Abstract
Ultraviolet radiation exposure is the dominant environmental determinant of all major forms of skin cancer, and the main cause of prematurely aged skin that is referred to as photoaging. Collagen type I (COL I) is expressed differently along with the dermis between healthy and pathological skin tissues. The aim of this study was to understand the impact of solar radiation in the dermis and assess the impact of solar radiation to COL I. The hematoxylin and eosin staining protocol was performed in tissue paraffin blocks and then they were stained immunohistochemically with the rabbit monoclonal anti-COL I antibody. A total of 270 slides were studied with an Olympus BX 41 microscope; we scored positively the expression of COL I in dermis and statistically analyzed with IBM SPSS Statistics. Based on our results, we observed that solar elastosis changes the structure of the skin’s collagen. In healthy tissues, COL I had a uniform expression along with the dermis. In tissues with aging, COL I expression was weaker and lost homogeneity. In pathological tissues (non-melanoma skin cancers, NMSCs), precancerous lesions, and benign skin lesions), the expression of COL I was observed to be almost weaker than tissues with aging in all body parts and much weaker below the lesions. The most severe solar elastosis was observed in the extremities. The degree of severity of the solar elastosis in relation to age did not appear to be completely affected. Solar radiation divides the collagen more rapidly than normal biological aging and solar elastosis was observed into the skin tissues with photoaging, which replaces the collagen fibers of the skin. These results confirm previous studies, which have shown that skin COL I decreases during aging, more in photoaging and even more in skin cancers. We conclude that skin COL I expression is reduced as a result of ultraviolet radiation and leading to negative impacts on the skin.
1. Introduction
It is now known that exposure to solar radiation can cause negative effects on the skin and human health. Sun damage is accumulative, so even a short exposure to the sun is added to the skin throughout a person’s life. The skin is a vital organ that permits the body’s communication with the environment. Radiation alters normal skin [1]. Ultraviolet radiation exposure is the dominant environmental determinant of all major forms of skin cancer, and the main cause of prematurely aged skin that is referred to as photoaging. Photoaging is also called actinic aging and can be caused by the breakdown of collagen, the formation of free radicals, and the interaction of DNA repair mechanisms and their inhibitory effect on immune mechanisms [2].
Solar elastosis is a degenerative condition of elastic tissue in the dermis due to prolonged sun exposure. There are a variety of clinical manifestations of solar elastosis; most commonly appearing as yellow, thickened, and coarsely wrinkled skin [3]. Solar elastosis and the degeneration of collagen can be observed histologically using hematoxylin and eosin staining (H&E) [4]. These changes are due to an imbalance between the production and degradation of the main proteins produced by fibroblasts [2]. Among these proteins, the most important is type I collagen (COL I, fibrillar). Total skin collagen is made of 80 to 85% of COL I [5].
Skin aging (biological aging and photoaging) is caused by both endogenous and exogenous factors [6]. Endogenous aging is a process that leads to thin, dry skin with fine wrinkles and gradual skin atrophy. [7] Exogenous aging is caused by environmental factors such as air pollution, smoking, poor nutrition and sun exposure, resulting in rough wrinkles, loss of elasticity, relaxation, and a rough look [8]. Ultraviolet (UV) radiation causes oxidative stress in skin cells, resulting in damaged cells with oxidized lipids activating complement systems and causing inflammation, leading to infiltration and activation of macrophages. Activated macrophages release uterine metalloproteinases (MMPs) which break down the extracellular matrix [9]. Repeated ultraviolet radiation inactivates the complement system, causing damage to the epidermis–dermis junction, in which macrophages are deposited and are overloaded with oxidized lipids. Overloaded macrophages release pro-inflammatory cytokines and reactive oxygen species (ROS), which cause chronic inflammation and long-term damage to the dermis [10].
Skin cancers represent the most common type of cancer worldwide. Non-melanoma skin cancer (NMSC) refers to a group of cancers that slowly develop in the upper layers of the skin [11]. The term non-melanoma distinguishes these more common types of skin cancer (99% are basal cell carcinomas, BCCs) and squamous cell carcinomas (SCCs) from less common skin cancers such as melanoma [12].
This study is based on the different expressions of COL I in the dermis between healthy and pathological tissues (e.g., aging, solar elastosis, NMSC, etc.). The aim was to assess the impact of solar radiation on COL I.
2. Materials and Methods
2.1. Tissue Samples
Biopsies of severe sun damaged skin (n = 135) recovered from the First Department of Pathology of Medicine School of the National and Kapodistrian University of Athens in Greece. Tissue samples (n = 88, NMSC, and n = 47, healthy skins that were used as controls) were fixed in buffered formalin, embedded into paraffin blocks, and then stained with hematoxylin and eosin.
2.2. Antibodies
The rabbit monoclonal anti-COL I antibody [EPR7785] IHC-P 1/1500 was used. It was performed using heat-mediated antigen retrieval with Thermo Scientific Pierce Tris-EDTA (TE) buffer, pH 9, before commencing with IHC staining for protocollagen.
2.3. Immunohistochemistry Microscopy Analysis
The microscope slides were evaluated by using an Olympus BX 41 microscope in magnification ×40 and ×100. The immunohistochemical report was performed by estimating with visual evaluation the percentage of COL I expression on a scale of 1 to 5, positively (weak +, weak to moderate ++, moderate +++, moderate to severe ++++, and severe +++++) [5].
2.4. Statistical and Data Analysis
All the data collected were entered into an electronic database created by Excel software. Data analysis was performed using IBM SPSS Statistics for Windows, version 26.0. Frequencies were calculated for qualitative variables. Categorical variables were gender, age categories, body part, and type of lesion. They were studied using chi-square (×2) and descriptive analysis, in relation to: (a) type of lesion, body part; (b) expression of COL I; and (c) the degree of severity of solar elastosis. One sample t-test was applied to determine the different expression of COL I in sun-damaged skins. The Kolmogorov–Smirnov test was applied to check normality. This relationship was accessed by the Kruskal Wallis test, providing the mean and standard deviation. Values of p < 0.05 were indicative of statistical significance.
3. Results
3.1. Characteristics of Tissue Samples
Healthy tissue samples (n = 47) were from, the abdomen (n = 4), face (n = 22), and breast (n = 21). In terms of the pathological specimens (n = 88), 40 were from the face, 14 from the back, 12 from the abdomen, and 22 from extremities. A total of 44 of them had aging and 3 were youth skin. Of the 88 pathological tissues (42 male, 46 female), 86 had solar elastosis and 66 of them had more lesions, concurrently. A total of 23 of the 66 had been diagnosed as benign lesions (seborrheic keratosis and nevus), 3 as precancerous skin lesions (dysplastic nevus and actinic keratosis), and 38 as NMSC (basal cell carcinomas and squamous cell carcinomas). The specimens were divided into 3 age groups (1st group = 66–85 years old, 2nd = 46–65 years old, 3rd = 25–45 years old). The largest specimen in our study with NMSC (n = 74) concerned the age group of 66–85. The study focused on COL I’s expression in three indexes. The first index was between the epidermis and solar elastosis (index A), the second was along the dermis (index B), and the last was below the cutaneous lesion (index C).
3.2. Healthy Tissue Samples
The results from the IHC microscopy analysis showed that the healthy skin samples had a uniform expression of COL I in the dermis. The expression of COL I in the healthy tissue samples with biological aging was uniform along the dermis and weaker than the expression of young skin. Moderate to intense (=4) expression was observed in the age group of 25–45, and moderate expression (=3) in the age group of 46–65, with a percentage of 65.96%. However, in chronological aging (in the age group of 66–85 years old), COL I’s I expression was moderate (=3) and a small percentage (2.65%) showed a weak (=1) expression (ages over 75 years old).
COL I staining confirmed that the collagen fibers were thin and loose in the papillary dermis and thicker in the reticular dermis. The healthy samples with youthful skin and chronological ageing appeared with collagen fibers that were thin and loose in the papillary dermis and were thicker in the reticular dermis. The distance of collagen fibers was bigger from each other in samples with ageing, compared with youthful skin samples. In the aging tissues, the keratin layer showed hyperplasia, skin atrophy, and reduction of the number of skin components except for sebaceous glands that were overgrown. Weaker expression of COL I was generally observed in relation to the skin at a younger age.
3.3. Photoaging
The specimens with photoaging were assessed according to the severity of the solar elastosis per body part. Then, it was compared with the degree of COL I expression. The results of the average expression of COL I per age group and body part are delineated in Table 1.

Table 1.
Average of the degree severity of solar elastosis per body part and average expression of COL I along the dermis (per body part, Index A, Index B).
In tissue samples with photoaging, the formation of a solar elastosis islet of elastin was observed beneath the skin, which replaced collagen. The average COL I expression between the epidermis and solar elastosis (index A) was weak to moderate and weak along with the dermis. Below the epidermis, it was observed that COL I was not expressed at all. The severest solar elastosis was observed in the extremities, then in the back, and less in the abdomen and face. Solar elastosis represented as a film-like distribution, except for four specimens with a very weak expression of COL I that was interrupted.
The degree of solar elastosis had a negative correlation with COL I index A, of the order of 42.3% (when the degree of elastosis increases by one unit, the effect of collagen decreases by 0.42 of the unit and vice versa; when the degree of elastosis decreases by one unit the effect of collagen increases by 0.42 of the unit).
Moreover, a negative correlation was observed with COL I index B, of the order of 16% (which means that when, for example, the degree of elastosis increases by one unit, the effect of collagen decreases by 0.16 of the unit and vice versa; when the degree of elastosis is reduced by one unit the effect of collagen increases by 0.16 of the unit).
Of the statistical analysis comparison of COL I’s expression in biological aging and photoaging, per age groups in the face to criterion B, the following was observed: In group 46–65 with biological aging, the expression of COL I was on average moderate; in specimens with photoaging, COL I’s expression was weaker than biological aging. It was observed that in ages over 75 years old, solar elastosis was milder than at the age of 65 years old (Figure 1).
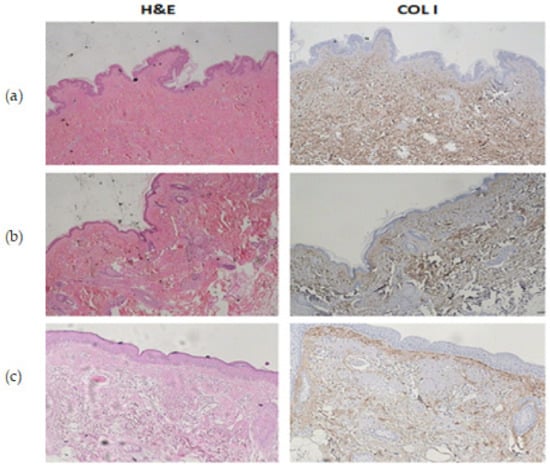
Figure 1.
(a) Histochemical staining of hematoxylin–eosin and anti-COL I in youthful tissue. (b) H&E and anti-COL I staining: biological aging. (c) H&E and anti-COL I staining: photoaging.
3.4. Non-Melanoma Skin Cancers
The results in benign lesions were with an average of COL I expression almost weak to moderate (index A = 1.82, index C = 1.85) in all areas of the body. The average expression of COL I along with the dermis (index B) was weak. Our sample number regarding the precancerous lesions were limited, thus, the results are under consideration. However, it was observed that the average of COL I was moderately expressed in index A and weakly in indexes B and C. The average expression of COL I in the NMSCs was weakly expressed along with the dermis (index B) and weaker below the lesions (index C), while it was weak to moderate between the epidermis and solar elastosis (index A) (Figure 2).
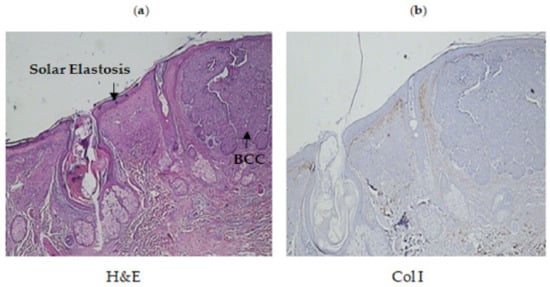
Figure 2.
(a) Histochemical staining of hematoxylin–eosin in pathological tissue from the hand of a 77 year old woman. She had been diagnosed with solar elastosis and basal cell carcinoma (BCC). (b) Immunohistochemistry: anti-Col I antibody. Absence of COL I in the positions of solar elastosis. Moderate expression of COL I observed between the epidermis and solar elastosis (film-like distribution) which was weak in the rest of the dermis. Absence of expression observed below the lesion.
The average expression of COL I was weak in almost all body parts, and the abdomen had the maximum expression of COL I compared with skin tissues from other body areas (average 2.67 for index A, 1.33 for index B).
4. Discussion
To understand the impact of solar radiation in the dermis and assess the impact of solar radiation on COL I, we studied biopsies from healthy and pathological tissues and assessed the expression of COL I in these samples. In healthy tissues, COL I staining confirmed that the collagen fibers were thin and loose in the papillary dermis and thicker with homogeneity. However, with aging they became weaker and lost their homogeneity [7].
UVA radiation is absorbed in a percentage of 20% by the dermis and 80% by the epidermis. Thus, solar elastosis appears superficially and can change the structure of collagen and elastin fibers in the skin [8]. The effect of sunlight on the dermis causes an increase in elastin in quantity and MMPs are produced in large quantities [13]. Under normal conditions, these enzymes repair the “wound” from the sun-damaged crust, making and reconstituting collagen. This process is not always 100% successful and some MMPs breaks down collagen, producing decomposed collagen fibers, resulting in “solar scars” [7,14]. As well as direct UVA irradiation in the dermis, UVB-irradiated keratinocytes can affect collagen formation (degradation) in dermal fibroblasts through secretory factors such as inflammatory cytokines including interleukin-1 (IL-1) and tumor necrosis factor-α (TNF-α) in the skin. TNF-α stimulates the chemotaxis of inflammatory cells to the skin and downregulates procollagen mRNA, and thus a blockade may be beneficial to the production of type I collagen [13].
In our specimens, it was observed that the solar elastosis was more severe in the extremities, back, and less in the face. The severity of the solar elastosis in relation to age did not appear to be completely affected. It was observed that in people over the age of 75, solar elastosis was milder than in the age of 65, and we would expect the reduction to be more severe in older skin. This could be a random finding observed in our samples owing to various factors, for example, lifestyle, location, duration of sun exposure, etc. [5]. Nevertheless, the possible cause could be the relation with the ozone layer, as the stratospheric ozone is an effective UV absorber. As the ozone layer becomes thinner, the protective filter provided by the atmosphere gradually decreases. As a result, every year humans and the environment are exposed to higher levels of UV radiation with more severe adverse effects at younger ages than in the past [5]. In pathological tissues of our study (NMSCs, precancerous lesions) and in benign skin lesions, the expression of COL I was almost weaker than skin tissues with aging in all body parts and much weaker below the lesion.
Fligiel, S.E., et.al. found collagen changes in photodamaged skin and changes in collagen structure in aged and photodamaged skin. They suggested that collagen fragmentation in vivo could underlie the loss of collagen synthesis in photodamaged skin and, to a lesser extent perhaps, in aged skin [15]. Solar radiation divides the collagen more rapidly than normal biological aging [15]. Solar elastosis was observed in the skin samples with photoaging, which replaced the collagen fibers of the skin [5,6]. Our results confirmed previous reports, which showed that in photodamaged skin COL I decreases and solar elastosis changes the structure of the skin’s collagen. In healthy tissues, COL I had a uniform expression in the dermis. In tissues with aging, COL I expression was weaker and lost homogeneity.
To our knowledge, this is the most multitudinous study in current literature to assess the impact of solar ultraviolet radiation in the expression of COL I in the dermis and compare its expression between healthy youth skin, aging, photoaging, benign skin lesions, and NMSCs. In conclusion, skin COL I expression is reduced as a result of ultraviolet radiation, which leads to negative impacts on the skin. COL I decreases during aging, more in photoaging, and even more in skin cancers.
Author Contributions
Conceptualization, F.B. and V.K.; methodology, A.C.L.; software, E.R.; validation, G.S., E.T. and N.K.; formal analysis, F.B.; investigation, F.B.; resources, A.S.; data curation, G.S.; writing—original draft preparation, F.B.; writing—review and editing, E.R. and A.S.; visualization, N.K.; supervision, A.C.L. and V.K.; and project administration; V.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We thank A. Kapranou and V. Papadopoulos for their help in IHC analysis.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Nguyen, A.V.; Soulika, A.M. The Dynamics of the Skin’s Immune System. Int. J. Mol. Sci. 2019, 20, 1811. [Google Scholar] [CrossRef] [PubMed]
- Lai-Cheong, J.E.; McGrath, J.A. Structure and function of skin, hair and nails. Medicine 2017, 45, 347. [Google Scholar] [CrossRef]
- Heng, J.K.; Aw, D.C.W.; Tan, K.B. Solar Elastosis in Its Papular Form: Uncommon, Mistakable. Case Rep. Dermatol. 2014, 6, 124–128. [Google Scholar] [CrossRef]
- Knapp, M.; Carpenter, C.E.; Shea, K.; Stowman, A.; Pierson, J. Focal Dermal Elastosis. A Proposed Update to the Nomenclature. Am. J. Dermatopathol. 2020, 42, 774–775. [Google Scholar] [CrossRef] [PubMed]
- Pain, S.; Berthélémy, N.; Naudin, C.; Degrave, V.; André-Frei, V. Understanding Solar Skin Elastosis-Cause and Treatment. J. Cosmet. Sci. 2018, 69, 175–185. [Google Scholar] [PubMed]
- Weihermann, A.C.; Lorencini, M.; Brohem, C.A.; de Carvalho, C.M. The structure of elastin and its involvement in photosynthesis of the skin. Int. J. Cosmet. Sci. Investig. 2017, 39, 241–247. [Google Scholar] [CrossRef]
- Zhang, S.; Duan, E. Fighting against Skin Ageing. The Way from Bench to Bedside. Available online: https://doi.org/10.2147/CCID.S191935 (accessed on 25 April 2018).
- Varvaresou, A.; Tsirivas, E.; Tsaoula, E.; Protopapa, E. Oxidative stress, photoageing and topical antioxidant protection. Rev. Clin. Pharmacol. Pharmacokinet. 2014, 18, 261–266. [Google Scholar]
- Shao, Y.; Qin, Z.; Alexander Wilks, J.; Balimunkwe, R.M.; Fisher, G.J.; Voorhees, J.J.; Quan, T. Physical properties of the photodamaged human skin dermis: Rougher Colsurface and stiffer/harder mechanical properties. Exp. Dermatol. 2019, 28, 914–921. [Google Scholar] [CrossRef] [PubMed]
- Trudel, D.; Desmeules, P.; Turcotte, S.; Plante, M.; Grégoire, P.; Renaud, M.C.; Orain, M.; Bairati, I.; Têtu, B. Visual and automated assessment of matrix metalloproteinase-25 tissue expression for the evaluation of ovarian cancer prognosis. Modern Pathol. 2014, 27, 1394–1404. [Google Scholar] [CrossRef]
- Saggini, A.; Cota, C.; Lora, V.; Kutzner, H.; Rütten, A.; Sangüeza, O.; Requena, L.; Cerroni, L. Uncommon Histopathological Variants of Malignant Melanoma. Part 2. Am. J. Dermatopathol. 2019, 41, 321–342. [Google Scholar] [CrossRef] [PubMed]
- Apalla, Z.; Nashan, D.; Weller, R.B.; Castellsagué, X. Skin Cancer: Epidemiology, Disease Burden, Pathophysiology, Diagnosis, and Therapeutic Approaches. Dermatol. Ther. 2017, 7, 5–19. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.R.; Mitrani, R.; Wertha, P. Effect of TNFα blockade on UVB-induced inflammatory cell migration and collagen loss in mice. J. Photochem. Photobiol. Biol. 2020, 213, 112072. [Google Scholar] [CrossRef] [PubMed]
- Gregorio, I.; Braghetta, P.; Bonaldo, P.; Cescon, M. ColVI in healthy and diseased nervous system. Cell Transplant. 2018, 27, 729–738. [Google Scholar]
- Fligiel, S.E.; Varani, J.; Datta, S.C.; Kang, S.; Fisher, G.J.; Voorhees, J.J. Col Degradation in Aged/Photodamaged Skin In Vivo and After Exposure to Matrix Metalloproteinase-1 In Vitro. J. Investig. Dermatol. 2003, 120, 842–884. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).