An Uncontrolled Case Series Using a Botanically Derived, β-Cyclodextrin Inclusion Complex in Two Androgenetic Alopecia-Affected Male Subjects †
Abstract
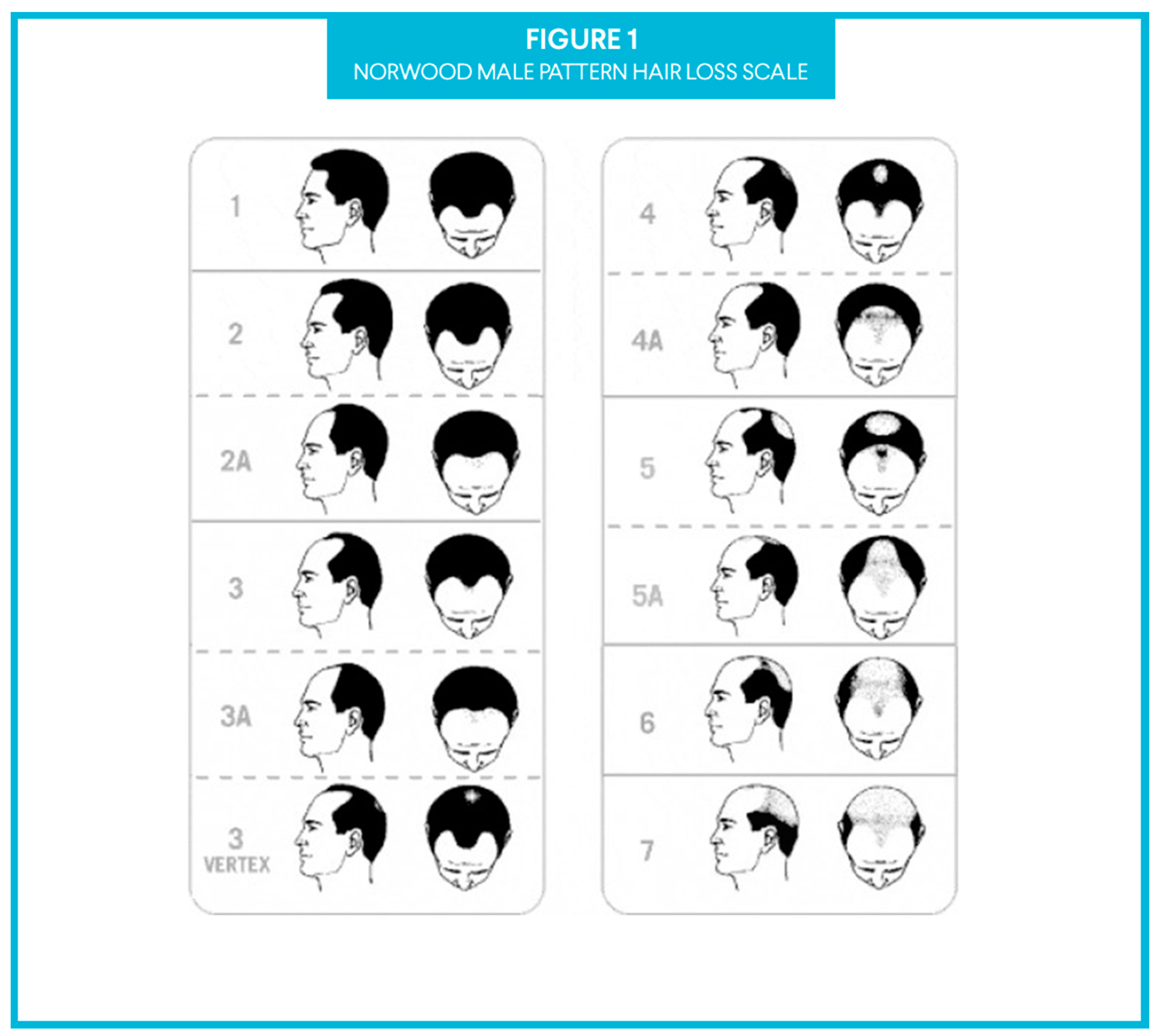
1. Introduction
1.1. Drug-Based Treatment Choices
1.1.1. Minoxidil
1.1.2. Finasteride
1.1.3. Combined Drug Therapy
1.2. Botanicals, Unique Potential, Formidable Challenges
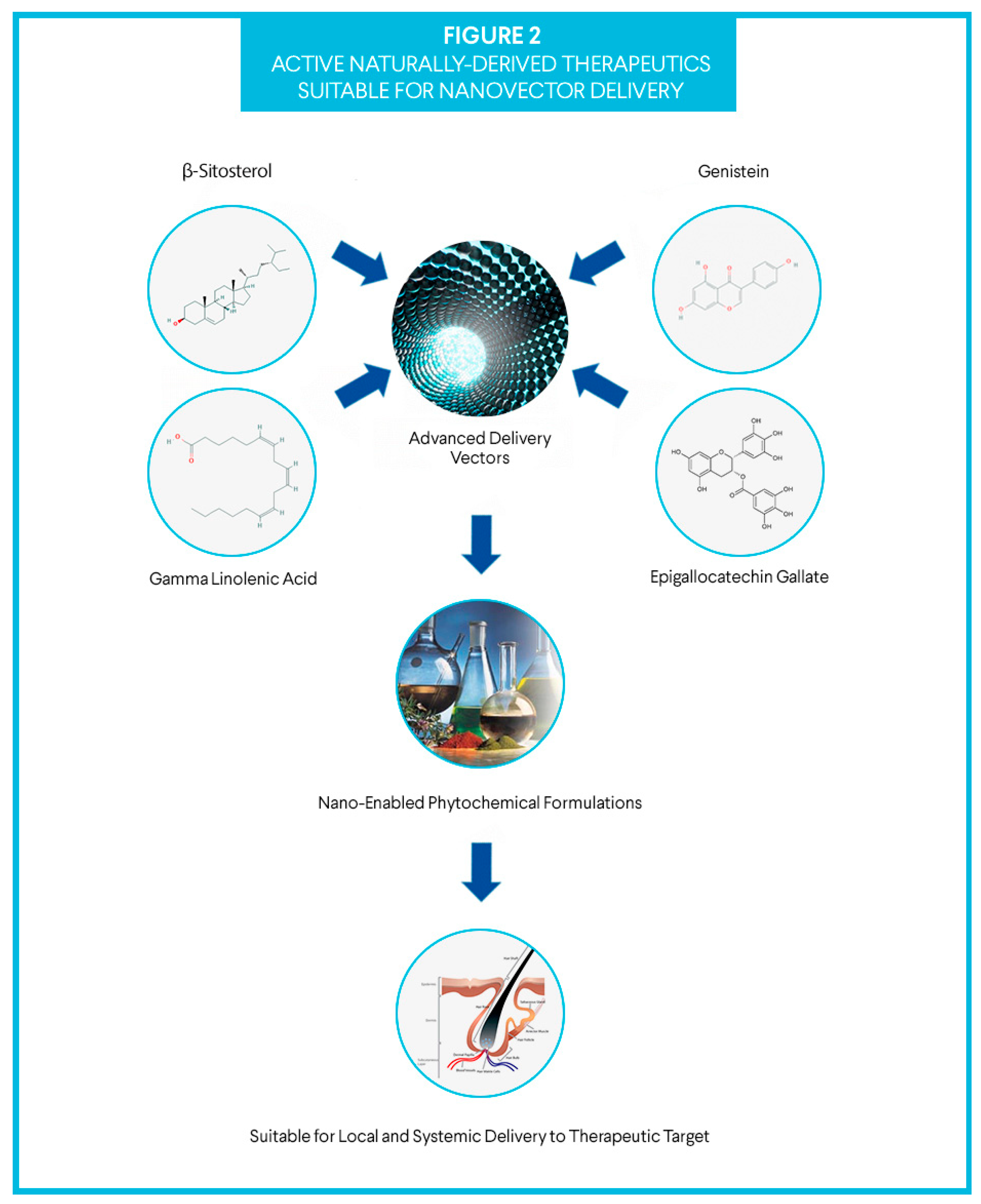
1.3. Active Compounds
1.3.1. γ linolenic acid
1.3.2. β-Sitosterol
1.3.3. Epigallocatechin Gallate
1.3.4. Genistein
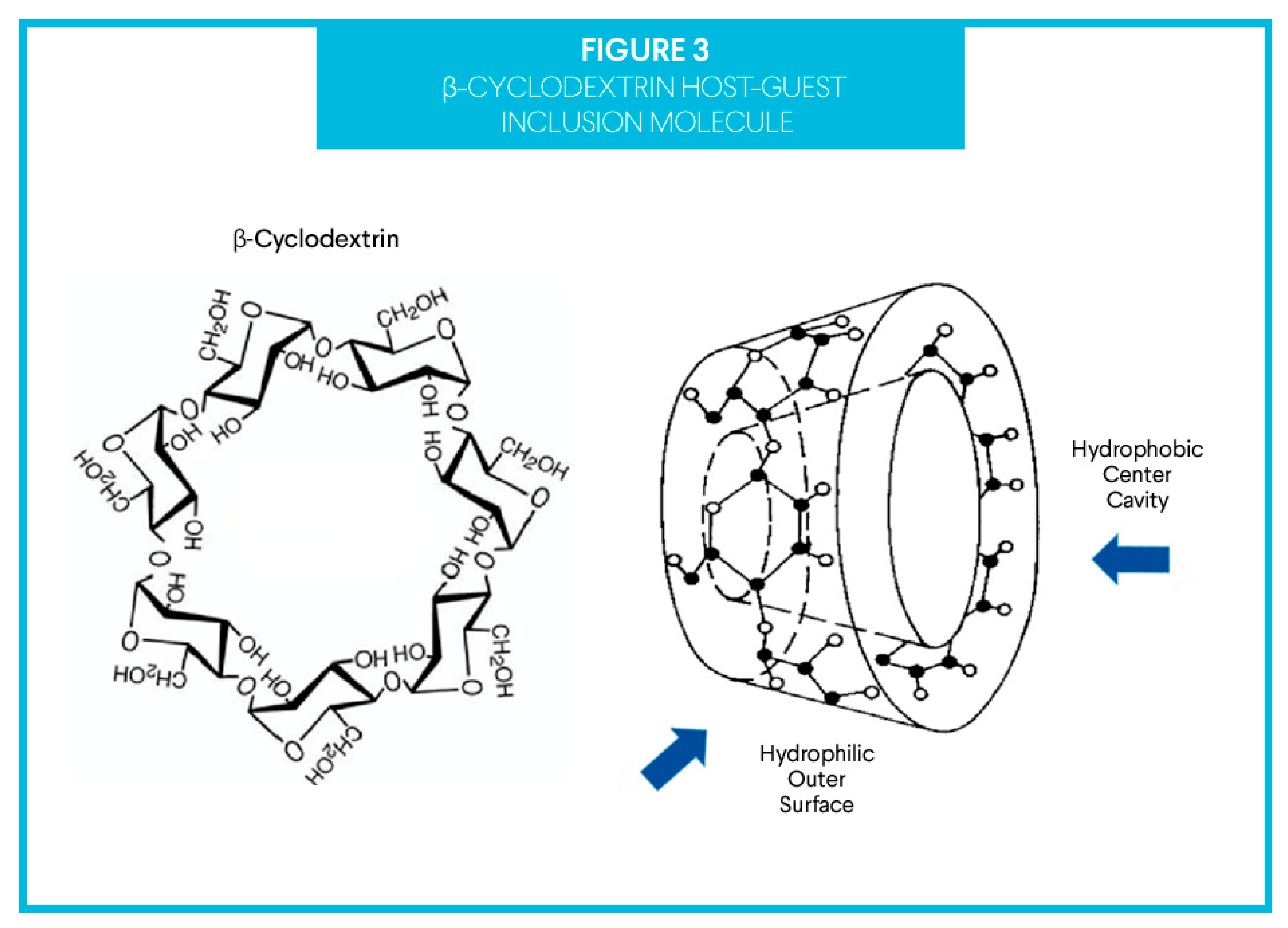
1.4. The Delivery Vector
2. Methods
2.1. Preparation of Treatment Materials
2.2. Uncontrolled, Two Patient Case Series
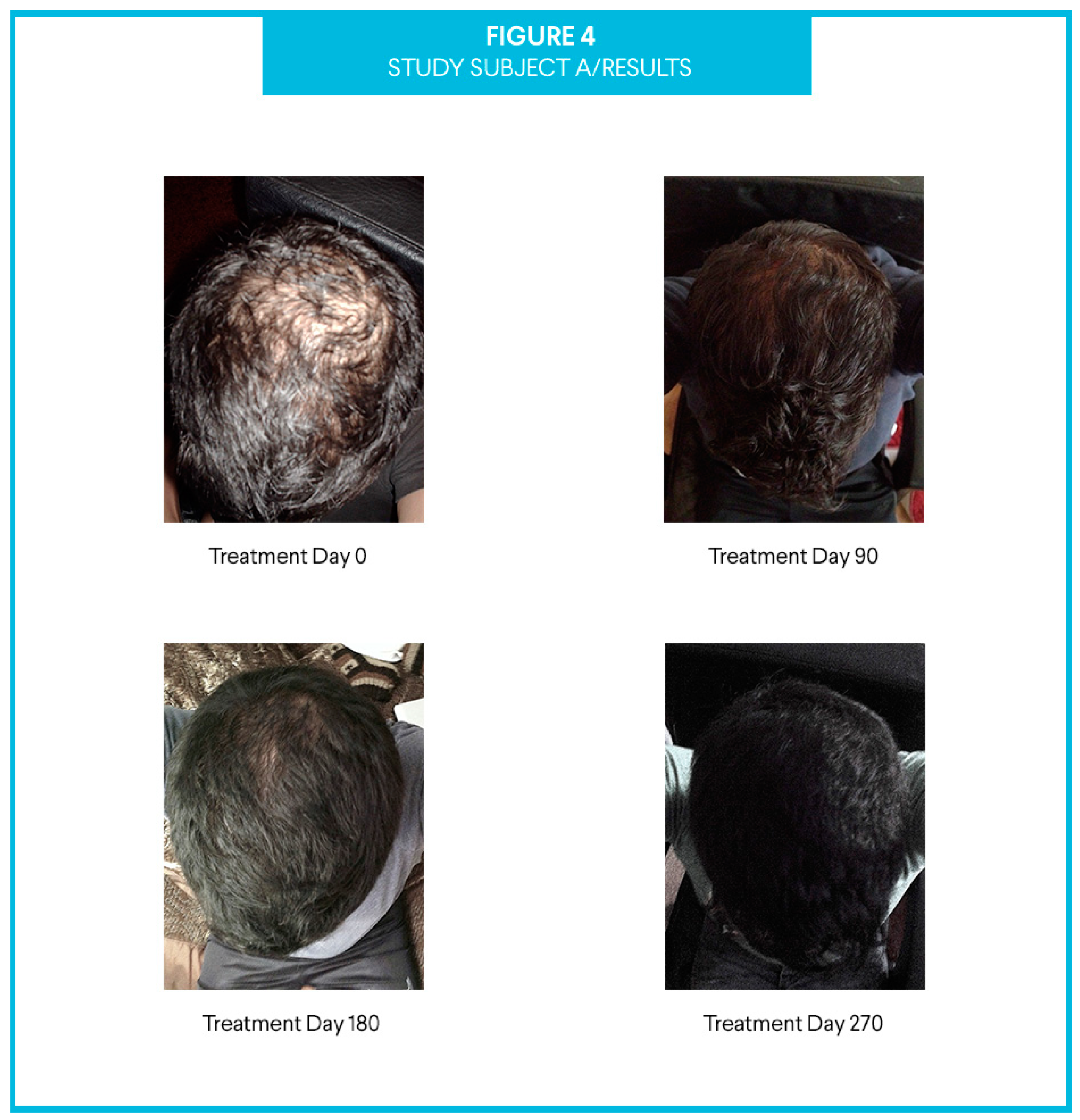
3. Results
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tai, T.; Kochhar, A. Physiology and Medical Treatments for Alopecia. Facial Plast. Surg. Clin. N. Am. 2020, 28, 149–159. [Google Scholar] [CrossRef]
- He, H.; Xie, B.; Xie, L. Male pattern baldness and incidence of prostate cancer: A systematic review and meta-analysis. Medicine 2018, 97, e11379. [Google Scholar] [CrossRef]
- Starace, M.; Orlando, G.; Alessandrini, A.; Piraccini, B.M. Female Androgenetic Alopecia: An Update on Diagnosis and Management. Am. J. Clin. Dermatol. 2020, 21, 69–84. [Google Scholar] [CrossRef]
- Martinez-Jacobo, L.; Villarreal-Villarreal, C.D.; Ortiz-López, R.; Ocampo-Candiani, J.; Rojas-Martínez, A. Genetic and molecular aspects of androgenetic alopecia. Indian J. Dermatol. Venereol. Leprol. 2018, 84, 263–268. [Google Scholar]
- Singh, S.; Ilyayeva, S. Androgen Insensitivity Syndrome; StatPearls: Treasure Island, FL, USA, 2020. [Google Scholar]
- Lolli, F.; Pallotti, F.; Rossi, A.; Fortuna, M.C.; Caro, G.; Lenzi, A.; Sansone, A.; Lombardo, F. Androgenetic alopecia: A review. Endocrine 2017, 57, 9–17. [Google Scholar] [CrossRef]
- Sinclair, R.; Torkamani, N.; Jones, L. Androgenetic alopecia: New insights into the pathogenesis and mechanism of hair loss. F1000Research 2015, 4, 585. [Google Scholar] [CrossRef]
- Said, M.A.; Mehta, A. The Impact of 5α-Reductase Inhibitor Use for Male Pattern Hair Loss on Men’s Health. Curr. Urol. Rep. 2018, 16, 65. [Google Scholar] [CrossRef]
- Badri, T.; Nessel, T.A.; Kumar, D.D. Minoxidil; StatPearls: Treasure Island, FL, USA, 2020. [Google Scholar]
- Suchonwanit, P.; Thammarucha, S.; Leerunyakul, K. Minoxidil and its use in hair disorders: A review. Drug Des. Dev. Ther. 2019, 13, 2777–2786. [Google Scholar] [CrossRef]
- Barbareschi, M. The use of minoxidil in the treatment of male and female androgenetic alopecia: A story of more than 30 years. G. Ital. Dermatol. Venereol. 2018, 153, 102–106. [Google Scholar]
- Colamarino, R.; Dubost, J.J.; Brun, P.; Flori, B.; Tournilhac, M.; Eschalier, A.; Sauvezie, B. Polymyalgia Induced by Minoxidil. Ann. Med. Interne 1990, 141, 425–428. [Google Scholar]
- Tennstedt, D.; Herman, A.; Lachapelle, J.M. Adverse effects of hair care in users. Ann. Dermatol. Venereol. 2018, 145, 521–531. [Google Scholar] [CrossRef]
- Lee, S.W.; Juhasz, M.; Mobasher, P.; Ekelem, C.; Mesinkovska, N.A. A Systematic Review of Topical Finasteride in the Treatment of Androgenetic Alopecia in Men and Women. J. Drugs Dermatol. 2018, 17, 457–463. [Google Scholar]
- Iamsumang, W.; Leerunyakul, K.; Suchonwanit, P. Finasteride and Its Potential for the Treatment of Female Pattern Hair Loss: Evidence to Date. Drug Des. Dev. Ther. 2020, 14, 951–959. [Google Scholar] [CrossRef]
- Traish, A.M. Post-finasteride syndrome: A surmountable challenge for clinicians. Fertil. Steril. 2020, 113, 21–50. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, C.; Qu, Q.; Zhang, C.; Wang, J.; Fan, Z.; Miao, Y.; Hu, Z. The effectiveness of combination therapies for androgenetic alopecia: A systematic review and meta-analysis. Dermatol. Ther. 2020, e13741. [Google Scholar] [CrossRef]
- York, K.; Meah, N.; Bhoyrul, B.; Sinclair, R. A review of the treatment of male pattern hair loss. Expert Opin. Pharmacother. 2020, 21, 603–612. [Google Scholar] [CrossRef]
- Katzer, T.; Leiter, A., Jr.; Beck, R.; Da Silva, C.; Silva, C.B. Physiopathology and current treatments of androgenetic alopecia: Going beyond androgens and anti-androgens. Dermatol. Ther. 2019, 32, e13059. [Google Scholar] [CrossRef]
- Shoji, Y.; Nakashima, H. Nutraceutics and Delivery Systems. J. Drug Target. 2004, 12, 385–391. [Google Scholar] [CrossRef]
- Jafari, S.M.; McClements, D.J. Nanotechnology Approaches for Increasing Nutrient Bioavailability. Adv. Food Nutr. Res. 2017, 81, 1–30. [Google Scholar]
- Speranza, B.; Petruzzi, L.; Bevilacqua, A.; Gallo, M.; Campaniello, D.; Sinigaglia, M.; Corbo, M. Encapsulation of Active Compounds in Fruit and Vegetable Juice Processing: Current State and Perspectives. J. Food Sci. 2017, 82, 1291–1301. [Google Scholar] [CrossRef]
- Balic, A.; Vlašić, D.; Žužul, K.; Marinović, B.; Mokos, Z.B. Omega-3 Versus Omega-6 Polyunsaturated Fatty Acids in the Prevention and Treatment of Inflammatory Skin Diseases. Int. J. Mol. Sci. 2020, 21, 741. [Google Scholar] [CrossRef]
- Cabeza, M.; Bratoeff, E.; Heuze, I.; Ramírez, E.; Sánchez, M.; Flores, E. Effect of beta-sitosterol as inhibitor of 5 alpha-reductase in hamster prostate. Proc. West. Pharmacol. Soc. 2003, 46, 153–155. [Google Scholar]
- Andreu-Fernández, V.; Serra-Delgado, M.; Pizarro, N.; Navarro-Tapia, E.; Gomez-Roig, M.D.; De La Torre, R.; García-Algar, O. Bioavailability of Epigallocatechin Gallate Administered with Different Nutritional Strategies in Healthy Volunteers. Antioxidants 2020, 9, 440. [Google Scholar] [CrossRef]
- Shin, S.; Kim, K.; Lee, M.J.; Lee, J.; Choi, S.; Kim, K.-S.; Ko, J.-M.; Han, H.; Kim, S.Y.; Youn, H.J.; et al. Epigallocatechin Gallate-Mediated Alteration of the MicroRNA Expression Profile in 5α-Dihydrotestosterone-Treated Human Dermal Papilla Cells. Ann. Dermatol. 2016, 28, 327–334. [Google Scholar] [CrossRef]
- Merlotto, M.R.; Ramos, P.M.; Miot, H.A. Pattern hair loss: Assessment of microinflammation in miniaturized and terminal hair follicles through horizontal histologic sections. J. Am. Acad. Dermatol. 2020. [Google Scholar] [CrossRef]
- Lippens, S.; Hoste, E.; Vandenabeele, P.; Agostinis, P.; Declercq, W. Cell death in the skin. Apoptosis 2009, 14, 549–569. [Google Scholar] [CrossRef]
- Bae, M.; Woo, M.; Kusuma, I.W.; Arung, E.T.; Yang, C.H.; Kim, Y.-U. Inhibitory Effects of Isoflavonoids on Rat Prostate Testosterone 5α-Reductase. J. Acupunct. Meridian Stud. 2012, 5, 319–322. [Google Scholar] [CrossRef][Green Version]
- Yao, Q.; Lin, M.T.; Lan, Q.H.; Huang, Z.W.; Zheng, Y.W.; Jiang, X.; Zhu, Y.D.; Kou, L.; Xu, H.L.; Zhao, Y.Z. In Vitro and in Vivo evaluation of didymin cyclodextrin inclusion complexes: Characterization and chemosensitization activity. Drug Deliv. 2020, 27, 54–65. [Google Scholar] [CrossRef]
- Frigerio, G.; Campo, L.; Mercadante, R.; Santos, P.M.; Missineo, P.; Polledri, E.; Fustinoni, S. Development and validation of a liquid chromatography/tandem mass spectrometry method to quantify metabolites of phthalates, including di-2-ethylhexyl terephthalate (DEHTP) and bisphenol A, in human urine. Rapid Commun. Mass Spectrom. 2020, 34, e8796. [Google Scholar] [CrossRef]
- Carneiro, B.; Duarte, F.; Heimfarth, L.; Siqueeira, J.; Quintens-Jr, L.; da Veiga, V.; Neves de Lima, A. Cyclodextrin–Drug Inclusion Complexes: In Vivo and In Vitro Approaches. Int. J. Mol. Sci. 2019, 20, 642. [Google Scholar] [CrossRef]
- Manca, A.; Cabboi, M.P.; Ortu, E.; Ginatempo, F.; Dragone, D.; Zarbo, I.R.; De Natale, E.R.; Mureddu, G.; Bua, G.; Deriu, F. Effect of Contralateral Strength Training on Muscle Weakness in People With Multiple Sclerosis: Proof-of-Concept Case Series. Phys. Ther. 2016, 96, 828–838. [Google Scholar] [CrossRef]
- Brotzu, G.; Fadda, A.M.; Manca, L.; Consolaro, F. A liposome-based formulation containing equol, dihomo-γ-linolenic acid (DGLA), and propionyl-L-carnitine to prevent and treat hair loss: A prospective investigation. Dermatol. Ther. 2019, 32, e12778. [Google Scholar] [CrossRef]

| Minoxidil | Finasteride | ||
|---|---|---|---|
| Positives | Negatives | Positives | Negatives |
|
|
|
|
| Study Type | Study Start Date |
|---|---|
| Interventional (Clinical Case Series) | 3 March 2019 |
| Enrollment | Study Completion Date |
| 2 Participants | 27 November 2019 |
| Intervention Model Description | Primary Outcome Measure |
| Uncontrolled, open label trial | Incidence of Adverse Events |
| Primary Purpose | Secondary Outcome Measure |
| Test of concept | Assessment of therapeutic results |
| Inclusion criteria | Exclusion criteria |
|
|
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marcovici, G.; Bauman, A. An Uncontrolled Case Series Using a Botanically Derived, β-Cyclodextrin Inclusion Complex in Two Androgenetic Alopecia-Affected Male Subjects. Cosmetics 2020, 7, 65. https://doi.org/10.3390/cosmetics7030065
Marcovici G, Bauman A. An Uncontrolled Case Series Using a Botanically Derived, β-Cyclodextrin Inclusion Complex in Two Androgenetic Alopecia-Affected Male Subjects. Cosmetics. 2020; 7(3):65. https://doi.org/10.3390/cosmetics7030065
Chicago/Turabian StyleMarcovici, Geno, and Alan Bauman. 2020. "An Uncontrolled Case Series Using a Botanically Derived, β-Cyclodextrin Inclusion Complex in Two Androgenetic Alopecia-Affected Male Subjects" Cosmetics 7, no. 3: 65. https://doi.org/10.3390/cosmetics7030065
APA StyleMarcovici, G., & Bauman, A. (2020). An Uncontrolled Case Series Using a Botanically Derived, β-Cyclodextrin Inclusion Complex in Two Androgenetic Alopecia-Affected Male Subjects. Cosmetics, 7(3), 65. https://doi.org/10.3390/cosmetics7030065






