1. Introduction
As humans age, the accumulation of damages caused by environmental factors such as reactive oxygen radicals, sun exposure, high stress levels, environmental pollution, cigarette smoking, alcohol, and drug abuse attribute to the effect of skin disease and skin aging. At the molecular level, environmental and chronic stress causes DNA damage, increases pro-inflammatory cytokine levels (e.g., interleukins) that causes skin inflammation. The skin becomes thinner, sweat- and oil- secreting glands in the skin decrease, leaving the skin dry and thin. Production of the proteins collagen and elastin in the skin also declines, leading to decreased firmness and formation of wrinkles. Melanin, the pigment that gives skin its color, becomes unevenly distributed causing freckles and age spots.
In order to understand the skin aging mechanism, active components and phytochemicals for skin care have been investigated extensively aiming to intervene with the exogenous and endogenous aging agents to slow down aging and other skin deterioration effects. One category of the notable phytochemicals that have been reported to provide such therapeutic skin care functions is polyphenols. Polyphenols (polyphenolic compounds), are known to be a major group of naturally occurring phytochemicals containing conjugated phenol ring(s) as a common molecular structure. A key feature of these polyphenolic compounds is their antioxidant function expressed by neutralizing reactive oxygen and nitrogen species (ROS/RNS, referred as ROS in this paper) facilitated by the conjugated phenol ring(s) [
1]. As an example, Resveratrol, a polyphenol from red wine, has demonstrated to be a front performer in prevention of photo-aging due to its strong antioxidant properties [
2]. Based on a flaky skin mouse model, researchers also demonstrated that green tea polyphenol reduced the symptoms of epidermal pathology in mouse skin [
3].
Sugarcane (
Saccharum officinarum), a perennial grass indigenous to tropical South Asia, Southeast Asia, and New Guinea, has mainly been used as a food source up until the recent discovery of polyphenols and other bioactives in the plant. Pharmacological studies revealed several polyphenolic compounds such as phenolic acids, flavonoids, and different glycosides in sugarcane juices and its unrefined products (e.g., molasses), as well as fatty acids in its stems and leaves [
4]. Since then, sugarcane has attracted much research attention in its potential therapeutic usage in a number of areas including diabetic management and neurological therapy [
5]. However, the use of sugarcane phytochemicals in skin care has not been fully explored.
We recently reported that a polyphenol rich sugarcane extract (PRSE) obtained from sugarcane molasses using a patented extraction process contains a high level of phenolic compounds including tricin, apigenin, and luteolin, and displays a full spectrum of antioxidant property that effectively neutralizes six different species of ROS [
6]. In another study, we also examined the anti-inflammatory effect of Officinol™ via its inhibitive effect on pro-inflammatory cytokine tumor necrosis factor (TNF-α) and activation effect on nuclear factor erythroid 2-related factor 2 (Nrf2), a redox-sensitive transcription factor deployed by a cellular system to counter oxidative stress and inflammation [
7].
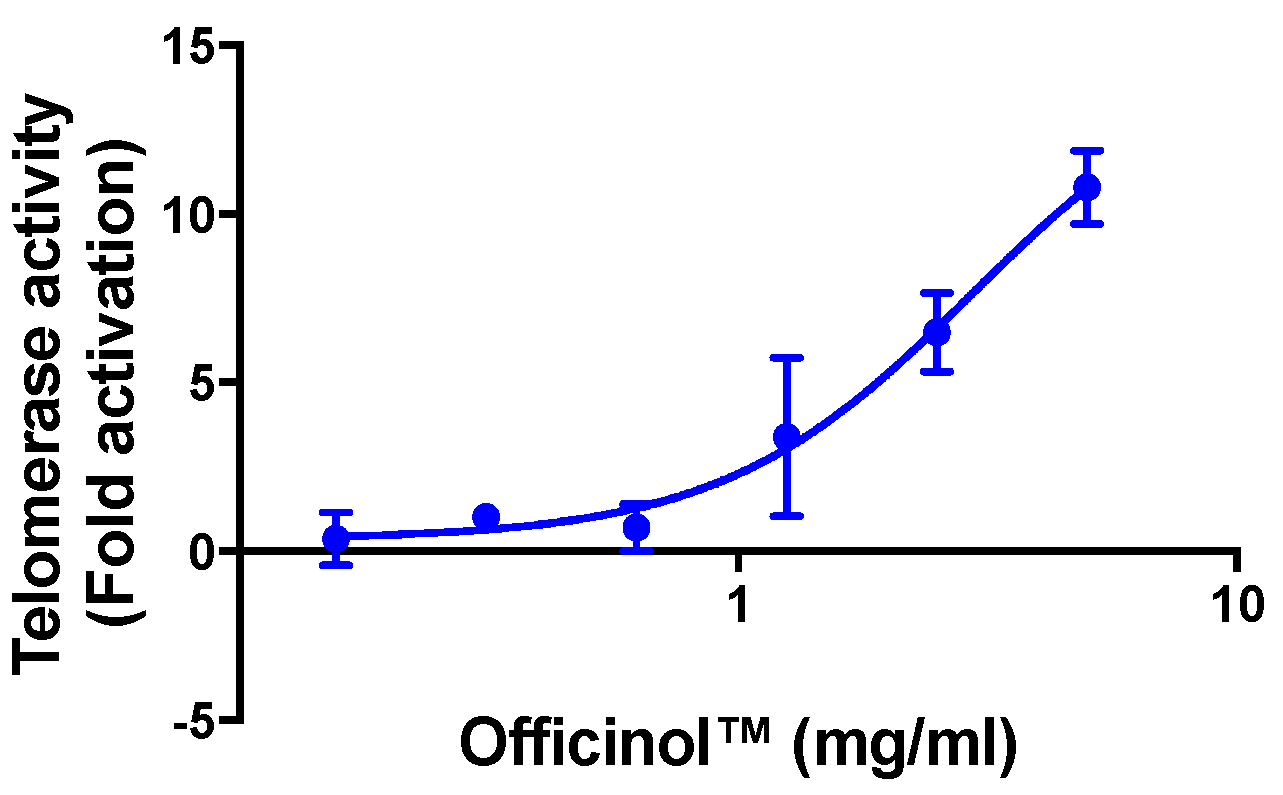
Based on the unique therapeutic functions we observed in PRSE, we theorized that the high level of polyphenols and its anti-oxidation and anti-inflammatory effect may render it a candidate as an active ingredient for skin care. In this study, we prepared a polyphenol-rich sugarcane concentrate (Officinol™) specifically for skin care application. We first examined the general functions of Officinol™ as an anti-aging agent, using cellular telomerase activity as biomarkers for aging. Telomerase is a ribonucleoprotein complex that contributes to the maintenance of telomeric structure and length that are associated with life span [
8]. We then undertook a range of preclinical studies to examine the specific effect of Officinol™ on the biology and function of skin related enzymes and cells. These include the effect of Officinol™ on: (1) UV protection and tissue integrity with matrix metalloproteinase-1 (MMP-1) as an indicator; (2) melanin modulation in melanocytes, and skin brightness based on activity of tyrosinase, the key enzyme that catalyzes the first step of melanin biosynthesis; (3) wrinkle prevention using elastase, an enzyme that facilities the reduction of skin elasticity, as the indicator.
At the conclusion of this investigation, we carried out a 10-person, clinical pre-study to examine the effect of Officinol™ on skin lightening and fine line and wrinkle reduction in human skin. The combination of the in vitro and clinical studies demonstrated that Officinol™ could provide significant preventative and protective functions including anti-aging, UV protection, wrinkle reduction, and skin brightening for human skin suffering from aging and other chronicle and environmental stresses. The findings are to be further verified via a larger scale clinical study at a later stage.
2. Materials and Methods
2.1. Reagents
Unless otherwise specified, all chemicals were obtained from Sigma-Aldrich, St. Louis, MO, USA. The cell culture, cell media, and other biological reagents were obtained from sources indicated in the method description below.
2.2. Study Material
A proprietary, polyphenol rich sugarcane plant concentrate (Officinol™) obtained from sugarcane molasses was used in this study. The material was developed and prepared by The Product Makers Pty Ltd. (Melbourne, Australia). In short, sugarcane molasses is diluted to 50 Brix in deionized water, mixed well in room temperature until homogeneous. To this feedstock, food grade 95% ethanol is added slowly while stirring until the final ethanol level is 75%. The clear yellow supernatant is aspirated and collected and then centrifuged at 2500× g for 15 min to remove any remaining particulate matter. The supernatant is collected and then filtered through a Whatman grade GF/A filter under vacuum and the filtrate collected. Extract is concentrated to 70 Brix in a Buchi Rotary Evaporator. Quantification of general antioxidant capacity with both chemical (6-radical Oxygen Radical Absorption Capacity assay, ORAC 6.0) and biological (Cellular antioxidant assay, CAA) quantification methods were also carried out as previously described (6).
For the following cell-based studies, Officinol™ was suspended in 50% EtOH to yield a 1g/mL mixture. The mixture was then thoroughly vortexed for 30 s. Subsequently the solution was ultrasonicated in an ice bath for 15 min and vortexed occasionally during this time. Following ultrasonication, the solution was vortexed again for 30 s and centrifuged at 1780× g (or 3900 rpm @105mm radius) under 4 °C for 15 min to separate out any residue in the mix. The supernatant was then collected as the stock extract (1 g/mL) and stored under 4 °C until use. For each in vitro study, a series of dilutions of the stock extract were used according to specific study requirements. A minimum 1:33 dilution of the stock extract in cellular media was used when adding the sample into cell cultures, making the final concentration of ethanol in cell cultures less than 1.5%.
2.3. Cellular Telomerase Activity Assay
Cellular telomerase activity assay was carried out basing on an established study model with modification [
9]. Human embryonic kidney cell line 293 T (HEK 293 T) from American Type Culture Collection (ATCC, Manassas, VA, USA) were cultured in Dulbecco’s modified Eagle medium (DMEM) supplemented with 0.5% fetal calf serum (FCS), 1 unit/mL of penicillin/streptomycin, and 2 mM glutamine. in a humidified atmosphere of 5% CO2. After 4–5 passages, the cells were counted and seeded at the density of 2.5 × 10
5 cells per 60-mm gelatin-coated well. Cells were then further cultivated, and assays were carried out when cells reached approximately 80–90% confluence in the wells. Unless otherwise indicated, cells were seeded with similar density and cell cultures reaching 80–90% confluence in culture wells were used for the rest of the cellular assays in this paper.
On the day of assay, the cells were treated with treated various concentrations of Officinol™, or cycloastragenol (used as the assay positive control), or phosphate-buffered saline (PBS) at pH 7.4 (negative control). After 24 h of treatment, a Telomerase Repeated Amplification Protocol (TRAP) assay [
10] was performed using the TRAPEZE XL telomerase detection kit (Millipore, Burlington, MA) according to the manufacturer’s instructions and measured in a fluorescence plate reader after transfer of the polymerase chain reaction (PCR) products into black 96-well plates. Briefly, cells were lysed with CHAPS lysis buffer containing 100–200 units/mL of RNase inhibitor. The cell pellet was washed once with PBS at pH 7.4, and re-suspended in 800 µL of CHAPS Lysis Buffer. The suspension was incubated on ice for 30 min, and spun in a microcentrifuge at 12,000×
g for 20 min at 4 °C. 500 µL of the supernatant was transferred into a fresh tube and the protein concentration determined. PCR amplification was then performed to determine the telomerase products. In short, aliquots of 48 µL of a TRAP reaction mix including telomerase substrate (TS) primer, a TS oligo (sequence: 5′-AAT CCG TCG AGC AGA GTT-3), ACX reverse primer (sequence: 5′-GCGCGGCTTACCCTTACCCTTACCCTAACC-3′) modified with proprietary fluorescent energy transfer dyes, and Taq polymerase were added into a RNase-free PCR tube. 2 µL of cell lysis extracts or controls were added into each tube. The tubes were placed in the thermocycler block, and incubated at 30 °C for 30 min. Then, in a thermocycler, a 4-step PCR (94 °C/30 s, 59 °C/30 s, 72 °C/1 min) was performed for 36 cycles followed by a 72 °C/3 min extension step and then at 55 °C/25 min, concluding with a 4 °C incubation. The yield of the PCR reaction was then determined by measuring the fluorescence in a spectrofluorometer. In each PCR reaction, a proprietary internal standard (K2) was also included which produced a detectable 56 bp sulforhodamine amplification product that served as a control for PCR amplification efficiency. The telomerase products of cells treated with Officinol™ or positive control were normalized against cells without treatments. Two biological replicates with a minimum two technical measurements per replicate were carried out for each assay in this paper.
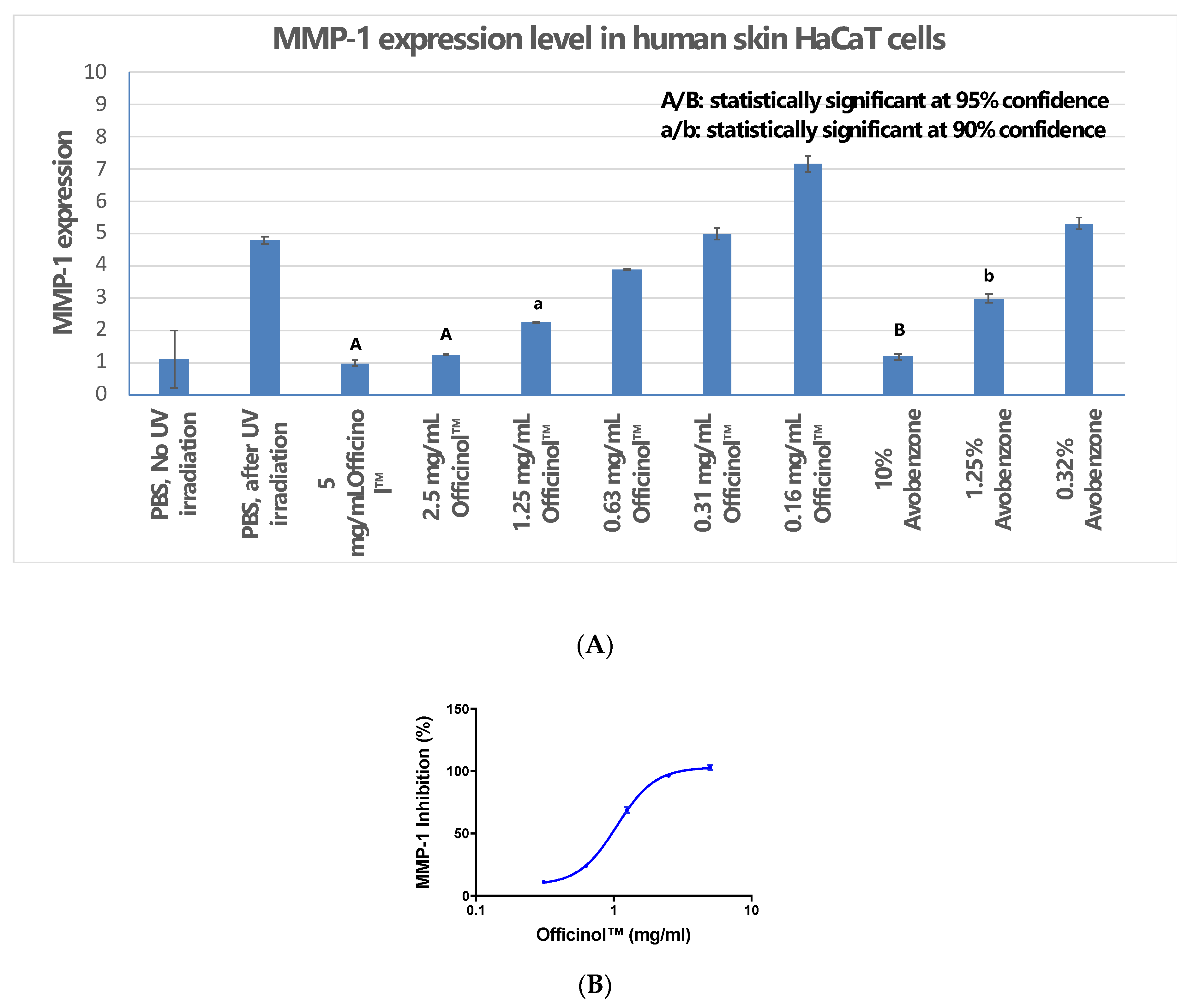
2.4. UV Protection Study: Cellular Matrix Metalloproteinase-1 (MMP-1) Assay
HaCaT cells, an immortalized human keratinocyte cell line (ATCC, Manassas, VA, USA), were cultured in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% heat-inactivated FBS and 1% antibiotic-antimycotic (GIBCO-BRL, Grand Island, NY, USA) at 37 °C under a humidified atmosphere of 95% air and 5% CO2.
On the day of assay, the cells were treated by various concentrations of Officinol™ or avobenzone (used as the assay positive control), or PBS at pH 7.4 (negative control) for 24 h. Then, the cells were exposed to UV irradiation of 50 mJ/cm2 generated by an UVB lamp. After UV irradiation, the cells were washed with PBS at pH 7.4, and incubated with serum-free DMEM for 24 h. Mock-irradiated controls followed the same schedule of medium changes without UVB irradiation.
Quantitative real time PCR (qRT-PCR) was then performed to determine the MMP-1 expression level in each treatment group. Extraction of total RNA was performed by using the MagMAX™-96 Total RNA Isolation Kit. cDNA was synthesized from total RNA by using High Capacity RNA-to-cDNA Kits and a Veriti thermal cycler. The primer pair used was: TCTGACGTTGATCCCAGAGAGCAG (forward) and CAGGGTGACACCAGTGACTGCAC (reverse). Peptidyl-prolyl cis-trans isomerasa (Ppia) was used as the house keeping gene. qRT-PCR was performed in Applied Biosystems Real-Time Quantitative PCR system. MMP-1 expression levels in human skin cells treated with Officinol™ and positive controls were normalized against cells without treatments.
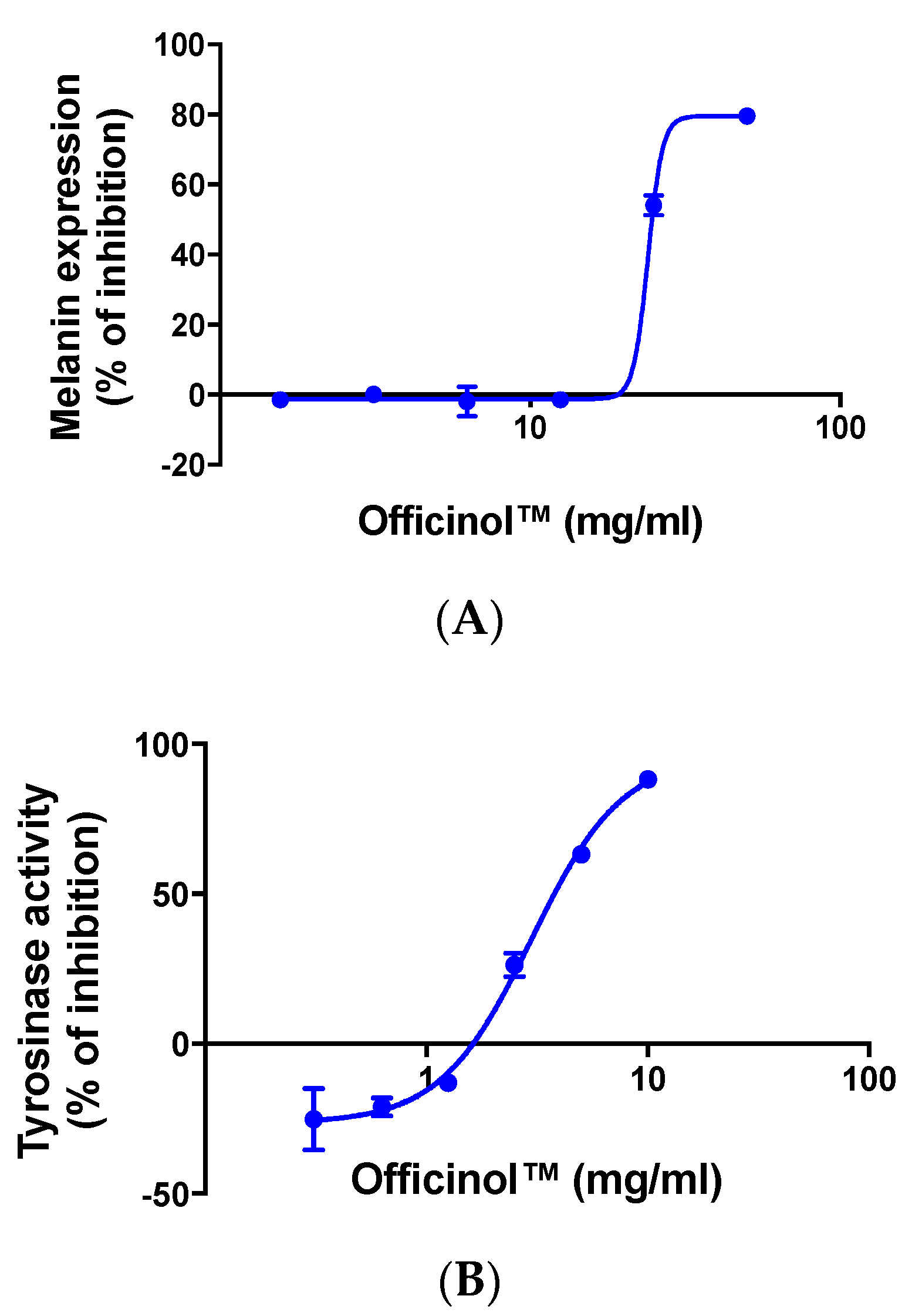
2.5. Cellular Melanin Pigmentation Inhibition
Cellular melanin pigmentation inhibition study was carried out according to a previously published procedure with minor adjustment [
11]. Briefly, human melanoma cells MNT-1 (Thermo Fisher Scientific, Waltham, MA, USA) were maintained in DMEM with 10% heat-inactivated FCS and a 1% antibiotic-antimycotic at 37 °C in a humidified 5% CO
2 atmosphere before use.
On the day of assay, the cells were treated by various concentrations of Officinol™ or chloroquine (used as the assay positive control), or PBS at pH 7.4 (negative control) for 72 h. The cells were then washed with PBS at pH 7.4, and lysed in 200 µL of 1M NaOH and boiled for 5 min to solubilize the melanin. 100 µL of each lysate was added into in a 96-well microplate, and the absorbance at 490 nm was measured with a microplate reader. The level of melanin pigmentation formation in human skin cells treated with Officinol™ and positive controls were normalized against that in the cells without treatments.
2.6. Enzymatic Tyrosinase Inhibition Assay
The inhibition of tyrosinase by Officinol™ was investigated using an enzymatic tyrosinase inhibitor screening assay kit (Abcam, Cambridge, UK) according to the manufacturer’s instruction, following an assay design tailored towards inhibition activity study. In principle, tyrosinase catalyzes the oxidation of tyrosine, producing a chromophore that can be detected at OD 510 nm. A series of concentration of Officinol™, or Kojic Acid (a reversible inhibitor of tyrosinase that is used as the assay control) were allowed to react with a mixture of tyrosinase and tyrosinase substrate for 10 min in room temperature (25 °C). The optical signal of each reaction mix was then recorded and normalized against that of the assay background. Inhibition curves and IC50s were then generated by Prism 8.0 (GraphPad Software, San Diego, CA, USA).
2.7. Enzymatic Elastase Inhibition Assay
The inhibition of elastase by Officinol™ was investigated using an enzymatic elastase assay kit (EnzChek®, Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s instruction, with a modified assay design tailored towards inhibition activity study. In brief, a soluble bovine neck ligament elastin that has been labeled with BODIPY® FL (Thermo Fisher Scientific, Waltham, MA, USA), a fluorescent dye, such that the conjugate’s fluorescence is quenched. When elastase is introduced, this non-fluorescent substrate can be digested by elastase to yield a highly fluorescent product.
A series of concentration of Officinol™, or N-Methoxysuccinyl-Ala-Ala-Pro-Val-chloromethyl ketone (a known inhibitor of elastase that is used as the assay control) were allowed to react with a mixture of the above non-fluorescent substrate and elastase for 30 min under room temperature (25 °C) in dark. Tej fluorescence signal of each reaction mix is then measured using a plate reader (Synergy HT, BioTek, Winooski, VT, USA) and normalized again the assay background control. The degree of the fluorescent signal change indicates the activity of elastase present in the reaction mix. Inhibition curves and IC50s were then generated by Prism 8.0 (GraphPad Software, San Diego, CA, USA).
2.8. Clinical Study
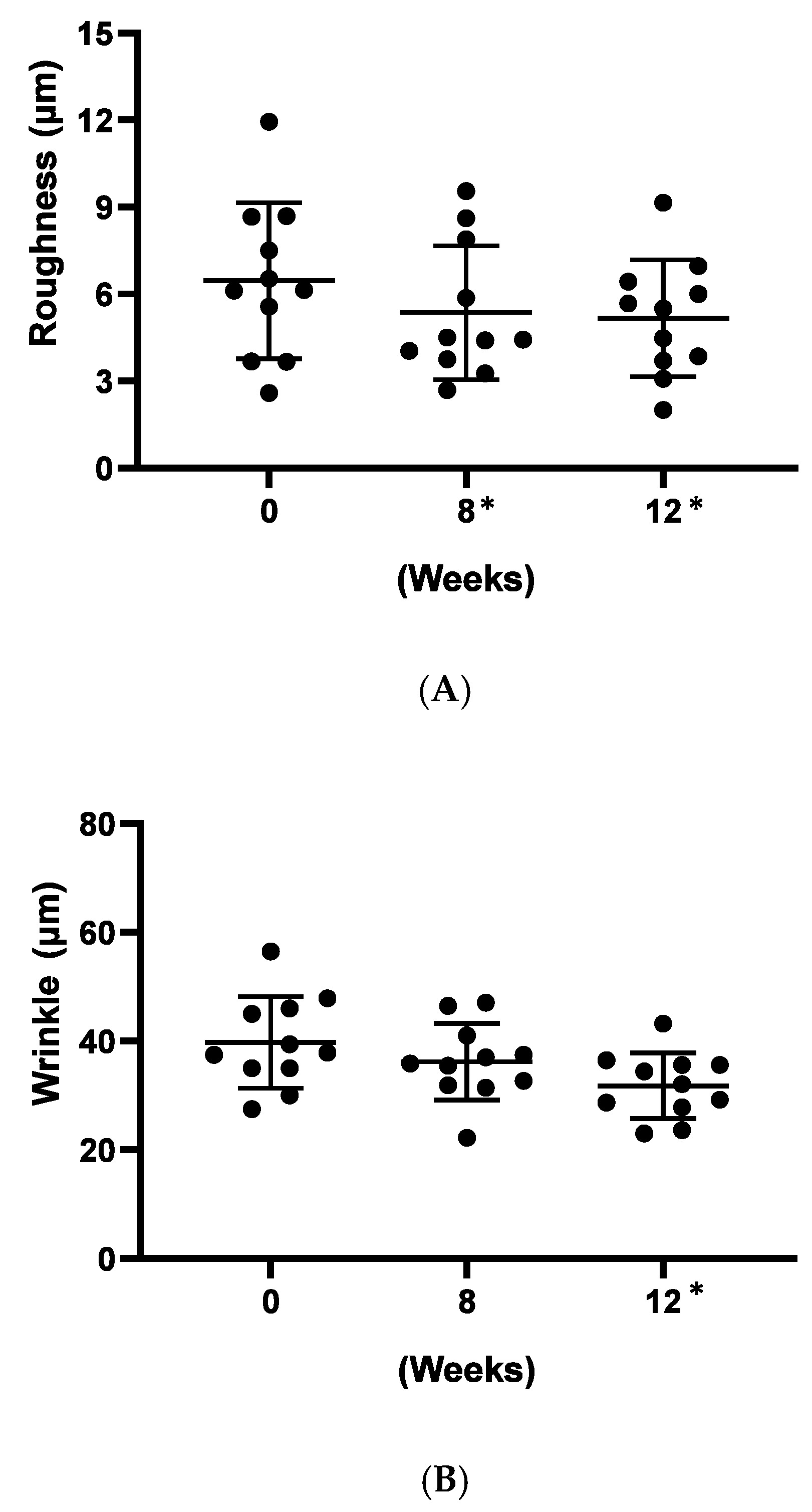
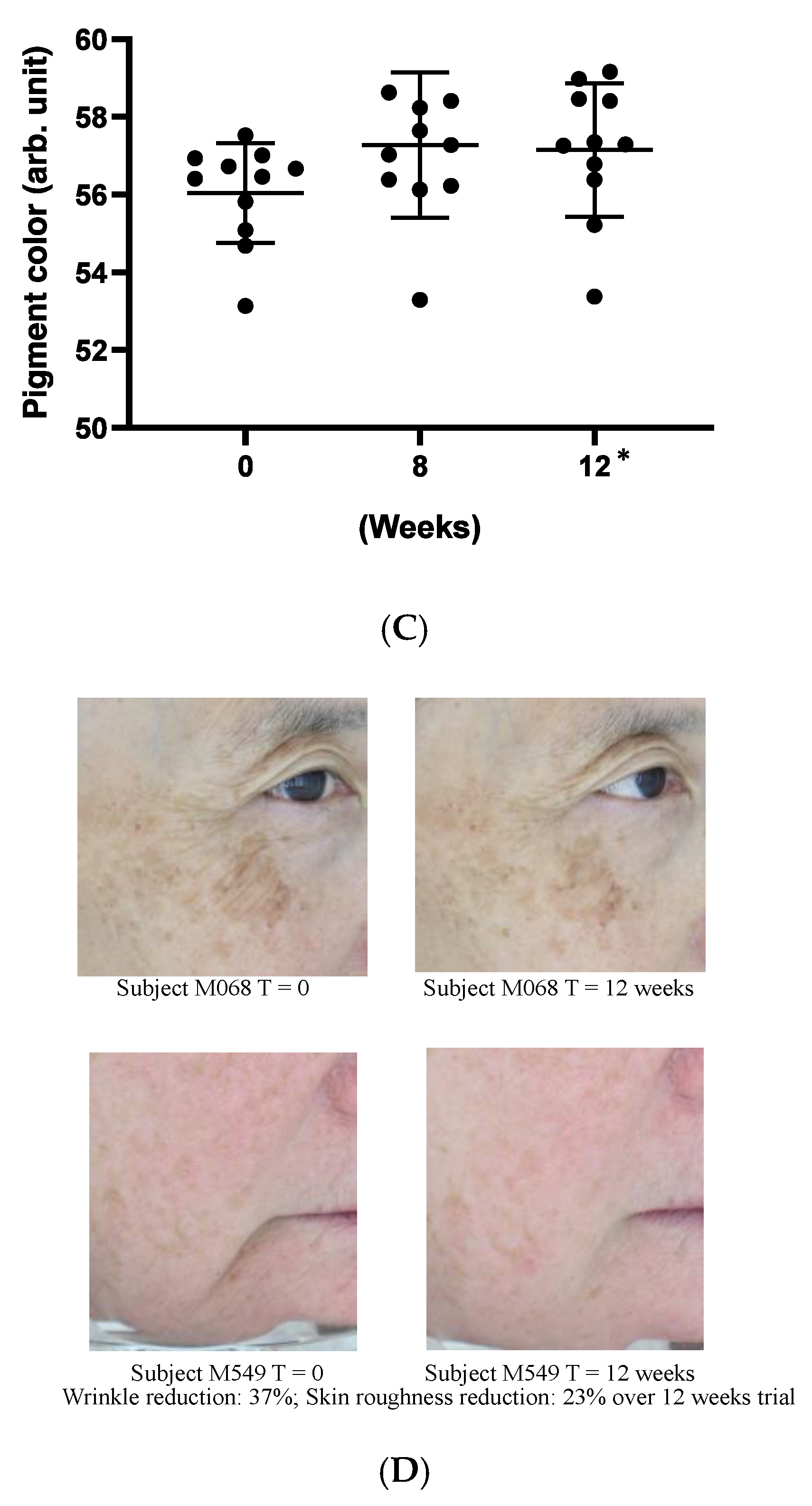
A clinical study was carried out to evaluate the in-use performance of Officinol™ after its application to the face. To facilitate the clinical study, a study product was prepared by adding Officinol™ at 1:20 (w/w) ratio into an inert carrier that possessed no active properties. The biophysical effects of the topically applied product were evaluated pre and post product application when used by the same test panelists. 11 subjects were recruited for the study, with results tabulated for all 10 who completed the study period of 12 weeks. All subjects gave their informed consent for inclusion before they participated in the study. The study was conducted in accordance with the guideline of Australian/New Zealand Standard (AS/NAS) 2604:2012, ISO 24444, the Declaration of Helsinki and the relevant Australian National Health and Medical Research Council (NHMRC) Act 1992. Wrinkle reduction was determined using silicone impressions and Profilometry and skin blemishes using a Minolta spectrophotometer. A photographic record was also taken of each test participant.
Healthy adult volunteers between the ages of 40–65 years were recruited for this study. The choice of the age criteria results from a combination consideration of the biological effect to be investigated, selection of a representative sample, and minimization of comorbidity. Inclusion criteria included the following: not taking medication or under the care of a physician for a period of one month prior to commencement and throughout the entire test period; free of any dermatological or systemic disorder that would interfere with the results, at the discretion of the Investigator; with self-described dry skin.
Exclusion criteria include: individuals who are under doctor’s care or are taking medication which in the opinion of the investigator would mask or interfere with the results; with any history of sensitivity to cosmetics in general and moisturizers in particular; with any form of skin cancer, or any disease that would interfere with the test results; diagnosed with chronic skin allergies; Female volunteers who indicate that they are pregnant or nursing an infant; Individuals with excessive hair on the test sites; Individuals with known hypersensitivity to cosmetic products.
Eleven participants had been recruited for the study, of which ten participants completed 12 weeks of study. The results were tabulated for all 10 who completed the full study period.
2.8.1. Study Protocol
After completing the informed consent forms, all participants were required to abstain from use of moisturizers and any other skin treatments on the test area for a period of 10 days prior to study commencement. At the completion of the 10 days ‘washout’ period, panelists were required to apply the study product (Officinol™) evenly applied to the face twice per day (Morning & Evening) for consecutive 12 weeks. During the 12 weeks test period, the participants were required to visit the clinic site for three times: week 0, week 8, week 12. At each site visit, following skin evaluation tests were conducted:
Instrumental Description Profilometry (Wrinkles and Roughness)
At each visit, a single silicone replica was made of the target area and a photographic record was kept of this target for subsequent relocation. The samples were stored in controlled conditions for comparative measurement. Comparative analysis of skin profilometry was conducted, using surface roughness and wrinkle depth analysis. Concurrent use of other moisturizer or skin care products did not occur in the skin area under study. The height of the replicated wrinkles were measured using Miyomoto Surftest profilometer. Ry (depth) and Ra (mean roughness) were recorded at each time of measuring operation. The area scanned from each sample was clearly mapped so as to determine the same area in respective two months sampling.
Instrumental Description L*a*b* Color Measurement
A Minolta Chromometer hand held spectrophotometer was utilized. This tri-stimulus instrument was in an unpigmented adjoining area. Specular component included (SCI) values are documented.
Digital Photography
At each time point, a series of high-resolution digital photographs were collected. The subject was presented with a clean face, with hair pulled off face, with no jewelry (unless permanent) and with a black drape used to standardize clothing. Subject positioning was reproduced upon return visit. A light booth was used so as to provide controlled reproducible light conditions. The booth consists of an array of eight equally spaced fluorescent tubes in a semicircular configuration. The software driven system allows the position and expression of the test subjects to be aligned to a high degree. Lux values were calibrated and documented.
2.9. Statistical Analysis
For the clinical study, a statistical analysis was performed on the data of roughness, wrinkle, and pigment color by analysis of variance (ANOVA). The analysis was conducted with time as the fixed effect and subject as the random effect, followed by the contrast test to compare the values obtained at weeks 0, 8, and 12 if the time effect was statistically significant. The statistical significance of the change of the data is determined by the probability value (p-value), i.e., probability that the difference of the data obtained at different time points being zero. When p-value was <0.05, the difference between the two sets of data was considered significant. The analysis was performed using JMP IN version 3 (SAS, Cary, NC).
5. Conclusions
The sugarcane concentrate, Officinol™, exhibits significant anti-aging properties, potentially through telomerase activation pathway. Further studies in human keratinocytes indicated that Officinol™ protects human keratinocytes from UV irradiation by inhibiting MMP-1 as a possible pathway, inhibits melanin pigmentation in the skin cells, possibly through tyrosinase inhibition, and inhibits elastase, a key biomarker for wrinkle production. A 10-person clinical study has also been carried out to investigate the effect of Officinol™ on human skins. The human trial demonstrated that Officinol™ formulated cosmetic gel provided significant improvements in skin roughness, wrinkle depth, and skin pigmentation of the test subjects.
Our findings are also in accordance with previous reports on the use of polyphenols extracted from plants to improve skin appearance including skin pigmentation and wrinkle reduction [
27]. One consideration in applying natural plant extracts is the possible synergistic or competing effects from various components in the extract. In a review article, Scheepens et al. discussed in depth designed synergies to improve oral bioavailability as well as efficacy of beneficial polyphenols. In this current study, we reported the combined effect from Officinol™, a mixture plant extract concentrate. While we have completed preliminary phytochemical profiling work to support the possible bioactives, we are carrying out further composition profiling and characterization studies to provide more insight on the key bioactive(s) in Officinol™.
We recognize that current study findings are based on human cells and a small human pre-study, which is still a distance away towards the translation into clinical success. Regardless, our work encourages further in vitro and in vivo studies to verify these potential therapeutic effects, and explore signal pathway in more depth. In our laboratory, a clinical study of minimum 20 subjects are being planned to further verify the study findings above. The success of these follow-up studies would provide an opportunity of discovering the skin care benefits of the bioactives extracted from sugar production, resulting in a new active skin care component based on a globally abundant crop.