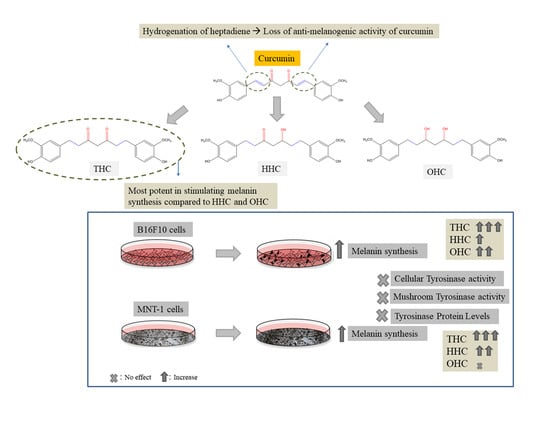
Comparative Study of Curcumin and Its Hydrogenated Metabolites, Tetrahydrocurcumin, Hexahydrocurcumin, and Octahydrocurcumin, on Melanogenesis in B16F10 and MNT-1 Cells
Abstract
1. Introduction
2. Results
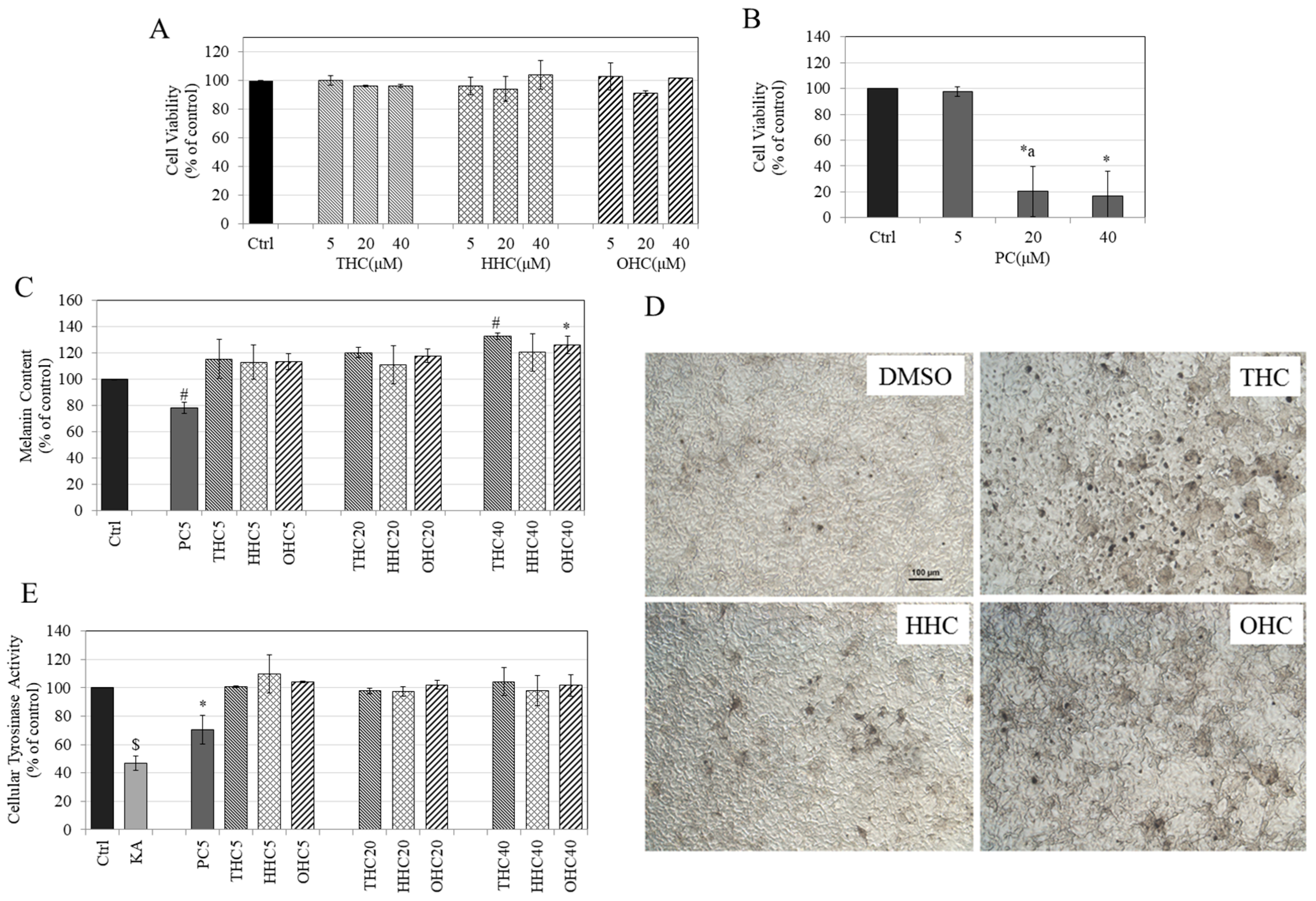
2.1. Effects of Compounds on Cell Viability in B16F10 Cells
2.2. Effects of Compounds on Melanin Synthesis in B16F10 Cells
2.3. Effects of Compounds on Intracellular Tyrosinase Activity in B16F10 Cells
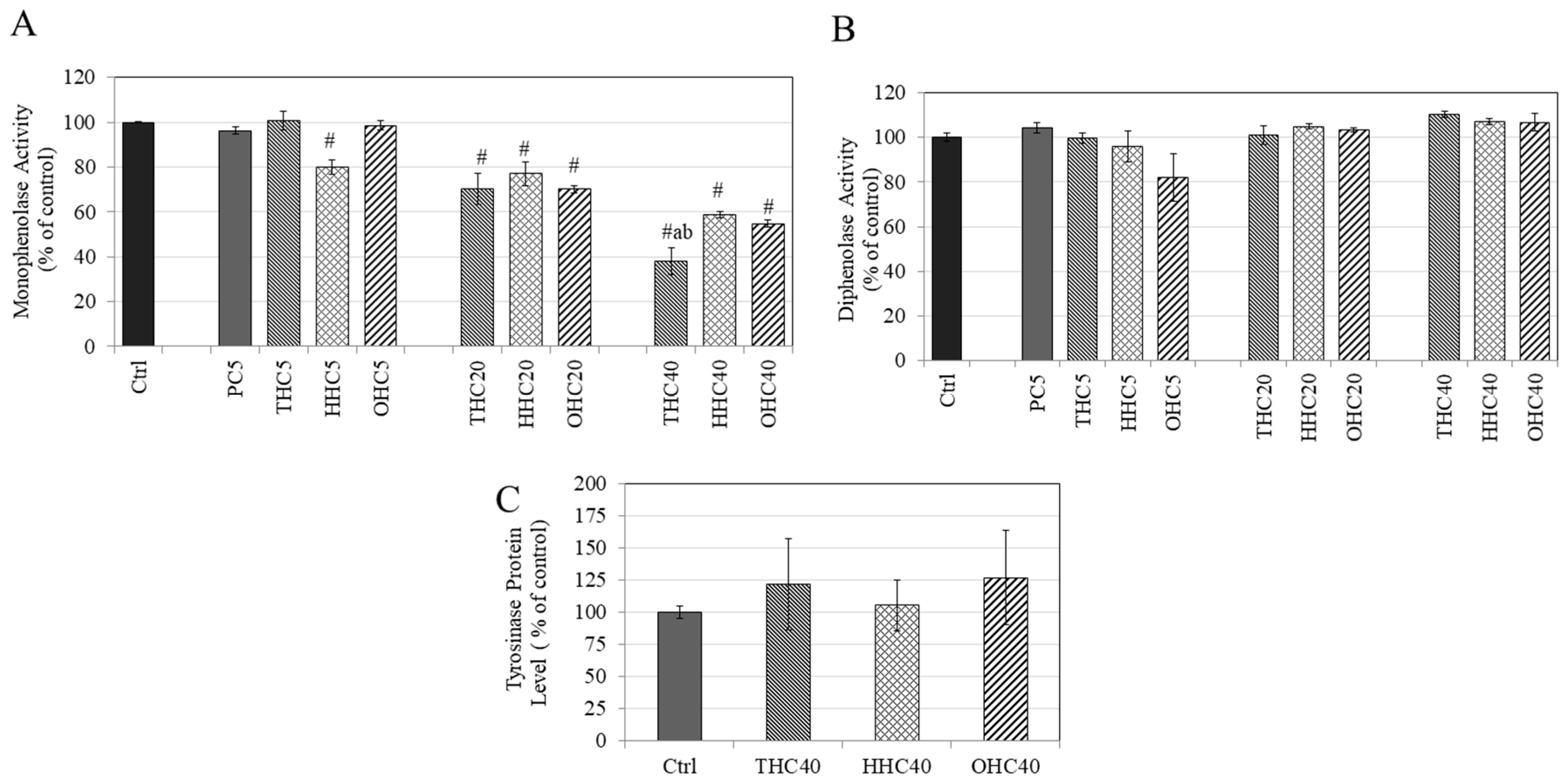
2.4. Effects of Compounds on L-DOPA and L-Tyrosine Oxidation in a Cell-Free System
2.5. Effects of Compounds on Tyrosinase Protein Expression in B16F10 Cells
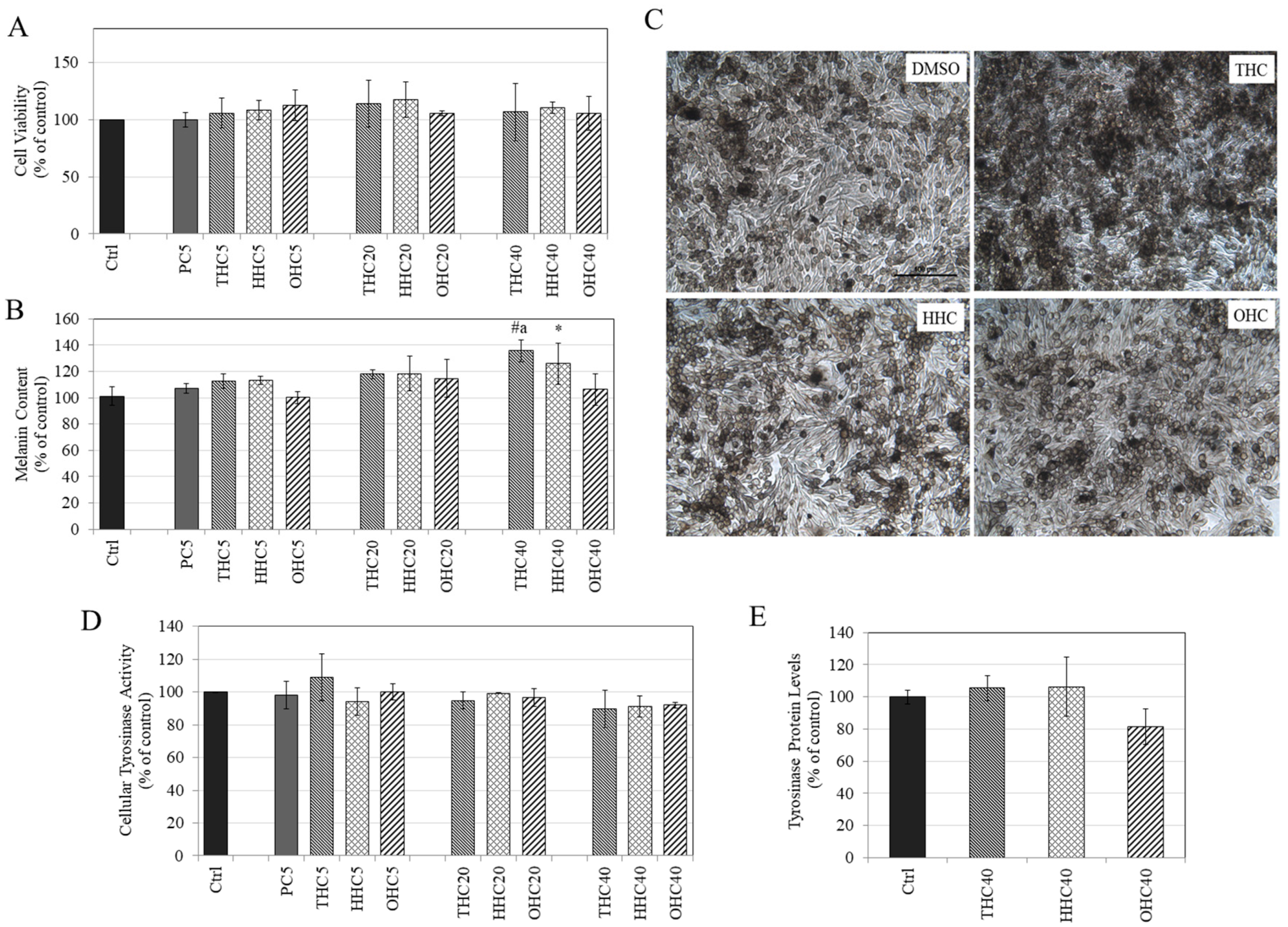
2.6. Effects of Compounds on Viability in MNT-1 Cells
2.7. Effects of Compounds on Melanin Synthesis in MNT-1 Cells
2.8. Effects of Compounds on Intracellular Tyrosinase Activity and Tyrosinase Protein Expression in MNT-1 Cells
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture
4.3. Testing in B16F10 and MNT-1 Cells
4.3.1. Cytotoxicity Assay
4.3.2. Melanogenesis Assay
4.3.3. Intracellular Tyrosinase Activity
4.3.4. Tyrosinase Protein Levels
4.4. Cell-Free Tyrosinase Activity Using L-TYR and L-DOPA Substrates
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SAR | Structure-Activity Relationship |
| PC | Pure Curcumin |
| HM | Hydrogenated Metabolite |
| THC | Tetrahydrocurcumin |
| HHC | Hexahydrocurcumin |
| OHC | Octahydrocurcumin |
| KA | Kojic Acid |
| DMEM | Dulbecco’s Modified Eagle Medium |
| MEM | Minimum Essential Medium |
| L-DOPA | L-Dihydroxyphenylalanine |
| TYR | Tyrosinase |
| ANOVA | Analysis of Variance |
| HBSS | Hank’s Balanced Salt Solution |
| HI-FBS | Heat-Inactivated Fetal Bovine Serum |
| ELISA | Enzyme-Linked Immunosorbent Assay |
References
- Brenner, M.; Hearing, V.J. The protective role of melanin against UV damage in human skin. Photochem. Photobiol. 2008, 84, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Herrling, T.; Jung, K.; Fuchs, J. The role of melanin as protector against free radicals in skin and its role as free radical indicator in hair. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2008, 69, 1429–1435. [Google Scholar] [CrossRef]
- Hong, Y.; Song, B.; Chen, H.D.; Gao, X.H. Melanocytes and Skin Immunity. J. Investig. Dermatol. Symp. Proc. 2015, 17, 37–39. [Google Scholar] [CrossRef] [PubMed]
- Hearing, V. Unraveling the melanocyte. Am. J. Hum. Genet. 1993, 52, 1. [Google Scholar] [PubMed]
- Ortonne, J.-P.; Bissett, D.L. Latest insights into skin hyperpigmentation. J. Investig. Dermatol. Symp. Proc. 2008, 13, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Nicolaidou, E.; Katsambas, A.D. Pigmentation disorders: Hyperpigmentation and hypopigmentation. Clin. Dermatol. 2014, 32, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Braun-Falco, O.; Plewig, G.; Wolff, H.H.; Burgdorf, W.H. Disorders of melanin pigmentation. In Dermatology; Springer: Berlin/Heidelberg, Germany, 2000; pp. 1013–1042. [Google Scholar]
- Draelos, Z.D. Skin lightening preparations and the hydroquinone controversy. Dermatol. Ther. 2007, 20, 308–313. [Google Scholar] [CrossRef]
- García-Gavín, J.; González-Vilas, D.; Fernández-Redondo, V.; Toribio, J. Pigmented contact dermatitis due to kojic acid. A paradoxical side effect of a skin lightener. Contact Dermat. 2010, 62, 63–64. [Google Scholar] [CrossRef]
- Jang, J.Y.; Lee, J.H.; Jeong, S.Y.; Chung, K.T.; Choi, Y.H.; Choi, B.T. Partially purified Curcuma longa inhibits alpha-melanocyte-stimulating hormone-stimulated melanogenesis through extracellular signal-regulated kinase or Akt activation-mediated signalling in B16F10 cells. Exp. Dermatol. 2009, 18, 689–694. [Google Scholar] [CrossRef]
- Lee, J.H.; Jang, J.Y.; Park, C.; Kim, B.W.; Choi, Y.H.; Choi, B.T. Curcumin suppresses alpha-melanocyte stimulating hormone-stimulated melanogenesis in B16F10 cells. Int. J. Mol. Med. 2010, 26, 101–106. [Google Scholar] [CrossRef]
- Park, S.Y.; Jin, M.L.; Kim, Y.H.; Kim, Y.; Lee, S.J. Aromatic-turmerone inhibits alpha-MSH and IBMX-induced melanogenesis by inactivating CREB and MITF signaling pathways. Arch. Dermatol. Res. 2011, 303, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Hosoya, T.; Nakata, A.; Yamasaki, F.; Abas, F.; Shaari, K.; Lajis, N.H.; Morita, H. Curcumin-like diarylpentanoid analogues as melanogenesis inhibitors. J. Nat. Med.-Tokyo 2012, 66, 166–176. [Google Scholar] [CrossRef] [PubMed]
- Mustarichie, R.; Levita, J.; Febriani, D. In-Silico Study of Curcumin, Demethoxycurcumin and Xanthorrizol as Skin Whitening Agents. World J. Pharm. Sci. 2013, 1, 72–80. [Google Scholar]
- Gopi, S.; Jacob, J.; George, R. Kinetic studies on the hydrogenation of curcuminoids isolated from Curcuma Longa by LC/MS. Res. J. Chem. Sci. ISSN 2015, 2231, 606X. [Google Scholar]
- Aggarwal, B.B.; Deb, L.; Prasad, S. Curcumin differs from tetrahydrocurcumin for molecular targets, signaling pathways and cellular responses. Molecules 2014, 20, 185–205. [Google Scholar] [CrossRef]
- Sugiyama, Y.; Kawakishi, S.; Osawa, T. Involvement of the beta-diketone moiety in the antioxidative mechanism of tetrahydrocurcumin. Biochem. Pharmacol. 1996, 52, 519–525. [Google Scholar] [CrossRef]
- Asawanonda, P.; Klahan, S.O. Tetrahydrocurcuminoid cream plus targeted narrowband UVB phototherapy for vitiligo: A preliminary randomized controlled study. Photomed. Laser Surg. 2010, 28, 679–684. [Google Scholar] [CrossRef]
- Zhao, F.; Gong, Y.; Hu, Y.; Lu, M.; Wang, J.; Dong, J.; Chen, D.; Chen, L.; Fu, F.; Qiu, F. Curcumin and its major metabolites inhibit the inflammatory response induced by lipopolysaccharide: Translocation of nuclear factor-kappaB as potential target. Mol. Med. Rep. 2015, 11, 3087–3093. [Google Scholar] [CrossRef]
- Park, C.H.; Song, J.H.; Kim, S.N.; Lee, J.H.; Lee, H.J.; Kang, K.S.; Lim, H.H. Neuroprotective Effects of Tetrahydrocurcumin against Glutamate-Induced Oxidative Stress in Hippocampal HT22 Cells. Molecules 2019, 25, 144. [Google Scholar] [CrossRef]
- Xi, J.; Luo, X.; Wang, Y.; Li, J.; Guo, L.; Wu, G.; Li, Q. Tetrahydrocurcumin protects against spinal cord injury and inhibits the oxidative stress response by regulating FOXO4 in model rats. Exp. Ther. Med. 2019, 18, 3681–3687. [Google Scholar] [CrossRef]
- Yuan, T.; Yin, Z.; Yan, Z.; Hao, Q.; Zeng, J.; Li, L.; Zhao, J. Tetrahydrocurcumin ameliorates diabetes profiles of db/db mice by altering the composition of gut microbiota and up-regulating the expression of GLP-1 in the pancreas. Fitoterapia 2020, 146, 104665. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.H.; Chen, J.W.; Kong, Z.L.; Wu, J.C.; Ho, C.T.; Lai, C.S. Attenuation by Tetrahydrocurcumin of Adiposity and Hepatic Steatosis in Mice with High-Fat-Diet-Induced Obesity. J. Agric. Food Chem. 2018, 66, 12685–12695. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.-S.; Ho, C.-T.; Pan, M.-H. The Cancer Chemopreventive and Therapeutic Potential of Tetrahydrocurcumin. Biomolecules 2020, 10, 831. [Google Scholar] [CrossRef] [PubMed]
- Srimuangwong, K.; Tocharus, C.; Tocharus, J.; Suksamrarn, A.; Chintana, P.Y. Effects of hexahydrocurcumin in combination with 5-fluorouracil on dimethylhydrazine-induced colon cancer in rats. World J. Gastroenterol. 2012, 18, 6951–6959. [Google Scholar] [CrossRef] [PubMed]
- Moohammadaree, A.; Changtam, C.; Wicha, P.; Suksamrarn, A.; Tocharus, J.; Tocharus, C. Mechanisms of Vasorelaxation Induced by Hexahydrocurcuminin Isolated Rat Thoracic Aorta. Phytother. Res. PTR 2015, 29, 1806–1813. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.N.; Chen, C.H.; Chen, S.N.; Huang, J.C.; Lai, L.J.; Lai, C.H.; Hung, C.H.; Lee, C.H.; Chen, C.Y. Anti-angiogenic effect of hexahydrocurcumin in rat corneal neovascularization. Int. Ophthalmol. 2018, 38, 747–756. [Google Scholar] [CrossRef]
- Dong, H.P.; Yang, R.C.; Chunag, I.C.; Huang, L.J.; Li, H.T.; Chen, H.L.; Chen, C.Y. Inhibitory effect of hexahydrocurcumin on human platelet aggregation. Nat. Prod. Commun. 2012, 7, 883–884. [Google Scholar] [CrossRef] [PubMed]
- Wicha, P.; Tocharus, J.; Janyou, A.; Jittiwat, J.; Chaichompoo, W.; Suksamrarn, A.; Tocharus, C. Hexahydrocurcumin alleviated blood-brain barrier dysfunction in cerebral ischemia/reperfusion rats. Pharmacol. Rep. 2020. [Google Scholar] [CrossRef]
- Zhang, Z.; Luo, D.; Xie, J.; Lin, G.; Zhou, J.; Liu, W.; Li, H.; Yi, T.; Su, Z.; Chen, J. Octahydrocurcumin, a final hydrogenated metabolite of curcumin, possesses superior anti-tumor activity through induction of cellular apoptosis. Food Funct. 2018, 9, 2005–2014. [Google Scholar] [CrossRef]
- Somparn, P.; Phisalaphong, C.; Nakornchai, S.; Unchern, S.; Morales, N.P. Comparative antioxidant activities of curcumin and its demethoxy and hydrogenated derivatives. Biol. Pharm. Bull. 2007, 30, 74–78. [Google Scholar] [CrossRef]
- Zhang, Z.-B.; Luo, D.-D.; Xie, J.-H.; Xian, Y.-F.; Lai, Z.-Q.; Liu, Y.-H.; Liu, W.-H.; Chen, J.-N.; Lai, X.-P.; Lin, Z.-X. Curcumin’s metabolites, tetrahydrocurcumin and octahydrocurcumin, possess superior anti-inflammatory effects in vivo through suppression of TAK1-NF-κB pathway. Front. Pharmacol. 2018, 9, 1181. [Google Scholar] [CrossRef] [PubMed]
- Boissy, R.E.; Sakai, C.; Zhao, H.; Kobayashi, T.; Hearing, V.J. Human tyrosinase related protein-1 (TRP-1) does not function as a DHICA oxidase activity in contrast to murine TRP-1. Exp. Dermatol. 1998, 7, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Olivares, C.; Jiménez-cervantes, C.; Lozano, J.A.; Solano, F.; García-borrón, J.C. The 5, 6-dihydroxyindole-2-carboxylic acid (DHICA) oxidase activity of human tyrosinase. Biochem. J. 2001, 354, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, M.K.; Gangwar, M.; Mondal, S.C.; Jana, S. Protective effects of tetrahydrocurcumin (THC) on fibroblast and melanoma cell lines in vitro: It’s implication for wound healing. J. Food Sci. Technol. 2017, 54, 1137–1145. [Google Scholar] [CrossRef] [PubMed]
- Ku, B.; Kim, D.; Choi, E.-M. Tetrahydrocurcumin Inhibits α-MSH-induced Melanogenesis via GSK3β Activation in B16F10 Melanoma Cells. Toxicol. Environ. Health Sci. 2019, 11, 210–218. [Google Scholar] [CrossRef]
- Candau, D. Self-Tanning Composition Containing a Tetrahydrocurcuminoid and a Self-Tanning Agent. Google Patents, U.S. Patent 6,875,426, 2005. [Google Scholar]
- Wolnicka-Glubisz, A.; Nogal, K.; Żądło, A.; Płonka, P.M. Curcumin does not switch melanin synthesis towards pheomelanin in B16F10 cells. Arch. Dermatol. Res. 2015, 307, 89–98. [Google Scholar] [CrossRef]
- Sato, K.; Toriyama, M. The inhibitory effect of non-steroidal anti-inflammatory drugs (NSAIDs) on the monophenolase and diphenolase activities of mushroom tyrosinase. Int. J. Mol. Sci. 2011, 12, 3998–4008. [Google Scholar] [CrossRef]
- Satooka, H.; Cerda, P.; Kim, H.-J.; Wood, W.F.; Kubo, I. Effects of matsutake mushroom scent compounds on tyrosinase and murine B16-F10 melanoma cells. Biochem. Biophys. Res. Commun. 2017, 487, 840–846. [Google Scholar] [CrossRef]
- Ali, A.; Ashraf, Z.; Kumar, N.; Rafiq, M.; Jabeen, F.; Park, J.H.; Choi, K.H.; Lee, S.; Seo, S.-Y.; Choi, E.H. Influence of plasma-activated compounds on melanogenesis and tyrosinase activity. Sci. Rep. 2016, 6, 1–20. [Google Scholar] [CrossRef]
- Mann, T.; Gerwat, W.; Batzer, J.; Eggers, K.; Scherner, C.; Wenck, H.; Stäb, F.; Hearing, V.J.; Röhm, K.-H.; Kolbe, L. Inhibition of Human Tyrosinase Requires Molecular Motifs Distinctively Different from Mushroom Tyrosinase. J. Investig. Dermatol. 2018, 138, 1601–1608. [Google Scholar] [CrossRef]
- Song, T.-Y.; Chen, C.-H.; Yang, N.-C.; Fu, C.-S. The correlation of in vitro mushroom tyrosinase activity with cellular tyrosinase activity and melanin formation in melanoma cells A2058. J. Food Drug Anal. 2009, 17, 156–162. [Google Scholar]
- Sugumaran, M.; Barek, H. Critical analysis of the melanogenic pathway in insects and higher animals. Int. J. Mol. Sci. 2016, 17, 1753. [Google Scholar] [CrossRef] [PubMed]
- Ancans, J.; Tobin, D.J.; Hoogduijn, M.J.; Smit, N.P.; Wakamatsu, K.; Thody, A.J. Melanosomal pH controls rate of melanogenesis, eumelanin/phaeomelanin ratio and melanosome maturation in melanocytes and melanoma cells. Exp. Cell Res. 2001, 268, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Cheli, Y. Alpha-MSH and cyclic-AMP elevating agents control melanosome pH through a PKA-independent mechanism. J. Biol. Chem. 2009, 284, 18699–18706. [Google Scholar] [CrossRef]
- Goenka, S.; Nagabhushanam, K.; Majeed, M.; Simon, S.R. Calebin-A, a Curcuminoid Analog Inhibits α-MSH-Induced Melanogenesis in B16F10 Mouse Melanoma Cells. Cosmetics 2019, 6, 51. [Google Scholar] [CrossRef]
- Yoshizaki, N.; Hashizume, R.; Masaki, H. A polymethoxyflavone mixture extracted from orange peels, mainly containing nobiletin, 3, 3′, 4′, 5, 6, 7, 8-heptamethoxyflavone and tangeretin, suppresses melanogenesis through the acidification of cell organelles, including melanosomes. J. Dermatol. Sci. 2017, 88, 78–84. [Google Scholar] [CrossRef]
- Al-Nasiry, S.; Geusens, N.; Hanssens, M.; Luyten, C.; Pijnenborg, R. The use of Alamar Blue assay for quantitative analysis of viability, migration and invasion of choriocarcinoma cells. Hum. Reprod. 2007, 22, 1304–1309. [Google Scholar] [CrossRef]
- McBride, J.; Ingram, P.R.; Henriquez, F.L.; Roberts, C.W. Development of colorimetric microtiter plate assay for assessment of antimicrobials against Acanthamoeba. J. Clin. Microbiol. 2005, 43, 629–634. [Google Scholar] [CrossRef]
- Khodashenas, S.; Khalili, S.; Moghadam, M.F. A cell ELISA based method for exosome detection in diagnostic and therapeutic applications. Biotechnol. Lett. 2019, 41, 523–531. [Google Scholar] [CrossRef]
- Spurgers, K.B.; Hurt, C.R.; Cohen, J.W.; Eccelston, L.T.; Lind, C.M.; Lingappa, V.R.; Glass, P.J. Validation of a cell-based ELISA as a screening tool identifying anti-alphavirus small-molecule inhibitors. J. Virol. Methods 2013, 193, 226–231. [Google Scholar] [CrossRef][Green Version]
- Goenka, S.; Ceccoli, J.; Simon, S.R. Anti-melanogenic activity of ellagitannin casuarictin in B16F10 mouse melanoma cells. Nat. Prod. Res. 2019, 1–6. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goenka, S.; Simon, S.R. Comparative Study of Curcumin and Its Hydrogenated Metabolites, Tetrahydrocurcumin, Hexahydrocurcumin, and Octahydrocurcumin, on Melanogenesis in B16F10 and MNT-1 Cells. Cosmetics 2021, 8, 4. https://doi.org/10.3390/cosmetics8010004
Goenka S, Simon SR. Comparative Study of Curcumin and Its Hydrogenated Metabolites, Tetrahydrocurcumin, Hexahydrocurcumin, and Octahydrocurcumin, on Melanogenesis in B16F10 and MNT-1 Cells. Cosmetics. 2021; 8(1):4. https://doi.org/10.3390/cosmetics8010004
Chicago/Turabian StyleGoenka, Shilpi, and Sanford R. Simon. 2021. "Comparative Study of Curcumin and Its Hydrogenated Metabolites, Tetrahydrocurcumin, Hexahydrocurcumin, and Octahydrocurcumin, on Melanogenesis in B16F10 and MNT-1 Cells" Cosmetics 8, no. 1: 4. https://doi.org/10.3390/cosmetics8010004
APA StyleGoenka, S., & Simon, S. R. (2021). Comparative Study of Curcumin and Its Hydrogenated Metabolites, Tetrahydrocurcumin, Hexahydrocurcumin, and Octahydrocurcumin, on Melanogenesis in B16F10 and MNT-1 Cells. Cosmetics, 8(1), 4. https://doi.org/10.3390/cosmetics8010004