration; (3) following single and repeated once-daily applications of the body wash gel to dry skin of the forearm for approximately 2 weeks, skin capacitance was numerically increased compared with the control area at all assessments and reached statistical significance at selected time points (e.g., day 7); (4) following single and repeated once-daily applications of the body wash gel to dry SLS-damaged skin of the forearm for approximately 2 weeks, skin capacitance was significantly increased at all time points later than 1 h postbaseline, suggesting long-lasting and long-term hydration; (5) following single and prolonged daily applications of both wash gels to dry SLS-undamaged and SLS-damaged skin of the forearm, and to dry skin of the face (face wash gel only) for approximately 2 weeks, no effects on the TEWL were observed, suggesting that both wash products exerted no adverse effects on skin barrier function and did not interfere with spontaneous barrier healing of experimentally damaged skin; (6) both wash gels were well tolerated and the cosmetic performances were appreciated by the overwhelming majority of subjects.
It can be inferred that both wash gels were effective cleansers without irritating the skin. At the same time, both wash gels acted as skin moisturizers without further compromising the skin’s natural protective skin barrier. This is an advantage compared to regular, soap-based products, which may cause SC damage and pronounced dryness [2,3,4]. The features of the new wash gels are particularly favorable for dry skin sufferers because xerotic skin already displays reduced skin hydration and damaged SC barrier function [6,7]. Hydration of the skin not only improves dryness but also reduces pruritus and helps to restore disturbed barrier function [21]. Therefore, the two new wash gels can be considered a significant contribution to a modern personal care regimen in dry skin conditions. The here-presented approach of adding ingredients, typically used in emollients, to body/face wash products can be claimed successful as it resulted in body and face wash gels showing moisturizing properties, which was thought to be impossible for a long time [9].
Our results are in accordance with previous trials that demonstrated beneficial effects (i.e., reduced loss of SC hydration and/or less adverse effects on skin barrier integrity) when studying body/face washes enriched with emollient ingredients versus regular cleansers [2,3,11,12]. In these studies, the cleansing washes contained a single emollient or a combination thereof, such as petrolatum, glycerin, fatty acids, and/or soybean oil. For the new wash gels tested in our two studies, mild non-ionic-based surfactants were combined with ingredients that belong to the key components of an ideal emollient: a non-physiological lipid (argan oil), a humectant (glycerin), an antipruritic/soothing agent (niacinamide), and a multifunctional ingredient (dexpanthenol) [7].
Considering recent evidence, it is thought that the skin-moisturizing effect and good cosmetic performance mediated by the two wash gels in our studies were triggered by the emollient ingredients added to the formulations.
The SC of dry skin reveals an altered lipid organization/composition and reduced lipid content [7,22]. Niacinamide, which is present in the wash gels tested in our studies, enhances the biosynthesis of natural SC lipids in addition to its antipruritic effects [23,24]. In a previous study in healthy subjects, the topical use of a niacinamide-containing emollient induced a significant increase in SC cholesterol and free fatty acids [14]. Thus, niacinamide may indirectly compensate for the reduced lipid content in the SC lipid matrix observed in dry skin conditions, thereby favorably influencing the dry skin lipid layers and SC hydration. The non-physiological lipid (argan oil) present in the new wash gels acts primarily via an occlusive effect. It creates a hydrophobic barrier on the skin’s surface, resulting in restricted evaporation (i.e., it works like a temporary dressing) [7]. Glycerin is a physiological humectant and plays an important role in keeping the SC hydrated. It is a small molecule (MW = 200–300 Da) and reaches the deeper layers of the SC. There, it restores water content and replicates the function of natural moisturizing factors [7,25]. Topical dexpanthenol, another key ingredient of the new wash gels, acts as a moisturizer. This activity is considered to be related to dexpanthenol’s hygroscopic properties and its capability to promote the retention of moisture [6]. In addition, dexpanthenol reduces the increased rigidity of the SC lipid lamellae and keratin filaments observed in dry skin via interactions with lipid segments of the extracellular lamellae and protein residues in the SC corneocytes [6]. Dexpanthenol is also able to generate properties of hydrated skin in dehydrated conditions [26].
In our studies, the wash gels did not interfere with spontaneous barrier healing of the experimentally damaged skin. This is considered another advantage compared to regular, soap-based products, which may have a significant disrupting effect on skin barrier function. The latter worsens with repeated washing without apparent signs of skin barrier repair [4].
The new wash gels tested in our studies did not accelerate spontaneous barrier healing of SLS-damaged skin (i.e., there was no expedited TEWL reduction). This was expected given the limited contact time of the study products with the skin.
Both wash gels were well tolerated in our trials. No cases of contact dermatitis were observed, as reported for other liquid body washes upon prolonged skin contact [27]. In fact, in the SLS-damaged skin area undergoing single and repeated daily usage of the body/face washes, fewer AEs with shorter durations were recorded than in the untreated SLS-damaged skin area. This may have been triggered by the emollient ingredients that were added to the body/face gel formulations.
A limitation of the study investigating the face wash gel (study 1) was the absence of control areas for the facial skin areas F1 and F2. Thus, we could not account for spontaneous changes in TEWL and SC moisturization status, respectively, over the 2-week study period.
5. Conclusions
Both dexpanthenol-containing wash gels investigated in our studies were found to be suitable for the personal care regimen in dry skin conditions. The study results suggest that their single and prolonged daily use was associated with significant skin-moisturizing effects without adversely affecting skin barrier function and barrier healing. The cosmetic performance (including cleansing effectiveness and self-assessment of dryness) of both the face and body wash gels was appreciated by the overwhelming majority of subjects. In addition, both wash gels were well tolerated by healthy subjects with dry skin in the 2-week trials. The results of our studies support the integration of emollient ingredients in liquid body/face cleansers.
Author Contributions
Conceptualization, H.S. and S.T.; methodology, H.S. and S.T.; validation, H.S., A.B.-G., and R.d.S.; formal analysis, I.D., R.O., E.A.N., and V.D.; investigation, R.O., E.A.N., and V.D.; data curation, R.O., E.A.N., and V.D.; writing—review and editing, A.B.-G., H.S., R.d.S., R.O., E.A.N., I.D., V.D., and S.T.; supervision, R.d.S. and E.A.N.; project administration, R.d.S. and E.A.N. All authors have read and agreed to the published version of the manuscript.
Funding
Both studies were funded by Bayer Consumer Care AG, Basel, Switzerland.
Institutional Review Board Statement
The studies were conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Ethics Committee of Eurofins Evic Romania (protocol codes: 20717 and 20718; dates of approval for both studies: 05.11.2019).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the studies.
Data Availability Statement
The data presented in this paper are available from the corresponding author upon reasonable request.
Acknowledgments
Drafting of the manuscript was carried out by Edgar A. Mueller, 3P Consulting; the authors were responsible for critical revisions of the manuscript and for important intellectual content.
Conflicts of Interest
S.T., R.d.S., and H.S. are employees of Bayer Consumer Care AG, Basel, Switzerland. A.B.G. is an employee of Bayer Hispania, S.L., Sant Joan Despí, Spain. The other authors report no conflicts of interest.
References
- Cowdell, F.; Jadotte, Y.T.; Ersser, S.J.; Danby, S.; Lawton, S.; Roberts, A.; Dyson, J. Hygiene and emollient interventions for maintaining skin integrity in older people in hospital and residential care settings. Cochrane Database Syst. Rev. 2020, 1, CD011377. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Edmunds, M.; Lei, X.; Ottaviani, M.F.; Ananthapadmanabhan, K.P.; Turro, N.J. Stearic acid delivery to corneum from a mild and moisturizing cleanser. J. Cosmet. Dermatol. 2010, 9, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Hawkins, S. Reduction of “ashiness” in skin of color with a lipid-rich moisturizing body wash. J. Clin. Aesthet. Dermatol. 2011, 4, 41–44. [Google Scholar] [PubMed]
- Voegeli, D. The effect of washing and drying practices on skin barrier function. J. Wound Ostomy Continence Nurs. 2008, 35, 84–90. [Google Scholar] [CrossRef]
- Rawlings, A.V.; Matts, P.J. Stratum corneum moisturization at the molecular level: An update in relation to the dry skin cycle. J. Invest. Dermatol. 2005, 124, 1099–1110. [Google Scholar] [CrossRef]
- Proksch, E.; De Bony, R.; Trapp, S.; Boudon, S. Topical use of dexpanthenol: A 70th anniversary article. J. Dermatol. Treat. 2017, 28, 766–773. [Google Scholar] [CrossRef] [PubMed]
- Proksch, E.; Berardesca, E.; Misery, L.; Engblom, J.; Bouwstra, J. Dry skin management: Practical approach in light of latest research on skin structure and function. J. Dermatol. Treat. 2020, 31, 716–722. [Google Scholar] [CrossRef] [PubMed]
- Augustin, M.; Kirsten, N.; Körber, A.; Wilsmann-Theis, D.; Itschert, G.; Staubach-Renz, P.; Maul, J.; Zander, N. Prevalence, predictors and comorbidity of dry skin in the general population. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 147–150. [Google Scholar] [CrossRef]
- Wei, K.; Stella, C.; Wehmeyer, K.; Christman, J.; Altemeier, A.; Spruell, R.; Wimalasena, R.; Fadayel, G.; Wickett, R.R. Effects of petrolatum, a petrolatum depositing body wash and a regular body wash on biomarkers and biophysical properties of the stratum corneum. Int. J. Cosmet. Sci. 2021, 43, 218–224. [Google Scholar] [CrossRef]
- Stettler, H.; de Salvo, R.; Olsavszky, R.; Nanu, E.A.; Dumitru, V.; Trapp, S. Performance and tolerability of a new topical dexpanthenol-containing emollient line in subjects with dry skin: Results from three randomized studies. Cosmetics 2021, 8, 18. [Google Scholar] [CrossRef]
- Abbas, S.; Goldberg, J.W.; Massaro, M. Personal cleanser technology and clinical performance. Dermatol. Ther. 2004, 17 (Suppl. 1), 35–42. [Google Scholar] [CrossRef]
- Hoffman, L.; Subramanyan, K.; Johnson, A.W.; Tharp, M.D. Benefits of an emollient body wash for patients with chronic winter dry skin. Dermatol. Ther. 2008, 21, 416–421. [Google Scholar] [CrossRef] [PubMed]
- EU Commission. Guidelines to Commission Regulation (EU) No 655/2013 Laying Down Common Criteria for the Justification of Claims Used in Relation to Cosmetic Products. Version July 2013. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32013R0655&from=EN (accessed on 20 March 2021).
- Stettler, H.; Kurka, P.; Lunau, N.; Manger, C.; Böhling, A.; Bielfeldt, S.; Wilhelm, K.-P.; Dähnhardt-Pfeiffer, S.; Dähnhardt, D.; Brill, F.H.; et al. A new topical panthenol-containing emollient: Results from two randomized controlled studies assessing its skin moisturization and barrier restoration potential, and the effect on skin microflora. J. Dermatol. Treat. 2017, 28, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, T.B. The validity and practicality of sun-reactive skin types I through VI. Arch. Dermatol. 1988, 124, 869–871. [Google Scholar] [CrossRef] [PubMed]
- Clarys, P.; Clijsen, R.; Taeymans, J.; Barel, A.O. Hydration measurements of the stratum corneum: Comparison between the capacitance method (digital version of the Corneometer CM 825®) and the impedance method (Skicon-200EX®). Skin Res. Technol. 2012, 18, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, U.; Koop, U.; Leneveu-Duchemin, M.-C.; Osterrieder, K.; Bielfeldt, S.; Chkarnat, C.; Degwert, J.; Hantschel, D.; Jaspers, S.; Nissen, H.-P.; et al. Multicentre comparison of skin hydration in terms of physical-, physiological- and product-dependent parameters by the capacitive method (Corneometer CM 825). Int. J. Cosmet. Sci. 2003, 25, 45–53. [Google Scholar] [CrossRef]
- Pinnagoda, J.; Tupker, R.A.; Agner, T.; Serup, J. Guidelines for transepidermal water loss (TEWL) measurement. A report from the Standardization Group of the European Society of Contact Dermatitis. Contact Dermat. 1990, 22, 164–178. [Google Scholar] [CrossRef]
- Antonov, D.; Schliemann, S.; Elsner, P. Methods for the assessment of barrier function. Sex. Transm. Dis. Adv. Diagn. Treat. 2016, 49, 61–70. [Google Scholar] [CrossRef]
- American Society for Testing and Materials E1958-16a. Standard Guide for Sensory Claim Substantiation; ASTM International: West Conshohocken, PA, USA, 2016.
- Hon, K.L.; Tsang, Y.C.; Pong, N.H.; Lee, V.W.; Luk, N.M.; Chow, C.M.; Leung, T.F. Patient acceptability, efficacy, and skin biophysiology of a cream and cleanser containing lipid complex with shea butter extract versus a ceramide product for eczema. Hong Kong Med. J. 2015, 21, 417–425. [Google Scholar] [CrossRef]
- van Smeden, J.; Bouwstra, J.A. Stratum corneum lipids: Their role for the skin barrier function in healthy subjects and atopic dermatitis patients. Curr. Probl. Dermatol. 2016, 49, 8–26. [Google Scholar] [CrossRef] [PubMed]
- Tanno, O.; Ota, Y.; Kitamura, N.; Katsube, T.; Inoue, S. Nicotinamide increases biosynthesis of ceramides as well as other stratum corneum lipids to improve the epidermal permeability barrier. Br. J. Dermatol. 2000, 143, 524–531. [Google Scholar] [CrossRef] [PubMed]
- Otte, N.; Borelli, C.; Korting, H.C. Nicotinamide—Biologic actions of an emerging cosmetic ingredient. Int. J. Cosmet. Sci. 2005, 27, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Giménez-Arnau, A. Standards for the protection of skin barrier function. Curr. Probl. Dermatol. 2016, 49, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Björklund, S.; Pham, Q.D.; Jensen, L.B.; Knudsen, N.Ø.; Nielsen, L.D.; Ekelund, K.; Ruzgas, T.; Engblom, J.; Sparr, E. The effects of polar excipients transcutol and dexpanthenol on molecular mobility, permeability, and electrical impedance of the skin barrier. J. Colloid Interface Sci. 2016, 479, 207–220. [Google Scholar] [CrossRef]
- Miller, M.A.; Borys, D.; Riggins, M.; Masneri, D.C.; Levsky, M.E. Two cases of contact dermatitis resulting from use of body wash as a skin moisturizer. Am. J. Emerg. Med. 2008, 26, 246.e1–246.e2. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).