1. Introduction
Although nappy rash, also known as irritant napkin dermatitis (IND) or irritant diaper dermatitis is not a life-threatening condition, it can cause significant discomfort to babies and anxiety for caregivers [
1,
2]. IND is primarily caused through repeated and prolonged exposure to irritants such as urine and feces. The urine/feces mixture causes disruption of the skin barrier through several mechanisms, including maceration (softening and weakening) of the skin, increased alkalinity, activation of fecal digestive enzymes and friction between nappy and skin [
3,
4,
5,
6,
7]. Most clinically diagnosed cases involve mild reddening and scaling, but cases of more severe skin lesions or fungal or bacterial secondary infection can develop in a small percentage (around 5%) of cases [
8,
9]. IND symptomatology typically consists of red patches, spots, pimples, or blisters in the diapered area, with skin that may look sore and feel hot to touch [
10]. These symptoms lead to discomfort and may negatively impact sleep and behavior of affected babies. However, each IND episode is short -typically 2–4 days. Hence most cases are not seen by a medical professional and are thus self-managed by the caregivers [
9,
11].
Literature estimates of IND rates vary greatly, ranging from 16% to 65% [
2], and it varies according to setting, hygiene practices, and age group [
3] As such, the focus of nappy rash management has gradually shifted toward prevention rather than treatment. Any practice that keeps urine and feces away from the skin and that reduces the abrasion will reduce the incidence of IND [
2]. The optimal prevention strategy should also consider the social circumstances of the baby and caregiver. Preventive strategies for IND include gentle cleansing, frequently changing nappies (especially at night), usage of superabsorbent nappy and the regular application of protective barrier topical product. In 2015, an expert committee in dermatology has defined the ideal properties of a topical nappy rash preparation for prevention of nappy rash [
7]. It was emphasized that not all products are equal and that some products may exhibit inadequate protection or even impair the skin barrier or worsen the skin condition. Nappy care products should contain ingredients chosen for their specific beneficial properties and proven lack of toxicities.
In Thailand, a recent large study (1153 participants) has estimated that the prevalence of IND was 36.1% among infants aged 1–24 months [
12]. The high prevalence of IND can be explained by the high using proportion (nearly 60% of participants) of cloth diapers but also by the low usage (13.6% of participants) of an effective nappy cream on the buttock area. The authors identified that nappy changing fewer than three times/night, using cloth diapers, and topical application of baby talcum powder to the diaper area are the main risk factors for IND in Thailand. The authors further recommended that barrier creams such as those containing dexpanthenol (B5) should be applied regularly on the buttock area, in place of baby talcum powder.
Topical application of formulas containing dexpanthenol is widely used in clinical practice for the treatment of skin lesions [
13]. Dexpanthenol is converted within the skin to pantothenic acid, a constituent of coenzyme A. Coenzyme A then catalyzes the synthesis of fatty acids and sphingolipids which are of crucial importance for skin lipid layers and cellular membrane integrity. Topical dexpanthenol acts like a moisturizer with barrier-improving properties; in addition, it exerts wound healing effects [
14,
15]. The mechanisms by which dexpanthenol restores and protects skin barrier function have not been fully elucidated. As the different layers of the skin undergo continual renewal, dexpanthenol-containing formulations provide an environment which promotes physiological processes (e.g., enzyme functioning) necessary for maintaining or restoring skin barrier function. It has also been suggested that dexpanthenol promotes epidermal regeneration by enhancing epidermal differentiation and lipid synthesis [
16]. In a context of IND management, the effect of dexpanthenol-based products have been evaluated as treatment or prevention product through multiple clinical studies [
17,
18,
19,
20,
21]. Another study in Thailand demonstrated the ability of 5% Dexpanthenol-based ointment to significantly decreased the transepidermal water loss (TEWL) upon an IND episode from acute diarrhea [
22]. This is supported by several other studies demonstrating the ability of dexpanthenol to decrease the TEWL significantly and increase skin moisturization in various skin conditions, regardless of the galenic of the investigational product [
23,
24,
25,
26].
Bepanthen
® Ointment consists of a water-in-oil preparation with high lipid content (more than 50%) combined with 5% dexpanthenol. The lipids mixture forms a lipophilic film on the skin surface that isolates the skin from irritants (urine and feces) and excess of moisture while replenishing the skin lipids. Water-in-oil preparation is considered by the experts as the preferred galenic formulation for IND preparations, it creates a barrier that is effective yet “breathable” [
27]. By not being fully occlusive, it helps maintain healthy moisture levels in the skin. Dexpanthenol contained in Bepanthen
® Ointment further confers to the product’s skin-regenerating properties. The efficacy of Bepanthen
® Ointment has been demonstrated through several in vitro, ex-vivo as well as in clinical studies in preventing and treating the symptoms of nappy rash [
28,
29]. However, the IND prevention effect of the product has been assessed only in controlled clinical settings with hospitalized infants in France There is a lack of understanding on the usage effects of Bepanthen Ointment in Asians, under a real-world setting.
The objectives of this observational real-world evidence study were to evaluate the usage satisfaction and perceived efficacy of Dexpanthenol in nappy care, from the perspectives of current users (Thai mothers). We report the findings from a study of 300 Thai mothers of babies 6–12 months old regarding the effect of Bepanthen® Ointment on babies’ nappy-related concerns, respondents’ satisfaction with Bepanthen® Ointment and its benefits and properties, as well as their perceptions of the product.
2. Materials and Methods
This online study was conducted in Thailand between September and October, 2019. The study was carried out using the ClaimIt Express online platform (ObvioHealth, Orlando, FL, USA), which was accessed via respondents’ digital devices (e.g., smartphones, laptops, or tablets). It was conducted in accordance with the Code of Conduct of the Market Research Society [
30]. As this was an observational study, ethical approval was not required.
In Thailand, Bepanthen Ointment is indicated for both prophylactic and symptomatic skincare use on the nappy area, nipples, and dry skin. It is recommended to be applied at every nappy change; on sore or cracked nipples after breastfeeding; and on dry cracked or chafed skin once daily or as often as needed.
2.1. Study Respondents
Respondents were mothers living in Thailand with babies between 6–12 months of age, who were decision-makers in their households with respect to buying nappy care products, who used disposable nappies for their babies, and had purchased and used Bepanthen® Ointment during the month prior to taking the study. Study respondents were recruited via internet advertisements on platforms including Facebook, Instagram, Google, and YouTube. These advertisements contained links to redirect potential respondents to a landing page with information on the study and registration/pre-screening process for those interested in participating. Potential respondents were presented with a pre-screening questionnaire. Those who met the inclusion criteria were invited to download the platform and complete the informed consent process before participating in the full study.
2.2. Study Design and Data Collection
Respondents provided their responses to the questionnaire via the ClaimIt Express platform. Questions included the types of nappy-related concerns experienced by respondents’ babies, how use of Bepanthen® Ointment addressed such concerns, how satisfied respondents were with the benefits of Bepanthen® Ointment, and respondents’ satisfaction with the use of Bepanthen® Ointment for nipple care. Only completed questionnaires were analyzed. Results were further analyzed with respect to two groups: frequent users, defined as mothers who used Bepanthen® Ointment every time they changed their baby’s nappy or at least four times a day, and as-needed users, who as used Bepanthen® Ointment on an as-needed basis. Responses were summarized using descriptive statistics, and differences in proportions between frequent and as-needed groups were calculated using a two-tailed z-test. The statistical significance of differences between groups was evaluated at the 5% significance level (α = 0.05). WinCross Version 18 (The Analytical Group Inc.; Scottsdale, Arizona) was used for all statistical analyses.
4. Discussion
This study of Thai mothers explored users’ perceptions, reported experiences and satisfaction with the dexpanthenol-containing nappy care product (Bepanthen® Ointment). Our findings showed that users perceived Bepanthen® Ointment to be effective in reducing nappy-related concerns in general, and, more specifically, were satisfied with its effectiveness, gentleness, and suitability as a barrier preparation. In general, frequent users of the product reported higher rates of satisfaction compared with as-needed users. This observational study confirms the consumer acceptance of using Bepanthen® Ointment as part of the preventive strategy of IND. These findings reflect the real-life situation in Thailand and may not be generalizable across other countries, but do provide additional insight to parents and caregivers, as well as front-line healthcare professionals caring for babies.
With the emergence and the democratization of Internet, the access to health and medicinal information became more straightforward than in the past. Two consequences of this easier access to medical knowledge are the constant development of the self-care globally and a better engagement of the patients in their health. In parallel, with the digitalization and the usage of social and digital media being more and more common, patients seem to be more willing and open to share their health data, knowledge, and experience about their personal preferences through these digital platform. A real-world observational study with digital recruitment leverages the new digital technology available to capture this new behavior of the patients while applying some criteria taken from the clinical studies activities to ensure generation of reliable data. This novel methodology has some weaknesses such as the lack of monitoring, guidance, and medical interventions; however, it stands as appropriate methodology to investigate product usage and the quality of life aspect especially for conditions which are managed without seeking medical advices. Indeed, by studying in real-life where patient populations and other variables cannot be controlled reveals the perceived benefits of a product for which the safety and efficacy have been properly evaluated a current methodology if used appropriately [
29].
We note several caveats in interpreting these results. Since only recent (past one month) and existing users of Bepanthen® Ointment were enrolled, the opinions of individuals who had used the product outside of that period would not have been captured. Respondents were recruited online through social media and were required to use their digital devices (e.g., smartphones, laptops, and tablets) to access the study. This could have excluded individuals with less access to these technologies, influencing the generalizability of the results.
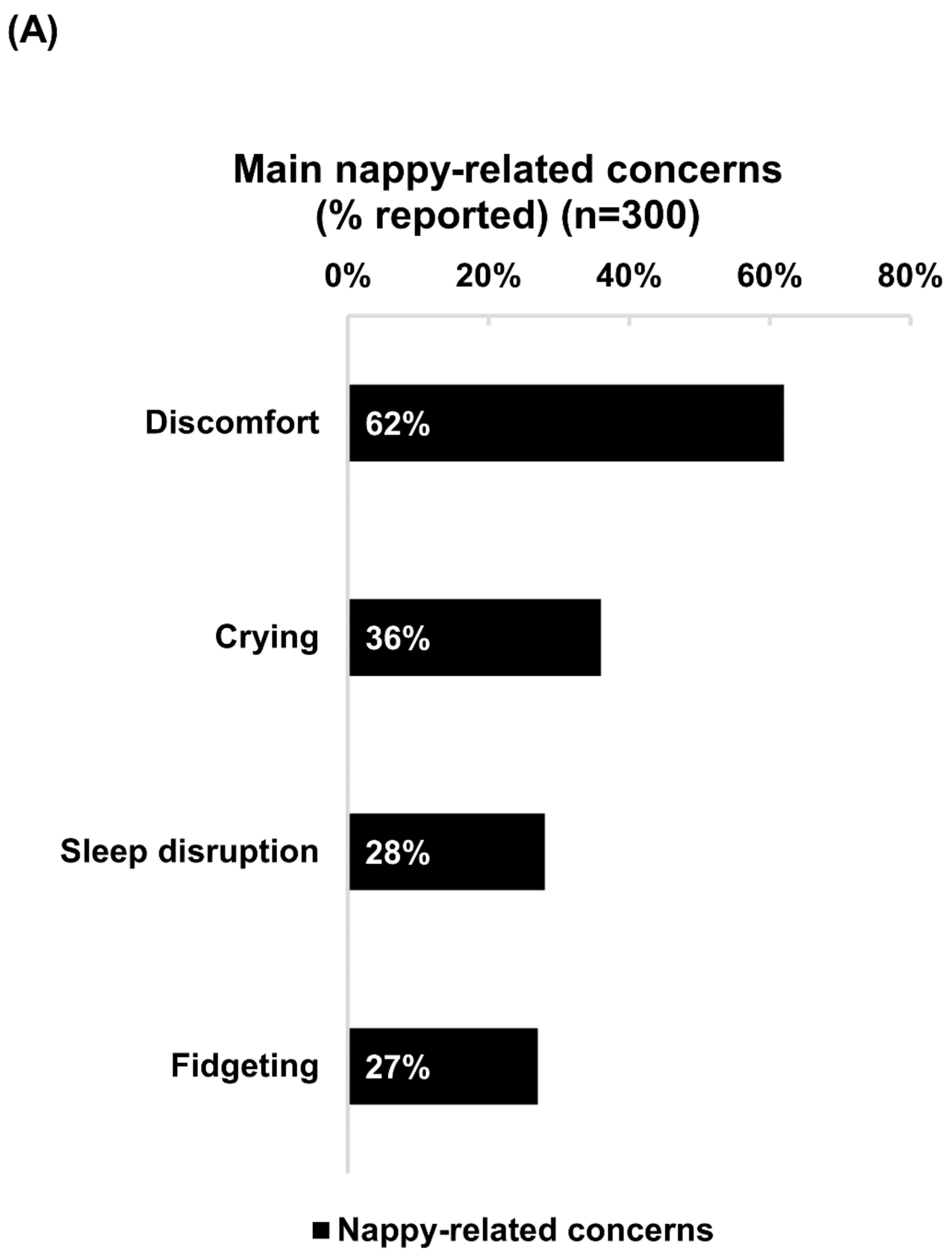
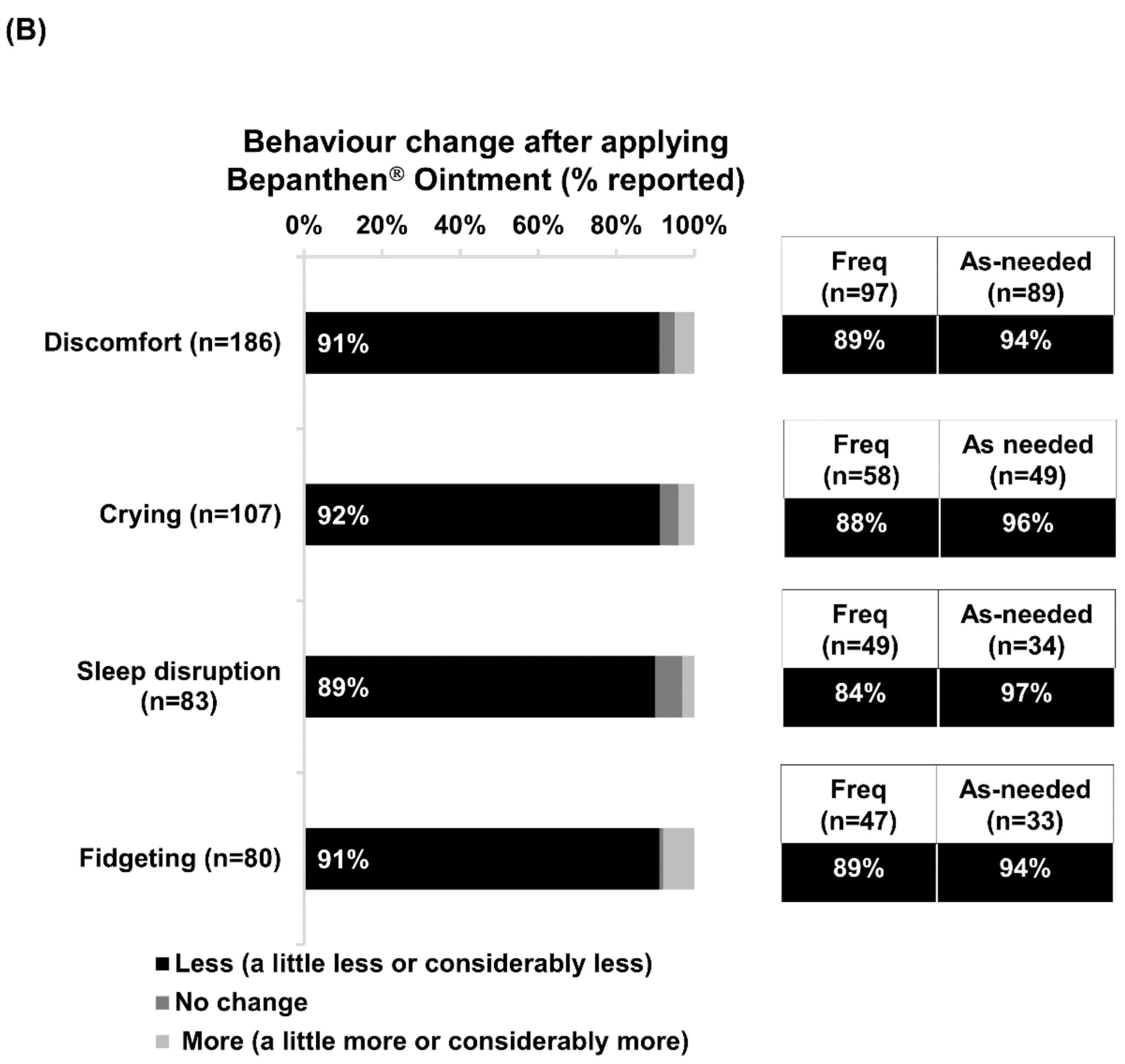
Over nine out of ten respondents reported that their babies experienced less nappy-related discomfort, crying, fidgeting and sleep disruption after applying the product. This correlates with previous clinical studies demonstrating that the application of dexpanthenol-containing products is associated with higher rates of alleviation of nappy rash symptoms, as well as significantly lower frequencies of nappy rash occurrence when compared with usual hygiene care alone [
27,
28]. Interestingly, a relatively higher proportion of as-needed users reported reductions in nappy-related discomfort, compared with frequent users. Although this was not investigated in our study, it is plausible that mothers/caregivers who used dexpanthenol only at the onset of their baby’s symptoms would be more likely to notice improvements than those who used it at every nappy change.
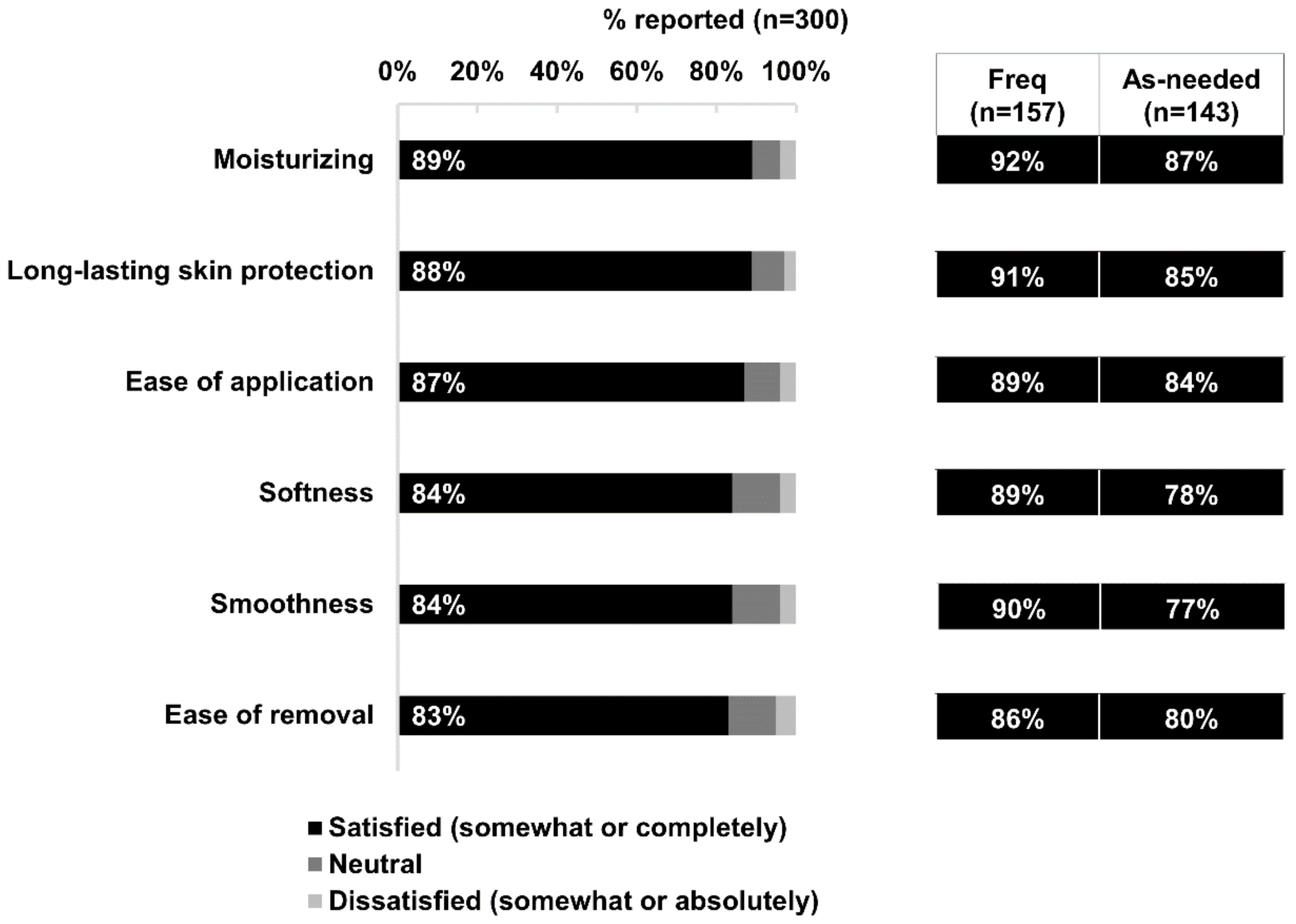
By exploring perceptions and specific reasons underlying real-world users’ satisfaction, we gained insight into how the clinical benefits of dexpanthenol (such as gentleness and skin hydration) translate to benefits for both mothers and babies. For example, the study demonstrated very high rates of user satisfaction with the moisture, smoothness, softness, and skin protection benefits of the investigated product. Specific to the Bepanthen
® Ointment formulation, the contained lipids create a protective yet non-occlusive layer, thus maintaining optimal moisture levels and preventing excessive maceration [
7]. Other lipids penetrate the stratum corneum, mimicking the effects of intracellular lipids. Together, these lipids help the skin to maintain its own natural barrier as further protection from external irritants [
30]. With its high lipid content and its emollient properties, Bepanthen
® Ointment further reduces friction and creates an homogenous and long-lasting protection over skin [
31]. This is in contrast to the use of talcum powder (36% of respondents in this study used baby powder as one of their nappy care products, data not shown) that was assessed by the experts as unable to provide a continuous barrier across the skin [
12]. In Thailand, the use of talcum power is based on the belief that it will capture the moisture and keep the skin dry. However, as shown by Sukhneewat and colleagues, talcum powder was associated with high risk to develop nappy rash in Thailand [
12]. The healing, gentle and protective properties of Bepanthen
® Ointment may also explain its popularity for mothers’ own use: half of all respondents have used the product for nipple care, with nine in ten being satisfied with it. Such perceptions of satisfaction complement clinical evidence for dexpanthenol’s efficacy in healing and preventing nipple fissures among nursing mothers [
32].
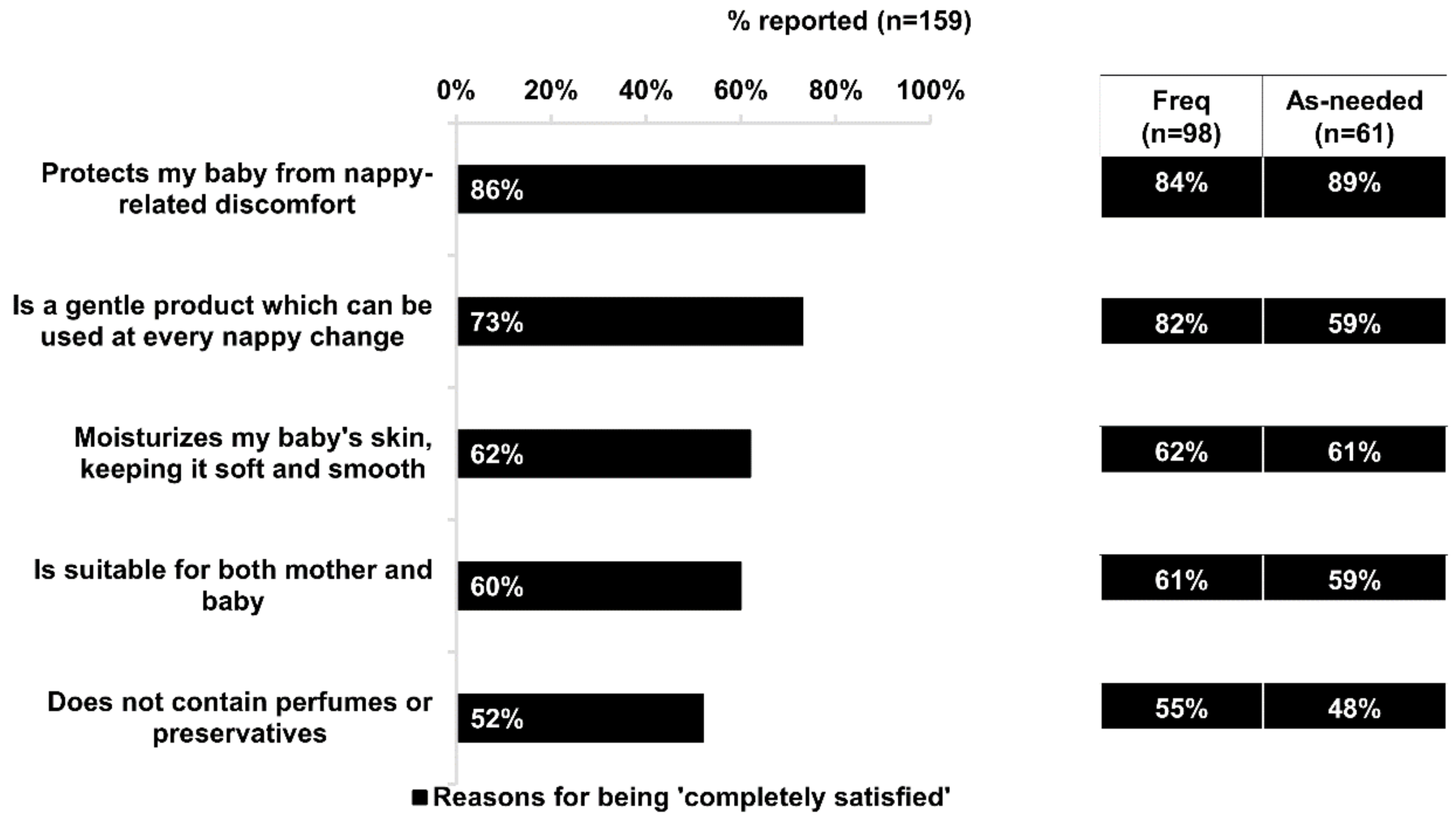
In addition to effectively relieving discomfort, almost all respondents agreed that the dexpanthenol-containing product was gentle on their baby’s skin and suitable for everyday nappy care. Respondents commonly cited benefits such as gentleness and suitability for every nappy change, and lack of perfumes or preservatives as reasons for their complete satisfaction. Bepanthen
® Ointment, is a formulation that further fulfills all nine standards of a safe and ideal IND preparation, contains no unnecessary, potentially toxic, or sensitizing ingredients [
7]. Notably, there were significantly more frequent users who were completely satisfied with Bepanthen
® Ointment’s gentleness and suitability for every nappy change compared with as-needed users. This suggests that besides the perceived effectiveness and overall suitability of Bepanthen
® Ointment, the gentleness of the product may be another important factor contributing to its continued and frequent use. The higher rates of satisfaction among frequent users in general might also be attributed to more frequent use of the product. Those who benefit more from the protective and emollient properties of Bepanthen
® Ointment through frequent and consistent usage are likely to perceive improvement and sustained quality of life and wellbeing over time and, accordingly, higher satisfaction.
Our study design included measures to minimize potential respondent bias for or against the target product. Only users who had purchased Bepanthen
® Ointment themselves were enrolled (that is, no product was provided to users for participation). Yet, among those studied, perceptions of Bepanthen
® Ointment were strongly positive, with nearly all agreeing it was their first choice for nappy care and that they were likely to recommend it to others. This high level of positive product perception is noteworthy, as Richins and colleagues noted that negative feedback tends to be top of mind when consumers communicate opinions about brands [
33]. The respondents’ high rates of satisfaction with Bepanthen
® Ointment may be further attributed to their perception of the product’s ease of application. Topical barrier preparations that are easy and pleasant to apply are likely to encourage consistent and sustained use, thus further enhancing their barrier effects [
7].