Abstract
Androgenetic alopecia (AGA) is a multifactorial and age-related condition characterized by substantial hair loss affecting both men and women. Conventional treatments include the use of topical minoxidil (MNX) formulations to stimulate hair growth and restore hair condition. However, those treatments are associated with limited performance and a lack of tolerability and compliance due to the emergence of adverse effects. Considering that the development of nanotechnology-based formulations as hair loss therapeutic strategies has been clearly growing, topical MNX delivery by means of these innovative formulations is known to enhance MNX skin permeation and depot formation into hair follicles, allowing for MNX-controlled release, increased MNX skin bioavailability and enhanced therapeutic efficacy with minimal adverse effects. This review highlights the potential of nanotechnology-based MNX delivery formulations for improved hair loss therapeutics, including a thorough assessment of their in vitro and in vivo performances, as well as regulatory and nanosafety considerations.
1. Introduction
Androgenetic alopecia (AGA) is a common hair-related disorder characterized by progressive hair loss patterns, affecting genetically predisposed men and women to dihydrotestosterone (DHT), a hormone responsible for follicular growth decrease [1,2]. AGA incidence is known to be age-associated, affecting 58% of men ca. 50 years old, and 73% of men and 57% women above the age of 80 years [2,3]. Even though AGA is a non-lethal disease, it is frequently associated with concerning psychological and social concerns [3,4]. The pathologic mechanism of AGA is not fully understood yet; however, it is known that AGA promotes the miniaturization of the hair follicles, diminishes the hair density and consequently enhances the emergence of vellus hair [1]. This mechanism is the result of the anagen phase’s shortening, leading to both a decrease in hair density (and the number of the hair follicles) and in hair follicle diameter (decreased thickness) [5]. Considering AGA treatment, four essential therapeutic groups can be outlined: growth factors, inhibitors of 5α- reductase (the hormone responsible for the transition of testosterone into DHT) and ATP-sensitive potassium channel agonists and antagonists of the androgen receptor [3]. Minoxidil (MXD) is a pyrimidine derivative and a peripheral vasodilator that appeared in clinical practice in the 1970s, and is able to decrease the blood pressure through the reduction of peripheral vascular resistance. It has been used as an antihypertensive drug when conventional therapy is not a viable option, owing to its vasodilating properties [6,7]. Additionally, MXD is also extensively used topically for AGA therapy, exerting its action as a potassium channel opener, releasing nitric oxide and increasing blood flow into the hair follicles, modifying the prostaglandins’ D2 and E2 pathways, thus supressing the pathway involved in AGA development [6]. Therefore, it has the ability to prolong the anagen phase in the hair follicles with efficient anti-hair loss results [4,8]. In fact, considering that several adenosine receptors are expressed in dermal papilla cells (DPCs), a study reported the relevance of adenosine as a mediator for MNX-induced vascular endothelial growth factor (VEGF) production in DPCs and further hair growth stimulation [9]. MXD has low water solubility, in keeping with its traditional topical formulations based in solutions comprising ethanol and propylene glycol, which, due to ethanol evaporation and consequent formation of MXD crystals, are responsible for adverse skin effects, including pruritus, rash, dandruff and allergic contact dermatitis [1,8,10]. The rise of adverse effects leads to treatment discontinuity and hinders patients’ compliance with the treatment, which limits the therapeutic success [1]. Considering that the absorption of MXD is regarded to be higher through the topical route, novel and suitable formulations are needed for MNX topical delivery [3,11]. The skin, besides functioning as a biological barrier for protecting the body against external aggressions and regulating key internal homeostatic mechanisms, consists of a particular route for drug delivery owing to the pronounced surface area and different delivery routes available. Both dermal and follicular drug delivery are the two main routes allowing drug localized effects, whereas transdermal delivery is aimed at systemic drug effects [12,13]. Skin drug delivery comprises the skin application of a drug-containing formulation destined at either penetrating and/or permeating through the skin layers, or achieving directly deeper skin strata penetration via the skin appendages. This comprises a potential alternative for systemic drug therapeutics, as well as the possibility of decreasing the overall necessary drug dose regarding skin conditions/diseases, and an effective way of reducing off-target adverse effects related to systemic drug administration [13]. Topical delivery of drugs often requires skin permeation, which depends on the drug’s lipophilic characteristics, as the presence of the stratum corneum restrains the drugs’ permeation [14]. Skin permeation can be achieved through other skin-related pathways, such as the hair follicle pathway (Figure 1) [15]. Skin permeation through the hair follicles is known as the transappendageal pathway, as hair appendages allow the drug’s absorption from the skin surface through ducts by an uninterrupted diffusion pathway, thus avoiding the stratum corneum barrier [16]. Hence, interest in the delivery of MXD through hair follicles for AGA therapeutic treatment has arisen in recent years, due to the hair follicle’s ability to supply a direct pathway for skin permeation [14]. The rationale for improved drug delivery via skin by means of nanotechnology-based formulations has to do with the small size and increased surface area of nanoparticles, which enable a close and extended contact with the stratum corneum. Moreover, the possibility of controlled drug release patterns allows for a drug controlled diffusion and deeper penetration into skin strata, minimizing both the required drug dosage and drug losses, as well as reducing topical adverse effects while increasing therapeutic efficacy [6,17]. Particularly, hair follicle-mediated nano-based delivery increase drug penetration and deposition into deeper skin layers [18], as drug-loaded nanoparticles can accumulate in hair follicles and favor drug penetration and release in deeper skin strata. Moreover, such hair follicles may function as drug reservoirs (drug depot formation) for controlled drug release, reducing adverse effects and enhancing therapeutic compliance [5,19] (Figure 1).
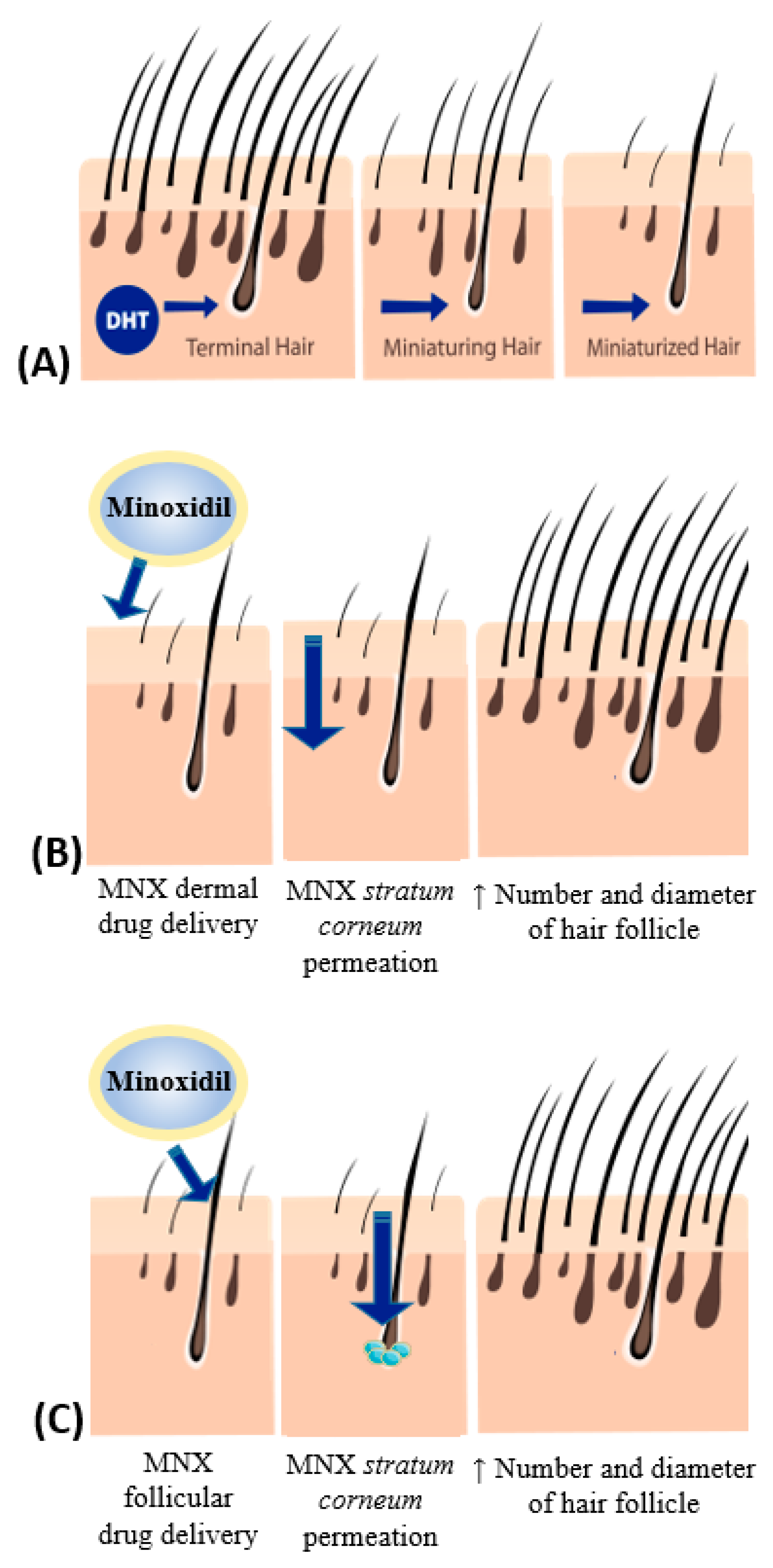
Figure 1.
Schematic illustration of: (A) hair miniaturization by dihydrotestosterone (DHT); (B) minoxidil (MNX) topical delivery pathway via MNX permeation through stratum corneum; (C) MNX topical delivery pathway via MNX penetration into the hair follicles with depot formation.
Additionally, preferential permeation of nanoparticles into the hair follicles when compared to the stratum corneum yields higher deposition and long-term accumulation of the drug, together with the ease of penetration related to the lack of differentiation and reduced size of interfollicular corneocytes. Besides local skin delivery, and once the basal section of the hair follicle is densely irrigated, this follicular delivery route could constitute a shortcut for systemic drug delivery [18]. The main nano-based formulations for topical drug delivery include natural and synthetic polymer-based nanoparticles and dendrimers; lipid-based nanoparticles, such as liposomes and lipid nanoparticles (solid lipid nanoparticles and nanostructured lipid carriers); and inorganic nanoparticles, such as metallic and silica nanoparticles [13]. MXD-loaded nanotechnology-based formulations in vitro and in vivo studies account for applications in vertical diffusion models, in order to characterize MXD’s skin permeation, and topical administration in laboratory animals for assessing pharmacokinetic/ pharmacodynamic profiles, respectively. However, for a better preclinical assessment of MXD-loaded nanotechnology-based formulations, concise regulation is required in order to provide guidance for the evaluation of toxic effects, especially long term, allowing us to establish their safety profiles (Figure 2).
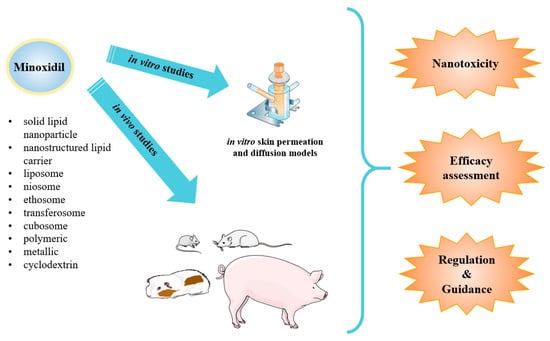
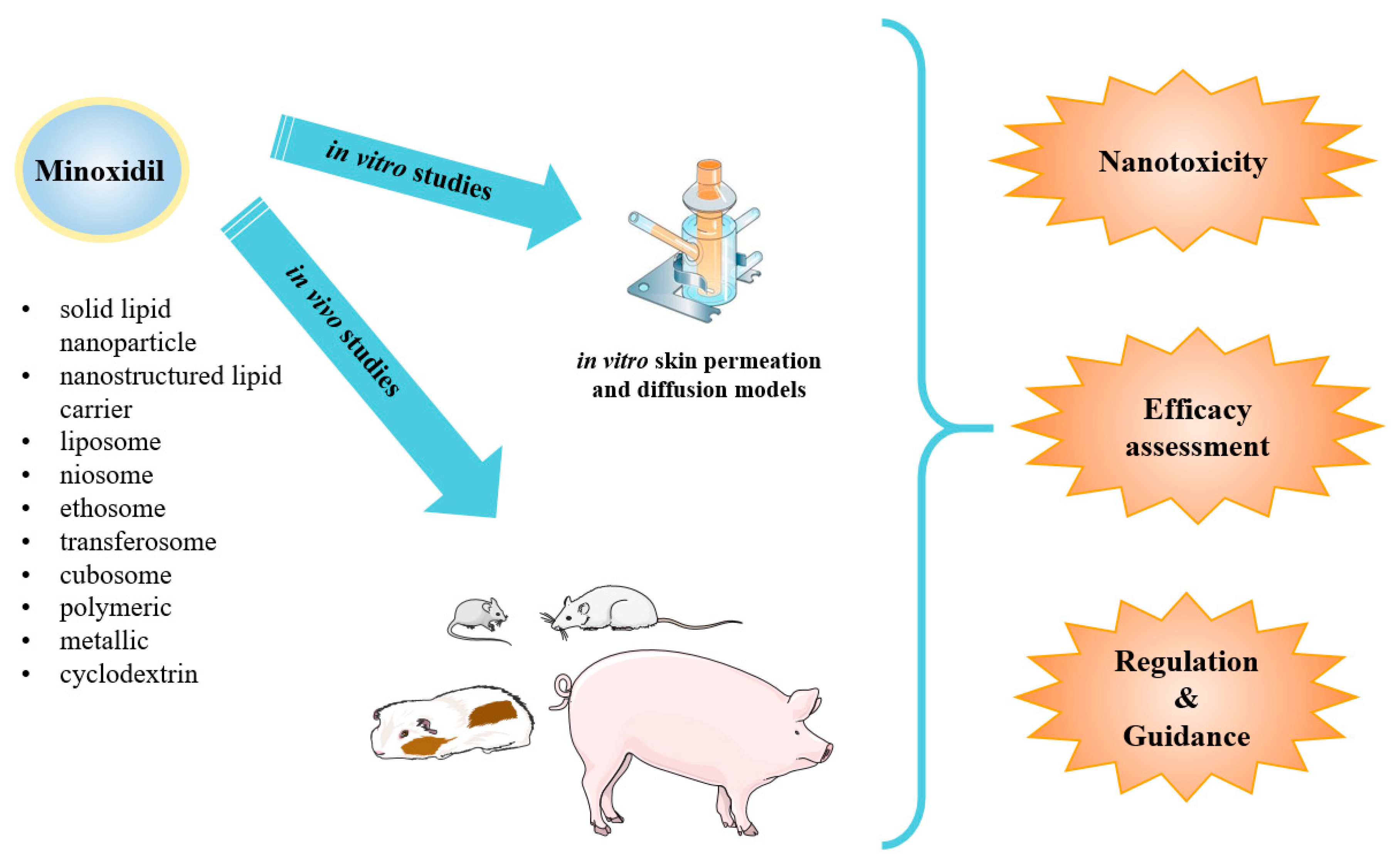
Figure 2.
Schematic presentation of minoxidil nanoparticle types applied as topical drug delivery systems for androgenic alopecia developed so far. Such systems have been submitted to in vitro and in vivo studies for toxicity, safety and efficacy profiles assessment under the regulation and guidance applied nowadays.
The main aim of this review is to describe and critically analyze the drug delivery strategies applied in AGA, with a focus on MXD topical delivery systems, including conventional and, particularly, nanotechnology-based formulations. Additionally, an overview of inherent in vitro and in vivo studies will be undertaken, as well as MXD toxicity issues and the regulatory affairs required for development and successful market introduction.
2. Minoxidil Topical Delivery Systems
2.1. Formulation Requirements for Topical Delivery of Minoxidil
The pursuit of drug delivery through the skin has been increasing in recent years due to the ability to provide both topical and systemic effects [17]. Conventional topical delivery of MXD is in part restrained by the skin’s physical barrier, which hampers MXD skin permeation through the skin layers. This requires higher daily doses that may result in the appearance of adverse effects [20]. Nevertheless, the topical MXD delivery by using nanotechnology-based formulations offers several advantages: (1) it avoids drug first passage metabolism, (2) allows the controlled release of MXD within the therapeutic window, without the rise of off-target adverse effects, thus (3) increasing the therapeutic adherence to the therapy, (4) as well as improving MXD’s overall effectiveness [20,21].
2.2. Conventional Formulations for Topical Delivery of Minoxidil
Conventional formulations for topical MXD delivery comprise 5% minoxidil solution for hair application [22], which is associated with high levels of drug loss during the application procedure and low patient compliance to the treatment, which ultimately may result in the unreliable control of the dosage [23]. Additionally, the use of ethanol or propylene glycol-based solutions for conventional MXD formulations, manufactured to increase MXD’s water solubility, can be associated with adverse skin effects, which may result from MXD’s reversion into crystal form upon the solvents’ evaporation [10]. The crystals of MXD are imparted and result in the emergence of pruritus, rash, dandruff and allergic contact dermatitis, which altogether significantly restrain AGA therapeutics [8,11]. Therefore, new topical formulations are required in order to increase MXD’s residence time on the scalp’s skin and hair follicle, thus decreasing the number of applications per day [23]. Among novel topical formulations, nanotechnology-based formulations have emerged as robust strategies for AGA management, owing to: (1) improved local accumulation of MXD; (2) minimized dermatological adverse effects and (3) the ability to permeate the stratum corneum [17,23,24].
2.3. Nanotechnology-Based Formulations for Topical Minoxidil Delivery
So far, nanotechnology has enabled the development of drug delivery systems that are able to increase (1) MXD’s stability, (2) skin pharmacokinetic and pharmacodynamics profiles, (3) permeation and formation of skin depots (4) therapeutic adherence, as well as (5) the decrease in MXD’s toxicity and treatment resistance [25,26] (Figure 3).
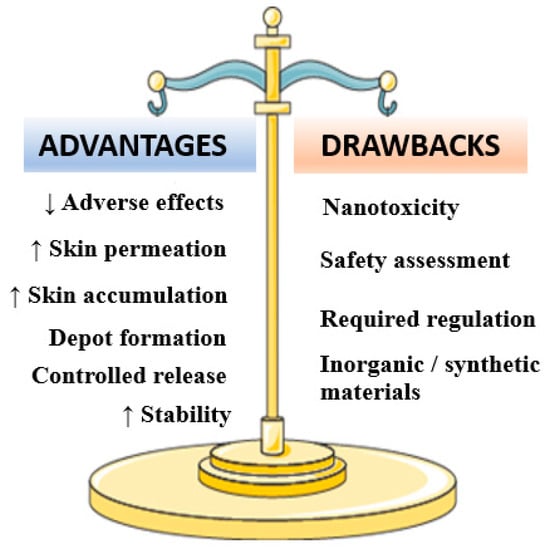
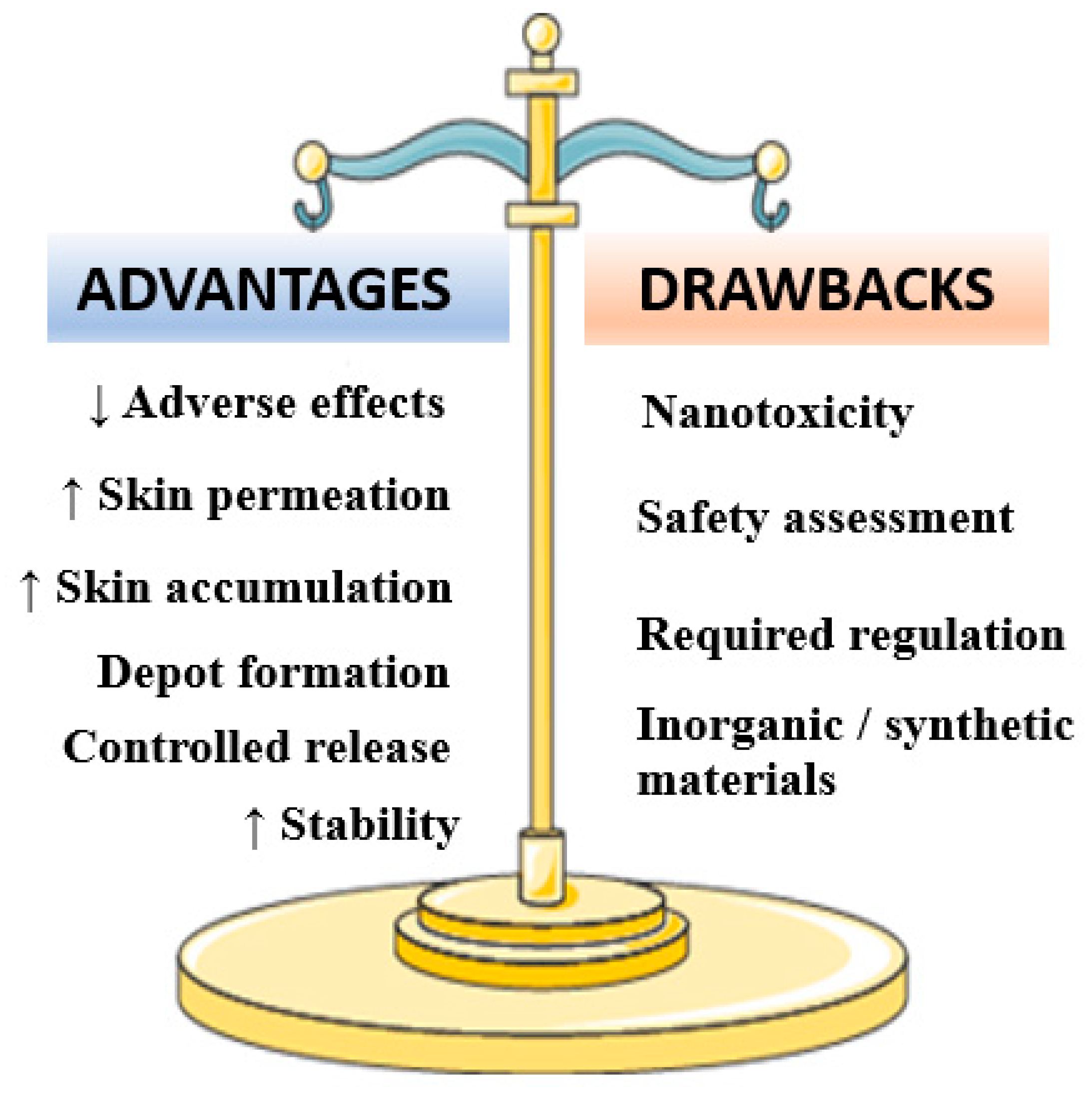
Figure 3.
Major advantages and drawbacks associated to minoxidil-loaded nanotechnology-based formulations.
Hence, nanotechnology-based formulations offer the possibility of smart MXD delivery at lower doses with maximized therapeutic effects and a more favorable release profile. MXD-loaded nanotechnology-based formulations include: (1) lipid, (2) polymer and (3) inorganic nanotechnology-based formulations. Table 1 shows the nanotechnology-based formulations developed or studied so far for MXD’s application in AGA, regarding their composition, method of preparation, particle size, zeta potential, encapsulation efficiency (EE) and stability. Furthermore, a critical analysis of the in vitro and in vivo studies for such nanotechnology-based formulations for topical MXD delivery are depicted in Table 2.

Table 1.
Nanotechnology-based formulations applied to topical administration of minoxidil.

Table 2.
In vitro and in vivo results overview of nanotechnology-based formulations for topical administration of minoxidil.
2.3.1. Lipid-Based Nanoparticles
Since the discovery of the first lipid-based nanotechnology-based formulations during the 1990s [47], the interest and research in this field has grown [48]. These formulations display enhanced surface areas and are able to enhance physic stabilization and topical tolerability, allowing the sustained and targeted release of compounds [49]. Therefore, lipid-based nanotechnology-based formulations enable the efficient targeted dermal delivery of MXD [48].
Lipid Nanoparticles
Lipid nanoparticles are colloidal systems that are easy to produce and allow a quick scale-up, emerging as sustainable and targeted MXD delivery systems [50]. Regarding these nanotechnology-based formulations, there are two major groups that may be categorized: solid lipid nanoparticles (SLNs), which appeared in the 1990s, and are constituted only by lipids in solid phase, and nanostructured lipid carriers (NLCs), which appeared after SLNs, as an improvement, in order to overcome SLNs’ issues regarding stability, drug leakage during storage and low drug loading [47,49,51]. The development of MXD-loaded lipid nanotechnology-based formulations are indeed a promising strategy for MXD dermal delivery regarding improved AGA treatment. MXD-loaded SLNs and NLCs interact with the lipids in the stratum corneum, promoting their reorganization, which results in enhanced permeation and in the improvement of MXD’s therapeutic efficacy. Additionally, these lipid nanoparticles do not compromise skin’s hydration upon topical application [49,52]. The application of SLNs and NLCs in MXD topical delivery systems has been widely investigated due to their ability to permeate the skin through the follicular pathway [53] (Table 1). However, based on their particle size, SLNs and NLCs may form MXD depots at the application site, or permeate the skin and enter the bloodstream [47]. Padois et al. developed SLN-based suspensions for MXD dermal delivery and hair follicle targeting. Despite efficacy results for the MXD-loaded SLNs being similar to the commercial MXD formulations, the MXD-loaded SLN was advantageous once the appearance of topical side effects (skin corrosion) was drastically reduced, as ex vivo studies showed [27]. Wang et al. reported the development of MXD-loaded NLC dispersions, composed of stearic acid as the solid lipid, and oleic acid as the liquid lipid. Both stearic acid and oleic acid were selected, owing to their performance on improving both MXD solubility and loading, and their overall stability. Besides, oleic acid acts as a skin permeation enhancer, potentiating MXD dermal permeation. The MXD-loaded NLCs exhibited faster MXD release compared to SLN derivatives, once MXD diffusion from the liquid lipid core was facilitated, as opposed to the solid lipid core of MXD-loaded SLNs. Additionally, MXD-loaded NLCs showed enhanced in vitro MXD skin permeation and retention (10.7-fold higher than MXD-SLNs) and stability [28]. In another study, Uprit et al. developed an MXD-loaded NLC gel formulation. Initially, different NLCs were prepared by altering the solid lipid (tristearin) and liquid lipid (oleic acid). The MXD-loaded tristearin:oleic acid (2:1) NLCs showed the lowest particle size as well as the highest MXD entrapment efficiency in the liquid lipid, showing the optimum ratio of the lipid mixtures. In vitro studies showed ca. 92.18% cumulative MXD release from NLC, as well as an initial burst release followed by sustained MXD release from the NLC gel, owing to increased dermal penetration and prolonged MXD action, respectively, being of utmost relevance considering dermal delivery [29]. Aljuffali and co-workers developed squalene-based NLCs designated as squarticles for topical delivery of MXD [33,34]. The squalene-based composition allows for the interaction between MXD and the hair follicles, as sebum contains squalene and is avidly secreted into the hair follicle duct by the sebaceous ducts. This lipidic nature and the nano-sized squarticles favor the interaction between squarticles and the squalene-containing sebum, ensuring fusion with sebum and facilitating targeted follicular delivery of MXD. Two different nanotechnology-based formulations were tested, namely MXD-containing nanoemulsions (NEs) and MXD-loaded NLCs. While in vitro skin delivery showed that both nanotechnology-based formulations could increase skin deposition compared to a control (30% propylene glycol (PG) in water), MXD deposition was significantly improved by the NLC formulation, once the crystalline lipid structure of NLCs ensured occlusive and hydration effects enhancing MXD absorption, contrary to the NEs-containing amorphous inner phase. The two nanotechnology-based formulations could effectively reduce MNX flux through the skin, thus indicating improvements in skin accumulation and reduced systemic absorption. The NLCs formulation showed higher MXD release when compared to the NE formulation, as well as accounting for the expressive increase in VEGF expression in dermal papilla cells. In vivo skin irritation tests showed no erythema formation for both NLCs and NEs formulations [33]. Overall, MXD-loaded squarticles showed improved skin and follicular MXD accumulation. DPCs are an active group of mesenchymal cells encountered at the base of hair follicles, imparting hair growth and regeneration. VEGF-mediated hair cycling and the growth process consists of an interesting target for different hair-related conditions. Further studies were reported, this time concerning antibody-conjugated squarticles for MXD-targeted delivery to hair follicles, more precisely DPCs. For achieving DPC targeting, the squarticles were conjugated with anti-platelet-derived growth factor (PDGF) receptor β antibody as an active targeting strategy for improved MXD delivery to the hair follicles and enhanced penetration into the DPCs [34]. The PDGF squarticles exhibited smaller particle size (194.5 ± 4.7 nm) when compared to the non-targeted ones (236.0 ± 3.3 nm). Overall, the MXD-loaded PDGF squarticles enhanced cellular internalization by the DPCs of the MXD-loaded nanoparticles, as well as the cell viability of human DPCs to a greater extent compared to non-targeted MXD-loaded squarticles and the MXD solution control. Both cell proliferation and VEGF production enhancements were reported, together with increased follicular uptake, as in vivo skin permeation studies in nude mouse skin models showed. Furthermore, MXD-loaded PDGF squarticles showed controlled and sustained MXD release. NLCs emerge this way as robust MXD delivery systems with optimized performance and improved therapeutic efficacy. Additional studies have reported high MXD retention in the skin, no erythema [28], faster onset yet prolonged MXD release [29,31], typical pseudoplastic behavior, and increased bioavailability towards skin delivery [29], which make MXD-NLC formulations suitable nanotechnology-based formulations for topical MXD administration. Besides, MXD-NLC formulations are an alternative to the conventional alcoholic solutions for MXD, therefore minimizing the risk of adverse effects, such as skin dryness and irritation [30]. Indeed, NLCs demonstrated attributes concerning the enhancement of tolerability and acceptance toward treatment, owing to enhanced tolerability and skin bioavailability when compared to commercial and SLN formulations of MXD. MXD-loaded lipid nanoparticles might be further emulsified with hydrofluoroalkane (HFA) and pluronic L62D surfactant to produce foams that lead to the release of MXD from the nanoparticles just upon application to the skin [32]. However, these dynamic foams have demonstrated premature dose dumping.
Vesicular Nanoparticles
Vesicular nanoparticles are a large group of lipid-based nanoparticles, which are attractive due to their capability to load either lipophilic and hydrophilic drugs, enhancing drug-targeted delivery and bioavailability [54]. These nanotechnology-based formulations have been widely explored as MXD topical delivery systems and are also considered to be permeation enhancers, due to their ability to solubilize MXD into the lipidic matrix, in order to promote localized depots and facilitate MXD’s skin absorption via the follicular pathway [55,56].
Liposomes
Liposomes are phospholipid bilayer systems with an aqueous core, which may be loaded with hydrophilic drugs in the core or lipophilic drugs trapped within the phospholipid bilayers [17,57]. Concerning their phospholipid constitution, liposomes are able to interact with the stratum corneum’s lipids, allowing MXD skin permeation, or through the hair follicles, creating MXD’s depots [58]. Liposomes have been explored for MXD topical delivery (Table 1). Liposomes are highly biocompatible and biodegradable, remaining in blood circulation for extended periods of time. Nonetheless, their applicability is restricted due to stability issues, namely aggregation, drug leakage, hydrolysis and particle size alterations [57,59]. MXD-loaded liposomes reportedly enhanced skin permeation through the stratum corneum pathway and depot formation with significant improvements, namely MXD sustained release and targeting features (Table 2). However, due to their ability to reach the bloodstream, liposomes may induce systemic adverse effects, such as headaches or hypotension [1]. Penetration enhancer-containing vesicles (PEVs), i.e., vesicles containing different penetration enhancers, are also able to increase MXD accumulation in the upper skin layers without reaching systemic circulation, improving cutaneous drug bioavailability. Particularly, MXD-loaded vesicles containing 2-(2-ethoxyethoxy) ethanol (Transcutol® (Trc)) as a penetration enhancer were developed [35]. The MXD-loaded PEVs containing 20% of Trc showed the highest skin accumulation, as in vitro penetration and permeation studies showed. A further study explored soy lecithin-based liposomes containing three different penetration enhancers, namely Trc, capryl-caproyl macrogol 8-glyceride (Labrasol®) and cineole. The inclusion of penetration enhancers—especially Labrasol® and cineole—increased the PEVs’ deformability and elasticity, enhancing skin MXD delivery compared to MXD-loaded lecithin liposomes [36]. Coating liposomes with cationic polymers, such as chitosan and Eudragit EPO©, increased MXD skin diffusion due to the charge differences between the skin surface and the cationic liposomes [38]. Additionally, the presence of the positively charged polymers might cause the disruption of the tight junctions of the skin, thus contributing to the improvement of MXD skin permeation.
Niosomes
Niosomes are non-ionic surfactant-based vesicular nanotechnology-based formulations capable of accommodating either hydrophilic or hydrophobic drugs. The first major use of niosomes were reported in the cosmetic field, with several applications concerning the development of niosome-based cosmetic formulations [14,51,60]. Typical compositions consist of cholesterol and surfactant molecules, assembled in a stable bilayered nanostructure with an aqueous inner compartment [61]. Niosomes can promote the targeted and sustained release of drugs through the stratum corneum and favoring local depot formation, enhancing drug skin permeation and bioavailability. In addition, niosomes exhibit increased stability and a better cost–effect ratio compared to liposomes [10,62], as well as biocompatible and biodegradable features. Skin irritation is also reported to be minimized, as these nanotechnology-based formulations have not been related so far to irritant or immunogenic responses, as opposed to conventional topical formulations, thereby allowing better therapeutic adherence due to a reduction in the oily sensation after application [63,64]. MXD entrapment into niosomes enhanced the ability to reach deeper skin layers and to form drug depots for MXD prolonged release. This is dependent on the particle size and lipid composition. However, and akin to liposomes, though to a lesser extent, niosomes may allow some MXD to reach the systemic bloodstream, leading to systemic adverse effects. Additional drug hydrolysis and drug leakage phenomena may also occur. In this regard, different MXD-loaded niosome formulations for topical delivery were studied, varying the surfactant composition and cholesterol–surfactant ratios [10]. Sorbitane monostearate (Span 60©) was the selected surfactant due to maximum encapsulation efficiency, whilst rendering stable niosomes with the lowest particle size. Additionally, cholesterol improved the encapsulation efficiency of MXD and the particle size. Moreover, and interestingly, by increasing cholesterol concentration, both MXD skin deposition and permeation yield significant improvements. In vitro skin permeation studies showed 43.68 ± 4.7% MXD permeation for the sorbitane monostearate/cholesterol (1:2) noisome-based formulation. MXD deposition was significantly higher (17.21 ± 3.2%) when compared to plain minoxidil gel (2.26 ± 1.3%) [10]. Furthermore, the noisome-based formulations produced by Balakrishnan and co-workers enhanced MXD bioavailability, proving to be auspicious substitutes for the commercial formulation (Minoxyl) [39].
Ethosomes
First developed in 1997, ethosomes comprise vesicular nanotechnology-based formulations composed by lipid contents and high ethanol concentrations. Several studies report the capabilities of ethosomes on improving drug skin permeation, due to their interaction with the stratum corneum’s lipids and enhancement of both the cellular membrane’s fluidity and permeability [40,65]. Ethosomes allow for deeper cutaneous drug permeation, enhanced drug delivery performance and stability compared to liposomes, the latter exhibiting lower skin penetration and hampering accumulation into deep skin layers [37,40,51]. MXD loading into ethosomes resulted in skin permeation enhancements, as it could reach deeper skin layers [37]. A study showed the potential of MXD-loaded ethosomes in management of hair loss disorders and reprogramming the hair growth cycle. Particularly, ethosomal MXD-topically treated C57BL/6 mice exhibited a telogen phase shortage, as well as stimulation of the premature anagen phase [41]. Despite their high content in ethanol (30–40%), MXD ethosomes did not show induced skin adverse effects, such as erythema or edema, surpassing the characteristic limitations of MXD’s conventional formulations.
Transferosomes
Transferosomes comprise an aqueous core surrounded by a phospholipid bilayer with edge activators. Those structures are characterized by their ability to deform and permeate through the skin layers, without suffering noticeable decrease in the particle size, hence reaching the stratum corneum’s deep layers [66]. The ability of transferosomes to deform is inherent in the edge activators, which act through the modification of the interfacial tension, allowing these vesicular nanoparticles to “squeeze” through intercellular spaces without losing their structure [24]. When compared to liposomes, transferosomes are more adaptable and may respond better in stress conditions [67]. The process of transferosome deformation when containing an hydrophilic drug, such as MXD, is challenging due to vesicular elasticity decrease, which may lead to transferosomal disruption [68]. This way, the application of this nanotechnology-based formulation to MXD topical delivery systems is not so common (Table 1 and Table 2). Ramezani and co-workers have developed a transferosome containing MXD and caffeine for the treatment of alopecia. The hair growth effects of the MXD and caffeine transferosomal formulation was evaluated in Wistar rats. The MXD transferosome enhanced MXD delivery into the rats’ hair follicles, promoting hair growth within 10 days after topical application [41].
Cubosomes
Cubosomes are self-assembled crystalline bilayer vesicular nanoparticles with a liquid honeycomb structure, comprised of two water canals on the inside and specific surfactants on the outside [69]. The honeycomb structure is a 3D bi-continuous structure which enhances their surface area. Their enhanced stability, biodegradability, and encapsulation efficiency attributes make cubosomes an promising strategy for sustained and targeted drug release [70]. MXD-loaded cubosomes have already been developed (Table 1) and have demonstrated their ability to decrease the barrier function of the skin, enhancing MXD skin permeation and hair follicle depots, allowing them to reach deeper skin layers and enabling the sustained release of MXD (Table 2). Nonetheless, due to the higher loading capability, cubosomes deliver higher MXD quantities, which may reach the systemic bloodstream and, consequently, promote intense systemic adverse effects, hindering the patient’s compliance and therapeutic adherence.
2.3.2. Polymeric Nanoparticles
Polymeric nanoparticles are colloidal systems constituted by natural or synthetic polymers, with a particle size between 200 to 300 nm and biocompatible properties [71]. Polymeric nanoparticles as topical administration strategies are among the most studied and developed topical drug delivery systems because of their ability to decrease drug degradation and to enhance drug skin permeation through the stratum corneum and follicular accumulation, thus forming drug depots which allow for sustained and targeted release of the drug. Polymeric nanoparticles are being developed and investigated for AGA treatment (Table 1 and Table 2) [4,51]. According to the polymeric components, polymeric nanoparticles may be configurated as nanocapsules or nanospheres. The former have an oil or water core surrounded by a polymeric matrix, allowing physical stability enhancement and the protection of the loaded drug [72,73]. MXD nanocapsules enhance the hair growth, due to the increment in number and length of hair follicles during the anagen phase. The formation of MXD depots is less reported for lipidic nanoparticles, which highlights the importance of nanocapsules in its targeted action enhancement in the hair follicles. Chitosan, a natural polymer derived from the chitin of crustaceans’ exoskeletons, has been extensively investigated in nanotechnology due to its biological compatibility, degradability, adhesiveness and renewability [14,74]. Moreover, its antimicrobial and anti-inflammatory activity, along with its ability to bind with lipids, comprise important features for application in topical therapeutic treatments [75,76]. In addition, MXD-loaded chitosan polymeric nanoparticles were endowed with MXD sustained release properties, enhanced skin permeation and hair follicle accumulation [8]. However, it is known that natural polymers have disadvantages regarding consistency, purity and inconsistent loading release between batches; thus, synthetic polymers are preferential for polymeric nanoparticle manufacturing [51]. These polymer-based synthetic nanotechnology-based formulations increase drug hair follicle accumulation and may carry the drug dispersed in the matrix, adsorbed at the surface or encapsulated [77]. The synthetic polymers used so far for MXD-loaded synthetic polymer-based nanotechnology-based formulation development include: poly (D,L-lactic acid) (PLA) [32], block copolymers (e.g., poly(ε-caprolactone)-block-poly(ethylene glycol)) [43], poly(lactic-co-glycolic acid) (PLGA) and poly(L-lactide-co-glycolide) (PLLGA) [44]. The poly(ε-caprolactone)-block-poly(ethylene glycol) and PLLGA nanoparticles enhanced MXD skin permeation in vivo via follicular route and have promoted mice hair growth [43,44] (Table 2). Further incorporation of the polymeric nanoparticles in topical dosage forms, e.g., dynamic foams, was already tested to enhance skin permeation and the patient’s compliance, however MXD release occurred during storage, limiting the foams’ efficacy [32].
2.3.3. Metallic Nanoparticles
Metallic nanoparticles are inorganic systems with a rigid composition, due to the incorporation of metal or metal oxide compounds, known for antimicrobial, anti-inflammatory, healing and antioxidant activities [14]. The drug may be adsorbed or conjugated at the surface. However, metallic nanoparticles are mostly employed in the cosmetic field in a wide pipeline of cosmetics as coloring agents or physical sun blockers, particularly silver, gold, zinc, titanium and silica [78]. In the present context of AGA treatment, Nagai and co-workers developed zircon-based nanoparticles for the delivery of MXD, which have promoted VEGF and IGF-1 gene expression and their respective mRNA levels. Consequently, significant hair growth with increased hair bulb retention, with no signs of erythema or inflammation, were observed [45] (Table 1 and Table 2).
2.3.4. Cyclodextrins
Cyclodextrins (CDs), cyclic polysaccharides with six to eight glucopyranose units, are an enzymatic product of starch degradation [14]. Three non-synthetic CDs exist namely, α, β and γ, only differing in the number of D-glucose units they possess—six, seven and eight, respectively [79]. Due to their ring shape, CDs may load a broad variety of drugs, creating CD–drug complexes, which are widely applied in a broad set of fields, such as pharmaceuticals or the cosmetics industry [14]. CDs promote the sustained and controlled release of a drug. Considering topical delivery, such as MXD delivery through the hair follicle pathway, CDs allow for drug stabilization and avoid oxidative, thermal and photolytic degradation, as well as improve MXD aqueous solubility [4,80]. CD/MXD inclusion complexes have been developed so far for enabling an enhanced MXD diffusion coefficient and influx through the skin, ultimately promoting MXD accumulation at the application site [1] (Table 1 and Table 2). Recently, alginate hydrogel-based CD formulations concerning topical delivery of MXD for AGA treatment were reported, consisting of hydroxypropyl-b-cyclodextrin (HP-β-CD) bearing two molar substitution degrees (MS) (0.65 and 0.85) [1]. The HP-β-CD derivative bearing 0.65 MS was the selected derivative, as it has allowed for the significant improvement in the aqueous solubility of MXD. The gel formulation of the HP-β-CD/MXD inclusion complexes showed adequate stability and biocompatibility, as well as superior performance when compared to specific gene targeting (e.g., enhanced expression of the ATP-sensitive potassium channel opener gene code (AKT2), which consequently potentiates the KATP/AKT2 signalling pathway, and stimulates hair growth), and overall hair quality, when compared to conventional topical MXD solutions. Additionally, an increase in both the number and diameter of the hair follicles was described for the HP-β-CD/MXD inclusion complex-containing gel [6]. In another study, a calcium alginate-based hydrogel formulation containing methyl-β-CD/MXD inclusion complexes was developed, and significant skin retention effects were observed and attributed to their bioadhesive properties [11]. In another study, in vitro skin permeation of HP-β-CD/MXD inclusion complexes entrapped in monoolein (MO) cubic phases was compared with the performance of conventional MXD propylene glycol/water/ethanol solution, exhibiting greater in vitro skin permeation performance. However, skin retention was higher for the MXD solution, probably owing to propylene glycol and ethanol activities as MXD co-solvents [46].
3. Toxicity Issues
The development of nanotechnology-based formulations for MXD topical delivery has introduced major improvements for AGA treatment due to the distinct physicochemical features of such formulations. Nonetheless, such formulations, as nanoscale-tailored materials, should be carefully assessed regarding their safety profile [81]. Indeed, the nanosized dimension is responsible for an increase in the surface area, hence increasing the surface contact area, potentiating the interaction between the nano sized MXD carrier and the biological systems. As such, the reactivity and toxicity when in contact with the human body should be cautioned, due to maximized exposure and/or the introduction of irritants and toxic particles, among others [82,83]. Therefore, for the development and further application of MXD nanotechnology-based formulations for hair treatment, an assessment of their pharmacological and toxicological profiles is required, together with their analytical and characterization evaluation in order to better understand and predict their suitability and potential degradation products [17]. Toxicity assessment is based upon the predicted toxic potential assessed in cell cultures (in vitro studies) and animal models (in vivo studies), which require detailed assessment of the nano-bio interactions [84]. Table 3 depicts several key points to be taken into consideration for a toxicological assessment.

Table 3.
Toxicological key points related to nanotechnology-based formulations.
Considering the increased rate of nanotechnology-based formulation production, regulatory organizations, such as the European Medicines Agency (EMA) or the U.S. Food and Drug Administration (FDA), are concerned with the creation of guidelines related to the assessment of the toxicological profiles of nano-based systems. Nowadays, there are only industry standards (ISO/TS 13830:2013 and ISO 19007:2018) for the assessment of toxicity in nanotechnology-based formulations, which were developed by the International Organization for Standardization (IOS) in association with the Organization for Economic Cooperation and Development (OECD), and may be applied in the industry [91,92]. Additionally, the FDA created a guidance draft for the industry regarding nanomaterials, however it does not enlighten us about the toxicity assessment, only mentioning the importance of establishing a safety profile [93]. Thus, it is urgent to continue the development of guidance regarding the toxicity assessment of such nanotechnology-based formulations in order to guarantee the patient’s safety.
4. Regulatory Affairs
The necessity of an adequate regulatory framework is required, namely the development of specific and concise guidance and regulation regarding the manufacturing and assessment of pharmacokinetic, pharmacodynamic and toxicological profiles, for guaranteeing the ultimate safety and efficiency of nanotechnology-based formulations [94,95]. This issue was addressed during an international nanomedicine workshop sponsored by EMA in 2010 [96]. The IOS defined a “nanomaterial” as a material with external dimension in the nanoscale or having an internal or surface structure in the nanoscale with a particle size range from 1 to 100 nm. However, not all regulatory parties agreed with this definition and developed other definitions regarding their own perspectives [97,98]. In addition, the problem of deciding if nanotechnology-based formulations for drug delivery are considered medical products or devices exists [95]. In Europe, medical products are regulated by EU Directive 2001/83/EC, and medical devices are regulated by EU Directive 93/42/EEC [99,100]. The basilar feature for accepting or denying regulatory approval is the toxicity assessment of the nanotechnology-based formulation, which provides the safety and efficacy results required [95]. Therefore, elucidation of the nanotechnology-based formulations regulatory framework is required, especially regarding the assessment of quality, non-clinical and clinical issues for market introduction approval.
5. Conclusions and Future Perspectives
MXD is a broadly used drug in AGA therapeutics for leveraging the anagen phase of the hair follicles and, therefore, inducing hair growth. Conventional MXD formulations consist of 5% MXD solutions of propylene glycol, ethanol and water topically administered. Such formulations are associated with topical adverse effects, decreasing patients’ tolerability towards AGA treatment and leading to therapeutic failure. In this regard, MXD nanotechnology-based formulations are able to both improve AGA treatment by potentiating the skin and follicular delivery of MXD, and decreasing MXD-related adverse effects of conventional formulations, allowing better compliance and treatment improvements. Moreover, the improvements in MXD bioavailability, hair follicle MXD accumulation and MXD skin permeation are ascribed to MXD delivery by means of nanotechnology-based formulations. Considering MXD release, the encapsulation of MXD in such nanotechnology-based systems enables sustained, controlled and targeted MXD delivery, contributing to MXD delivery optimization and overall performance enhancements. However, the lack of data related to the characterization of such nanotechnology-based formulations, such as stability assays, constitutes a clear constraint that needs further attention. In addition, the amount of information related to in vitro and in vivo assays should get a deeper exploration and insights in order to extract the maximum amount of relevant information and level up the overall knowledge available. Moreover, the amount of evidence collected by in vitro/ex vivo assays are confirmed by the in vivo results, in this way reinforcing the reliability of the replacement of in vivo models with in vitro permeation models. At the same time, the establishment of a nanosafety profile is of utmost importance and requires international guidance and regulation, which nowadays is still scarce, hampering market approval and clinical translation. Attending to the aforementioned issues, international authorities must develop guidance in order to provide enlightenment on correct manufacturing processes and underlying toxicity assessments, especially concerning the chronic exposure to nanotechnology-based therapies, and, particularly, to AGA topical therapy.
Author Contributions
Conceptualization, A.C.S. and M.P.-S.; methodology, A.C.S. and M.P.-S.; formal analysis, A.C.S., M.P.-S., D.C., D.P., I.P. (Irina Pereira) and I.P. (Inês Pita); resources, A.C.S., A.J.R. and F.V.; data curation, A.C.S., M.P.-S., D.C., D.P., I.P. (Irina Pereira) and I.P. (Inês Pita); writing—original draft preparation, A.C.S., M.P.-S., D.C., D.P., I.P. (Irina Pereira) and I.P. (Inês Pita); writing—review and editing, A.C.S., M.P.-S., D.C., D.P., I.P. (Irina Pereira) and I.P. (Inês Pita) visualization, A.C.S., A.J.R. and F.V.; supervision, A.C.S., A.J.R. and F.V.; project administration, A.C.S., A.J.R. and F.V.; funding acquisition, A.C.S., A.J.R. and F.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Fundação Para a Ciência e a Tecnologia (FCT) and Programa Operacional Capital Humano (POCH), grants number SFRH/BD/148771/2019 (M. P.-S.) and SFRH/BD/136892/2018 (I.P.).
Acknowledgments
M.P.-S. and I.P. acknowledge the PhD research grants, respectively, with the references SFRH/BD/148771/2019 and SFRH/BD/136892/2018 funded by Fundação para a Ciência e a Tecnologia (FCT) and Programa Operacional Capital Humano (POCH).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lopedota, A.; Denora, N.; Laquintana, V.; Cutrignelli, A.; Lopalco, A.; Tricarico, D.; Maqoud, F.; Curci, A.; Mastrodonato, M.; la Forgia, F.; et al. Alginate-Based Hydrogel Containing Minoxidil/Hydroxypropyl-beta-Cyclodextrin Inclusion Complex for Topical Alopecia Treatment. J. Pharm. Sci. 2018, 107, 1046–1054. [Google Scholar] [CrossRef] [PubMed]
- Adil, A.; Godwin, M. The effectiveness of treatments for androgenetic alopecia: A systematic review and meta-analysis. J. Am. Acad. Dermatol. 2017, 77, 136–141.e5. [Google Scholar] [CrossRef]
- Santos, Z.; Avci, P.; Hamblin, M.R. Drug discovery for alopecia: Gone today, hair tomorrow. Expert Opin. Drug Discov. 2015, 10, 269–292. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.-L.; Aljuffali, I.A.; Li, Y.-C.; Fang, J.-Y. Delivery and targeting of nanoparticles into hair follicles. Ther. Deliv. 2014, 5, 991–1006. [Google Scholar] [CrossRef] [PubMed]
- Tsujimoto, H.; Hara, K.; Tsukada, Y.; Huang, C.C.; Kawashima, Y.; Araki, M.; Okayasu, H.; Minura, H.; Miwa, N. Evaluation of the permeability of hair growing ingredient encapsulated PLGA nanospheres to hair follicles and their hair growing effects. Bioorg. Med. Chem. Lett. 2007, 17, 4771–4777. [Google Scholar] [CrossRef]
- Tricarico, D.; Maqoud, F.; Curci, A.; Camerino, G.; Zizzo, N.; Denora, N.; Cutrignelli, A.; Laquintana, V.; Lopalco, A.; la Forgia, F. Characterization of minoxidil/hydroxypropyl-beta-cyclodextrin inclusion complex in aqueous alginate gel useful for alopecia management: Efficacy evaluation in male rat. Eur. J. Pharm. Biopharm. 2018, 122, 146–157. [Google Scholar] [CrossRef]
- Chandrashekar, B.S.; Nandhini, T.; Vasanth, V.; Sriram, R.; Navale, S. Topical minoxidil fortified with finasteride: An account of maintenance of hair density after replacing oral finasteride. Indian Dermatol. Online J. 2015, 6, 17–20. [Google Scholar] [CrossRef]
- Matos, B.N.; Reis, T.A.; Gratieri, T.; Gelfuso, G.M. Chitosan nanoparticles for targeting and sustaining minoxidil sulphate delivery to hair follicles. Int. J. Biol. Macromol. 2015, 75, 225–229. [Google Scholar] [CrossRef]
- Li, M.; Marubayashi, A.; Nakaya, Y.; Fukui, K.; Arase, S. Minoxidil-Induced Hair Growth is Mediated by Adenosine in Cultured Dermal Papilla Cells: Possible Involvement of Sulfonylurea Receptor 2B as a Target of Minoxidil. J. Investig. Dermatol. 2001, 117, 1594–1600. [Google Scholar]
- Mali, N.; Darandale, S.; Vavia, P. Niosomes as a vesicular carrier for topical administration of minoxidil: Formulation and in vitro assessment. Drug Deliv. Transl. Res. 2013, 3, 587–592. [Google Scholar] [CrossRef]
- Lopedota, A.; Cutrignelli, A.; Denora, N.; Laquintana, V.; Lopalco, A.; Selva, S.; Ragni, L.; Tongiani, S.; Franco, M. New ethanol and propylene glycol free gel formulations containing a minoxidil-methyl-beta-cyclodextrin complex as promising tools for alopecia treatment. Drug Dev. Ind. Pharm. 2015, 41, 728–736. [Google Scholar] [CrossRef] [PubMed]
- Vogt, A.; Wischke, C.; Neffe, A.T.; Ma, N.; Alexiev, U.; Lendlein, A. Nanocarriers for drug delivery into and through the skin—Do existing technologies match clinical challenges? J. Control. Release 2016, 242, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Goyal, R.; Macri, L.K.; Kaplan, H.M.; Kohn, J. Nanoparticles and nanofibers for topical drug delivery. J. Control. Release 2016, 240, 77–92. [Google Scholar] [CrossRef]
- Sakamoto, K.; Lochhead, R.; Maibach, H.; Yamashita, Y. Cosmetic Science and Technology: Theoretical Principles and Applications; Elsevier: Amsterdam, Netherlands, 2017; Volume 813. [Google Scholar]
- Hadgraft, J. Skin, the final frontier. J. Pharm. 2001, 224, 1–18. [Google Scholar] [CrossRef]
- Benson, H.A.E.; Watkinson, A.C. Topical and Transdermal Drug Delivery: Principles and Practice; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012; Volume 448. [Google Scholar]
- Escobar-Chavez, J.; Díaz-Torres, R.; Rodríguez-Cruz, I.; Domínguez-Delgado, C.; Morales, R.; Ángeles-Anguiano, E.; Melgoza-Contreras, L. Nanocarriers for transdermal drug delivery. Res. Rep. Transdermal. Drug Deliv. 2012, 1, 3–17. [Google Scholar] [CrossRef]
- Palmer, B.C.; DeLouise, L.A. Nanoparticle-Enabled Transdermal Drug Delivery Systems for Enhanced Dose Control and Tissue Targeting. Molecules 2016, 21, 1719. [Google Scholar] [CrossRef] [PubMed]
- Glowka, E.; Wosicka-Frackowiak, H.; Hyla, K.; Stefanowska, J.; Jastrzebska, K.; Klapiszewski, L.; Jesionowski, T.; Cal, K. Polymeric nanoparticles-embedded organogel for roxithromycin delivery to hair follicles. Eur. J. Pharm. Biopharm. 2014, 88, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Hillery, A.M.; Lloyd, A.W.; Swarbrick, J. Drug Delivery and Targeting for Pharmacists and Phamaceutical Scientists; Francis, T., Ed.; Taylor & Francis: London, UK, 2001. [Google Scholar]
- Jayaneththi, V.R.; Aw, K.; Sharma, M.; Wen, J.; Svirskis, D.; McDaid, A.J. Controlled transdermal drug delivery using a wireless magnetic microneedle patch: Preclinical device development. Sens. Actuators B Chem. 2019, 297, 126708. [Google Scholar] [CrossRef]
- Suchonwanit, P.; Thammarucha, S.; Leerunyakul, K. Minoxidil and its use in hair disorders: A review. Drug Des. Devel. Ther. 2019, 13, 2777–2786. [Google Scholar] [CrossRef]
- Herrmann, S.; Daniels, R.; Lunter, D. Methods for the determination of the substantivity of topical formulations. Pharm. Dev. Technol. 2017, 22, 487–491. [Google Scholar] [CrossRef]
- Bibi, N.; Ahmed, N.; Khan, G.M. Nanostructures in Transdermal drug Delivery Systems; Elsevier: Amsterdam, Netherlands, 2017; pp. 639–668. [Google Scholar]
- Gupta, S.; Bansal, R.; Gupta, S.; Jindal, N.; Jindal, A. Nanocarriers and nanoparticles for skin care and dermatological treatments. Indian Dermatol. Online J. 2013, 4, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Benson, H.A.E.; Mohammed, Y.; Grice, J.E.; Roberts, M.S. Formulation Effects on Topical Nanoparticle Penetration; Academic Press: Amsterdam, Netherlands, 2016; pp. 115–126. [Google Scholar]
- Padois, K.; Cantiéni, C.; Bertholle, V.; Bardel, C.; Pirot, F.; Falson, F. Solid lipid nanoparticles suspension versus commercial solutions for dermal delivery of minoxidil. Int. J. Pharm. 2011, 416, 300–304. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Chen, L.; Huang, X.; Shao, A. Preparation and Characterization of Minoxidil Loaded Nanostructured Lipid Carriers. AAPS PharmSciTech 2017, 18, 509–516. [Google Scholar] [CrossRef]
- Uprit, S.; Sahu, R.K.; Roy, A.; Pare, A. Preparation and characterization of minoxidil loaded nanostructured lipid carrier gel for effective treatment of alopecia. Saudi Pharm. J. 2013, 21, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.C.; Santos, D.; Ferreira, D.C.; Souto, E.B. Minoxidil-loaded nanostructured lipid carriers (NLC): Characterization and rheological behaviour of topical formulations. Pharmazie 2009, 64, 177–182. [Google Scholar] [PubMed]
- Gomes, M.J.; Martins, S.; Ferreira, D.; Segundo, M.A.; Reis, S. Lipid nanoparticles for topical and transdermal application for alopecia treatment: Development, physicochemical characterization, and in vitro release and penetration studies. Int. J. Nanomed. 2014, 9, 1231–1242. [Google Scholar]
- Zhao, Y.; Brown, M.B.; Jones, S.A. The effects of particle properties on nanoparticle drug retention and release in dynamic minoxidil foams. Int. J. Pharm. 2010, 383, 277–284. [Google Scholar] [CrossRef]
- Aljuffali, I.A.; Sung, C.T.; Shen, F.-M.; Huang, C.-T.; Fanf, J.-Y. Squarticles as a lipid nanocarrier for delivering diphencyprone and minoxidil to hair follicles and human dermal papilla cells. AAPS J. 2014, 16, 140–150. [Google Scholar] [CrossRef]
- Aljuffali, I.A.; Pan, T.-L.; Sung, C.T.; Chang, S.-H.; Fang, J.-Y. Anti-PDGF receptor beta antibody-conjugated squarticles loaded with minoxidil for alopecia treatment by targeting hair follicles and dermal papilla cells. Nanomedicine 2015, 11, 1321–1330. [Google Scholar] [CrossRef]
- Mura, S.; Pirot, F.; Manconi, M.; Falson, F.; Fadda, A.M. Liposomes and niosomes as potential carriers for dermal delivery of minoxidil. J. Drug Target. 2007, 15, 101–108. [Google Scholar] [CrossRef]
- Mura, S.; Manconi, M.; Sinico, C.; Valenti, D.; Fadda, A.M. Penetration enhancer-containing vesicles (PEVs) as carriers for cutaneous delivery of minoxidil. Int. J. Pharm. 2009, 380, 72–79. [Google Scholar] [CrossRef]
- Lopez-Pinto, J.M.; Gonzalez-Rodriguez, M.L.; Rabasco, A.M. Effect of cholesterol and ethanol on dermal delivery from DPPC liposomes. Int. J. Pharm. 2005, 298, 1–12. [Google Scholar] [CrossRef]
- Hasanovic, A.; Hollick, C.; Fischinger, K.; Valenta, C. Improvement in physicochemical parameters of DPPC liposomes and increase in skin permeation of aciclovir and minoxidil by the addition of cationic polymers. Eur. J. Pharm. Biopharm. 2010, 75, 148–153. [Google Scholar] [CrossRef]
- Balakrishnan, P.; Shanmugam, S.; Lee, W.S.; Lee, W.M.; Kim, J.O.; Oh, D.H.; Kim, D.-D.; Kim, J.S.; Yoo, B.K.; Choi, H.-G.; et al. Formulation and in vitro assessment of minoxidil niosomes for enhanced skin delivery. Int. J. Pharm. 2009, 377, 1–8. [Google Scholar] [CrossRef]
- Touitou, E.; Dayan, N.; Bergelson, L.; Godin, B.; Eliaz, M. Ethosomes—Novel vesicular carriers for enhanced delivery: Characterization and skin penetration properties. J. Control. Release 2000, 65, 403–418. [Google Scholar] [CrossRef]
- Jun-Bo, T.; Zhuang-Qun, Y.; Xi-Jing, H.; Ying, X.; Yong, S.; Zhe, X.; Tao, C. Effect of ethosomal minoxidil on dermal delivery and hair cycle of C57BL/6 mice. J. Dermatol. Sci. 2007, 45, 135–137. [Google Scholar] [CrossRef]
- Ramezani, V.; Honarvar, M.; Seyedabadi, M.; Karimollah, A.; Ranjbar, A.M.; Hashemi, M. Formulation and optimization of transfersome containing minoxidil and caffeine. J. Drug Deliv. Sci. Technol. 2018, 44, 129–135. [Google Scholar] [CrossRef]
- Shim, J.; Kang, H.S.; Park, W.-S.; Han, S.-H.; Kim, J.; Chang, I.-S. Transdermal delivery of mixnoxidil with block copolymer nanoparticles. J. Control. Release 2004, 97, 477–484. [Google Scholar] [CrossRef]
- Takeuchi, I.; Hida, Y.; Makino, K. Minoxidil-encapsulated poly(L-lactide-co-glycolide) nanoparticles with hair follicle delivery properties prepared using W/O/W solvent evaporation and sonication. Biomed. Mater. Eng. 2018, 29, 217–228. [Google Scholar] [CrossRef]
- Nagai, N.; Iwai, Y.; Sakamoto, A.; Otake, H.; Oaku, Y.; Abe, A.; Nagahama, T. Drug Delivery System Based On Minoxidil Nanoparticles Promotes Hair Growth In C57BL/6 Mice. Int. J. Nanomed. 2019, 14, 7921–7931. [Google Scholar] [CrossRef] [PubMed]
- Kwon, T.K.; Kim, J.C. In vitro skin permeation of monoolein nanoparticles containing hydroxypropyl beta-cyclodextrin/minoxidil complex. Int. J. Pharm. 2010, 392, 268–273. [Google Scholar] [CrossRef]
- Garces, A.; Amaral, M.H.; Lobo, J.M.S.; Silva, A.C. Formulations based on solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) for cutaneous use: A review. Eur. J. Pharm. Sci. 2018, 112, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Roberts, M.S.; Mohammed, Y.; Pastore, M.N.; Namjoshi, S.; Yousef, S.; Alinaghi, A.; Haridass, I.N.; Abd, E.; Leite-Silva, V.R.; Benson, H.; et al. Topical and cutaneous delivery using nanosystems. J. Control. Release 2017, 247, 86–105. [Google Scholar] [CrossRef] [PubMed]
- Müller, R.H.; Shegokar, R.; Keck, C.M. 20 Years of Lipid Nanoparticles (SLN & NLC): Present State of Development & Industrial Applications. Curr. Drug Discov. Technol. 2011, 8, 207–227. [Google Scholar] [PubMed]
- Charoenputtakun, P.; Pamornpathomkul, B.; Opanasopit, P.; Rojanarata, T.; Ngawhirunpat, T. Terpene Composited Lipid Nanoparticles for Enhanced Dermal Delivery of All-trans-Retinoic Acids. Biol. Pharm. Bull. 2014, 37, 1139–1148. [Google Scholar] [CrossRef] [PubMed]
- Carter, P.; Narasimhan, B.; Wang, Q. Biocompatible nanoparticles and vesicular systems in transdermal drug delivery for various skin diseases. Int. J. Pharm. 2019, 555, 49–62. [Google Scholar] [CrossRef]
- Muller, R.H.; Petersen, R.D.; Hommoss, A.; Pardeike, J. Nanostructured lipid carriers (NLC) in cosmetic dermal products. Adv. Drug Deliv. Rev. 2007, 59, 522–530. [Google Scholar] [CrossRef]
- Souto, E.B.; Baldim, I.; Oliveira, W.P.; Rao, R.; Yadav, N.; Gama, F.M.; Mahant, S. SLN and NLC for topical, dermal, and transdermal drug delivery. Expert Opin. Drug Deliv. 2020, 17, 357–377. [Google Scholar] [CrossRef]
- Modi, C.D.; Bharadia, P.D. Transfersomes: New Dominants for Transdermal Drug Delivery. Am. J. Pharm. Tech. Res. 2012, 2, 71–91. [Google Scholar]
- Muzzalupo, R.; Tavano, L.; Cassano, R.; Trombino, S.; Ferrarelli, T.; Picci, N. A new approach for the evaluation of niosomes as effective transdermal drug delivery systems. Eur. J. Pharm. Biopharm. 2011, 79, 28–35. [Google Scholar] [CrossRef]
- Choi, M.J.; Maibach, H.I. Liposomes and niosomes as topical drug delivery systems. Skin Pharmacol. Physiol. 2005, 18, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Mottaleb, M.M.A.; Try, C.; Pellequer, Y.; Lamprecht, A. Nanomedicine strategies for targeting skin inflamation. Nanomedicine 2014, 9, 1727–1743. [Google Scholar] [CrossRef] [PubMed]
- Barua, S.; Mitragotri, S. Challenges associated with Penetration of Nanoparticles across Cell and Tissue Barriers: A Review of Current Status and Future Prospects. Nano Today 2014, 9, 223–243. [Google Scholar] [CrossRef] [PubMed]
- Bakowsky, H.; Richter, T.; Kneuer, C.; Hoekstra, D.; Rothe, U.; Bendas, G.; Ehrhardt, C.; Bakowsky, U. Adhesion characteristics and stability assessment of lectin-modified liposomes for site-specific drug delivery. Biochim. Biophys. Acta 2008, 1778, 242–249. [Google Scholar] [CrossRef]
- Toshimitsu, Y.; Florence, A.T. Vesicle (niosome)-in-water-in-oil (v/w/o) emulsions: An in vitro study. Int. J. Pharm. 1994, 1018, 117–123. [Google Scholar] [CrossRef]
- Bhardwaj, P.; Tripathi, P.; Gupta, R.; Pandey, S. Niosomes: A review on niosomal research in the last decade. J. Drug Deliv. Sci. Technol. 2020, 56, 101581. [Google Scholar] [CrossRef]
- Moghassemi, S.; Hadjizadeh, A. Nano-niosomes as nanoscale drug delivery systems: An illustrated review. J. Control. Release 2014, 185, 22–36. [Google Scholar] [CrossRef]
- Uchechi, O.; Ogbonna, J.D.N.; Attama, A.A. Nanoparticles for Dermal and Transdermal Drug Delivery. Appl. Nanotechnol. Drug Deliv. 2014, 4, 193–227. [Google Scholar]
- Rajera, R.; Nagpal, K.; Singh, S.K.; Mishra, D.N. Niosomes: A Controlled and Novel Drug Delivery System. Biol. Pharm. Bull. 2011, 34, 945–953. [Google Scholar] [CrossRef]
- Touitou, E.; Godin, B.; Weiss, C. Enhanced Delivery of Drugs Into and Across the Skin by Ethosomal Carriers. Drug Dev. Res. 2000, 50, 406–415. [Google Scholar] [CrossRef]
- Hua, S. Lipid-based nano-delivery systems for skin delivery of drugs and bioactives. Front. Pharmacol. 2015, 6, 219. [Google Scholar] [CrossRef] [PubMed]
- Rajan, R.; Jose, S.; Mukung, V.P.B.; Vasudevan, D.T. Transferosomes—A vesicular transdermal delivery system for enhanced drug permeation. J. Adv. Pharm. Technol. Res. 2011, 2, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Patel, N.; Shah, M.K.; Khatri, P.; Vora, N. Recent Advances in Lipid-Based Vesicles and Particulate Carriers for Topical and Transdermal Application. J. Pharm. Sci. 2017, 106, 423–445. [Google Scholar] [CrossRef] [PubMed]
- Karami, Z.; Hamidi, M. Cubosomes: Remarkable drug delivery potential. Drug Discov. Today 2016, 21, 789–801. [Google Scholar] [CrossRef]
- Pan, X.; Han, K.; Peng, X.; Yang, Z.; Qin, L.; Zhu, C.; Huang, X.; Shi, X.; Dian, L.; Lu, M.; et al. Nanostructured cubosomes as advanced drug delivery system. Curr. Pharm. Des. 2013, 19, 6290–6297. [Google Scholar] [CrossRef]
- Alvarez-Roman, R.; Naik, A.; Kalia, Y.N.; Guy, R.H.; Fessi, H. Skin penetration and distribution of polymeric nanoparticles. J. Control. Release 2004, 99, 53–62. [Google Scholar] [CrossRef]
- Li, J.; Qiao, Y.; Wu, Z. Nanosystem trends in drug delivery using quality-by-design concept. J. Control. Release 2017, 256, 9–18. [Google Scholar] [CrossRef]
- Ramezanli, T.; Kilfoyle, B.E.; Zhang, Z.; Michniak-Kohn, B.B. Polymeric nanospheres for topical delivery of vitamin D3. Int. J. Pharm. 2017, 516, 196–203. [Google Scholar] [CrossRef]
- Zhao, D.; Yu, S.; Sun, B.; Gao, S.; Guo, S.; Zhao, K. Biomedical Applications of Chitosan and Its Derivative Nanoparticles. Polymers (Basel) 2018, 10, 462. [Google Scholar] [CrossRef]
- Hu, X.; Tang, Y.; Wang, Q.; Li, Y.; Yang, J.; Du, Y.; Kennedy, J.F. Rheological behaviour of chitin in NaOH/urea aqueous solution. Carbohydr. Polym. 2011, 83, 1128–1133. [Google Scholar] [CrossRef]
- Gutha, Y.; Pathak, J.L.; Zhang, W.; Zhang, Y.; Jiao, X. Antibacterial and wound healing properties of chitosan/poly(vinyl alcohol)/zinc oxide beads (CS/PVA/ZnO). Int. J. Biol. Macromol. 2017, 103, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Tsai, P.-C.; Ramezanli, T.; Michniak-Kohn, B.B. Polymeric nanoparticles-based topical delivery systems for the treatment of dermatological diseases. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2013, 5, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Borowska, S.; Brzoska, M.M. Metals in cosmetics: Implications for human health. J. Appl. Toxicol. 2015, 35, 551–572. [Google Scholar] [CrossRef]
- Arora, N.; Agarwal, S.; Murthy, R.S.R. Latest Technology Advances in Cosmaceuticals. Int. J. Pharm. Sci. Drug Res. 2012, 4, 168–182. [Google Scholar]
- Tarimci, N. Cyclodextrins in the Cosmetic Field, in Cyclodextrins in Pharmaceutics, Cosmetics, and Biomedicine: Current and Future Industrial Applications; Bilensoy, E., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011. [Google Scholar]
- Isigonis, P.; Hristozov, D.; Benighaus, C.; Giubilato, E.; Grieger, K.; Pizzol, L.; Semenzin, E.; Linkov, I.; Zabeo, A.; Marcomini, A. Risk Governance of Nanomaterials: Review of Criteria and Tools for Risk Communication, Evaluation, and Mitigation. Nanomaterials (Basel) 2019, 9, 696. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Agrawal, U.; Vyas, S.P. Nanocarrier-based topical drug delivery for the treatment of skin diseases. Expert Opin. Drug Deliv. 2012, 9, 783–804. [Google Scholar] [CrossRef] [PubMed]
- Nel, A.; Xia, T.; Madler, L.; Li, N. Toxic potential of materials at the nanolevel. Science 2006, 311, 622–627. [Google Scholar] [CrossRef]
- Monteiro-Riviere, N.A.; Inman, A.O.; Zhang, L.W. Limitations and relative utility of screening assays to assess engineered nanoparticle toxicity in a human cell line. Toxicol. Appl. Pharmacol. 2009, 234, 222–235. [Google Scholar] [CrossRef]
- Pourmand, A.; Abdollahi, M. Current Opinion on Nanotoxicolog. Daru 2012, 20, 95–98. [Google Scholar] [CrossRef]
- Bahadar, H.; Maqbool, F.; Niaz, K.; Abdollahi, M. Toxicity of Nanoparticles and an Overview of Current Experimental Models. Iran. Biomed. J. 2016, 20, 1. [Google Scholar]
- Wang, S.; Su, R.; Nie, S.; Sun, M.; Zhang, J.; Wu, D.; Moustaid-Moussa, N. Application of nanotechnology in improving bioavailability and bioactivity of diet-derived phytochemicals. J. Nutr. Biochem. 2014, 25, 363–376. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.L.; Xu, Z.Z.; Fan, M.X.; Liu, H.Y.; Xie, Y.H.; Zhu, T. Study on characteristics and harm of surfactants. J. Chem. Pharm. Res. 2014, 6, 2233–2237. [Google Scholar]
- Moustafalou, S.; Mohammadi, H.; Ramazani, A.; Abdollahi, M. Different biokinetics of nanomedicines linking to their toxicity; an overview. DARU J. Pharm. Sci. 2013, 21, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Duncan, R.; Gaspar, R. Nanomedicine(s) under the microscope. Mol. Pharm. 2011, 8, 2101–2141. [Google Scholar] [CrossRef] [PubMed]
- International Organization for Standardization. ISO/TS 13830:2013—Guidance on Voluntary Labelling for Consumer Products Containing Manufactured Nano-Objects. 2013. Available online: https://www.iso.org/standard/54315.html (accessed on 21 November 2019).
- International Organization for Standardization. ISO 19007:2018—In vitro MTS Assay for Measuring the Cytotoxic Effect of Nanoparticles. 2018. Available online: https://www.iso.org/standard/63698.html (accessed on 21 November 2019).
- U. S. Food and Drug Administration. Drug Products, Including Biological Products, that Contain Nanomaterials—Draft Guidance for Industry; Office of Medical Products and Tobacco: Rockville, MD, USA, 2017. [Google Scholar]
- Hofmann-Amtenbrink, M.; Hofmann, H.; Hool, A.; Roubert, F. Nanotechnology in medicine: European research and its implications. Swiss Med. Wkly. 2014, 144, w14044. [Google Scholar] [CrossRef] [PubMed]
- Kelly, B. Nanomedicines: Regulatory Challenges and Risks Ahead; Regulatory Affairs Pharma: London, UK, 2010. [Google Scholar]
- European Medicines Agency. European Medicines Agency’s Workshop on Nanomedicines. 2010. Available online: https://www.ema.europa.eu/en/events/european-medicines-agencys-workshop-nanomedicines (accessed on 21 November 2019).
- Beer, C. Nanotoxicology and Regulatory Affairs; Springer: New York, NY, USA, 2016; pp. 279–310. [Google Scholar]
- Patravale, V.; Dandekar, P.; Jain, R. Regulatory Aspects of Nanoparticulate Drug Delivery Systems; Elsevier: Amsterdam, Netherlands, 2012; pp. 157–190. [Google Scholar]
- European Parliament. EU Directive 2001/83/EC—Community Code Relating to Medicinal Products for human Use. Available online: https://eur-lex.europa.eu/legal-content/en/ALL/?uri=CELEX%3A32001L0083 (accessed on 27 March 2020).
- European Council. EU Directive 93/42/EEC—Concerning Medical Devices. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A31993L0042 (accessed on 27 March 2020).
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).