Abstract
Novel nanotechnologies represent the most attractive and innovative tools to date exploited by cosmetic companies to improve the effectiveness of their formulations. In this context, nanoliposomes have had a great impact in topical preparations and dermocosmetics, allowing the transcutaneous penetration and absorption of several active ingredients and improving the stability of sensitive molecules. Despite the recent boom of this class of delivery systems, their industrial production is still limited by the lack of easily scalable production techniques. In this work, nanoliposomes for the topical administration of vitamin D3, K2, E, and curcumin, molecules with high antioxidant and skin curative properties but unstable and poorly absorbable, were produced through a novel simil-microfluidic technique. The developed high-yield semi continuous method is proposed as an alternative to face the problems linked with low productive conventional methods in order to produce antioxidant formulations with improved features. The novel technique has allowed to obtain a massive production of stable antioxidant vesicles of an 84–145 nm size range, negatively charged, and characterized by high loads and encapsulation efficiencies. The obtained products as well as the developed high-performance technology make the achieved formulations very interesting for potential topical applications in the cosmetics/cosmeceutical field.
1. Introduction
Nanotechnology is considered the most powerful and promising strategy of the 21st century providing innovative solutions and new opportunities to modern cosmetic dermatology through the investigation of the unique properties of matter at the nanoscale [1,2,3,4]. In particular, nanoparticles, recognized for their potential to penetrate human skin, are increasingly used to enhance the topical delivery of cosmetic ingredients and also to give stability to formulations that contain easily degradable materials [5,6,7].
Recently, the production of formulations containing nanoparticles has been disciplined by the Regulation (EC) n. 1223/2009 of the European Parliament and of the Council of 30 November 2009 on cosmetic products [8], subsequently replaced by Directive 76/768/EEC on 11 July 2013 [9], while at a global level, the International Cooperation on Cosmetics Regulation (ICCR) has produced key reports related to the characterization procedures of nanomaterials and their safety in cosmetics [10,11].
Among nanoparticles, a prominent place is occupied by nanoliposomes for cosmetic and cosmeceutical preparations, used for the formulation of anti-aging creams, moisturizers, sun lotions, facial beauty masks, for the treatment of hair loss, and many other applications [1,12]. Since 1986, the year in which the first liposomal cosmetic product, the anti-aging cream “Capture” launched by Christian Dior, appeared on the market, countless cosmetic formulations containing nanoliposomes have been patented (Supplementary Materials Figure S1).
Nanoliposomes (often also denominated small unilamellar vesicles, SUV) are vesicles of nanometric size characterized by an aqueous core surrounded by an hydrophobic bilayer, which can accommodate, inside their structure, active molecules of a different nature [13] (representation in Supplementary Materials Figure S2). Composed of phospholipids, nanoliposomes have high affinity for the stratum corneum (SC), or the horny layer of the skin, a characteristic which allows a more efficient uptake of the encapsulated active molecule with respect to conventional dosage forms [14]. Phospholipids composing liposomes are of GRAS (generally recognized as safe) type ingredients, therefore, materials safe for human health: the resulting carriers are biocompatible, biodegradable, and non-toxic. Moreover, nanoliposomes offer many favorable features to hydrophilic, amphiphilic, and lipophilic molecules, such as an improved solubility, stability, and pharmacokinetic/pharmacodynamics properties, target selectively, reduced toxicity, protection from external reacting materials, improved tolerability of the skin to the substances reducing the risk of irritation, allowing their slow release, and prolonging their beneficial effect. Finally, being more effective, the liposomal formulation requires the use of less amounts of product resulting in an economic saving [2,14,15].
In order to take advantage of these properties, in this work, vitamin D3, K2, E, and curcumin, all molecules joined by a strong antioxidant activity but poor bioavailability, solubility, absorption, low stability, and rapid metabolism/systemic elimination in their naked form [16,17,18,19], were encapsulated inside nanoliposomes to be used, all together or as separate ingredients, as topical formulations for the treatment of skin aging and several dermatological disorders. In particular, recent studies have shown that vitamin D has beneficial effects in repairing skin cell damage caused by UV, in erythemo-papulo-squamous disorders like psoriasis vulgaris, in skin hydration, in the treatment of vitiligo, and facial seborrheic dermatitis [16,20,21]; vitamin K is useful in suppressing pigmentation and resolving bruising of skin, in limiting the occurrence of acne, and in promoting wound healing [18,22]; vitamin E is effective in reducing the formation of erythema induced by UV radiation, in treating atopic dermatitis, psoriasis, and other dermatological diseases, in resolving cutaneous ulcers, and in improving the wound healing and scarring [23,24,25]; finally, curcumin is used in UV radiation protection, for treatment of skin aging, psoriasis, acne, skin inflammation, and cancer [26,27,28].
Although widely studied in the scientific literature and extensively exploited at the industrial level, productions of liposomal formulations for dermatological uses are nowadays limited to the use of conventional techniques such as the thin film hydration or Bangham method, emulsification, reverse-phase evaporation, detergent removal, hot/cold homogenization, spray drying, solvent injection, freeze thaw, sonication, and extrusion, as reported in numerous works and patents [29,30,31,32,33,34]. Among these, the most used in cosmetics are the thin film hydration and the ethanol injection methods. Due to the difficulty in scaling up the processes, which are discontinuous and not controllable, these bulk methods are all unsuitable for the industrial large-scale production of nanoliposomes, besides requiring the use of large amounts of solvent, extreme process conditions such as temperature and pressure, returning small output volumes of products [35]. A relatively new technology consists in the production of liposomes by means of microfluidic hydrodynamic focusing (MHF) chips. Although the method allows to avoid the use of toxic solvents, offering a precise control over the dimensional features of the particles, it has the disadvantage of high manufacturing costs and small production volumes [31,36]. It results as evident that new techniques appropriate for the growing “nanocosmeceutical” field are indispensable at the industrial level.
Embracing this problem, in this study, antioxidant-nanoliposomal formulations were produced through the recently developed and patented simil-microfluidic manufacturing procedure which, compared to the traditional techniques, gives the possibility to have a tight control on the physicochemical characteristics of the nanoparticles, at the same time obtaining their massive and rapid production through a sustainable and continuous process without the use of drastic conditions and special micro-fabricated devices [37].
In particular, in this work, after a brief discussion about the developed simil-microfluidic method, by contextualizing the process among those used in cosmetics at industrial and laboratory scale, a description of the new technique used for the production of nanoliposomal formulations containing antioxidants is addressed. Then characterization of carriers loaded with vitamins D3, K2, E, and curcumin in terms of morphology, mean diameter size, polydispersity index, superficial charge, encapsulation efficiency, and load is presented. Finally, a physicochemical characterization of aged particles after one month storage at 4 °C is also proposed testing the carriers’ ability to remain stable under preservation conditions.
2. Materials and Methods
2.1. Antioxidant Nanoliposomes Production through the Simil-Microfluidic Apparatus
2.1.1. Materials
L-a-Phosphatidylcholine (PC) from soybean, type II-S, 14–23% choline basis (CAS no. 8002-43-5), cholesterol (CHOL) (CAS no. 57-88-5), ethanol of analytical grade (CAS no. 64-17-5), vitamin D3 or cholecalciferol (CAS no. 67-97-0), vitamin K2 or menaquinone 4 (CAS no. 863-61-6), curcumin (Cur) (CAS no. C1386), vitamin E or α-tocopherol (CAS n. 10191-41-0), and Triton X-100 (CAS no. 9002-93-1) were purchased from Sigma Aldrich (Milan, Italy).
2.1.2. Manufacturing Technique
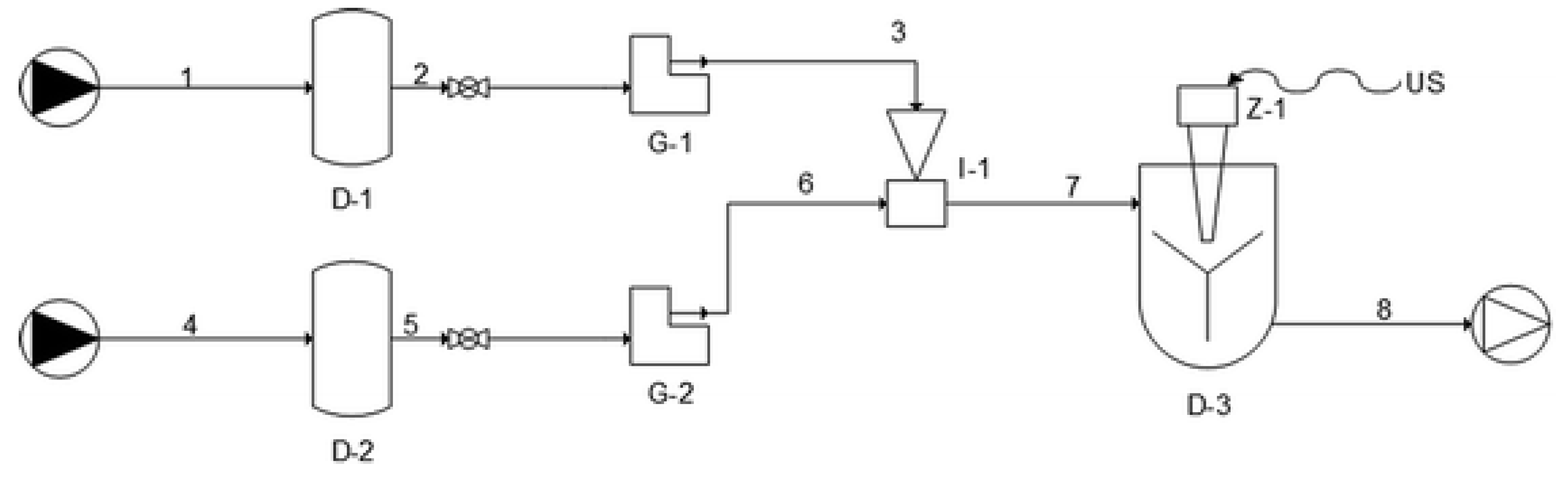
In order to produce antioxidant-loaded liposomal vesicles, a novel semicontinuous simil-microfluidic apparatus (piping representation in Figure 1 [38]), the layout, operative conditions, and phenomenological aspects of which are detailed in [37,38,39], was used. Briefly, it consists of two feed solutions (lipids/ethanol and water) which are pushed through peristaltic pumps (into the production section, a millimetric tubular device where the interdiffusion of the two flows leads to the formation of liposomes directly at nanometric scale. In particular, all antioxidant-loaded nanoliposomes formulations were produced by using the conditions of 10:1 (Vhs/Vls) hydration solution volumetric flow rate (Vhs) to lipid solution volumetric flow rate (Vls) and 5 mg/mL lipids in the final hydroalcoholic solution. First, for vitamin D3 vesicles production, a lipid/ethanol solution was prepared by dissolving 940 mg of PC, 188 mg of cholesterol, and 129.6 mg of vitamin D3 in 20 mL of ethanol. As a hydration solution, 200 mL of deionized water was used. After the complete dissolution of the components, the two solutions were put in contact in the simil-microfuidic set-up. Subsequently, keeping the amount of lipids used and the volumes of ethanol and water constant, the same steps followed for vitamin D3 nanoliposomes were repeated for the production of vesicles loaded with vitamin K2, vitamin E, and curcumin. In particular, vitamin K2 (129.6 mg), vitamin E (216 mg), and curcumin (140.6 mg) were added instead of the vitamin D3 in the organic phase. For each formulation, unloaded liposomes control samples were also prepared for comparison. Through the described method, vitamin D3- and K2-loaded vesicles were previously produced for potential nutraceutical and pharmaceutical applications as described in a precedent work [40], while vitamin E and curcumin nanoliposomes were explored for the first time.
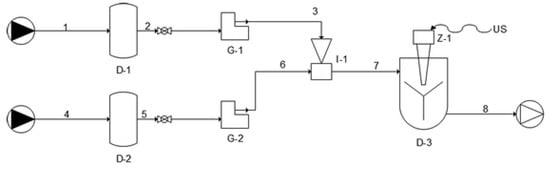
Figure 1.
Piping representation for the experimental setup for the simil-microfluidic method: (1–2–3) lipids/ethanol feed line; (4–5–6) water feed line; (D-1 and D-2) feed tanks; (G-1 and G-2) peristaltic pumps; (I-1) injector (production section); (7–8) water/ethanol nanoliposomes suspension; (D-3) recovering/homogenizing tank (from [38], published by The Royal Society of Chemistry).
2.2. Vesicles Characterization
2.2.1. Morphology
Morphological characterization of unloaded and antioxidants loaded nanoliposomal vesicles was performed by transmission electron microscopy, TEM (EM 208, Philips), equipped with a camera Olympus Quemesa (EMSIS GmbH and Software RADIUS). About 10 µL of samples, diluted with distilled water at 1:10 v/v, were deposited on a carbon support on copper specimen grid mesh 200 (Electron Microscopy Sciences) and negatively stained with 1% w/v of uranyl acetate solution.
2.2.2. Size and Zeta Potential
Size and zeta potential determinations of unloaded and antioxidants loaded vesicles were performed by dynamic light scattering (DLS) method, using the Zetasizer Nano ZS (Malvern, UK) with noninvasive backscatter (NIBS) optics and a detection angle of 173 degrees.
DLS measurements were performed at room temperature applying a dilution, for each sample, of 1/10 with pure water (by mixing 100 µL of nanoliposomes suspension with 900 µL of pure water). For the numerical size distribution, the number of particles versus the particle size was plotted.
The polydispersity index (PDI) and the Z-average values were calculated for all the preparations, and all the measurements were performed in triplicate.
2.2.3. Encapsulation Efficiency (e.e.) and Effective Load
In order to measure the real encapsulated and the un-encapsulated amounts of antioxidants, from all the samples, aliquots were taken and spectrophotometrically analyzed. In order to remove the supernatant (containing the un-encapsulated active molecule) from the precipitated nanoliposomes (pellet), 3 mL aliquots of the samples containing vesicles loaded with vitamin D3, K2, E, and curcumin were centrifuged (Beckman Optima L-90K, SW 55 Ti rotor) at 35,000 rpm (118,443 x g) for 1 h at 4 ℃ under vacuum. The supernatant volume, gently removed from the centrifuged samples and stored for the subsequent spectrophotometric determination, was measured and replaced with the same volume of Triton X100 at 1% (v/v) or pure ethanol in order to lyse the nanoliposomes pellet and to analyze the encapsulated active molecule. In particular, for vesicles loaded with vitamin K2, E, and curcumin, the pellet was incubated with 3 mL of Triton X-100 at 1% (v/v), while for the rupture of D3 loaded liposomes 3 mL of ethanol were used, as Triton X-100 absorbs in the same range as vitamin D3 disturbing the UV-VIS quantification.
After 30 min of incubation and 1 min of sonication at 100% amplitude (VCX 130 PB Ultrasonic Processors, 130 W, frequency 20 kHz, Sonics & Materials Inc., CT, USA), the pellet and the previously preserved supernatants of each sample were submitted to UV-VIS spectrophotometric analysis (Lambda 35, PerkinElmer, Monza, Italy).
An absorption spectrum from 200 nm to 600 nm was investigated for all the samples, and the maximal wavelengths of 276 nm for vitamin D3, 330 nm for vitamin K2, 292 nm for vitamin E, and 426 nm for curcumin were considered.
Encapsulation efficiency (e.e.) was determined as the percentage of antioxidants (vitamin D3, K2, E, and curcumin) encapsulated in nanoliposomes to the initial amount of antioxidants included in the formulation and was calculated using the equation:
Theoretical load is referred to as the initial amount of antioxidant (AO) included in the formulation divided by the total mass, i.e., PC, CHOL, antioxidants (vitamin D3 or K2 or E or curcumin), while the effective load was determined as the encapsulation efficiency multiplied by the theoretical load, using the following equations:
2.2.4. Stability
To evaluate the preservation of liposomal suspension features and the integrity of liposomal vesicles (i.e., aggregation and/or segregation phenomena, structure disintegration, load leakages), fresh products underwent an aging period. In particular, the stability of loaded vesicles was investigated by maintaining the samples at 4 °C in deionized water, protected from light, for 1 month (aged samples). After this time, aliquots from all the formulations were taken and analyzed for size, PDI, zeta-potential, encapsulation efficiency, and morphology following the methodologies described for the fresh sample characterization.
2.2.5. Statistical Evaluation
All kinds of measurements (size, PDI, zeta-potential, and encapsulation efficiency on fresh and aged samples) were performed in triplicate. Results were expressed as average values with the standard deviation (SD). The student t-test was used for the comparison of two mean values with their associated standard deviations. A level of significance of 5% was considered acceptable. An Excel data sheet was used to manage experimental achieved values.
3. Results and Discussions
3.1. Manufacturing Issues
Considering the social impact of skin care on life quality (and on financial context), it is not surprising that cosmetic industries such as Lancôme, Christian Dior, Estee Lauder, Shiseido, Johnsons & Johnsons, and many others are investing in the engineered nanomaterials for makeup products and alternative formulations for the management of different dermatological diseases [41,42]. Although an evident and increasing incorporation of nanotechnology in most of their manufacturing processes, the scaling-up to achieve greater production is the major difficulty faced by industries, limiting them to the use of conventional techniques, e.g., the patented “Gaulin method” of Johnson & Johnson Company exploits a high shear homogenizer equipment, working at high pressure and temperature, for the production of liposomes for cosmetic/diagnostic/pharmaceutical applications [43]. It is well known that the main disadvantages of the high-pressure homogenization technique reside in the use of drastic conditions, such as high-shear forces, elevated pressure, and temperature, submitting active molecules to strong mechanical/chemical stresses. Moreover, particles coalescence can occur due to their high kinetic energy, leading to the engendering of heterogeneous vesicles.
Apart from industrial production, on a laboratory scale, the bulk thin film hydration (TFH) and ethanol injection (EI) techniques are the most exploited for liposomes fabrication to be used in cosmetics [35]. The THF method, through the use of a rotary evaporator, provides the dissolution of the lipids in an organic solvent and its consequent evaporation under vacuum leading to the formation of a lipid film which, after a hydration step, gives lipid micrometric vesicles. Through a discontinuous process and long fabrication times, the technique allows the production of small volumes of lipid vesicles (of not controllable dimensions) which must undergo a further sizing process to obtain nanometric particles. The EI method, instead, involves the dissolution of lipids in an ethanol phase which is instantaneously injected in the aqueous phase (though a syringe), leading to the production of nanometric vesicles. Despite being characterized by a faster process, the EI is a discontinuous bulk technique that does not allow to have a control over the dimensional features of the particles, whose production volumes are furthermore reduced and linked to those of the used syringes.
In that regard, in a work of Pamunuwa and collaborators, in which the skin deposition ability of liposomes loaded with curcumin was studied, the TFH method followed by sonication sizing was used, producing 10 mL of suspension (nanoliposomes of 225 nm to 285 nm in size and 88% e.e. of curcumin) in more than 24 h [17]. A similar study was carried out by Chen and coworkers with the aim to investigate the in vitro skin permeation and in vivo antineoplastic effect of curcumin by using liposomes as transdermal drug-delivery systems. The conventional TFH technique followed by sonication was exploited by producing just 1 mL of liposomal suspension (nanoliposomes of 82 nm in size and 82% e.e. of curcumin) in a long time (only the solvent evaporation step usually needed 3–4 h) [27]. In another work of Bi and collaborators, a liposomal vitamin D3 formulation to be used as skin anti-aging agent was produced by means of the TFH and EI techniques obtaining, with the last method, more homogeneous vesicles (92 nm in size and 80% e.e. of vitamin D3) but always in small volumes (100 mL) [16].
Another technique to produce nanoliposomes, even if still little explored in cosmetics, is the microfluidic method. This is a relatively new technology that allows the production of liposomes directly on the nanometer scale, with a good control over nanoparticle dimensions, through the hydrodynamic focusing of a lipid/ethanol stream in a flow of aqueous buffer. Despite overcoming several limits of conventional techniques, this method is characterized by small amounts of product in output besides being obtainable often through the use of expensive micro-devices. Recently, through the microfluidic technique, Hood and collaborators have synthesized nanoliposomes to be used for transdermal drug delivery with good dimensional properties but in very small amounts (volumetric flow rates of about 100 μL/min) [44].
In order to overcome several limitations characterizing the available techniques for nanoliposomes manufacturing, such as the use of often drastic conditions (i.e., high temperature/pressure and toxic solvents), the additional post-processing steps (i.e., extrusion, sonication, and freezing-thawing often required to homogenize the heterogeneous lipid vesicles), the poorly controlled process conditions, the low output volume of products, and the high microfabrication costs of microfluidic devices, a new simil-microfluidic technology was tested for the production of antioxidant-loaded nanoliposomes.
The novel method, characterized by laminar flow conditions and diffusive mass transfer, allows the formation of homogeneous liposomes directly at nanometric scale as a consequence of the molecular interdiffusion between the water and the lipid/ethanol phases, as described in depth in [37,39]. In particular, by using a volumetric flow rate ratio of 10:1 (Vhs/Vls) and 5 mg/mL lipids in the final hydroalcoholic solution, it was possible to achieve a massive production of uniform nanometric vesicles (84 nm–145 nm size range) with high encapsulation efficiencies of vitamin D3, K2, E, and curcumin (88–98% range e.e.) in just one step. Indeed, with respect to the small and finite output volumes obtainable by the use of conventional methods characterized by long process times, here, one batch of 1 L of nanoliposomal suspension was produced in about 20 min, with the possibility to indefinitely keep on the process according to the production needs, with a yield equal to 3 L/h. The simil-microfluidic method developed is a simple and easy-to-transfer technology which gives a massive output with a minimum of energy and time. Not least the fact that the novel setup, characterized by the assembly of cheap components, breaks down the microfabrication costs of microfluidic devices as well as being a sustainable production process, avoiding the use of toxic solvents and drastic conditions.
3.2. Liposomes Characterization
3.2.1. Morphology
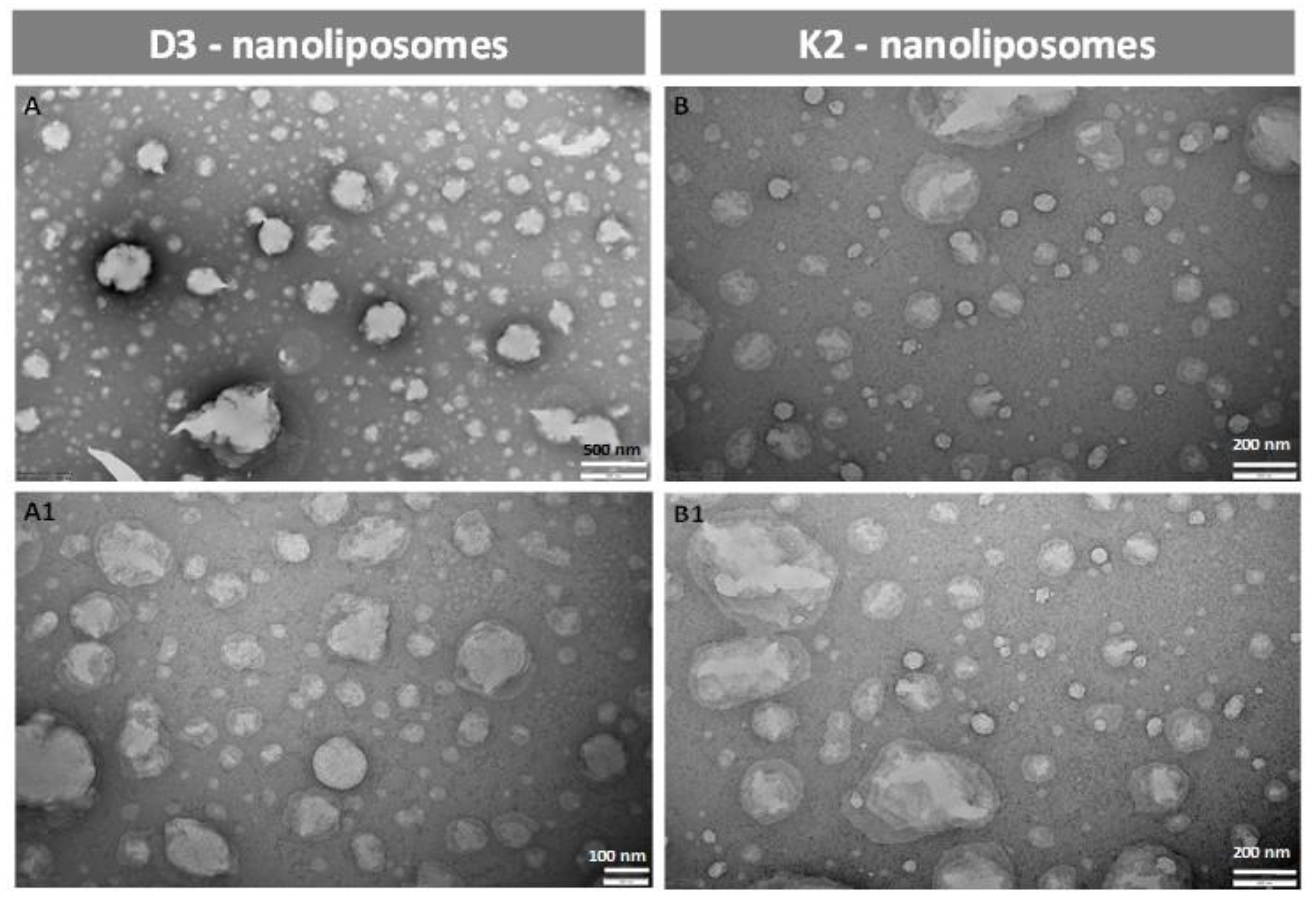
In order to obtain information about particle morphology, transmission electron microscopy TEM was used. Micrographs of D3- and K2-loaded vesicles were reported in Figure 2 as examples of part of the produced formulations. Through the simil-microfluidic technique employed, spherical and well-separated antioxidant-loaded nanoliposomes were achieved, without signs of aggregation. Moreover, the particle shape has remained unchanged during one month in which the samples were kept at 4 °C protected from light, demonstrating the ability of these carriers in keeping their structure intact during storage, in accordance with the data obtained from the characterization analyses (particle dimension, zeta potential, and encapsulation efficiency) reported in Section 3.2.4.
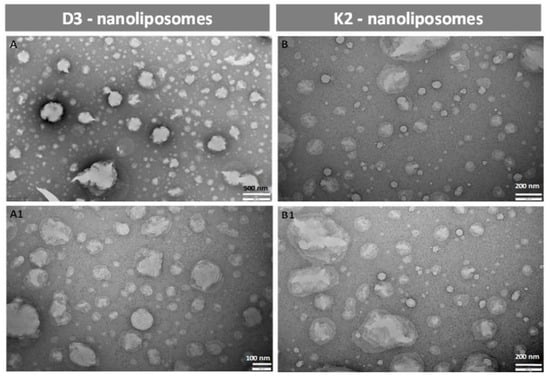
Figure 2.
Transmission electron microscopy (TEM) images of D3 and K2 nanoliposomes fresh samples (A and B, respectively) and after 1 month of storage (A1 and B1, respectively).
3.2.2. Size and Zeta Potential
The ability of liposomes to penetrate the stratum corneum enhancing the skin bioavailability of drug substances mostly depends on the physicochemical properties of these carriers. In particular, their nanometric size ensures a closer contact with the SC, thus increasing the amount of incorporated active ingredients reaching the site of action [45]. In general, the mechanisms proposed for nanoliposomes penetration through the skin are different: liposome components can act as penetration-enhancing factors which increase the permeability of the SC to the active molecules; vesicles “fuse” with the lipid components of the SC; vesicles penetrate intact into skin layers; liposomes penetrate through follicle invaginations (called the shunt route) [46]. According to the shunt route via follicles, which seems to be the preferred pathway of nanoparticle skin penetration, if vesicles are of nanometric size, they can reach the infundibulum where they accumulate and act as a drug reservoir: it was observed an increased follicular penetration by particles smaller than 300 nm [47]. A size-dependent penetration of nanoparticles into/through the cutaneous barrier was also demonstrated by Alvarez-Romàn and collaborators who have shown that carriers (20–200 nm size range) accumulated preferentially in the follicular openings, and their follicular localization is favored by smaller particle size [48].
In this work, for all the four formulations tested, liposomes of nanometric size were obtained through the simil-microfluidic method. In Table 1, the results for the analysis of size (numerical and Z-average) and PDI are shown. It can be observed that the numerical size value of unloaded nanoliposomes (about 90 nm) is similar to that of the vitamin D3-, K2-, E-, and curcumin-loaded forms (p > 0.05) which are about 87 nm, 145 nm, 118 nm, and 84 nm in size, respectively, showing the repeatability of the production process. The zeta average values of vitamin D3- and curcumin-loaded liposomes are slightly higher than those of the other formulations (p <0.05) demonstrating the possible formation of some aggregate. This is more evident for curcumin-loaded vesicles which also have a PDI (0.67) higher than that of the other formulations (0.31–0.38 PDI range). Apart from the possible liposomes aggregation, a more heterogeneous formulation can be explained by the perpendicular orientation of curcumin with respect to the normal bilayer that creates more space between lipids of vesicles, allowing water molecules to enter into the lipid bilayers as reported in [49].

Table 1.
Numerical size, Z-average, polydispersity index (PDI), and zeta potential of unloaded vesicles and nanoliposomes loaded with vitamin D3, vitamin K2, vitamin E, and curcumin at time zero and after 1 month storage (aged samples) at 4 °C. Results are expressed as average of three determinations and reported along with the standard deviation (SD).
In general, good dimensional properties have been obtained for all the tested formulations which, due to their nanometric size, can be considered valid carriers in penetrating skin layers for cosmetic/cosmeceutical applications.
In addition to the dimension, the surface charge of liposomes is another key feature influencing the transdermal drug delivery of lipid vesicles. In a work of Gillet and collaborators, the penetration-enhancing ability of charged liposomes was tested ex vivo using the pig ear skin as a model membrane. In particular, they demonstrated that negatively charged liposomes significantly enhance the skin crossing of betamethasone (used as model drug) in which penetration into the epidermis was increased 9.3 times compared with the free drug, 2.5 times compared with neutral liposomes, and 2.7 times compared with positively charged vesicles [50]. A penetration-dependent behavior was also studied by Sinico and coworkers for tretinoin (TRA)-loaded liposomes, finding that negatively charged liposomes strongly improve newborn pig skin hydration and TRA retention in SC [51].
The zeta potential also determines whether dispersions are stable or destined to aggregate. Generally, zeta potential absolute values above the 30 mV limit are required for particle stability [52,53]. In that regard, the obtained results show zeta potential values of about −35 mV, −38.5 mV, −36 mV, and −26.5 for unloaded and vitamin D3-, vitamin K2-, and vitamin E-loaded vesicles, respectively, indicating that no significant change occurred on charge value of liposomes after the encapsulation of antioxidant compounds, which, due to their high lipophilicity, are completely entrapped in the liposomal bilayer structure (Table 1) [54,55]. Curcumin-nanoliposomes, instead, have a less negative zeta potential value (−18 mV) than those of the other formulations. This may be due to the fact that curcumin is localized in the hydrophobic acyl chain region very close to the glycerol group of the lipids of the liposome bilayer [56]. In particular, curcumin, having two hydroxyl groups (one at each end of the molecule), interacts favorably both with water oxygen and lipid headgroup oxygen atoms, continuously fluctuating from the center to the outer interface of the liposomal membrane [49]. It can be supposed that the portion of curcumin at the interface between the lipid bilayer and the external aqueous solution, orienting so as to make hydrogen bonds with bulk water, can cover the negative charge of PC, increasing vesicles’ zeta potential.
Overall, it can be stated that the produced antioxidant-loaded vesicles, formulated by using PC and CHOL as lipid components, are homogeneous dispersions and potential efficient carriers in penetrating the cutaneous barrier due to their nanometric size and negative superficial charge.
3.2.3. Encapsulation Efficiency and Effective Load
The preparation method of liposome is one of the key factor affecting the incorporation efficiency of active materials. In this work, through the simil-microfluidic apparatus, loaded nanoliposomes characterized by high antioxidant encapsulation efficiencies and elevated loads were achieved. Theoretical and effective loads and encapsulation efficiency experienced on fresh and aged samples are summarized in Table 2.

Table 2.
Vitamin D3-, vitamin K2-, vitamin E-, and curcumin-loaded nanoliposome characterization in terms of theoretical load, effective load, and encapsulation efficiency (e.e.) at time zero and after 1 month storage (aged samples) at 4 °C. Measured data are expressed as average of three determinations and reported along with the standard deviation (SD).
In particular, encapsulation efficiencies of about 88%, 95%, 93%, and 98% were achieved for vitamin D3, K2, E, and curcumin nanoliposomes, respectively, with effective loads higher than 9%. The results obtained are in agreement with several literature works in which elevated incorporation efficiencies were also found for the antioxidants here explored. Some examples are the 86% e.e. found by Bi and collaborators for vitamin D3 entrapped in liposomal vesicles about 100 nm in size to be used as an anti-aging agent for the skin [16]; a 97% e.e. found by Qu and coworkers for vitamin E encapsulated in nanoliposomes about 230 nm in size to be embedded into a chitosan hydrogel and used as a tissue-engineered scaffold [57]; an 82% e.e. of curcumin obtained by Chen and collaborators for about 82 nm liposomal vesicles to be used as transdermal drug delivery systems [27]. The congruence of the obtained encapsulation efficiencies with those found in the scientific literature highlights the robustness and reliability of the new simil-microfluidic nanotechnology that permits to obtain the same high liposomal antioxidant entrapment efficiencies while allowing their massive production.
3.2.4. Stability
Nanoliposomes produced by the simil-microfluidic method were maintained at 4 °C for one month in order to test their stability during storage. For all the formulations produced, thus with the proposed formulations, the numerical size, Z-average, PDI, and zeta potential values were kept unchanged (p > 0.05) with respect to those found at time zero (Table 1), revealing the production of highly stable vesicles containing antioxidants. As reported above, TEM images of several samples also showed the morphology preservation (Section 3.2.1).
Moreover, after one month, vitamin leakages were observed by monitoring the encapsulation efficiencies parameter. All the kinds of loaded liposomal suspensions (i.e., liposomes with vitamin D3, K2, E, and curcumin) did not change their e.e. with respect to those assayed for the fresh samples (p > 0.05), confirming the ability of these carriers to keep their content intact during storage (Table 2).
4. Conclusions
Nanoliposomes provide new opportunities for cosmetic dermatology reversibly modulating the skin barrier normally hardly accessible allowing the penetration of active ingredients across the stratum corneum. Albeit widely exploited by giant cosmetic companies to ameliorate the characteristics of their products, the production of these delivery systems is actually based on conventional methods characterized by low production yields and drastic process conditions. To cope with these problems, in this work, a high-yield semi continuous method, based on microfluidic principles transposed on a millimeter scale, was developed for the production of nanoliposomes containing vitamin D3, K2, E, and curcumin antioxidants to be used in topical formulations. Pointing out that the nanometric dimension and the negative superficial charge are the main features improving liposomes skin crossing performance, highly penetrating antioxidant carriers have been produced, with negative zeta potential values of −38.5 mV, −36 mV, −26.5 mV, and −18 mV for vitamin D3, K2, E, and curcumin vesicles, respectively, and nanometric dimensions of about 87 nm, 145 nm, 118 nm, and 84 nm, respectively, for the loaded vesicles in the previous order.
Moreover, stable and highly loaded vesicles with elevated encapsulation efficiencies (88%, 95%, 93%, and 98% e.e. for vitamin D3, K2, E, and curcumin, respectively) were obtained. The simil-microfluidic method allowed the one-step production of 220 mL antioxidant vesicle suspension in just 5 min through a sustainable, economic, and highly productive process of high potential interest for cosmetic companies.
5. Patent
Barba, A.A., Lamberti, G., D’amore, M., Bochicchio, S., Dalmoro, A., 2018. Process for Preparing Nanoliposomes Comprising Micronutrients and Food Products Comprising Said Nanoliposomes, Italy, WO2019049186.
Supplementary Materials
The following are available online at https://www.mdpi.com/2079-9284/7/2/22/s1.
Author Contributions
Conceptualization: A.A.B. and S.B.; methodology: S.B., A.D., V.D.S.; investigation: S.B., A.D., V.D.S. and P.B.; data curation: A.D., V.D.S.; writing—original draft preparation: S.B.; writing—review and editing S.B., A.A.B.; supervision: A.A.B., G.L.; funding acquisition: A.A.B., G.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
The authors thank the Centro di Microscopia Elettronica–University of Trieste, Italy.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kaul, S.; Gulati, N.; Verma, D.; Mukherjee, S.; Nagaich, U. Role of nanotechnology in cosmeceuticals: A review of recent advances. J. Pharm. 2018, 2018. [Google Scholar] [CrossRef] [PubMed]
- Fakhravar, Z.; Ebrahimnejad, P.; Daraee, H.; Akbarzadeh, A. Nanoliposomes: Synthesis methods and applications in cosmetics. J. Cosmet. Laser Ther. 2016, 18, 174–181. [Google Scholar] [CrossRef]
- Rosen, J.; Landriscina, A.; Friedman, A.J. Nanotechnology-based cosmetics for hair care. Cosmetics 2015, 2, 211–224. [Google Scholar] [CrossRef]
- Lin, L.L.; Nufer, K.L.; Tomihara, S.; Prow, T.W. Non-invasive nanoparticle imaging technologies for cosmetic and skin care products. Cosmetics 2015, 2, 196–210. [Google Scholar] [CrossRef]
- Katz, L.M.; Dewan, K.; Bronaugh, R.L. Nanotechnology in cosmetics. Food Chem. Toxicol. 2015, 85, 127–137. [Google Scholar] [CrossRef]
- Landriscina, A.; Rosen, J.; Friedman, A.J. Nanotechnology, inflammation and the skin barrier: Innovative approaches for skin health and cosmesis. Cosmetics 2015, 2, 177–186. [Google Scholar] [CrossRef]
- Morganti, P.; Palombo, M.; Tishchenko, G.; Yudin, V.E.; Guarneri, F.; Cardillo, M.; Del Ciotto, P.; Carezzi, F.; Morganti, G.; Fabrizi, G. Chitin-hyaluronan nanoparticles: A multifunctional carrier to deliver anti-aging active ingredients through the skin. Cosmetics 2014, 1, 140–158. [Google Scholar] [CrossRef]
- UNION, P. Regulation (EC) No 1223/2009 of the european parliament and of the council. Off. J. Eur. Union L 2009, 342, 59. [Google Scholar]
- Preud’homme, L.; Depues, A.; Noiset, S. Cosmetic regulatory writing. Med. Writ. 2014, 23, 186–189. [Google Scholar] [CrossRef]
- SCCS. Guidance on the Safety Assessment of Nanomaterials in Cosmetics. 2012. Available online: https://ec.europa.eu/health/sites/health/files/scientific_committees/consumer_safety/docs/sccs_o_233.pdf (accessed on 2 April 2020).
- Ansell, J.; Rauscher, H. Report of the Joint Regulator-Industry Ad Hoc Working Group: Currently Available Methods for Characterization of Nanomaterials; International Cooperation on Cosmetics Regulation (ICCR-5): Paris, France, 2011; Available online: http://ec.europa.eu/consumers/sectors/cosmetics/files/pdf/iccr5_char_nano_en.pdf (accessed on 2 April 2020).
- Zoghi, A.; Khosravi-Darani, K.; Omri, A. Process variables and design of experiments in liposome and nanoliposome research. Mini Rev. Med. Chem. 2018, 18, 324–344. [Google Scholar] [CrossRef]
- Li, M.; Du, C.; Guo, N.; Teng, Y.; Meng, X.; Sun, H.; Li, S.; Yu, P.; Galons, H. Composition design and medical application of liposomes. Eur. J. Med. Chem. 2019, 164, 640–653. [Google Scholar] [CrossRef] [PubMed]
- Ashtiani, H.R.A.; Bishe, P.; Lashgari, N.A.; Nilforoushzadeh, M.A.; Zare, S. Liposomes in cosmetics. J. Skin Stem Cell 2016, 3, e65815. [Google Scholar] [CrossRef]
- Salvetová, E.; Muthný, T. Delivery systems in cosmetics. Chemistry 2014, 9, 3. [Google Scholar]
- Bi, Y.; Xia, H.; Li, L.; Lee, R.J.; Xie, J.; Liu, Z.; Qiu, Z.; Teng, L. Liposomal Vitamin D3 as an Anti-Aging Agent for the Skin. Pharmaceutics 2019, 11, 311. [Google Scholar] [CrossRef] [PubMed]
- Pamunuwa, G.; Karunaratne, V.; Karunaratne, D. Effect of lipid composition on in vitro release and skin deposition of curcumin encapsulated liposomes. J. Nanomater. 2016, 2016. [Google Scholar] [CrossRef]
- Goto, S.; Setoguchi, S.; Yamakawa, H.; Watase, D.; Terada, K.; Matsunaga, K.; Karube, Y.; Takata, J. Prodrugs for Skin Delivery of Menahydroquinone-4, an Active Form of Vitamin K2 (20), Could Overcome the Photoinstability and Phototoxicity of Vitamin K2 (20). Int. J. Mol. Sci. 2019, 20, 2548. [Google Scholar] [CrossRef]
- Vinardell, M.P.; Mitjans, M. Nanocarriers for delivery of antioxidants on the skin. Cosmetics 2015, 2, 342–354. [Google Scholar] [CrossRef]
- Dimitrova, J. Therapeutic efficacy of local choleclaciferol in facial seborrheic dermatitis. Proc. ARSA-Adv. Res. Sci. Areas 2016. [Google Scholar] [CrossRef]
- Philips, N.; Ding, X.; Kandalai, P.; Marte, I.; Krawczyk, H.; Richardson, R. The Beneficial Regulation of Extracellular Matrix and Heat Shock Proteins, and the Inhibition of Cellular Oxidative Stress Effects and Inflammatory Cytokines by 1α, 25 dihydroxyvitaminD3 in Non-Irradiated and Ultraviolet Radiated Dermal Fibroblasts. Cosmetics 2019, 6, 46. [Google Scholar] [CrossRef]
- Hemmati, A.A.; Houshmand, G.; Ghorbanzadeh, B.; Nemati, M.; Behmanesh, M.A. Topical vitamin K1 promotes repair of full thickness wound in rat. Indian J. Pharmacol. 2014, 46, 409. [Google Scholar]
- Keen, M.A.; Hassan, I. Vitamin E in dermatology. Indian Dermatol. Online J. 2016, 7, 311. [Google Scholar] [CrossRef] [PubMed]
- Addor, F.A.S.A. Antioxidants in dermatology. An. Bras. Dermatol. 2017, 92, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Aziz, A.A.; Taher, Z.M.; Muda, R.; Aziz, R. Cosmeceuticals and Natural Cosmetics; Recent Trends in Research into Malaysian Medicinal Plants Research; Penerbit UTM Press: Johor, Malaysia, 2017; pp. 126–175. [Google Scholar]
- Panahi, Y.; Fazlolahzadeh, O.; Atkin, S.L.; Majeed, M.; Butler, A.E.; Johnston, T.P.; Sahebkar, A. Evidence of curcumin and curcumin analogue effects in skin diseases: A narrative review. J. Cell. Physiol. 2019, 234, 1165–1178. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wu, Q.; Zhang, Z.; Yuan, L.; Liu, X.; Zhou, L. Preparation of curcumin-loaded liposomes and evaluation of their skin permeation and pharmacodynamics. Molecules 2012, 17, 5972–5987. [Google Scholar] [CrossRef]
- Fuller, B. Role of PGE-2 and Other Inflammatory Mediators in Skin Aging and Their Inhibition by Topical Natural Anti-Inflammatories. Cosmetics 2019, 6, 6. [Google Scholar] [CrossRef]
- Gyamera, B.; Kim, Y.-H. Preparation and Characterization of Liposomes Containing Green Tea and Roselle Extracts to be Used in Cosmetics. J. Int. Dev. Coop. 2019, 14, 131–160. [Google Scholar] [CrossRef]
- Panahi, Y.; Farshbaf, M.; Mohammadhosseini, M.; Mirahadi, M.; Khalilov, R.; Saghfi, S.; Akbarzadeh, A. Recent advances on liposomal nanoparticles: Synthesis, characterization and biomedical applications. Artif. Cells Nanomed. Biotechnol. 2017, 45, 788–799. [Google Scholar] [CrossRef]
- Costa, R.; Santos, L. Delivery systems for cosmetics-From manufacturing to the skin of natural antioxidants. Powder Technol. 2017, 322, 402–416. [Google Scholar] [CrossRef]
- Heinrich, H.; Ursula, F. Improved Liposomal Formulations of Lipophilic Compounds; European Patent Office: Munich, Germany, 2010. [Google Scholar]
- Imanaka, H.; Ando, H.; Makino, T. Skin-Whitening Cosmetic. Google Patents US006669932B2, 30 December 2003. [Google Scholar]
- Barba, A.A.; Bochicchio, S.; Dalmoro, A.; Caccavo, D.; Cascone, S.; Lamberti, G. Polymeric and Lipid-Based Systems for Controlled Drug Release: An Engineering Point of View. In Nanomaterials for Drug Delivery and Therapy; Elsevier: Amsterdam, The Netherlands, 2019; pp. 267–304. [Google Scholar]
- Van Tran, V.; Moon, J.-Y.; Lee, Y.-C. Liposomes for delivery of antioxidants in cosmeceuticals: Challenges and development strategies. J. Control. Release 2019, 300, 114–140. [Google Scholar] [CrossRef]
- Carugo, D.; Bottaro, E.; Owen, J.; Stride, E.; Nastruzzi, C. Liposome production by microfluidics: Potential and limiting factors. Sci. Rep. 2016, 6, 25876. [Google Scholar] [CrossRef]
- Bochicchio, S.; Dalmoro, A.; Recupido, F.; Lamberti, G.; Barba, A.A. Nanoliposomes production by a protocol based on a simil-microfluidic approach. In Advances in Bionanomaterials; Springer: Berlin, Germany, 2018; pp. 3–10. [Google Scholar]
- Bochicchio, S.; Dalmoro, A.; Bertoncin, P.; Lamberti, G.; Moustafine, R.I.; Barba, A.A. Design and production of hybrid nanoparticles with polymeric-lipid shell–core structures: Conventional and next-generation approaches. RSC Adv. 2018, 8, 34614–34624. [Google Scholar] [CrossRef]
- Dalmoro, A.; Bochicchio, S.; Nasibullin, S.F.; Bertoncin, P.; Lamberti, G.; Barba, A.A.; Moustafine, R.I. Polymer-lipid hybrid nanoparticles as enhanced indomethacin delivery systems. Eur. J. Pharm. Sci. 2018, 121, 16–28. [Google Scholar] [CrossRef] [PubMed]
- Dalmoro, A.; Bochicchio, S.; Lamberti, G.; Bertoncin, P.; Janssens, B.; Barba, A.A. Micronutrients encapsulation in enhanced nanoliposomal carriers by a novel preparative technology. RSC Adv. 2019, 9, 19800–19812. [Google Scholar] [CrossRef]
- Melo, A.; Amadeu, M.S.; Lancellotti, M.; Hollanda, L.M.D.; Machado, D. The role of nanomaterials in cosmetics: National and international legislative aspects. Quím. Nova 2015, 38, 599–603. [Google Scholar] [CrossRef]
- Sharma, N.; Singh, S.; Kanojia, N.; Grewal, A.S.; Arora, S. Nanotechnology: A Modern Contraption in Cosmetics and Dermatology. Appl. Clin. Res. Clin. Trials Regul. Aff. 2018, 5, 147–158. [Google Scholar] [CrossRef]
- Niemiec, S.; Nystrand, G.; Wang, J. Method of Manufacturing Liposomes. Google Patents 462,218,8, 11 November 1986. [Google Scholar]
- Hood, R.R.; Kendall, E.L.; DeVoe, D.L.; Quezado, Z.; Junqueira, M.; Finkel, J.C.; Vreeland, W.N. Microfluidic formation of nanoscale liposomes for passive transdermal drug delivery. In Proceedings of the 2013 Microsystems for Measurement and Instrumentation: Fulfilling the Promise (MAMNA), Gaithersburg, MD, USA, 14 May 2013. [Google Scholar]
- Morganti, P. Use and potential of nanotechnology in cosmetic dermatology. Clin. Cosmet. Investig. Dermatol. CCID 2010, 3, 5. [Google Scholar] [CrossRef]
- El Maghraby, G.M.; Barry, B.W.; Williams, A.C. Liposomes and skin: From drug delivery to model membranes. Eur. J. Pharm. Sci. 2008, 34, 203–222. [Google Scholar] [CrossRef]
- Bolzinger, M.-A.; Briançon, S.; Pelletier, J.; Chevalier, Y. Penetration of drugs through skin, a complex rate-controlling membrane. Curr. Opin. Colloid Interface Sci. 2012, 17, 156–165. [Google Scholar] [CrossRef]
- Alvarez-Román, R.; Naik, A.; Kalia, Y.N.; Guy, R.H.; Fessi, H. Skin penetration and distribution of polymeric nanoparticles. J. Control. Release 2004, 99, 53–62. [Google Scholar] [CrossRef]
- Jalili, S.; Saeedi, M. Study of curcumin behavior in two different lipid bilayer models of liposomal curcumin using molecular dynamics simulation. J. Biomol. Struct. Dyn. 2016, 34, 327–340. [Google Scholar] [CrossRef]
- Gillet, A.; Compère, P.; Lecomte, F.; Hubert, P.; Ducat, E.; Evrard, B.; Piel, G. Liposome surface charge influence on skin penetration behaviour. Int. J. Pharm. 2011, 411, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Sinico, C.; Manconi, M.; Peppi, M.; Lai, F.; Valenti, D.; Fadda, A.M. Liposomes as carriers for dermal delivery of tretinoin: In vitro evaluation of drug permeation and vesicle–skin interaction. J. Control. Release 2005, 103, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Duman, G.; Aslan, İ.; Yekta Özer, A.; İnanç, İ.; Taralp, A. Liposome, gel and lipogelosome formulations containing sodium hyaluronate. J. Liposome Res. 2014, 24, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Budai, L.; Kaszás, N.; Gróf, P.; Lenti, K.; Maghami, K.; Antal, I.; Klebovich, I.; Petrikovics, I.; Budai, M. Liposomes for topical use: A physico-chemical comparison of vesicles prepared from egg or soy lecithin. Sci. Pharm. 2013, 81, 1151–1166. [Google Scholar] [CrossRef] [PubMed]
- Ausili, A.; Clemente, J.; Pons-Belda, Ó.D.; deGodos, A.M.; Corbalan-Garcia, S.; Torrecillas, A.; Teruel, J.A.; Gomez-Fernandez, J.C. Interaction of vitamin K1 and vitamin K2 with DMPC and their location in the membrane. Langmuir 2020, 36, 1062–1073. [Google Scholar] [CrossRef]
- Mohammadi, M.; Ghanbarzadeh, B.; Hamishehkar, H. Formulation of nanoliposomal vitamin D3 for potential application in beverage fortification. Adv. Pharm. Bull. 2014, 4 (Suppl. 2), 569. [Google Scholar]
- Karewicz, A.; Bielska, D.; Gzyl-Malcher, B.; Kepczynski, M.; Lach, R.; Nowakowska, M. Interaction of curcumin with lipid monolayers and liposomal bilayers. Colloids Surf. B Biointerfaces 2011, 88, 231–239. [Google Scholar] [CrossRef]
- Qu, Y.; Tang, J.; Liu, L.; Song, L.; Chen, S.; Gao, Y. α-Tocopherol liposome loaded chitosan hydrogel to suppress oxidative stress injury in cardiomyocytes. Int. J. Biol. Macromol. 2019, 125, 1192–1202. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).