Fractions of Concern: Challenges and Strategies for the Safety Assessment of Biological Matter in Cosmetics
Abstract
1. Introduction
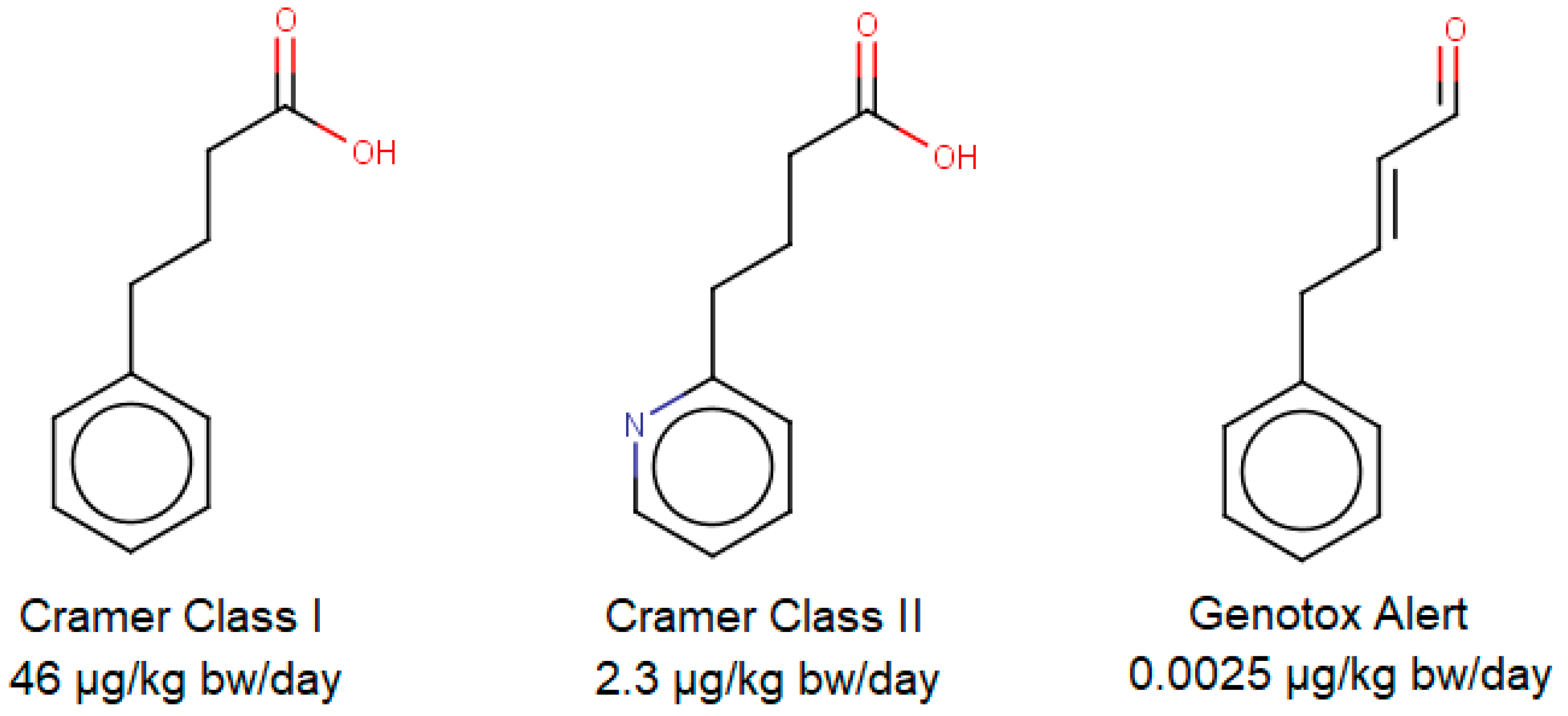
2. Threshold Approaches
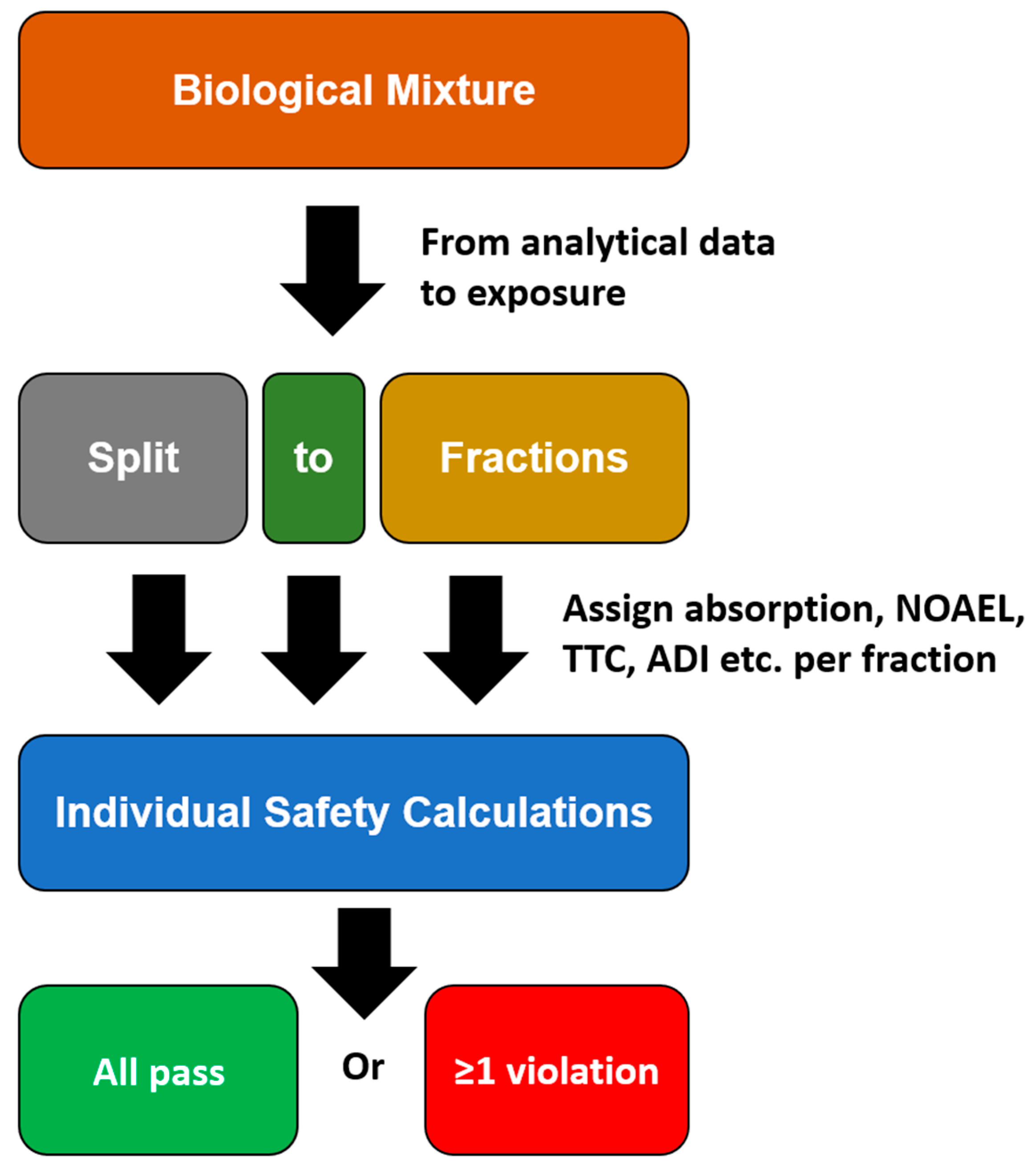
3. Fractions of Concern
4. Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rinaldi, A. Healing beauty? More biotechnology cosmetic products that claim drug-like properties reach the market. EMBO Rep. 2008, 9, 1073–1077. [Google Scholar] [CrossRef]
- Allemann, I.B.; Bauman, L. Botanicals in skin care products. Int. J. Dermatol. 2009, 48, 923–934. [Google Scholar] [CrossRef]
- Becker, M.; Tickner, J.A. Driving safer products through collaborative innovation: Lessons learned from the Green Chemistry & Commerce Council’s collaborative innovation challenge for safe and effective preservatives for consumer products. Sustain. Chem. Pharm. 2020, 18, 100330. [Google Scholar]
- Regulation (EC) No. 1223/2009 of the European Parliament and of the Council of 30 November 2009 on cosmetic products (recast). Off. J. Eur. Union Luxemb. 2009, 342, 59–209.
- Bernauer, U.; Bodin, L.; Chaudry, Q.; Coenraads, P.J.; Dusinka, M.; Ezendam, J.; Gaffet, E.; Galli, C.L.; Granum, B.; Rogiers, V.; et al. The SCCS Notes of Guidance for the Testing of Cosmetic Ingredients and Their Safety Evaluation, 10th ed.; SCCS: Brussels, Belgium, 2019. [Google Scholar]
- Díez-Sales, O.; Nácher, A.; Merino, M.; Merino, V. Chapter 17-Alternative Methods to Animal Testing in Safety Evaluation of Cosmetic Products. Analysis of Cosmetic Products, 2nd ed.; Salvador, A., Chisvert, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 551–584. [Google Scholar]
- Constable, A.; Jonas, D.; Cockburn, A.; Davi, A.; Edwards, G.; Hepburn, P.; Herouet-Guicheney, C.; Knowles, M.; Moseley, B.; Oberdörfer, R.; et al. History of safe use as applied to the safety assessment of novel foods and foods derived from genetically modified organisms. Food Chem. Toxicol. 2007, 45, 2513–2525. [Google Scholar] [CrossRef]
- ECHA. Read-Across Assessment Framework (RAAF); ECHA: Helsinki, Finland, 2017. [CrossRef]
- Munro, I.C.; Ford, R.A.; Kennepohl, E.; Sprenger, J.G. Correlation of a structural class with No-Observed-Effect-Levels: A proposal for establishing a threshold of concern. Food Chem. Toxicol. 1996, 34, 829–867. [Google Scholar] [CrossRef]
- Yang, C.; Barlow, S.M.; Jacobs, K.L.M.; Vitcheva, V.; Boobis, A.R.; Felter, S.P.; Arvidson, K.B.; Keller, D.; Cronin, M.T.D.; Enoch, S.; et al. Thresholds of Toxicological Concern for cosmetics-related substances: New database, thresholds, and enrichment of chemical space. Food Chem. Toxicol. 2017, 109, 170–193. [Google Scholar] [CrossRef] [PubMed]
- Cramer, G.M.; Ford, R.A.; Hall, R.L. Estimation of toxic hazard-a decision tree approach. Food Cosmet. Toxicol. 1978, 16, 255–276. [Google Scholar] [CrossRef]
- ToxTree v3.1.0. Toxic Hazard Estimation by Decision Tree Approach. Available online: http://toxtree.sourceforge.net/ (accessed on 11 November 2018).
- OECD 4.4.1. OECD QSAR Toolbox 4.4.1. Available online: https://qsartoolbox.org/download/ (accessed on 11 January 2021).
- Kroes, R.; Renwick, A.G.; Cheeseman, M.; Kleiner, J.; Mangelsdorf, I.; Piersma, A.; Schilter, B.; Schlatter, J.; van Schothorst, F.; Vos, J.G.; et al. Structure-based thresholds of toxicological concern (TTC): Guidance for application to substances present at low levels in the diet. Food Chem. Toxicol. 2004, 42, 65–83. [Google Scholar] [CrossRef]
- Payne, M.P.; Walsh, P.T. Structure-activity relationships for skin sensitization potential: Development of structural alerts for use in knowledge-based toxicity prediction systems. J. Chem. Inf. Comput. Sci. 1994, 34, 154–161. [Google Scholar] [CrossRef]
- Roberts, D.W.; Api, A.M.; Safford, R.J.; Lalko, J.F. Principles for identification of High Potency Category Chemicals for which the Dermal Sensitisation Threshold (DST) approach should not be applied. Regul. Toxicol. Pharmacol. 2015, 72, 683–693. [Google Scholar] [CrossRef]
- Safford, R.J.; Aptula, A.O.; Gilmour, N. Refinement of the Dermal Sensitisation Threshold (DST) approach using a larger dataset and incorporating mechanistic chemistry domains. Regul. Toxicol. Pharmacol. 2011, 60, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Safford, R.J.; Api, A.M.; Roberts, D.W.; Lalko, J.F. Extension of the Dermal Sensitisation Threshold (DST) approach to incorporate chemicals classified as reactive. Regul. Toxicol. Pharmacol. 2015, 72, 694–701. [Google Scholar] [CrossRef] [PubMed]
- Kawamoto, T.; Fuchs, A.; Fautz, R.; Morita, O. Threshold of Toxicological Concern (TTC) for Botanical Extracts (Botanical-TTC) derived from a meta-analysis of repeated-dose toxicity studies. Toxicol. Lett. 2019, 316, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Chacko, S.M.; Thambi, P.T.; Kuttan, R.; Nishigaki, I. Beneficial effects of green tea: A literature review. Chin. Med. 2010, 5, 13. [Google Scholar] [CrossRef]
- Reto, M.; Figueira, M.E.; Filipe, H.M.; Almeida, C.M. Chemical composition of green tea (Camellia sinensis) infusions commercialized in Portugal. Plant. Foods Hum. Nutr. 2007, 62, 139–144. [Google Scholar] [CrossRef]
- Prasanth, M.I.; Sivamaruthi, B.S.; Chaiyasut, C.; Tencomnao, T. A Review of the Role of Green Tea (Camellia sinensis) in Antiphotoaging, Stress Resistance, Neuroprotection, and Autophagy. Nutrients 2019, 11, 474. [Google Scholar] [CrossRef]
- SCHER; SCCS; SCENIHR. Opinion on the Toxicity and Assessment of Chemical Mixtures. 2012. Available online: https://ec.europa.eu/health/scientific_committees/environmental_risks/docs/scher_o_155.pdf (accessed on 11 February 2021).
- Boobis, A.; Budinsky, R.; Collie, S.; Crofton, K.; Embry, M.; Felter, S.; Hertzberg, R.; Kopp, D.; Mihlan, G.; Mumtaz, M.; et al. Critical analysis of literature on low-dose synergy for use in screening chemical mixtures for risk assessment. Crit. Rev. Toxicol. 2011, 41, 369–383. [Google Scholar] [CrossRef]
- Kortenkamp, A.; Faust, M.; Scholze, M.; Backhaus, T. Low-level exposure to multiple chemicals: Reason for human health concerns? Environ. Health Perspect. 2007, 115 (Suppl. 1), 106–114. [Google Scholar] [CrossRef]
- Martin, O.; Scholze, M.; Ermler, S.; McPhie, J.; Bopp, S.K.; Kienzler, A.; Parissis, N.; Kortenkamp, A. Ten years of research on synergisms and antagonisms in chemical mixtures: A systematic review and quantitative reappraisal of mixture studies. Environ. Int. 2021, 146, 106206. [Google Scholar] [CrossRef]
- Antignac, E.; Nohynek, G.J.; Re, T.; Clouzeau, J.; Toutain, H. Safety of botanical ingredients in personal care products/cosmetics. Food and chemical toxicology. Food Chem. Toxicol. 2011, 49, 324–341. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Steinmetz, F.P.; Wakefield, J.C.; Boughton, R.M. Fractions of Concern: Challenges and Strategies for the Safety Assessment of Biological Matter in Cosmetics. Cosmetics 2021, 8, 34. https://doi.org/10.3390/cosmetics8020034
Steinmetz FP, Wakefield JC, Boughton RM. Fractions of Concern: Challenges and Strategies for the Safety Assessment of Biological Matter in Cosmetics. Cosmetics. 2021; 8(2):34. https://doi.org/10.3390/cosmetics8020034
Chicago/Turabian StyleSteinmetz, Fabian P., James C. Wakefield, and Ray M. Boughton. 2021. "Fractions of Concern: Challenges and Strategies for the Safety Assessment of Biological Matter in Cosmetics" Cosmetics 8, no. 2: 34. https://doi.org/10.3390/cosmetics8020034
APA StyleSteinmetz, F. P., Wakefield, J. C., & Boughton, R. M. (2021). Fractions of Concern: Challenges and Strategies for the Safety Assessment of Biological Matter in Cosmetics. Cosmetics, 8(2), 34. https://doi.org/10.3390/cosmetics8020034




