Natural and Bioinspired Phenolic Compounds as Tyrosinase Inhibitors for the Treatment of Skin Hyperpigmentation: Recent Advances
Abstract
:1. Introduction
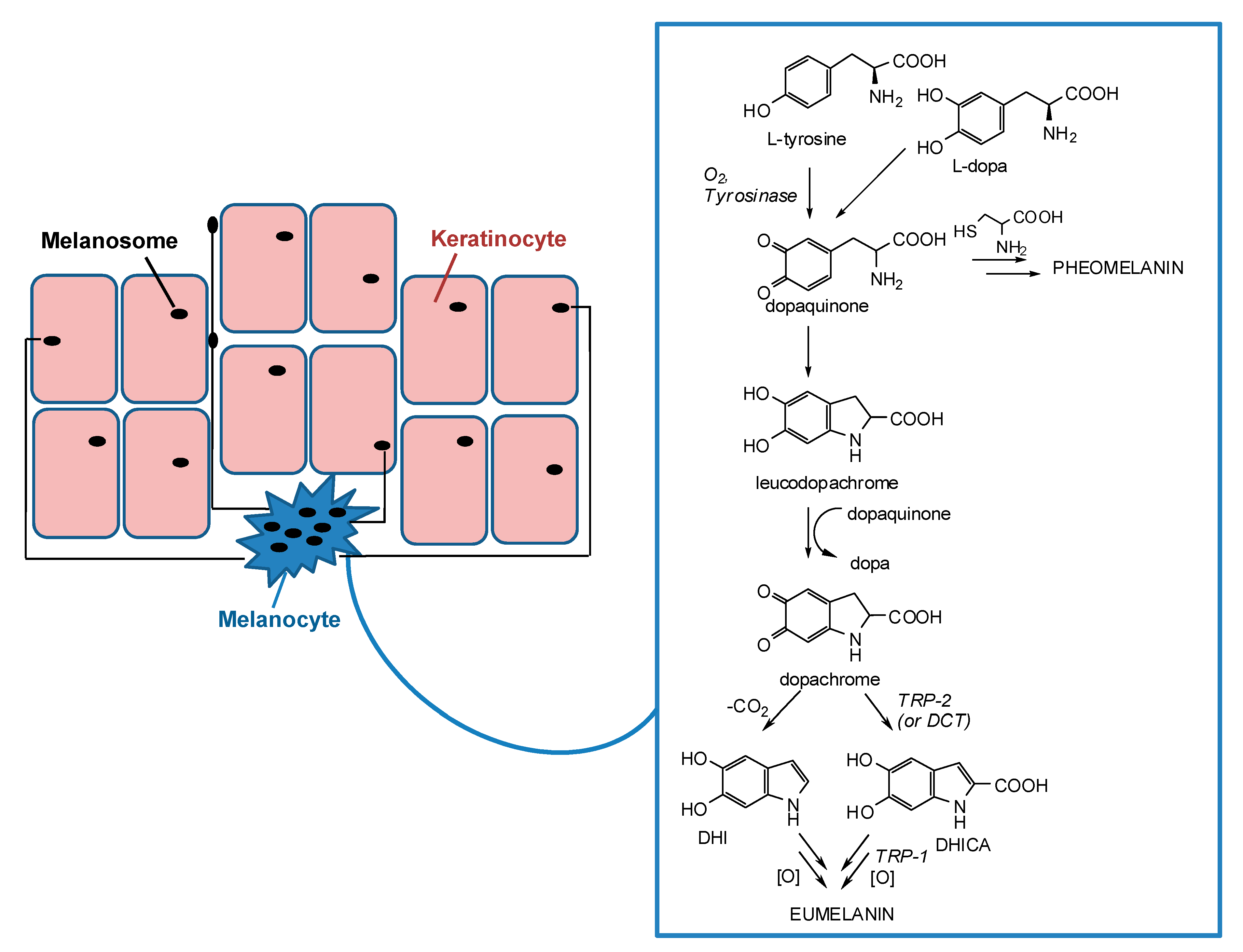
2. Skin Pigmentation and Hyperpigmentary Disorders
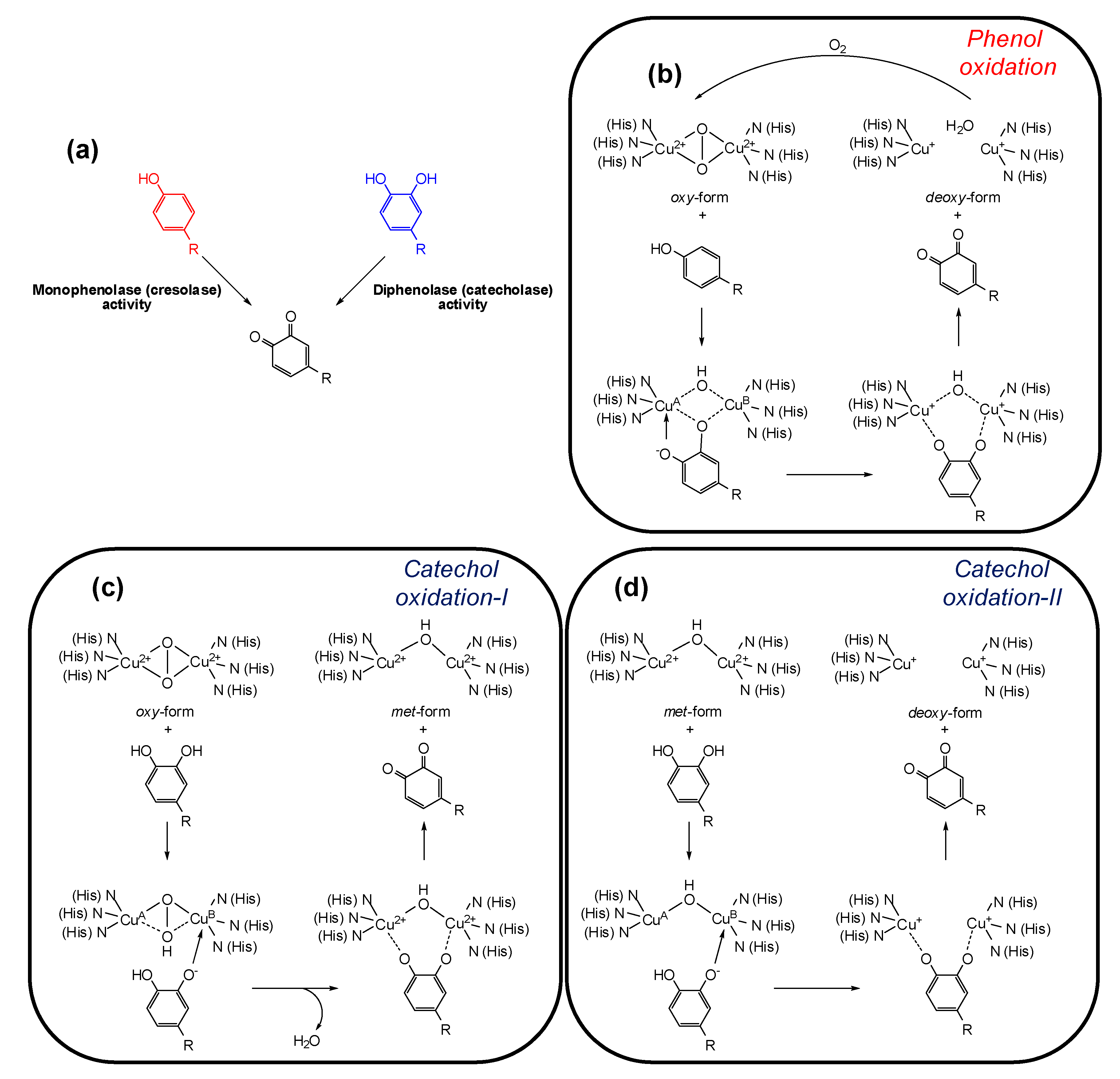
3. Tyrosinase and its Key Role in Melanin Synthesis
4. Tyrosinase Inhibition Mechanisms
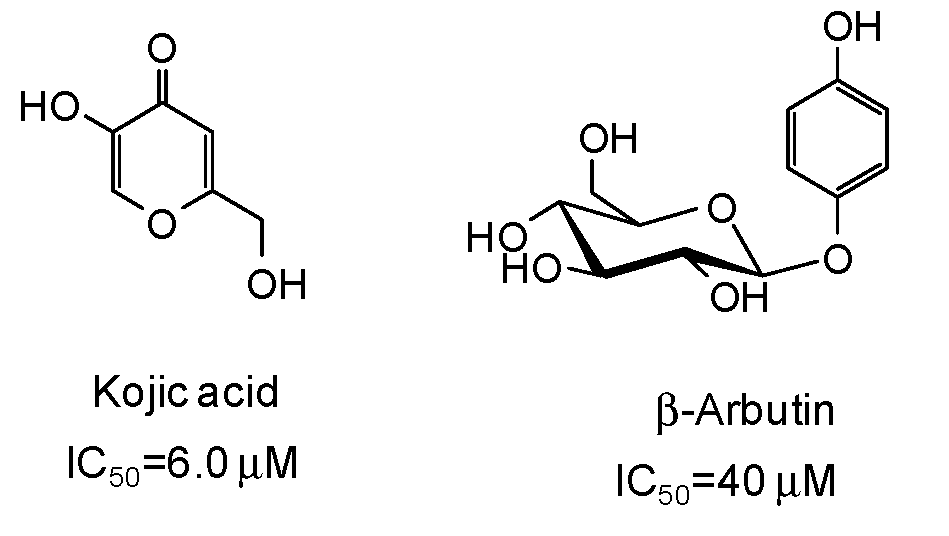
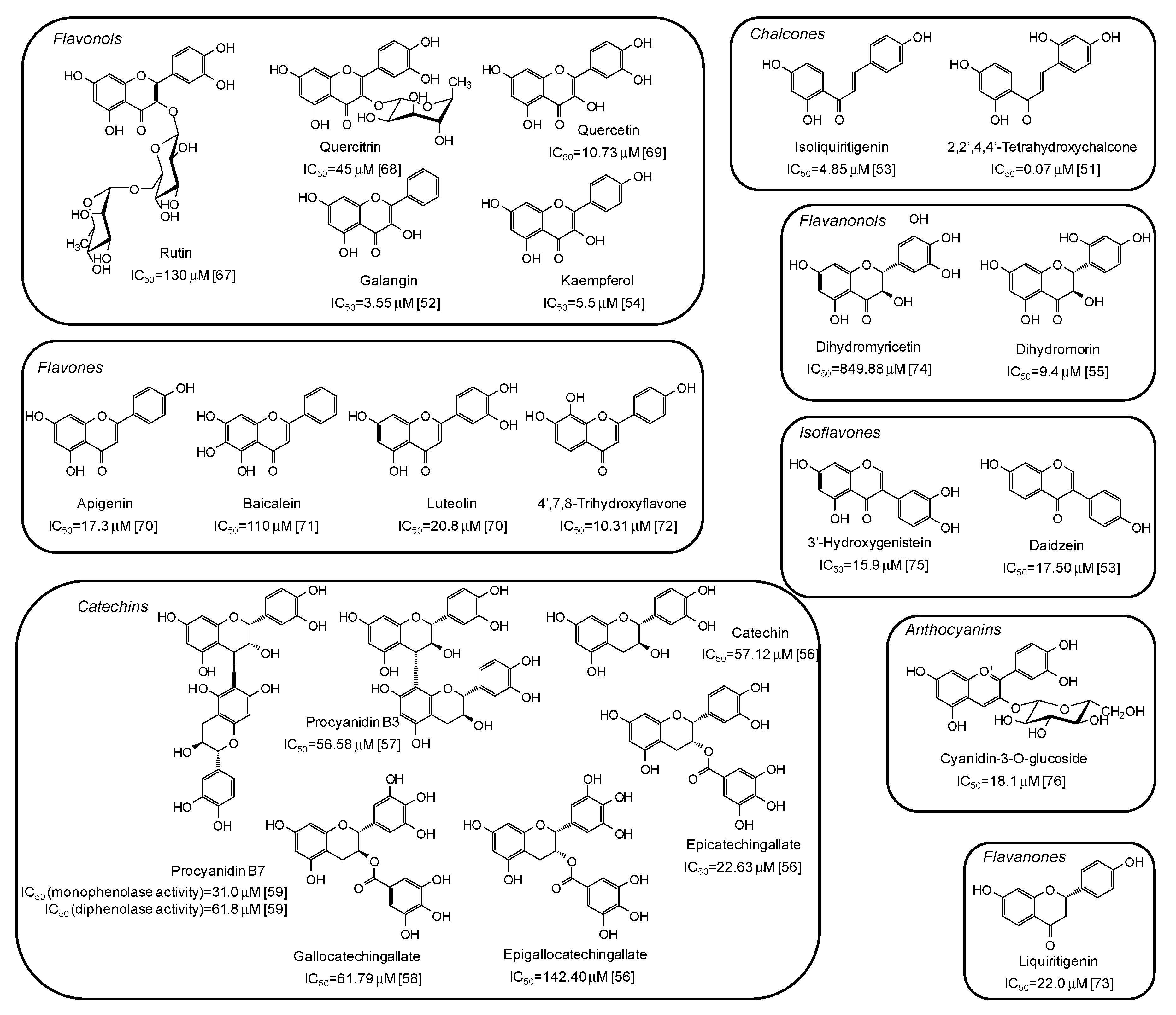
5. Natural Phenolic Inhibitors of Mushroom Tyrosinase
6. Synthetic Phenolic Inhibitors of Mushroom Tyrosinase
7. Human and Animal Tyrosinase Phenolic Inhibitors
8. Structural Requirements for the Design of Tyrosinase Inhibitors
9. New Trends in the Search for Tyrosinase Inhibitors
10. Conclusions and Perspectives
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zolghadri, S.; Bahrami, A.; Hassan Khan, M.T.; Munoz-Munoz, J.; Garcia-Molina, F.; Garcia-Canovas, F.; Saboury, A.A. A comprehensive review on tyrosinase inhibitors. J. Enzyme Inhib. Med. Chem. 2019, 34, 279–309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumari, S.; Tien Guan Thng, S.; Kumar Verma, N.; Gautam, H.K. Melanogenesis inhibitors. Acta Dermatol. Venereol. 2018, 98, 924–931. [Google Scholar] [CrossRef] [PubMed]
- Pillaiyar, T.; Namasivayam, V.; Manickam, M.; Jung, S.H. Inhibitors of melanogenesis: An updated review. J. Med. Chem. 2018, 61, 7395–7418. [Google Scholar] [CrossRef] [PubMed]
- Gunia-Krzyżak, A.; Popiol, J.; Marona, H. Melanogenesis inhibitors: Strategies for searching for and evaluation of active compounds. Curr. Med. Chem. 2016, 23, 3548–3574. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Baek, N.; Nam, T.G. Natural, semisynthetic and synthetic tyrosinase inhibitors. J. Enzyme Inhib. Med. Chem. 2016, 31, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Wakamatsu, K. Biochemical mechanism of rhododendrol-induced leukoderma. Int. J. Mol. Sci. 2018, 19, 552. [Google Scholar]
- Gabe, Y.; Miyaji, A.; Kohno, M.; Hachiya, A.; Moriwaki, S.; Baba, T. Substantial evidence for the rhododendrol-induced generation of hydroxyl radicals that causes melanocyte cytotoxicity and induces chemical leukoderma. J. Dermatol. Sci. 2018, 91, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Abe, Y.; Okamura, K.; Kawaguchi, M.; Hozumi, Y.; Aoki, H.; Kunisada, T.; Ito, S.; Wakamatsu, K.; Matsunaga, K.; Suzuki, T. Rhododenol-induced leukoderma in a mouse model mimicking Japanese skin. J. Dermatol. Sci. 2016, 81, 35–43. [Google Scholar] [CrossRef]
- Mann, T.; Gerwat, W.; Batzer, J.; Eggers, K.; Scherner, C.; Wenck, H.; Stäb, F.; Hearing, V.J.; Röhm, K.H.; Kolbe, L. Inhibition of human tyrosinase requires molecular motifs distinctively different from mushroom tyrosinase. J. Investig. Dermatol. 2018, 138, 1601–1608. [Google Scholar] [CrossRef]
- Ito, S.; Wakamatsu, K.; d’Ischia, M.; Napolitano, A.; Pezzella, A. Structure of melanins. In Melanins and Melanosomes: Biosynthesis, Biogenesis, Physiological, and Pathological Functions; Riley, P.A., Borovansky, J., Eds.; Wiley-VCH: Weinheim, Germany, 2011; pp. 167–185. [Google Scholar]
- Panzella, L.; Ebato, A.; Napolitano, A.; Koike, K. The late stages of melanogenesis: Exploring the chemical facets and the application opportunities. Int. J. Mol. Sci. 2018, 19, 1753. [Google Scholar] [CrossRef]
- Serre, C.; Busuttil, V.; Botto, J.M. Intrinsic and extrinsic regulation of human skin melanogenesis and pigmentation. Int. J. Cosmet. Sci. 2018, 40, 328–347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hurbain, I.; Romao, M.; Sextius, P.; Bourreau, E.; Marchal, C.; Bernerd, F.; Duval, C.; Raposo, G. Melanosome distribution in keratinocytes in different skin types: Melanosome clusters are not degradative organelles. J. Investig. Dermatol. 2018, 138, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Tadokoro, R.; Takahashi, Y. Intercellular transfer of organelles during body pigmentation. Curr. Opin. Genet. Dev. 2017, 45, 132–138. [Google Scholar] [CrossRef]
- Thong, H.Y.; Jee, S.H.; Sun, C.C.; Boissy, R.E. The patterns of melanosome distribution in keratinocytes of human skin as one determining factor of skin colour. Br. J. Dermatol. 2003, 149, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Brenner, M.; Hearing, V.J. The protective role of melanin against UV damage in human skin. Photochem. Photobiol. 2008, 84, 539–549. [Google Scholar] [CrossRef]
- Zubair, R.; Lyons, A.B.; Vellaichamy, G.; Peacock, A.; Hamzavi, I. What’s new in pigmentary disorders. Dermatol. Clin. 2019, 37, 175–181. [Google Scholar] [CrossRef]
- Maeda, K. Large melanosome complex is increased in keratinocytes of solar lentigo. Cosmetics 2017, 4, 49. [Google Scholar] [CrossRef]
- Plensdorf, S.; Livieratos, M.; Dada, N. Pigmentation disorders: Diagnosis and management. Am. Fam. Physician 2017, 96, 797–804. [Google Scholar]
- Ferreira Cestari, T.; Pinheiro Dantas, L.; Catucci Boza, J. Acquired hyperpigmentations. An. Bras. Dermatol. 2014, 89, 11–25. [Google Scholar] [CrossRef] [Green Version]
- Yamaguchi, Y.; Hearing, V.J. Melanocytes and their diseases. Cold Spring Harb. Perspect. Med. 2014, 4, a017046. [Google Scholar] [CrossRef]
- Cardinali, G.; Kovacs, D.; Picardo, M. Mechanisms underlying post-inflammatory hyperpigmentation: Lessons from solar lentigo. Ann. Dermatol. Venereol. 2012, 139, S148–S152. [Google Scholar] [CrossRef]
- Raper, H.S. The tyrosinase-tyrosine reaction. Production from tyrosine of 5,6-dihydroxyindole and 5,6-dihydroxyindole-2-carboxylic acid—The precursors of melanin. Biochem. J. 1927, 21, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Mason, H.S. The chemistry of melanin. Mechanism of the oxidation of dihydroxyphenylalanine by tyrosinase. J. Biol. Chem. 1948, 172, 83–99. [Google Scholar] [PubMed]
- Ito, S.; IFPCS. The IFPCS presidential lecture: A chemist’s view of melanogenesis. Pigment Cell Res. 2003, 16, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Micillo, R.; Panzella, L.; Koike, K.; Monfrecola, G.; Napolitano, A.; d’Ischia, M. “Fifty shades” of black and red or how carboxyl groups fine tune eumelanin and pheomelanin properties. Int. J. Mol. Sci. 2016, 17, 746. [Google Scholar] [CrossRef] [PubMed]
- Panzella, L.; Napolitano, A.; d’Ischia, M. Is DHICA the key to dopachrome tautomerase and melanocyte functions? Pigment Cell Melanoma Res. 2011, 24, 248–249. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Cervantes, C.; Solano, F.; Kobayashi, T.; Urabe, K.; Hearing, V.J.; Lozano, J.A.; Garcia-Borron, J.C. A new enzymatic function in the melanogenic pathway. The 5,6-dihydroxyindole-2-carboxylic acid oxidase activity of tyrosinase related protein-1 (TRP1). J. Biol. Chem. 1994, 269, 17993–18001. [Google Scholar] [PubMed]
- Olivares, C.; Solano, F. New insights into the active site structure and catalytic mechanism of tyrosinase and its related proteins. Pigment Cell Melanoma Res. 2009, 22, 750–760. [Google Scholar] [CrossRef] [PubMed]
- Niu, C.; Aisa, H.A. Upregulation of melanogenesis and tyrosinase activity: Potential agents for vitiligo. Molecules 2017, 22, 1303. [Google Scholar] [CrossRef] [PubMed]
- Pillaiyar, T.; Manickam, M.; Namasivayam, V. Skin whitening agents: Medicinal chemistry perspective of tyrosinase inhibitors. J. Enzyme Inhib. Med. Chem. 2017, 32, 403–425. [Google Scholar] [CrossRef] [PubMed]
- Solano, F. On the metal cofactor in the tyrosinase family. Int. J. Mol. Sci. 2018, 19, 633. [Google Scholar] [CrossRef] [PubMed]
- Citek, C.; Lyons, C.T.; Wasinger, E.C.; Stack, T.D.P. Self-assembly of the oxy-tyrosinase core and the fundamental components of phenolic hydroxylation. Nat. Chem. 2012, 4, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Fujieda, N.; Yabuta, S.; Ikeda, T.; Oyama, T.; Muraki, N.; Kurisu, G.; Itoh, S. Crystal structures of copper-depleted and copper-bound fungal pro-tyrosinase: Insights into endogenous cysteine-dependent copper incorporation. J. Biol. Chem. 2013, 288, 22128–22140. [Google Scholar] [CrossRef] [PubMed]
- Ramsden, C.A.; Riley, P.A. Tyrosinase: The four oxidation states of the active site and their relevance to enzymatic activation, oxidation and inactivation. Bioorg. Med. Chem. 2014, 15, 2388–2395. [Google Scholar] [CrossRef]
- Hernández-Romero, D.; Sanchez-Amat, A.; Solano, F. A tyrosinase with an abnormally high tyrosine hydroxylase/dopa oxidase ratio. FEBS J. 2006, 273, 257–270. [Google Scholar] [CrossRef] [PubMed]
- Vanitha, M.; Soundhari, C. Isolation and characterisation of mushroom tyrosinase and screening of herbal extracts for anti-tyrosinase activity. Int. J. ChemTech Res. 2017, 10, 1156–1167. [Google Scholar]
- Ito, S.; Wakamatsu, K. A convenient screening method to differentiate phenolic skin whitening tyrosinase inhibitors from leukoderma-inducing phenols. J. Dermatol. Sci. 2015, 80, 18–24. [Google Scholar] [CrossRef]
- Ioniţă, E.; Aprodu, I.; Stănciuc, N.; Râpeanu, G.; Bahrim, G. Advances in structure-function relationships of tyrosinase from Agaricus bisporus—Investigation on heat-induced conformational changes. Food Chem. 2014, 156, 129–136. [Google Scholar] [CrossRef]
- Ismaya, W.T.; Tandrasasmita, O.M.; Sundari, S.; Diana; Lai, X.; Retnoningrum, D.S.; Dijkstra, B.W.; Tjandrawinata, R.R.; Rachmawati, H. The light subunit of mushroom Agaricus bisporus tyrosinase: Its biological characteristics and implications. Int. J. Biol. Macromol. 2017, 102, 308–314. [Google Scholar] [CrossRef]
- Ismaya, W.T.; Rozeboom, H.J.; Weijn, A.; Mes, J.J.; Fusetti, F.; Wichers, H.J.; Dijkstra, B.W. Crystal structure of Agaricus bisporus mushroom tyrosinase: Identity of the tetramer subunits and interaction with tropolone. Biochemistry 2011, 50, 5477–5486. [Google Scholar] [CrossRef]
- Bourquelot, E.; Bertrand, G. La laccase dans les champignons. Compt. Rendus. 1895, 121, 783–786. [Google Scholar]
- Muñoz-Muñoz, J.L.; Garcia-Molina, F.; Varon, R.; Garcia-Ruíz, P.A.; Tudela, J.; Garcia-Cánovas, F.; Rodríguez-López, J.N. Suicide inactivation of the diphenolase and monophenolase activities of tyrosinase. IUBMB Life 2010, 62, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Haghbeen, K.; Saboury, A.A.; Karbassi, F. Substrate share in the suicide inactivation of mushroom tyrosinase. Biochim. Biophys. Acta 2004, 1675, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Land, E.J.; Ramsden, C.A.; Riley, P.A. The mechanism of suicide-inactivation of tyrosinase: A substrate structure investigation. Tohoku J. Exp. Med. 2007, 212, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.S. An updated review of tyrosinase inhibitors. Int. J. Mol. Sci 2009, 10, 2440–2475. [Google Scholar] [CrossRef] [PubMed]
- Solano, F.; Briganti, S.; Picardo, M.; Ghanem, G. Hypopigmenting agents: An updated review on biological, chemical and clinical aspects. Pigment Cell Res. 2006, 195, 550–571. [Google Scholar] [CrossRef] [PubMed]
- Hamed, S.H.; Sriwiriyanont, P.; deLong, M.A.; Visscher, M.O.; Wickett, R.R.; Boissy, R.E. Comparative efficacy and safety of deoxyarbutin, a new tyrosinase inhibiting agent. J. Cosmet. Sci. 2006, 57, 291–308. [Google Scholar] [PubMed]
- Tofani, R.P.; Sumirtapura, Y.C.; Darijanto, S.T. Formulation, characterisation, and in vitro skin diffusion of nanostructured lipid carriers for deoxyarbutin compared to a nanoemulsion and conventional cream. Sci. Pharm. 2016, 84, 634–645. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Jimenez, A.; Teruel-Puche, J.A.; Garcia-Ruiz, P.A.; Saura-Sanmartin, A.; Berna, J.; Garcia-Canovas, F.; Rodriguez-Lopez, J.N. Structural and kinetic considerations on the catalysis of deoxyarbutin by tyrosinase. PLoS ONE 2017, 12, e0187845. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Tao, G.; Chen, J.; Zheng, Z.P. Characterization of a new flavone and tyrosinase inhibition constituents from the twigs of Morus Alba L. Molecules 2016, 21, 1130. [Google Scholar] [CrossRef]
- Chung, K.W.; Jeong, H.O.; Lee, E.K.; Kim, S.J.; Chun, P.; Chung, H.Y.; Moon, H.R. Evaluation of antimelanogenic activity and mechanism of galangin in silico and in vivo. Biol. Pharm. Bull. 2018, 41, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Morgan, A.M.A.; Jeon, M.N.; Jeong, M.H.; Yang, S.Y.; Kim, Y.H. Chemical components from the stems of Pueraria lobata and their tyrosinase inhibitory activity. Nat. Prod. Sci. 2016, 22, 111–116. [Google Scholar] [CrossRef]
- Solimine, J.; Garo, E.; Wedler, J.; Rusanov, K.; Fertig, O.; Hamburger, M.; Atanassov, I.; Butterweck, V. Tyrosinase inhibitory constituents from a polyphenol enriched fraction of rose oil distillation wastewater. Fitoterapia 2016, 108, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Chaita, E.; Lambrinidis, G.; Cheimonidi, C.; Agalou, A.; Beis, D.; Trougakos, I.; Mikros, E.; Skaltsounis, A.L.; Aligiannis, N. Anti-melanogenic properties of Greek plants. A novel depigmenting agent from Morus alba wood. Molecules 2017, 22, 514. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Cui, F.; Li, H.; Huang, Q.; Li, Y. Understanding the inhibitory mechanism of tea polyphenols against tyrosinase using fluorescence spectroscopy, cyclic voltammetry, oximetry, and molecular simulations. RSC Adv. 2018, 8, 8310–8318. [Google Scholar] [CrossRef] [Green Version]
- Renda, G.; Ozel, A.; Barut, B.; Korkmaz, B.; Soral, M.; Kandemir, U.; Liptaj, T. Bioassay guided isolation of active compounds from Alchemilla barbatiflora Juz. Rec. Nat. Prod. 2018, 12, 76–85. [Google Scholar] [CrossRef]
- Lall, N.; Kishore, N.; Momtaz, S.; Hussein, A.; Naidoo, S.; Nqephe, M.; Crampton, B. Extract from Ceratonia siliqua exhibits depigmentation properties. Phytother. Res. 2015, 29, 1729–1736. [Google Scholar] [CrossRef] [PubMed]
- Kakumu, Y.; Yamauchi, K.; Mitsunaga, T. Identification of chemical constituents from the bark of Larix kaempferi and their tyrosinase inhibitory effect. Holzforschung 2019, 73, 637–643. [Google Scholar] [CrossRef]
- Uesugi, D.; Hamada, H.; Shimoda, K.; Kubota, N.; Ozaki, S.; Nagatani, N. Synthesis, oxygen radical absorbance capacity, and tyrosinase inhibitory activity of glycosides of resveratrol, pterostilbene, and pinostilbene. Biosci. Biotech. Biochem. 2017, 81, 226–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Popova, I.E.; Morra, M.J. Sinapis alba seed meal as a feedstock for extracting the natural tyrosinase inhibitor 4-hydroxybenzyl alcohol. Ind. Crops Prod. 2018, 124, 505–509. [Google Scholar] [CrossRef]
- Zuo, A.R.; Cao, S.W.; Zuo, A.R.; Dong, H.H.; Shu, Q.L.; Zheng, L.X.; Yu, X.Y.; Yu, Y.Y.; Cao, S.W. The antityrosinase and antioxidant activities of flavonoids dominated by the number and location of phenolic hydroxyl groups. Chin. Med. 2018, 13, 51. [Google Scholar] [CrossRef] [PubMed]
- Hagiwara, K.; Okura, M.; Sumikawa, Y.; Hida, T.; Kuno, A.; Horio, Y.; Yamashita, T. Biochemical effects of the flavanol-rich lychee fruit extract on the melanin biosynthesis and reactive oxygen species. J. Dermatol. 2016, 43, 1174–1183. [Google Scholar] [CrossRef] [PubMed]
- Pham, V.C.; Kim, O.; Lee, J.H.; Min, B.S.; Kim, J.A. Inhibitory effects of phloroglucinols from the roots of Dryopteris crassirhizoma on melanogenesis. Phytochem. Lett. 2017, 21, 51–56. [Google Scholar] [CrossRef]
- Luyen, B.T.T.; Thao, N.P.; Widowati, W.; Fauziah, N.; Maesaroh, M.; Herlina, T.; Kim, Y.H. Chemical constituents of Piper aduncum and their inhibitory effects on soluble epoxide hydrolase and tyrosinase. Med. Chem. Res. 2017, 26, 220–226. [Google Scholar] [CrossRef]
- Crespo, M.I.; Chaban, M.F.; Lanza, P.A.; Joray, M.B.; Palacios, S.M.; Vera, D.M.A.; Carpinella, M.C. Inhibitory effects of compounds isolated from Lepechinia meyenii on tyrosinase. Food Chem. Toxicol. 2019, 125, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Kishore, N.; Twilley, D.; Blom van Staden, A.; Verma, P.; Singh, B.; Cardinali, G.; Kovacs, D.; Picardo, M.; Kumar, V.; Lall, N. Isolation of flavonoids and flavonoid glycosides from Myrsine africana and their inhibitory activities against mushroom tyrosinase. J. Nat. Prod. 2018, 81, 49–56. [Google Scholar] [CrossRef]
- Abed, S.A.; Sirat, H.M.; Taber, M. Tyrosinase inhibition, anti-acetylcholinesterase, and antimicrobial activities of the phytochemicals from Gynotroches axillaris Blume. Pak. J. Pharm. Sci. 2016, 29, 2071–2078. [Google Scholar] [PubMed]
- Omar, S.H.; Scott, C.J.; Hamlin, A.S.; Obied, H.K. Biophenols: Enzymes (β-secretase, cholinesterases, histone deacetylase and tyrosinase) inhibitors from olive (Olea europaea L.). Fitoterapia 2018, 128, 118–129. [Google Scholar] [CrossRef]
- Kim, D.H.; Lee, J.H. Comparative evaluation of phenolic phytochemicals from perilla seeds of diverse species and screening for their tyrosinase inhibitory and antioxidant properties. S. Afr. J. Bot. 2019, 123, 341–350. [Google Scholar] [CrossRef]
- Guo, N.; Wang, C.; Shang, C.; You, X.; Zhang, L.; Liu, W. Integrated study of the mechanism of tyrosinase inhibition by baicalein using kinetic, multispectroscopic and computational simulation analyses. Int. J. Biol. Macromol. 2018, 118, 57–68. [Google Scholar] [CrossRef]
- Shang, C.; Zhang, Y.; You, X.; Guo, N.; Wang, Y.; Fan, Y.; Liu, W. The effect of 7,8,4’-trihydroxyflavone on tyrosinase activity and conformation: Spectroscopy and docking studies. Luminescence 2018, 33, 681–691. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.X.; Nguyen, N.T.; Nguyen, M.H.K.; Le, T.H.; Do, T.N.V.; Hung, T.M.; Nguyen, M.T.T. Tyrosinase inhibitory activity of flavonoids from Artocarpus heterophyllous. Chem. Cent. J. 2016, 10, 2. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, S.; Huang, Z.; Huang, W.; Li, Q.; Ye, Y. Molecular inhibitory mechanism of dihydromyricetin on mushroom tyrosinase. J. Biomol. Struct. Dyn. 2018, 36, 3740–3752. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.Y.; Chiang, C.M.; Tzeng, W.M.; Chang, T.S. Identification of 3′-hydroxygenistein as a potent melanogenesis inhibitor from biotransformation of genistein by recombinant Pichia pastoris. Process. Biochem. 2015, 50, 1614–1617. [Google Scholar] [CrossRef]
- Casedas, G.; Les, F.; Gonzalez-Burgos, E.; Gomez-Serranillos, M.P.; Smith, C.; Lopez, V. Cyanidin-3-O-glucoside inhibits different enzymes involved in central nervous system pathologies and type-2 diabetes. S. Afr. J. Bot. 2019, 120, 241–246. [Google Scholar] [CrossRef]
- Xiong, S.L.; Lim, G.T.; Yin, S.J.; Lee, J.; Si, Y.X.; Yang, J.M.; Park, Y.D.; Qian, G.Y. The inhibitory effect of pyrogallol on tyrosinase activity and structure: Integration study of inhibition kinetics with molecular dynamics simulation. Int. J. Biol. Macromol. 2019, 121, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Boghrati, Z.; Naseri, M.; Rezaie, M.; Pham, N.; Quinn, R.J.; Tayarani-Najaran, Z.; Iranshahi, M. Tyrosinase inhibitory properties of phenylpropanoid glycosides and flavonoids from Teucrium polium L. var. gnaphalodes. Iran. J. Basic Med. Sci. 2016, 19, 804–811. [Google Scholar]
- Zeng, H.J.; Liu, Z.; Hu, G.Z.; Qu, L.B.; Yang, R. Investigation on the binding of aloe-emodin with tyrosinase by spectral analysis and molecular docking. Spectrochim. Acta Mol. Biomol. Spectrosc. 2019, 211, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.M.; Ko, H.H. A new anthraquinone glycoside from Rhamnus nakaharai and anti-tyrosinase effect of 6-methoxysorigenin. Nat. Prod. Res. 2016, 30, 2655–2661. [Google Scholar] [CrossRef]
- Kim, S.S.; Park, K.J.; JooAn, H.; Choi, Y.H. Inhibitory kinetics of enterolactone on mushroom tyrosinase. Der. Pharma Chem. 2016, 8, 124–130. [Google Scholar]
- Magid, A.A.; Schmitt, M.; Prin, P.C.; Pasquier, L.; Voutquenne-Nazabadioko, L. In vitro tyrosinase inhibitory and antioxidant activities of extracts and constituents of Paeonia lactiflora Pall. flowers. Nat. Prod. J. 2017, 7, 237–245. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, L.Y.; Liu, X.; Feng, L.H.; Zhou, Z.W.; He, X.R.; Ren, G. Artopithecins A-D, prenylated 2-arylbenzofurans from the twigs of Artocarpus pithecogallus and their tyrosinase inhibitory activities. Chem. Pharm Bull. 2018, 66, 1199–1202. [Google Scholar] [CrossRef]
- Abdullah, S.A.; Jamil, S.; Basar, N.; Abdul, L.; Siti, M.; Mohd Arriffin, N. Flavonoids from the leaves and heartwoods of Artocarpus lowii King and their bioactivities. Nat. Prod. Res. 2017, 31, 1113–1120. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.J.; Lin, C.C.; Lu, T.M.; Li, J.H.; Chen, I.S.; Kuo, Y.H.; Ko, H.H. Chemical constituents derived from Artocarpus xanthocarpus as inhibitors of melanin biosynthesis. Phytochemistry 2015, 117, 424–435. [Google Scholar] [CrossRef]
- Lathiff, S.M.A.; Jemaon, N.; Abdullah, S.A.; Jamil, S. Flavonoids from Artocarpus anisophyllus and their bioactivities. Nat. Prod. Commun. 2015, 10, 393–396. [Google Scholar] [CrossRef]
- Nguyen, M.T.T.; Le, T.H.; Nguyen, H.X.; Dang, P.H.; Do, T.N.V.; Abe, M.; Takagi, R.; Nguyen, N.T. Artocarmins G-M, prenylated 4-chromenones from the stems of Artocarpus rigida and their tyrosinase inhibitory activities. J. Nat. Prod. 2017, 80, 3172–3178. [Google Scholar] [CrossRef] [PubMed]
- Duc, L.V.; Thanh, T.B.; Thu, H.L.T.; Bach, N.V. Chemical constituents and tyrosinase inhibitory activity of aqueous fraction of the leaves of Morus alba L. from Vietnam. Int. J. Pharmacogn. 2018, 5, 399–403. [Google Scholar]
- Koirala, P.; Seong, S.H.; Zhou, Y.; Shrestha, S.; Jung, H.A.; Choi, J.S. Structure-activity relationship of the tyrosinase inhibitors kuwanon G, mulberrofuran G, and albanol B from Morus species: A kinetics and molecular docking study. Molecules 2018, 23, 1413. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Huang, C.; Wu, C.; Huang, T.; Jia, A.; Hu, X. Chiral separation, absolute configuration, and bioactivity of two pairs of flavonoid enantiomers from Morus nigra. Phytochemistry 2019, 16333–16337. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Yu, M.H.; Yan, G.R.; Wang, H.Y.; Hou, A.J.; Lei, C. Isoprenylated phenolic compounds with tyrosinase inhibition from Morus nigra. J. Asian Nat. Prod. Res. 2018, 20, 488–493. [Google Scholar] [CrossRef]
- Tian, J.L.; Liu, T.L.; Xue, J.J.; Hong, W.; Zhang, Y.; Zhang, D.X.; Cui, C.C.; Liu, M.C.; Niu, S.L. Flavanoids derivatives from the root bark of Broussonetia papyrifera as a tyrosinase inhibitor. Ind. Crops Prod. 2019, 138, 111445. [Google Scholar] [CrossRef]
- Santi, M.D.; Peralta, M.A.; Mendoza, C.S.; Cabrera, J.L.; Ortega, M.G. Chemical and bioactivity of flavanones obtained from roots of Dalea pazensis Rusby. Bioorg. Med. Chem. Lett. 2017, 27, 1789–1794. [Google Scholar] [CrossRef] [PubMed]
- Sabudak, T.; Ozturk, M.; Alpay, E. New bioflavonoids from Solanum nigrum L. by anticholinesterase and anti-tyrosinase activities-guided fractionation. Rec. Nat. Prod. 2017, 11, 130–140. [Google Scholar]
- Promden, W.; Viriyabancha, W.; Monthakantirat, O.; Umehara, K.; Noguchi, H.; Umehara, K.; Noguchi, H.; De-Eknamkul, W. Correlation between the potency of flavonoids on mushroom tyrosinase inhibitory activity and melanin synthesis in melanocytes. Molecules 2018, 9, 1403. [Google Scholar] [CrossRef] [PubMed]
- Chai, W.M.; Wei, Q.M.; Deng, W.L.; Zheng, Y.L.; Chen, X.Y.; Huang, Q.; Chong, O.Y.; Peng, Y.Y. Anti-melanogenesis properties of condensed tannins from Vigna angularis seeds with potent antioxidant and DNA damage protection activities. Food Funct. 2019, 10, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Song, W.; Sun, K.K.; Du, H.W.; Wei, S.D. Structure elucidation and evaluation of antioxidant and tyrosinase inhibitory effect and mechanism of proanthocyanidins from leaf and fruit of Leucaena leucocephala. J. Wood Chem. Technol. 2018, 38, 430–444. [Google Scholar] [CrossRef]
- Chai, W.M.; Ou-Yang, C.; Huang, Q.; Lin, M.Z.; Wang, Y.X.; Xu, K.L.; Huang, W.Y.; Pang, D.D. Antityrosinase and antioxidant properties of mung bean seed proanthocyanidins: Novel insights into the inhibitory mechanism. Food Chem. 2018, 260, 27–36. [Google Scholar] [CrossRef]
- Chai, W.M.; Huang, Q.; Lin, M.Z.; Chong, O.Y.; Huang, W.Y.; Wang, Y.X.; Xu, K.L.; Feng, H.L. Condensed tannins from Longan bark as inhibitor of tyrosinase: Structure, activity, and mechanism. J. Agric. Food Chem. 2018, 66, 908–917. [Google Scholar] [CrossRef]
- Song, W.; Qin, S.T.; Fang, F.X.; Gao, Z.J.; Liang, D.D.; Liu, L.L.; Tian, H.T.; Yang, H.B. Isolation and purification of condensed tannin from the leaves and branches of Prunus cerasifera and its structure and bioactivities. Appl. Biochem. Biotechnol. 2018, 185, 464–475. [Google Scholar] [CrossRef]
- Chai, W.M.; Lin, M.Z.; Wang, Y.X.; Xu, K.L.; Huang, W.Y.; Pan, D.D.; Zou, Z.R.; Peng, Y.Y. Inhibition of tyrosinase by cherimoya pericarp proanthocyanidins: Structural characterization, inhibitory activity and mechanism. Food Res. Int. 2017, 100, 731–739. [Google Scholar] [CrossRef]
- Chai, W.M.; Lin, M.Z.; Feng, H.L.; Zou, Z.R.; Wang, Y.X. Proanthocyanidins purified from fruit pericarp of Clausena lansium (Lour.) Skeels as efficient tyrosinase inhibitors: Structure evaluation, inhibitory activity and molecular mechanism. Food Funct. 2017, 8, 1043–1051. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Zhu, X.F.; Ding, X.D.; Yang, H.B.; Qin, S.T.; Chen, H.; Wei, S.D. Structural features, antioxidant and tyrosinase inhibitory activities of proanthocyanidins in leaves of two tea cultivars. Int. J. Food Prop. 2017, 20, 1348–1358. [Google Scholar] [CrossRef]
- Deng, Y.T.; Liang, G.; Shi, Y.; Li, H.L.; Zhang, J.; Mao, X.M.; Fu, Q.R.; Peng, W.X.; Chen, Q.X.; Shen, D.Y. Condensed tannins from Ficus altissima leaves: Structural, antioxidant, and antityrosinase properties. Process. Biochem. 2016, 51, 1092–1099. [Google Scholar] [CrossRef]
- Chai, W.M.; Wang, R.; Wei, M.K.; Zou, Z.R.; Deng, R.G.; Liu, W.S.; Peng, Y.Y. Proanthocyanidins extracted from Rhododendron pulchrum leaves as source of tyrosinase inhibitors: Structure, activity, and mechanism. PLoS ONE 2015, 10, e0145483. [Google Scholar] [CrossRef] [PubMed]
- Chai, W.M.; Wei, M.K.; Wang, R.; Deng, R.G.; Zou, Z.R.; Peng, Y.Y. Avocado proanthocyanidins as a source of tyrosinase inhibitors: Structure characterization, inhibitory activity, and mechanism. J. Agric. Food Chem. 2015, 63, 7381–7387. [Google Scholar] [CrossRef]
- Gong, C.F.; Wang, Y.X.; Wang, M.L.; Su, W.C.; Wang, Q.; Chen, Q.X.; Shi, Y. Evaluation of the structure and biological activities of condensed tannins from Acanthus ilicifolius Linn and their effect on fresh-cut fuji apples. Appl. Biochem. Biotechnol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Kuang, Y.; Li, K.; Wang, S.; Song, W.; Qiao, X.; Sabir, G.; Ye, M. Screening for bioactive natural products from a 67-compound library of Glycyrrhiza inflate. Bioorg. Med. Chem. 2017, 25, 3706–3713. [Google Scholar] [CrossRef]
- Li, K.; Ji, S.; Song, W.; Kuang, Y.; Lin, Y.; Tang, S.; Cui, Z.; Qiao, X.; Yu, S.; Ye, M. Glycybridins A-K, bioactive phenolic compounds from Glycyrrhiza glabra. J. Nat. Prod. 2017, 80, 334–346. [Google Scholar] [CrossRef]
- Ji, S.; Li, Z.; Song, W.; Wang, Y.; Liang, W.; Li, K.; Tang, S.; Wang, Q.; Qiao, X.; Zhou, D.; et al. Bioactive constituents of Glycyrrhiza uralensis (licorice): Discovery of the effective components of a traditional herbal medicine. J. Nat. Prod. 2016, 79, 281–292. [Google Scholar] [CrossRef]
- Tao, Y.; Su, D.; Du, Y.; Li, W.; Cai, B.; Di, L.; Shi, L.; Hu, L. Magnetic solid-phase extraction coupled with HPLC-Q-TOF-MS for rapid analysis of tyrosinase binders from San-Bai decoction by Box-Behnken statistical design. RSC Adv. 2016, 6, 109730–109741. [Google Scholar] [CrossRef]
- Magid, A.A.; Abdellah, A.; Pecher, V.; Pasquier, L.; Harakat, D.; Voutquenne-Nazabadioko, L. Flavonol glycosides and lignans from the leaves of Opilia amentacea. Phytochem. Lett. 2017, 21, 84–89. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kim, J.Y.; Jenis, J.; Li, Z.P.; Ban, Y.J.; Baiseitova, A.; Park, K.H. Tyrosinase inhibitory study of flavonolignans from the seeds of Silybum marianum (Milk thistle). Bioorg. Med. Chem. 2019, 27, 2499–2507. [Google Scholar] [CrossRef] [PubMed]
- Setyawati, A.; Hirabayashi, K.; Yamauchi, K.; Hattori, H.; Mitsunaga, T.; Batubara, I.; Heryanto, R.; Hashimoto, H.; Hotta, M. Melanogenesis inhibitory activity of components from Salam leaf (Syzygium polyanthum) extract. J. Nat. Med. 2018, 72, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.W.; Woo, H.S.; Kim, J.Y.; Ryuk, J.A.; Park, K.H.; Ko, B.S. Phenols displaying tyrosinase inhibition from Humulus lupulus. J. Enzyme Inhib. Med. Chem. 2016, 31, 742–747. [Google Scholar] [PubMed]
- Sasaki, A.; Yamano, Y.; Sugimoto, S.; Otsuka, H.; Matsunami, K.; Shinzato, T. Phenolic compounds from the leaves of Breynia officinalis and their tyrosinase and melanogenesis inhibitory activities. J. Nat. Med. 2018, 72, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.G.; Cha, B.J.; Seo, W.D.; Jeong, R.H.; Shrestha, S.; Kim, J.Y.; Kang, H.C.; Baek, N.I. Feruloyl sucrose esters from Oryza sativa roots and their tyrosinase inhibition activity. Chem. Nat. Compd. 2015, 51, 1094–1098. [Google Scholar] [CrossRef]
- Peng, W.W.; Wang, Z.Q.; Ji, M.Y.; Liao, Z.L.; Liu, Z.Q.; Wu, P. Tyrosinase inhibitory activity of three new glycosides from Breynia fruticosa. Phytochem. Lett. 2017, 22, 1–5. [Google Scholar] [CrossRef]
- Kim, S.B.; Liu, Q.; Ahn, J.H.; Jo, Y.H.; Turk, A.; Hong, I.P.; Han, S.M.; Hwang, B.Y.; Lee, M.K. Polyamine derivatives from the bee pollen of Quercus mongolica with tyrosinase inhibitory activity. Bioorg. Chem. 2018, 81, 127–133. [Google Scholar] [CrossRef]
- Yang, L.; Yang, Y.L.; Dong, W.H.; Li, W.; Wang, P.; Cao, X.; Yuan, J.Z.; Chen, H.Q.; Mei, W.L.; Dai, H.F. Sesquiterpenoids and 2-(2-phenylethyl)chromones respectively acting as α-glucosidase and tyrosinase inhibitors from agarwood of an Aquilaria plant. J. Enzyme Inhib. Med. Chem. 2019, 34, 853–862. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.M.; Yang, L.; Dong, W.H.; Li, W.; Chen, H.Q.; Wang, H.; Cai, C.H.; Gai, C.J.; Mei, W.L.; Dai, H.F. Three new 2-(2-phenylethyl)chromone derivatives from agarwood of Aquilaria crassna Pierre ex Lecomte (Thymelaeaceae) in Laos. Phytochem. Lett. 2019, 32, 134–137. [Google Scholar] [CrossRef]
- Lee, G.Y.; Cho, B.O.; Shin, J.Y.; Jang, S.I.; Cho, I.S.; Kim, H.Y.; Park, J.S.; Cho, C.W.; Kang, J.S.; Kim, J.H.; et al. Tyrosinase inhibitory components from the seeds of Cassia tora. Arch. Pharm. Res. 2018, 41, 490–496. [Google Scholar] [CrossRef] [PubMed]
- Ullah, S.; Park, Y.; Park, C.; Lee, S.; Kang, D.; Yang, J.; Akter, J.; Chun, P.; Moon, H.R. Antioxidant, anti-tyrosinase and anti-melanogenic effects of (E)-2,3-diphenylacrylic acid derivatives. Bioorg. Med. Chem. 2019, 27, 2192–2200. [Google Scholar] [CrossRef] [PubMed]
- Gur, Z.T.; Senol, F.S.; Shekfeh, S.; Orhan, I.E.; Banoglu, E.; Caliskan, B. Novel piperazine amides of cinnamic acid derivatives as tyrosinase inhibitors. Lett. Drug Des. Discov. 2019, 16, 36–44. [Google Scholar] [CrossRef]
- Kim, S.J.; Yang, J.; Lee, S.; Park, C.; Kang, D.; Akter, J.; Ullah, S.; Kim, Y.J.; Chun, P.; Moon, H.R. The tyrosinase inhibitory effects of isoxazolone derivatives with a (Z)-β-phenyl-α, β-unsaturated carbonyl scaffold. Bioorg. Med. Chem. 2018, 26, 3882–3889. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.K.; Lee, E.; Hwang, I.J.; Yim, D.B.; Han, J.; Lee, Y.S.; Kim, J.H. β-Lactoglobulin peptide fragments conjugated with caffeic acid displaying dual activities for tyrosinase inhibition and antioxidant effect. Bioconjugate Chem. 2018, 29, 1000–1005. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.J.; Lee, M.J.; Park, Y.J.; Noh, S.G.; Lee, A.K.; Moon, K.M.; Lee, E.K.; Bang, E.J.; Park, Y.J.; Kim, S.J.; et al. A novel synthetic compound, (Z)-5-(3-hydroxy-4-methoxybenzylidene)-2-iminothiazolidin-4-one (MHY773) inhibits mushroom tyrosinase. Biosci. Biotech. Biochem. 2018, 82, 759–767. [Google Scholar] [CrossRef] [PubMed]
- Bang, E.; Lee, E.K.; Noh, S.G.; Jung, H.J.; Moon, K.M.; Park, M.H.; Park, Y.J.; Hyun, M.K.; Lee, A.K.; Kim, S.J.; et al. In vitro and in vivo evidence of tyrosinase inhibitory activity of a synthesized (Z)-5-(3-hydroxy-4-methoxybenzylidene)-2-thioxothiazolidin-4-one (5-HMT). Exp. Dermatol. 2019, 28, 734–737. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.H.; Lee, B.; Son, S.; Yun, H.Y.; Moon, K.M.; Jeong, H.O.; Kim, D.H.; Lee, E.K.; Choi, Y.J.; Kim, D.H.; et al. (Z)-2-(Benzo[d]thiazol-2-ylamino)-5-(substituted benzylidene)thiazol-4(5H)-one derivatives as novel tyrosinase inhibitors. Biol. Pharm. Bull. 2015, 38, 1227–1233. [Google Scholar] [CrossRef]
- Abbas, Q.; Ashraf, Z.; Hassan, M.; Nadeem, H.; Latif, M.; Afzal, S.; Seo, S.Y. Development of highly potent melanogenesis inhibitor by in vitro, in vivo and computational studies. Drug Des. Devel. Ther. 2017, 11, 2029–2046. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, Z.; Rafiq, M.; Seo, S.Y.; Babar, M.M.; Zaidi, N.U. Synthesis, kinetic mechanism and docking studies of vanillin derivatives as inhibitors of mushroom tyrosinase. Bioorg. Med. Chem. 2015, 23, 5870–5880. [Google Scholar] [CrossRef]
- Ullah, S.; Park, C.; Ikram, M.; Kang, D.; Lee, S.; Yang, J.; Park, Y.; Yoon, S.; Chun, P.; Moon, H.R. Tyrosinase inhibition and anti-melanin generation effect of cinnamamide analogues. Bioorg. Chem. 2019, 87, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Ullah, S.; Kang, D.; Lee, S.; Ikram, M.; Park, C.; Park, Y.; Yoon, S.; Chun, P.; Moon, H.R. Synthesis of cinnamic amide derivatives and their anti-melanogenic effect in α-MSH-stimulated B16F10 melanoma cells. Eur. J. Med. Chem. 2019, 161, 78–92. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.S.; Noh, S.G.; Park, Y.; Kang, D.; Chun, P.; Chung, H.Y.; Jung, H.J.; Moon, H.R. A potent tyrosinase inhibitor, (E)-3-(2,4-dihydroxyphenyl)-1-(thiophen-2-yl)prop-2-en-1-one, with anti-melanogenesis properties in α-MSH and IBMX-induced B16F10 melanoma cells. Molecules 2018, 23, 2725. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.J.; Lee, A.K.; Park, Y.J.; Lee, S.; Kang, D.; Jung, Y.S.; Chung, H.Y.; Moon, H.R. (2E,5E)-2,5-Bis(3-hydroxy-4-methoxybenzylidene) cyclopentanone exerts anti-melanogenesis and anti-wrinkle activities in B16F10 melanoma and Hs27 fibroblast cells. Molecules 2018, 23, 1415. [Google Scholar] [CrossRef] [PubMed]
- Micillo, R.; Sires-Campos, J.; Garcia-Borron, J.C.; Panzella, L.; Napolitano, A.; Olivares, C. Conjugation with dihydrolipoic acid imparts caffeic acid ester potent inhibitory effect on dopa oxidase activity of human tyrosinase. Int. J. Mol. Sci. 2018, 19, 2156. [Google Scholar] [CrossRef]
- Micillo, R.; Pistorio, V.; Pizzo, E.; Panzella, L.; Napolitano, A.; d’Ischia, M. 2-S-lipoylcaffeic acid, a natural product-based entry to tyrosinase inhibition via catechol manipulation. Biomimetics 2017, 2, 15. [Google Scholar] [CrossRef]
- Bang, E.; Noh, S.G.; Ha, S.; Jung, H.J.; Kim, D.H.; Lee, A.K.; Hyun, M.K.; Kang, D.; Lee, S.; Park, C.; et al. Evaluation of the novel synthetic tyrosinase inhibitor (Z)-3-(3-bromo-4-hydroxybenzylidene) thiochroman-4-one (MHY1498) in vitro and in silico. Molecules 2018, 23, 3307. [Google Scholar] [CrossRef]
- Ha, J.H.; Park, S.N. Dimeric cinnamoylamide analogues for regulation of tyrosinase activity in melanoma cells: A role of diamide-link chain length. Bioorg. Med. Chem. 2018, 26, 6015–6022. [Google Scholar] [CrossRef]
- Sheng, Z.; Ge, S.; Xu, X.; Zhang, Y.; Wu, P.; Zhang, K.; Xu, X.; Li, C.; Zhao, D.; Tang, X. Design, synthesis and evaluation of cinnamic acid ester derivatives as mushroom tyrosinase inhibitors. Medchemcomm 2018, 9, 853–861. [Google Scholar] [CrossRef]
- Sheng, Z.; Ge, S.; Xu, X.; Zhang, Y.; Wu, P.; Zhang, K.; Xu, X.; Li, C.; Zhao, D.; Tang, X. Correction: Design, synthesis and evaluation of cinnamic acid ester derivatives as mushroom tyrosinase inhibitors. Medchemcomm 2018, 9, 897. [Google Scholar] [CrossRef]
- Aliabadi, R.S.; Mahmoodi, N.O.; Ghafoori, H.; Roohi, H.; Pourghasem, V. Design and synthesis of novel bis-hydroxychalcones with consideration of their biological activities. Res. Chem. Intermediat. 2018, 44, 2999–3015. [Google Scholar] [CrossRef]
- Suthar, S.K.; Bansal, S.; Narkhede, N.; Guleria, M.; Alex, A.T.; Joseph, A. Design, synthesis and biological evaluation of oxindole-based chalcones as small-molecule inhibitors of melanogenic tyrosinase. Chem. Pharm. Bull. 2017, 65, 833–839. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, S.K.; Shimmon, R.G.; Conn, C.; Baker, A.T. Inhibitory kinetics of azachalcones and their oximes on mushroom tyrosinase: A facile solid-state synthesis. Chem. Biodivers. 2016, 13, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, S.; Shimmon, R.; Conn, C.; Baker, A. Development of hydroxylated naphthylchalcones as polyphenol oxidase inhibitors: Synthesis, biochemistry and molecular docking studies. Bioorg. Chem. 2015, 63, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, S.; Shimmon, R.; Conn, C.; Baker, A. Integrated kinetic studies and computational analysis on naphthyl chalcones as mushroom tyrosinase inhibitors. Bioorg. Med. Chem. Lett. 2015, 25, 4085–4091. [Google Scholar] [CrossRef] [PubMed]
- Suthar, S.K.; Aggarwal, V.; Chauhan, M.; Sharma, A.; Bansal, S.; Sharma, M. Molecular docking and biological evaluation of hydroxy-substituted (Z)-3-benzylideneindolin-2-one chalcones for the lead identification as tyrosinase inhibitors. Med. Chem. Res. 2015, 24, 1331–1341. [Google Scholar] [CrossRef]
- Zheng, Z.P.; Zhang, Y.N.; Zhang, S.; Chen, J. One-pot green synthesis of 1,3,5-triarylpentane-1,5-dione and triarylmethane derivatives as a new class of tyrosinase inhibitors. Bioorg. Med. Chem. Lett. 2016, 26, 795–798. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Zhang, Y.; He, J.L.; Zhang, S.; Zeng, M.M.; Chen, J.; Zheng, Z.P. Preparation of tyrosinase inhibitors and antibrowning agents using green technology. Food Chem. 2016, 197, 589–596. [Google Scholar] [CrossRef]
- Radhakrishnan, S.K.; Shimmon, R.G.; Conn, C.; Baker, A.T. Azachalcones: A new class of potent polyphenol oxidase inhibitors. Bioorg. Med. Chem. Lett. 2015, 25, 1753–1756. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, S.K.; Shimmon, R.G.; Conn, C.; Baker, A.T. Evaluation of novel chalcone oximes as inhibitors of tyrosinase and melanin formation in B16 cells. Archiv der Pharmazie 2016, 349, 20–29. [Google Scholar] [CrossRef]
- Qin, H.L.; Shang, Z.P.; Jantan, I.; Tan, O.U.; Hussain, M.A.; Sher, M.; Bukhari, S.N.A. Molecular docking studies and biological evaluation of chalcone based pyrazolines as tyrosinase inhibitors and potential anticancer agents. RSC Adv. 2015, 5, 46330–46338. [Google Scholar] [CrossRef]
- Kim, B.H.; Park, K.C.; Park, J.H.; Lee, C.G.; Ye, S.K.; Park, J.Y. Inhibition of tyrosinase activity and melanin production by the chalcone derivative 1-(2-cyclohexylmethoxy-6-hydroxy-phenyl)-3-(4-hydroxymethyl- phenyl)-propenone. Biochem. Biophys. Res. Commun. 2016, 480, 648–654. [Google Scholar] [CrossRef] [PubMed]
- Ranjbar, S.; Akbari, A.; Edraki, N.; Khoshneviszadeh, M.; Hemmatian, H.; Firuzi, O.; Khoshneviszadeh, M. 6-Methoxy-3,4-dihydronaphthalenone chalcone-like derivatives as potent tyrosinase inhibitors and radical scavengers. Lett. Drug Des. Discov. 2018, 15, 1170–1179. [Google Scholar] [CrossRef]
- Radhakrishnan, S.; Shimmon, R.; Conn, C.; Baker, A. Design, synthesis and biological evaluation of hydroxy substituted amino chalcone compounds for antityrosinase activity in B16 cells. Bioorg. Chem. 2015, 62, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, S.; Shimmon, R.; Conn, C.; Baker, A. Inhibitory kinetics of novel 2,3-dihydro-1H-inden-1-one chalcone-like derivatives on mushroom tyrosinase. Bioorg. Med. Chem. Lett. 2015, 25, 5495–5499. [Google Scholar] [CrossRef]
- Brotzman, N.; Xu, Y.; Graybill, A.; Cocolas, A.; Ressler, A.; Seeram, N.P.; Ma, H.; Henry, G.E. Synthesis and tyrosinase inhibitory activities of 4-oxobutanoate derivatives of carvacrol and thymol. Bioorg. Med. Chem. Lett. 2019, 29, 56–58. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, Z.; Rafiq, M.; Seo, S.Y.; Kwon, K.S.; Babar, M.M.; Zaidi, N.U. Kinetic and in silico studies of novel hydroxy-based thymol analogues as inhibitors of mushroom tyrosinase. Eur. J. Med. Chem. 2015, 98, 203–211. [Google Scholar] [CrossRef]
- Ashraf, Z.; Rafiq, M.; Nadeem, H.; Hassan, M.; Afzal, S.; Waseem, M.; Afzal, K.; Latip, J. Carvacrol derivatives as mushroom tyrosinase inhibitors; synthesis, kinetics mechanism and molecular docking studies. PLoS ONE 2017, 12, e0178069. [Google Scholar] [CrossRef]
- Iwadate, T.; Nihei, K. Rhododendrol glycosides as stereospecific tyrosinase inhibitors. Bioorg. Med. Chem. 2015, 23, 6650–6658. [Google Scholar] [CrossRef] [Green Version]
- Ali, A.; Ashraf, Z.; Kumar, N.; Rafiq, M.; Jabeen, F.; Park, J.H.; Choi, K.H.; Lee, S.; Seo, S.Y.; Choi, E.H.; et al. Influence of plasma-activated compounds on melanogenesis and tyrosinase activity. Sci. Rep. 2016, 6, 21779. [Google Scholar] [CrossRef]
- Matsumoto, T.; Nakajima, T.; Iwadate, T.; Nihei, K. Chemical synthesis and tyrosinase-inhibitory activity of isotachioside and its related glycosides. Carbohydr. Res. 2018, 465, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Kim, S.J.; Ullah, S.; Yun, H.Y.; Chun, P.; Moon, H.R. Design, synthesis, and antimelanogenic effects of (2-substituted phenyl-1,3-dithiolan-4-yl) methanol derivatives. Drug Des. Devel. Ther. 2017, 11, 827–836. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hao, M.M.; Sun, Y.; Wang, L.F.; Wang, H.; Zhang, Y.J.; Li, H.Y.; Zhuang, P.W.; Yang, Z. Synergistic promotion on tyrosinase inhibition by antioxidants. Molecules 2018, 23, 106. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Yang, H.; Mo, J.; Sun, H.; Chen, Y.; Wu, Y.; Kang, C.; Sun, Y. Identification by shape-based virtual screening and evaluation of new tyrosinase inhibitors. PeerJ 2018, 6, e4206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishioka, W.; Oonuki, S.; Iwadate, T.; Nihei, K. Resorcinol alkyl glucosides as potent tyrosinase inhibitors. Bioorg. Med. Chem. Lett. 2019, 29, 313–316. [Google Scholar] [CrossRef]
- Garcia-Jimenez, A.; Teruel-Puche, J.A.; Ortiz-Ruiz, C.V.; Berna, J.; Tudela, J.; Garcia-Canovas, F. 4-n-butylresorcinol, a depigmenting agent used in cosmetics, reacts with tyrosinase. IUBMB Life 2016, 68, 663–672. [Google Scholar] [CrossRef]
- Ortiz-Ruiz, C.V.; Berna, J.; Rodriguez-Lopez, J.N.; Tomas, V.; Garcia-Canovas, F. Tyrosinase-catalyzed hydroxylation of 4-hexylresorcinol, an antibrowning and depigmenting agent: A kinetic study. J. Agric. Food Chem. 2015, 63, 7032–7040. [Google Scholar] [CrossRef] [PubMed]
- Ismail, T.; Shafi, S.; Srinivas, J.; Sarkar, D.; Qurishi, Y.; Khazir, J.; Alam, M.S.; Kumar, H.M.S. Synthesis and tyrosinase inhibition activity of trans-stilbene derivatives. Bioorg. Chem. 2016, 64, 97–102. [Google Scholar] [CrossRef]
- Tanaka, Y.; Suzuki, M.; Kodachi, Y.; Nihei, K. Molecular design of potent, hydrophilic tyrosinase inhibitors based on the natural dihydrooxyresveratrol skeleton. Carbohydr. Res. 2019, 472, 42–49. [Google Scholar] [CrossRef]
- Ashraf, Z.; Rafiq, M.; Seo, S.Y.; Babar, M.M.; Zaidi, N.U. Design, synthesis and bioevaluation of novel umbelliferone analogues as potential mushroom tyrosinase inhibitors. J. Enzyme Inhib. Med. Chem. 2015, 30, 874–883. [Google Scholar] [CrossRef] [Green Version]
- Kamauchi, H.; Noji, M.; Kinoshita, K.; Takanami, T.; Koyama, K. Coumarins with an unprecedented tetracyclic skeleton and coumarin dimers from chemically engineered extracts of a marine-derived fungus. Tetrahedron 2018, 74, 2846–2856. [Google Scholar] [CrossRef]
- Pintus, F.; Matos, M.J.; Vilar, S.; Hripcsak, G.; Varela, C.; Uriarte, E.; Santana, L.; Borges, F.; Medda, R.; Di Petrillo, A.; et al. New insights into highly potent tyrosinase inhibitors based on 3-heteroarylcoumarins: Anti-melanogenesis and antioxidant activities, and computational molecular modeling studies. Bioorg. Med. Chem. 2017, 25, 1687–1695. [Google Scholar] [CrossRef] [PubMed]
- Molnar, M.; Kovac, T.; Strelec, I. Umbelliferone-thiazolidinedione hybrids as potent mushroom tyrosinase inhibitors. Int. J. Pharm. Res. Allied Sci. 2016, 5, 305–310. [Google Scholar]
- Santi, M.D.; Peralta, M.A.; Puiatti, M.; Cabrera, J.L.; Ortega, M.G. Melanogenic inhibitory effects of triangularin in B16F0 melanoma cells, in vitro and molecular docking studies. Bioorg. Med. Chem. 2019, 27, 3722–3728. [Google Scholar] [CrossRef]
- Lee, J.Y.; Cho, Y.R.; Park, J.H.; Ahn, E.K.; Jeong, W.; Shin, H.S.; Kim, M.S.; Yang, S.H.; Oh, J.S. Anti-melanogenic and anti-oxidant activities of ethanol extract of Kummerowia striata: Kummerowia striata regulate anti-melanogenic activity through down-regulation of TRP-1, TRP-2 and MITF expression. Toxicol. Rep. 2019, 6, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, H.; Kawakami, F.; Lwin, T.T.; Imai, M.; Shamsa, F. Biochemical characterization of ferulic acid and caffeic acid which effectively inhibit melanin synthesis via different mechanisms in B16 melanoma cells. Biol. Pharm. Bull. 2018, 41, 806–810. [Google Scholar] [CrossRef] [PubMed]
- Goenka, S.; Ceccoli, J.; Simon, S.R. Anti-melanogenic activity of ellagitannin casuarictin in B16F10 mouse melanoma cells. Nat. Prod. Res. 2019, 1–6. [Google Scholar] [CrossRef]
- Kang, M.; Park, S.H.; Oh, S.W.; Lee, S.E.; Yoo, J.A.; Nho, Y.H.; Lee, S.; Han, B.S.; Cho, J.Y.; Lee, J. Anti-melanogenic effects of resorcinol are mediated by suppression of cAMP signaling and activation of p38 MAPK signaling. Biosci. Biotech. Biochem. 2018, 82, 1188–1196. [Google Scholar] [CrossRef] [Green Version]
- Wu, Q.Y.; Wong, Z.C.F.; Wang, C.; Fung, A.H.Y.; Wong, E.O.Y.; Chan, G.K.L.; Dong, T.T.X.; Chen, Y.; Tsim, K.W.K. Isoorientin derived from Gentiana veitchiorum Hemsl. flowers inhibits melanogenesis by down-regulating MITF-induced tyrosinase expression. Phytomedicine 2019, 57, 129–136. [Google Scholar]
- Li, H.X.; Park, J.U.; Su, X.D.; Kim, K.T.; Kang, J.S.; Kim, Y.R.; Kim, Y.H.; Yang, S.Y. Identification of anti-melanogenesis constituents from Morus alba L. leaves. Molecules 2018, 23, 2559. [Google Scholar] [CrossRef]
- Park, S.; Jegal, J.; Chung, K.W.; Jung, H.J.; Noh, S.G.; Chung, H.Y.; Ahn, J.; Kim, J.; Yang, M.H. Isolation of tyrosinase and melanogenesis inhibitory flavonoids from Juniperus chinensis fruits. Biosci. Biotech. Biochem. 2018, 82, 2041–2048. [Google Scholar] [CrossRef] [PubMed]
- Ha, J.H.; Park, S.N. Mechanism underlying inhibitory effect of six dicaffeoylquinic acid isomers on melanogenesis and the computational molecular modeling studies. Bioorg. Med. Chem. 2018, 26, 4201–4208. [Google Scholar] [CrossRef] [PubMed]
- Oh, T.I.; Yun, J.M.; Park, E.J.; Kim, Y.S.; Lee, Y.M.; Lim, J.H. Plumbagin suppresses α-MSH-induced melanogenesis in B16F10 mouse melanoma cells by inhibiting tyrosinase activity. Int. J. Mol. Sci. 2017, 18, 320. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.T.; Chang, W.C.; Hsu, C.; Su, N.W. Antimelanogenic effect of urolithin A and urolithin B, the colonic metabolites of ellagic acid, in B16 melanoma cells. J. Agric. Food Chem. 2017, 65, 6870–6876. [Google Scholar] [CrossRef] [PubMed]
- Abdul Hamid, M.; Abu Bakar, N.; Park, C.S.; Ramli, F.B.; Sulaiman, W.R.W. Inhibitory effect and molecular mechanism of an anti-tyrosinase in B16F1 melanoma cells by α-mangostin, isolated from mangosteen pericarp. J. Food Nutr. Res. 2016, 4, 820–827. [Google Scholar]
- Zhu, L.; Lu, Y.; Yu, W.G.; Zhao, X.; Lu, Y.H. Anti-photoageing and anti-melanogenesis activities of chrysin. Pharm. Biol. 2016, 54, 2692–2700. [Google Scholar] [CrossRef] [Green Version]
- Nasr, B.N.; Chaabane, F.; Sassi, A.; Chekir-Ghedira, L.; Ghedira, K. Effect of apigenin-7-glucoside, genkwanin and naringenin on tyrosinase activity and melanin synthesis in B16F10 melanoma cells. Life Sci. 2016, 144, 80–85. [Google Scholar] [CrossRef]
- Campos, P.M.; Prudente, A.S.; Horinouchi, C.D.; Cechinel-Filho, V.; Favero, G.M.; Cabrini, D.A.; Otuki, M.F. Inhibitory effect of GB-2a (I3-naringenin-II8-eriodictyol) on melanogenesis. J. Ethnopharmacol. 2015, 174, 224–229. [Google Scholar] [CrossRef]
- Hridya, H.; Amrita, A.; Sankari, M.; George Priya Doss, C.; Gopalakrishnan, M.; Gopalakrishnan, C.; Siva, R. Inhibitory effect of brazilein on tyrosinase and melanin synthesis: Kinetics and in silico approach. Int. J. Biol. Macromol. 2015, 81, 228–234. [Google Scholar] [CrossRef]
- Yamauchi, K.; Mitsunaga, T.; Itakura, Y.; Batubara, I. Extracellular melanogenesis inhibitory activity and the structure-activity relationships of ugonins from Helminthostachys zeylanica roots. Fitoterapia 2015, 104, 69–74. [Google Scholar] [CrossRef]
- Imen, M.; Chaabane, F.; Nadia, M.; Soumaya, K.; Kamel, G.; Leila, C.G. Anti-melanogenesis and antigenotoxic activities of eriodictyol in murine melanoma (B16-F10) and primary human keratinocyte cells. Life Sci. 2015, 135, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.Y.; Liu, Q.; Kim, S.B.; Jo, Y.H.; Mo, E.J.; Yang, H.H.; Song, D.H.; Hwang, B.Y.; Lee, M.K. Characterization of melanogenesis inhibitory constituents of Morus alba leaves and optimization of extraction conditions using response surface methodology. Molecules 2015, 20, 8730–8741. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.M.; Chen, Y.S.; Lin, C.C.; Chen, K.H. Hair dyes resorcinol and lawsone reduce production of melanin in melanoma cells by tyrosinase activity inhibition and decreasing tyrosinase and microphthalmia-associated transcription factor (MITF) expression. Int. J. Mol. Sci. 2015, 16, 1495–1508. [Google Scholar] [CrossRef] [PubMed]
- Mustapha, N.; Bzeouich, I.M.; Ghedira, K.; Hennebelle, T.; Chekir-Ghedira, L. Compounds isolated from the aerial part of Crataegus azarolus inhibit growth of B16F10 melanoma cells and exert a potent inhibition of the melanin synthesis. Biomed. Pharmacother. 2015, 69, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.G.; Huh, J.; Jeong, R.H.; Cha, B.J.; Shrestha, S.; Lee, D.G.; Kang, H.C.; Kim, J.Y.; Baek, N.I. Inhibition effect of phenyl compounds from the Oryza sativa roots on melanin production in murine B16-F10 melanoma cells. Nat. Prod. Res. 2015, 29, 1052–1054. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.H.; Chen, C.C.; Wu, P.Y.; Wu, C.S.; Sung, P.J.; Lin, C.Y.; Chiang, H.M. N-(4-methoxyphenyl) caffeamide-induced melanogenesis inhibition mechanisms. BMC Complement. Altern. Med. 2017, 17, 71. [Google Scholar] [CrossRef] [PubMed]
- Ullah, S.; Park, Y.; Ikram, M.; Lee, S.; Park, C.; Kang, D.; Yang, J.; Akter, J.; Yoon, S.; Chun, P.; et al. Design, synthesis and anti-melanogenic effect of cinnamamide derivatives. Bioorg. Med. Chem. 2018, 26, 5672–5681. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Son, Y.H.; Lee, K.B.; Lee, J.H.; Kim, H.J.; Jeong, E.M.; Park, S.C.; Kim, I.G. 4-n-butylresorcinol enhances proteolytic degradation of tyrosinase in B16F10 melanoma cells. Int. J. Cosmet.Sci. 2017, 39, 248–255. [Google Scholar] [CrossRef]
- Ullah, S.; Akter, J.; Kim, S.J.; Yang, J.; Park, Y.; Chun, P.; Moon, H.R. The tyrosinase-inhibitory effects of 2-phenyl-1,4-naphthoquinone analogs: Importance of the (E)-β-phenyl-α, β-unsaturated carbonyl scaffold of an endomethylene type. Med. Chem. Res. 2019, 28, 95–103. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, Y.; Nakashima, S.; Wang, T.; Yoshikawa, M.; Matsuda, H. Inhibition of melanin production by anthracenone dimer glycosides isolated from Cassia auriculata seeds. J. Nat. Med. 2019, 73, 439–449. [Google Scholar] [CrossRef]
- Son, S.; Kim, H.; Yun, H.Y.; Kim, D.H.; Ullah, S.; Kim, S.J.; Kim, Y.J.; Kim, M.S.; Yoo, J.W.; Chun, P.; et al. (E)-2-Cyano-3-(substituted phenyl)acrylamide analogs as potent inhibitors of tyrosinase: A linear β-phenyl-α,β-unsaturated carbonyl scaffold. Bioorg. Med. Chem. 2015, 23, 7728–7734. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.H.; Chen, C.C.; Lin, P.; You, Y.J.; Chiang, H.M. N-(4-bromophenethyl) caffeamide inhibits melanogenesis by regulating AKT/glycogen synthase kinase 3 beta/microphthalmia-associated transcription factor and tyrosinase-related protein 1/tyrosinase. Curr. Pharm. Biotechnol. 2015, 16, 1111–1119. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.M.; Ko, H.H.; Ng, L.T.; Hsieh, Y.P. Free-radical-scavenging, antityrosinase, and cellular melanogenesis inhibitory activities of synthetic isoflavones. Chem. Biodivers. 2015, 12, 963–979. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.J.; Lee, C.S.; Choi, J.; Hong, Y.D.; Shin, S.S.; Park, J.S.; Lee, J.H.; Lee, S.; Yoon, K.D.; Ko, J. Flavokawains B and C, melanogenesis inhibitors, isolated from the root of Piper methysticum and synthesis of analogs. Bioorg. Med. Chem. Lett. 2015, 25, 799–802. [Google Scholar] [CrossRef] [PubMed]
- Veselinovic, J.B.; Veselinovic, A.M.; Ilic-Tomic, T.; Davis, R.; O’Connor, K.; Pavic, A.; Nikodinovic-Runic, J. Potent anti-melanogenic activity and favorable toxicity profile of selected 4-phenyl hydroxycoumarins in the zebrafish model and the computational molecular modeling studies. Bioorg. Med. Chem. 2017, 25, 6286–6296. [Google Scholar] [CrossRef] [PubMed]
- Haudecoeur, R.; Carotti, M.; Gouron, A.; Maresca, M.; Buitrago, E.; Hardre, R.; Bergantino, E.; Jamet, H.; Belle, C.; Reglier, M.; et al. 2-Hydroxypyridine-N-oxide-embedded aurones as potent human tyrosinase inhibitors. ACS Med. Chem. Lett. 2017, 8, 55–60. [Google Scholar] [CrossRef]
- Zhou, Z.; Hou, J.; Xiong, J.; Li, M. Characterization of sulfuretin as a depigmenting agent. Fundam. Clin. Pharmacol. 2019, 33, 208–215. [Google Scholar] [CrossRef]
- Chen, W.C.; Tseng, T.S.; Hsiao, N.W.; Lin, Y.L.; Wen, Z.H.; Tsai, C.C.; Lee, Y.C.; Lin, H.H.; Tsai, K.C. Discovery of highly potent tyrosinase inhibitor, T1, with significant anti-melanogenesis ability by zebrafish in vivo assay and computational molecular modeling. Sci. Rep. 2015, 5, 7995. [Google Scholar] [CrossRef]
- Kwak, J.Y.; Seok, J.K.; Suh, H.J.; Choi, Y.H.; Hong, S.S.; Kim, D.S.; Boo, Y.C. Antimelanogenic effects of luteolin 7-sulfate isolated from Phyllospadix iwatensis Makino. Br. J. Dermatol. 2016, 175, 501–511. [Google Scholar] [CrossRef]
- Kwak, J.Y.; Park, S.; Seok, J.K.; Liu, K.H.; Boo, Y.C. Ascorbyl coumarates as multifunctional cosmeceutical agents that inhibit melanogenesis and enhance collagen synthesis. Arch. Dermatol. Res. 2015, 307, 635–643. [Google Scholar] [CrossRef]
- Moon, K.M.; Hwang, Y.H.; Yang, J.H.; Ma, J.Y.; Lee, B. Spinosin is a flavonoid in the seed of Ziziphus jujuba that prevents skin pigmentation in a human skin model. J. Funct. Foods 2019, 54, 449–456. [Google Scholar] [CrossRef]
- Lee, S.; Lee, D.H.; Kim, J.C.; Um, B.H.; Sung, S.H.; Jeong, L.S.; Kim, Y.K.; Kim, S.N. Pectolinarigenin, an aglycone of pectolinarin, has more potent inhibitory activities on melanogenesis than pectolinarin. Biochem. Biophys. Res. Commun. 2017, 493, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Moon, K.M.; Lee, B.S.; Yang, J.H.; Park, K.I.; Cho, W.K.; Ma, J.Y. Swertiajaponin inhibits skin pigmentation by dual mechanisms to suppress tyrosinase. Oncotarget 2017, 8, 95530–95541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, B.; Moon, K.M.; Lim, J.S.; Park, Y.; Kim, D.H.; Son, S.; Jeong, H.O.; Kim, D.H.; Lee, E.K.; Chung, K.W.; et al. 2-(3,4-dihydroxybenzylidene)malononitrile as a novel anti-melanogenic compound. Oncotarget 2017, 8, 91481–91493. [Google Scholar] [CrossRef]
- Ha, J.H.; Jeong, Y.J.; Xuan, S.H.; Lee, J.Y.; Park, J.; Park, S.N. Methyl-2-acetylamino-3-(4-hydroxyl-3,5- dimethoxybenzoylthio) propanoate suppresses melanogenesis through ERK signaling pathway mediated MITF proteasomal degradation. J. Dermatol. Sci. 2018, 91, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Arrowitz, C.; Weber, T.; Schoelermann, A.M.; Mann, T.; Jiang, L.I.; Kolbe, L. Effective tyrosinase inhibition by thiamidol results in significant improvement of mild to moderate melasma. J. Investig. Dermatol. 2019, 139, 1691–1698. [Google Scholar] [CrossRef] [PubMed]
- Hornyak, T.J. Next time, save mushrooms for the pizza! J. Investig. Dermatol. 2018, 138, 1470–1472. [Google Scholar] [CrossRef]
- Mayr, F.; Sturm, S.; Ganzera, M.; Waltenberger, B.; Martens, S.; Schwaiger, S.; Schuster, D.; Stuppner, H. Mushroom tyrosinase-based enzyme inhibition assays are not suitable for bioactivity-guided fractionation of extracts. J. Nat. Prod. 2019, 82, 136–147. [Google Scholar] [CrossRef]
- Gasowska-Bajger, B.; Wojtasek, H. Reactions of flavonoids with o-quinones interfere with the spectrophotometric assay of tyrosinase activity. J. Agric. Food Chem. 2016, 64, 5417–5427. [Google Scholar] [CrossRef]
- Deng, H.H.; Lin, X.L.; He, S.B.; Wu, G.W.; Wu, W.H.; Yang, Y.; Lin, Z.; Peng, H.P.; Xia, X.H.; Chen, W. Colorimetric tyrosinase assay based on catechol inhibition of the oxidase-mimicking activity of chitosan-stabilized platinum nanoparticles. Mikrochim. Acta 2019, 186, 1–7. [Google Scholar] [CrossRef]
- Vandeput, M.; Patris, S.; Silva, H.; Parsajoo, C.; Dejaeghere, B.; Martinez, J.A.; Kauffmann, J.M. Application of a tyrosinase microreactor-detector in a flow injection configuration for the determination of affinity and dynamics of inhibitor binding. Sens. Actuator. B Chem. 2017, 248, 385–394. [Google Scholar] [CrossRef]
- Cheng, M.; Chen, Z. Screening of tyrosinase inhibitors by capillary electrophoresis with immobilized enzyme microreactor and molecular docking. Electrophoresis 2017, 38, 486–493. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Zhang, W.; Zhao, H.; Chen, Z. Tyrosinase inhibitor screening in traditional Chinese medicines by electrophoretically mediated microanalysis. J. Sep. Sci. 2015, 38, 2887–2892. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Tang, Q.; Wu, T.; Cheng, Z. Improved TLC bioautographic assay for qualitative and quantitative estimation of tyrosinase inhibitors in natural products. Phytochem. Anal. 2017, 28, 115–124. [Google Scholar] [CrossRef]
- Wang, Z.; Hwang, S.H.; Huang, B.; Lim, S.S. Identification of tyrosinase specific inhibitors from Xanthium strumarium fruit extract using ultrafiltration-high performance liquid chromatography. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2015, 1002, 319–328. [Google Scholar] [CrossRef]
- Chai, L.; Zhou, J.; Feng, H.; Tang, C.; Huang, Y.; Qian, Z. Functionalized carbon quantum dots with dopamine for tyrosinase activity monitoring and inhibitor screening: In vitro and intracellular investigation. ACS Appl. Mater. Interfaces 2015, 7, 23564–23574. [Google Scholar] [CrossRef]
- Markova, E.; Kotik, M.; Krenkova, A.; Man, P.; Haudecoeur, R.; Boumendjel, A.; Hardre, R.; Mekmouche, Y.; Courvoisier-Dezord, E.; Reglier, M.; et al. Recombinant tyrosinase from Polyporus arcularius: Overproduction in Escherichia coli, characterization, and use in a study of aurones as tyrosinase effectors. J. Agric. Food Chem. 2016, 64, 2925–2931. [Google Scholar] [CrossRef]
- Ortiz-Ruiz, C.V.; Garcia-Molina, M.; Serrano, J.T.; Tomas-Martinez, V.; Garcia-Canovas, F. Discrimination between alternative substrates and inhibitors of tyrosinase. J. Agric. Food Chem. 2015, 63, 2162–2171. [Google Scholar] [CrossRef]
- Crescenzi, O.; Napolitano, A.; Prota, G.; Peter, M.G. Oxidative coupling of dopa with resorcinol and phloroglucinol: Isolation of adducts with an unusual tetrahydromethanobenzofuro [2,3-d] azocine skeleton. Tetrahedron 1991, 47, 6243–6250. [Google Scholar] [CrossRef]
- Napolitano, A.; Micillo, R.; Monfrecola, G. Melanin pigmentation control by 1,3-thiazolidines: Does NO scavenging play a critical role? Exp. Dermatol. 2016, 25, 596–597. [Google Scholar] [CrossRef]
- Shin, H.J.; Beak, H.S.; Il Kim, S.; Joo, Y.H.; Choi, J. Development and evaluation of topical formulations for a novel skin whitening agent (AP736) using Hansen solubility parameters and PEG-PCL polymers. Int. J. Pharm. 2018, 552, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.H.; Na, Y.G.; Lee, H.K.; Cho, C.W. Hybrid polymeric microspheres for enhancing the encapsulation of phenylethyl resorcinol. J. Microencapsul. 2019, 36, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Limsuwan, T.; Boonme, P.; Khongkow, P.; Amnuaikit, T. Ethosomes of phenylethyl resorcinol as vesicular delivery system for skin lightening applications. BioMed Res. Int. 2017, 8310979. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.S.; Na, Y.G.; Choi, J.H.; Kim, I.; Lee, E.; Kim, S.Y.; Lee, J.Y.; Cho, C.W. The improvement of skin whitening of phenylethyl resorcinol by nanostructured lipid carriers. Nanomaterials 2017, 7, 241. [Google Scholar] [CrossRef]
- Koepke, D.; Mueller, R.H.; Pyo, S.M. Phenylethyl resorcinol smartLipids for skin brightening—Increased loading & chemical stability. Eur. J. Pharm. Sci. 2019, 137, 104992. [Google Scholar]
- Hespeler, D.; Kaltenbach, J.; Pyo, S.M. Glabridin smartPearls—Silica selection, production, amorphous stability and enhanced solubility. Int. J. Pharm. 2019, 561, 228–235. [Google Scholar] [CrossRef]
- Wei, Y.; Zhang, J.; Zhou, Y.; Bei, W.; Li, Y.; Yuan, Q.; Liang, H. Characterization of glabridin/hydroxypropyl-β-cyclodextrin inclusion complex with robust solubility and enhanced bioactivity. Carbohydr. Polym. 2017, 159, 152–160. [Google Scholar] [CrossRef]
- Chaikul, P.; Khat-udomkiri, N.; Iangthanarat, K.; Manosroi, J.; Manosroi, A. Characteristics and in vitro anti-skin aging activity of gallic acid loaded in cationic CTAB noisome. Eur. J. Pharm. Sci. 2019, 131, 39–49. [Google Scholar] [CrossRef]
- Schlich, M.; Fornasier, M.; Nieddu, M.; Sinico, C.; Murgia, S.; Rescigno, A. 3-hydroxycoumarin loaded vesicles for recombinant human tyrosinase inhibition in topical applications. Colloids Surf. B Biointerfaces 2018, 171, 675–681. [Google Scholar] [CrossRef]
- Fachinetti, N.; Rigon, R.B.; Eloy, J.O.; Sato, M.R.; dos Santos, K.C.; Chorilli, M. Comparative study of glyceryl behenate or polyoxyethylene 40 stearate-based lipid carriers for trans-resveratrol delivery: Development, characterization and evaluation of the in vitro tyrosinase inhibition. AAPS PharmSciTech 2018, 19, 1401–1409. [Google Scholar] [CrossRef]
- Rigon, R.B.; Fachinetti, N.; Chorilli, M.; Severino, P.; Santana, M.H.A. Skin delivery and in vitro biological evaluation of trans-resveratrol-loaded solid lipid nanoparticles for skin disorder therapies. Molecules 2016, 21, 116. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.H.; Kang, J.H.; Seo, J.O.; Baek, S.H.; Moh, S.H.; Chae, J.K.; Park, Y.U.; Ko, Y.T.; Kim, S.Y. Anti-melanogenic potentials of nanoparticles from calli of resveratrol-enriched rice against UVB-induced hyperpigmentation in guinea pig skin. Biomol. Ther. 2016, 24, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Shrotriya, S.; Ranpise, N.; Satpute, P.; Vidhate, B. Skin targeting of curcumin solid lipid nanoparticles-engrossed topical gel for the treatment of pigmentation and irritant contact dermatitis. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1471–1482. [Google Scholar] [CrossRef] [PubMed]
- Al-Amin, M.; Cao, J.; Naeem, M.; Banna, H.; Kim, M.S.; Jung, Y.; Chung, H.Y.; Moon, H.R.; Yoo, J.W. Increased therapeutic efficacy of a newly synthesized tyrosinase inhibitor by solid lipid nanoparticles in the topical treatment of hyperpigmentation. Drug Des. Devel. Ther. 2016, 10, 3947–3957. [Google Scholar] [CrossRef]
- Parisi, O.I.; Malivindi, R.; Amone, F.; Ruffo, M.; Malanchin, R.; Carlomagno, F.; Piangiolino, C.; Nobile, V.; Pezzi, V.; Scrivano, L.; et al. Safety and efficacy of dextran-rosmarinic acid conjugates as innovative polymeric antioxidants in skin whitening: What is the evidence? Cosmetics 2017, 4, 28. [Google Scholar] [CrossRef]
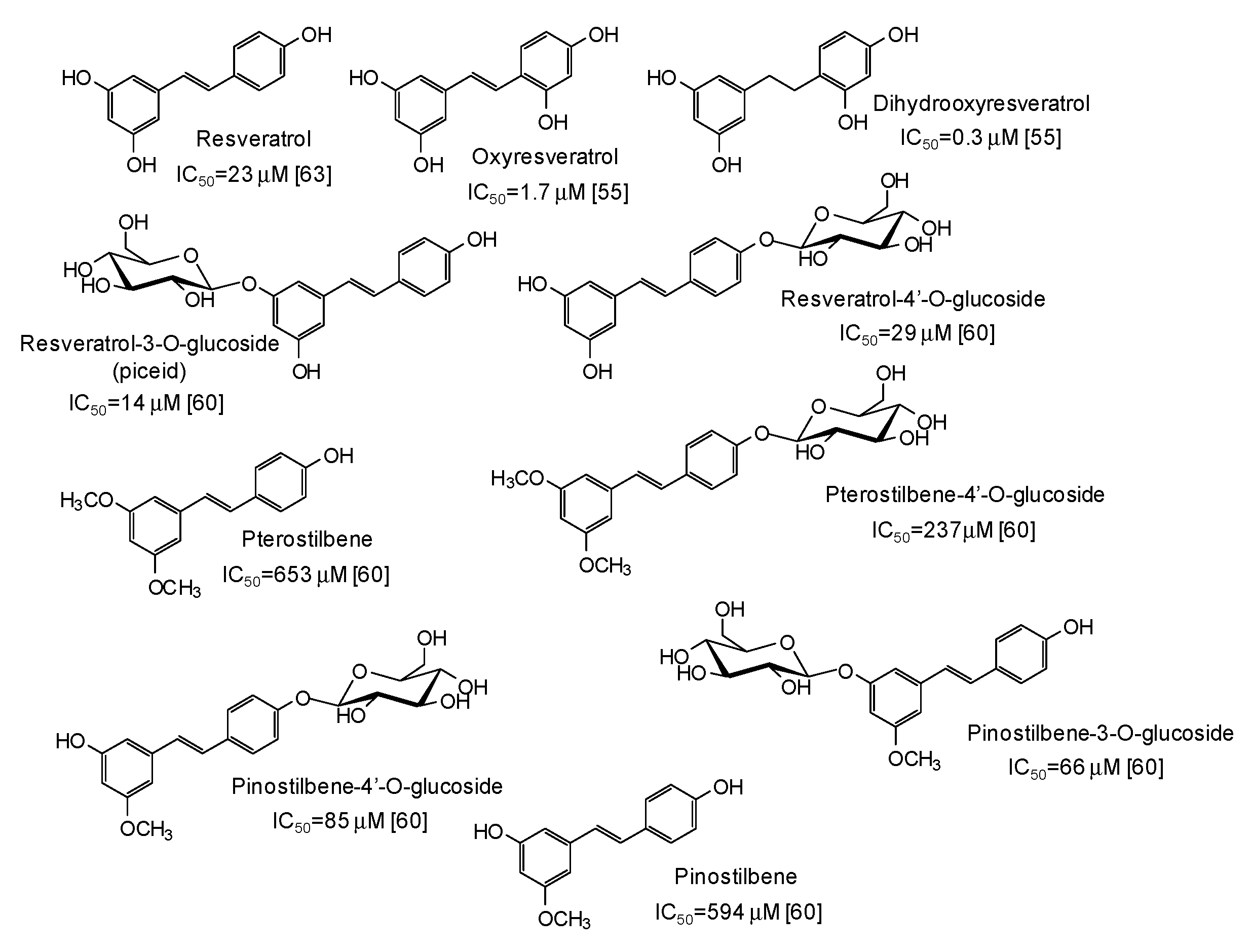
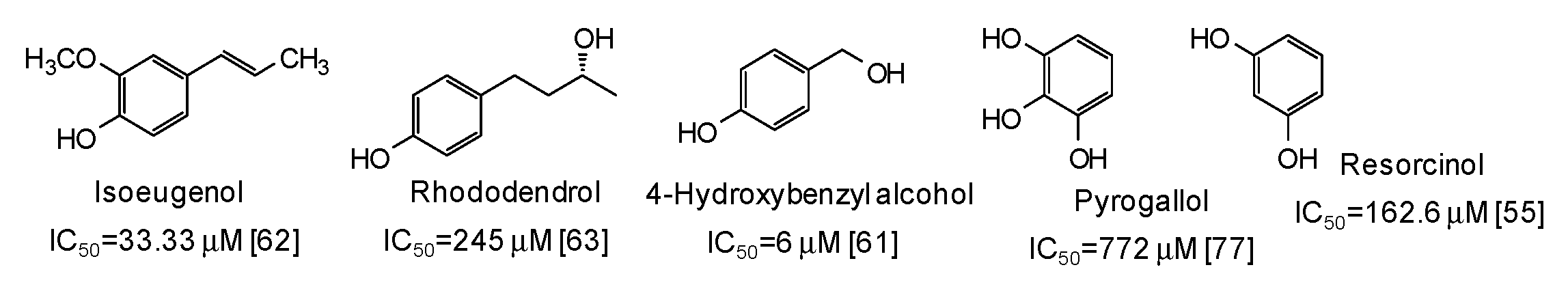
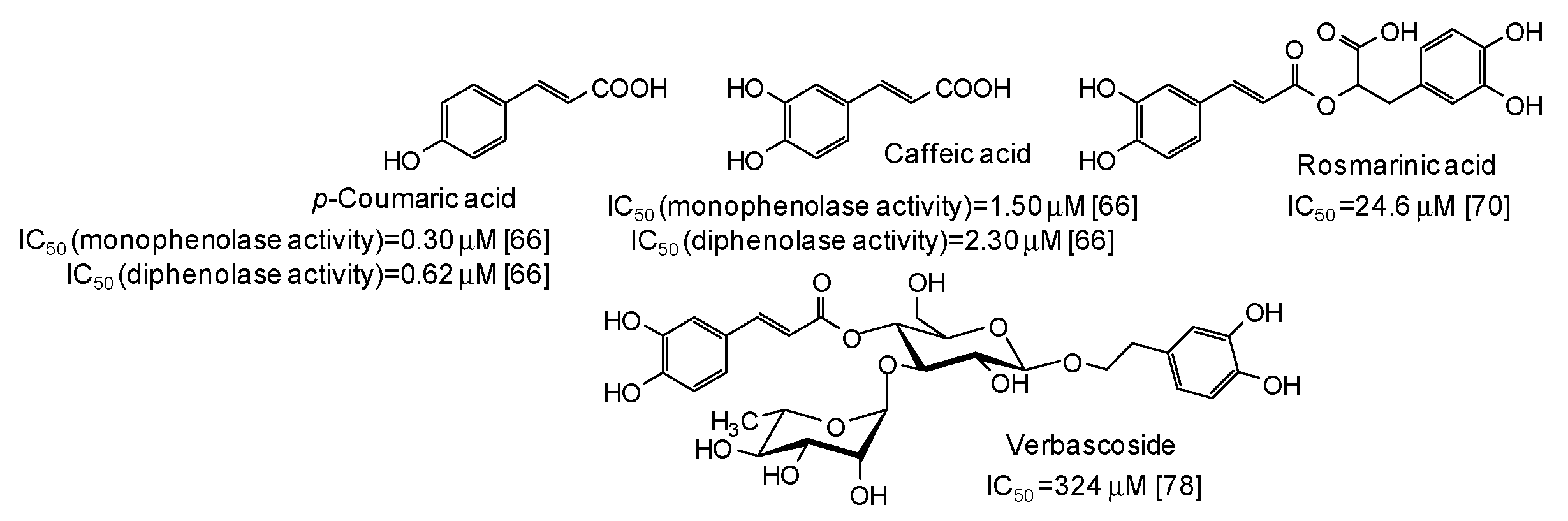
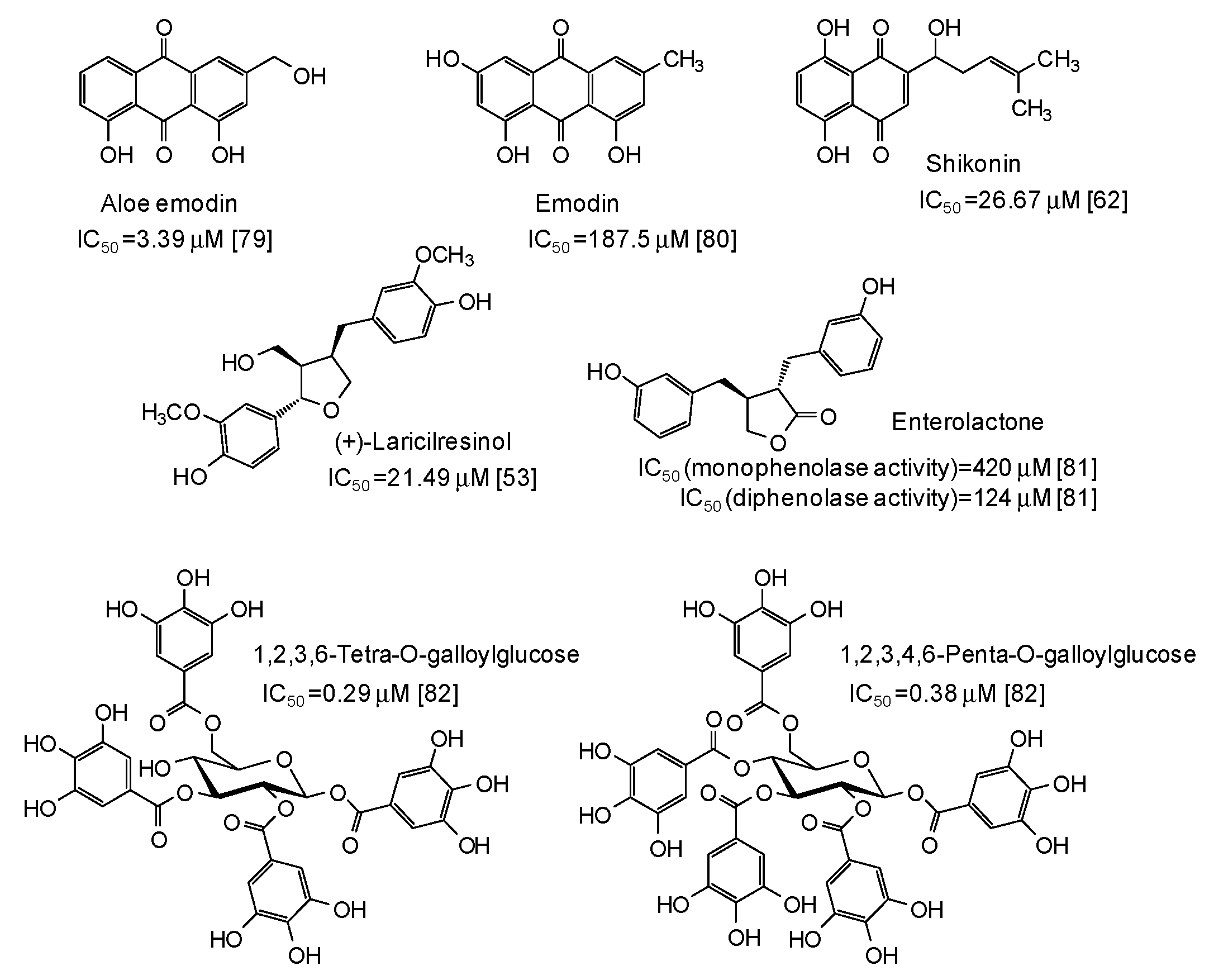
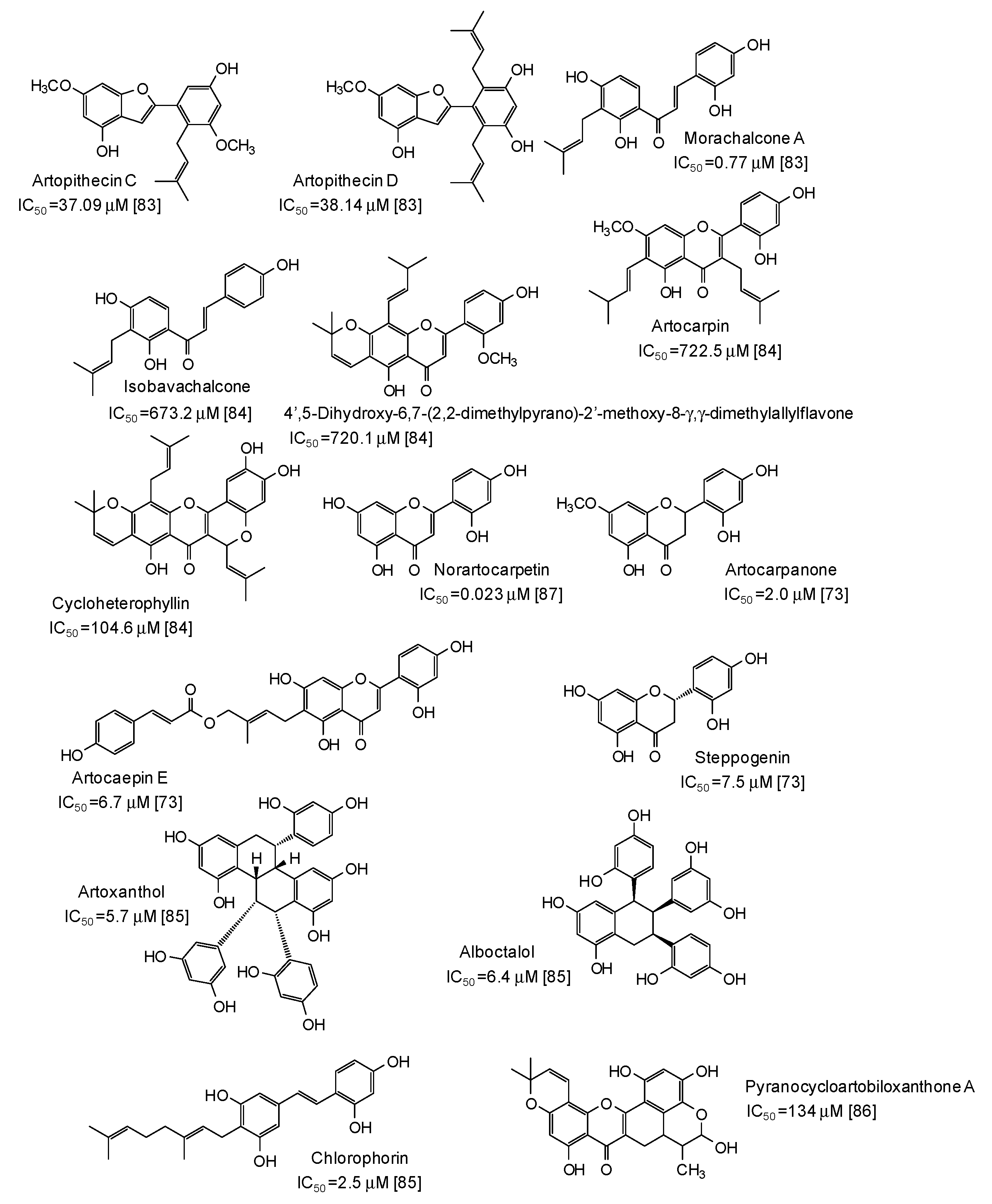
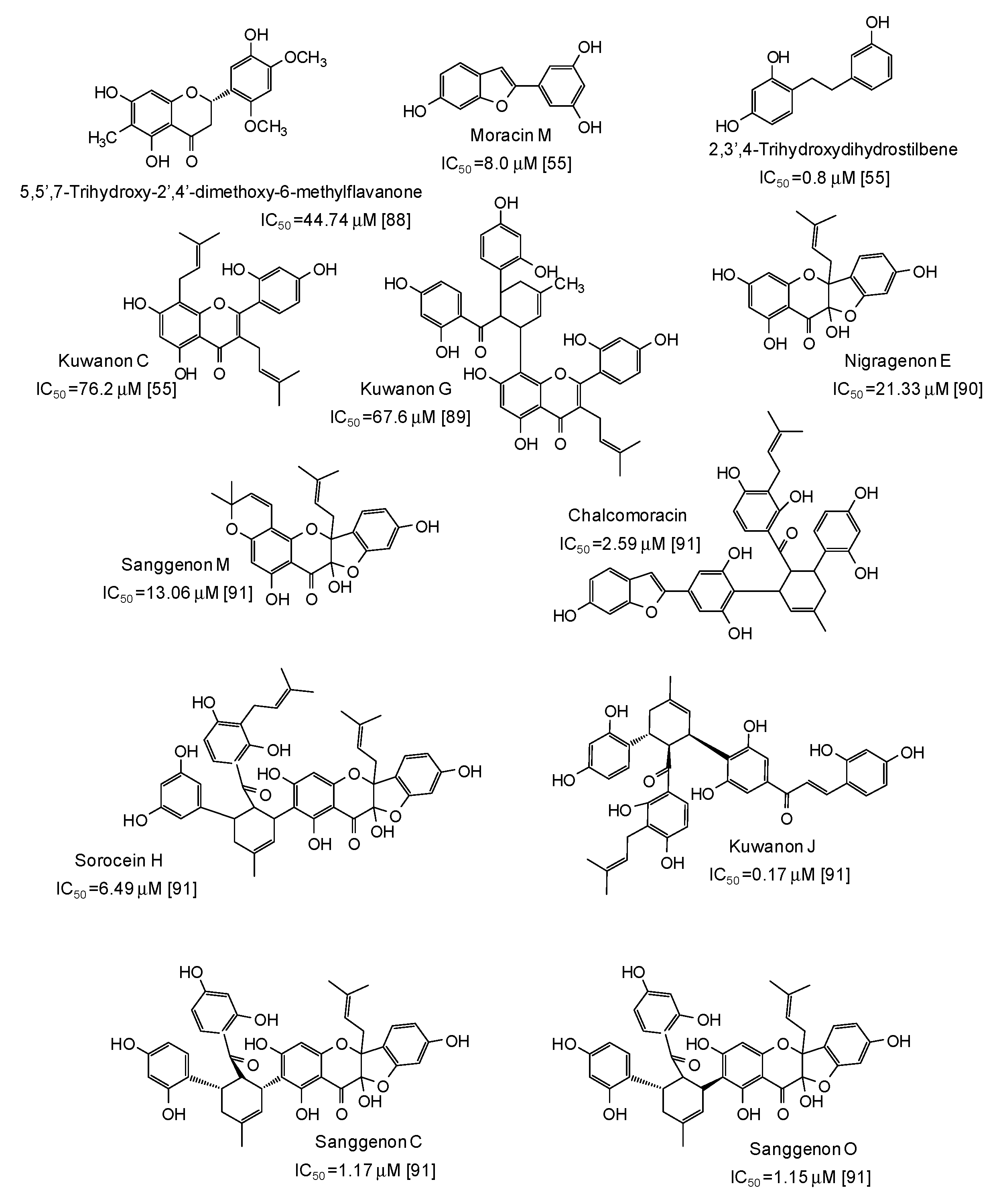


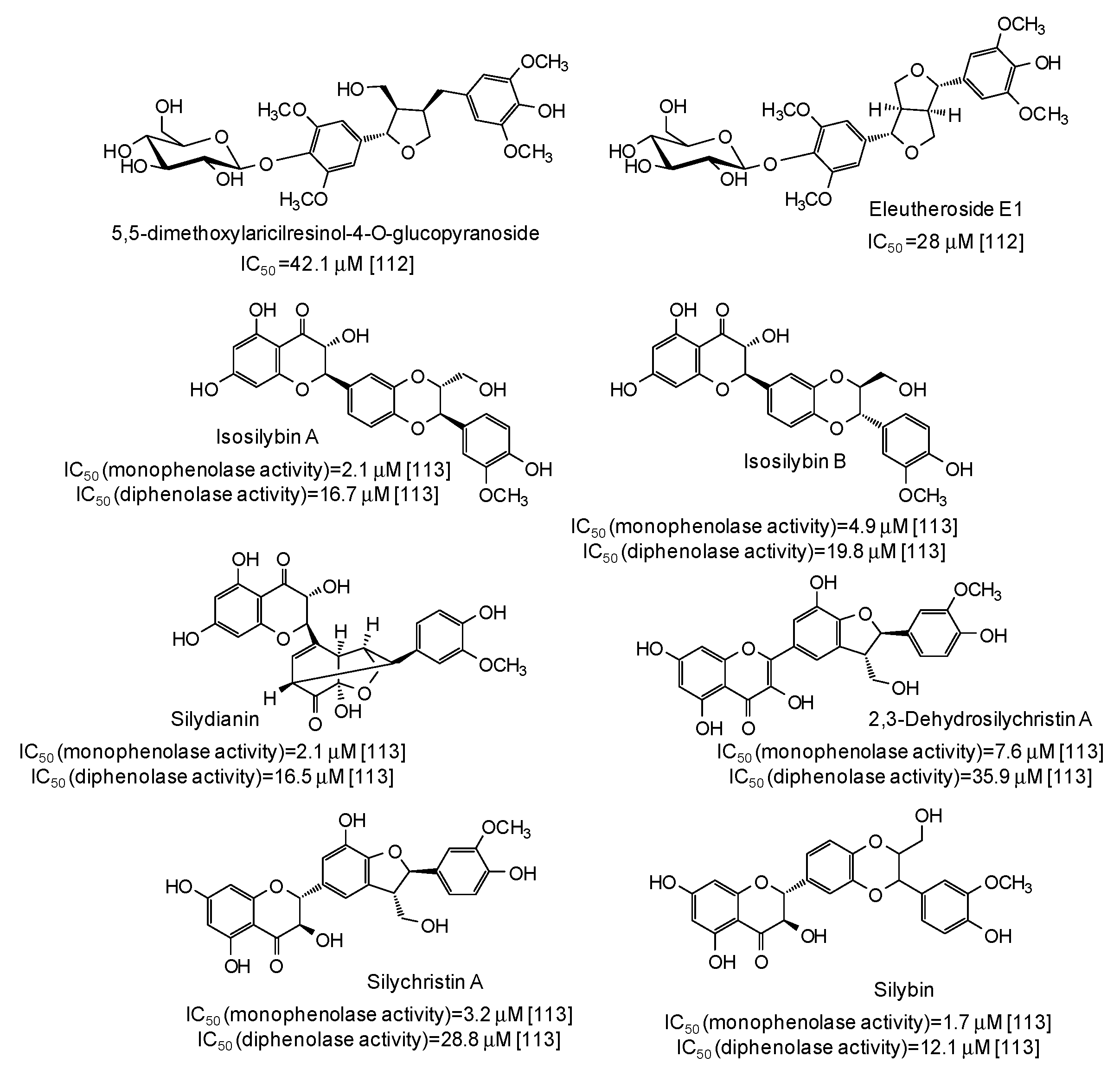
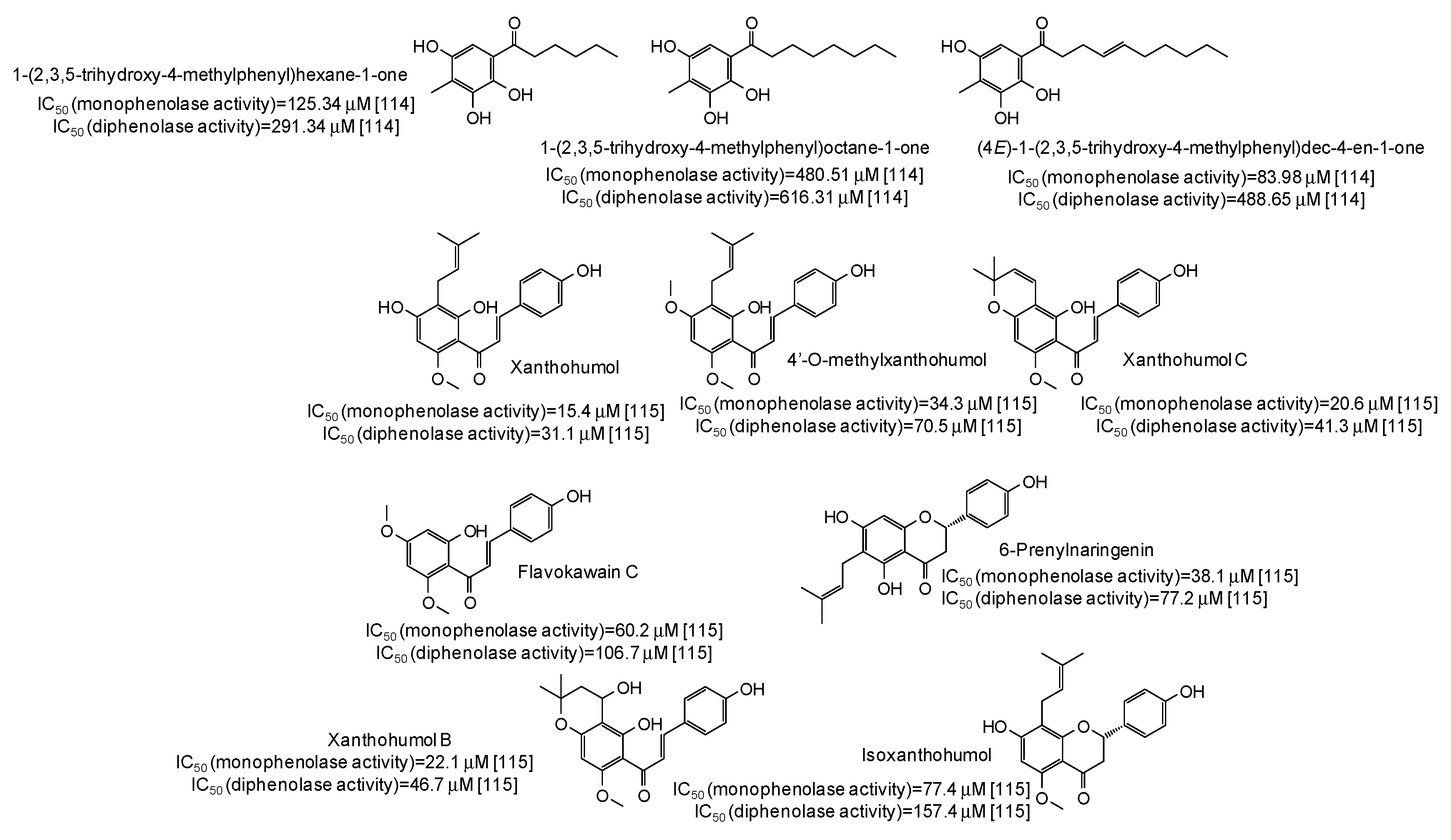
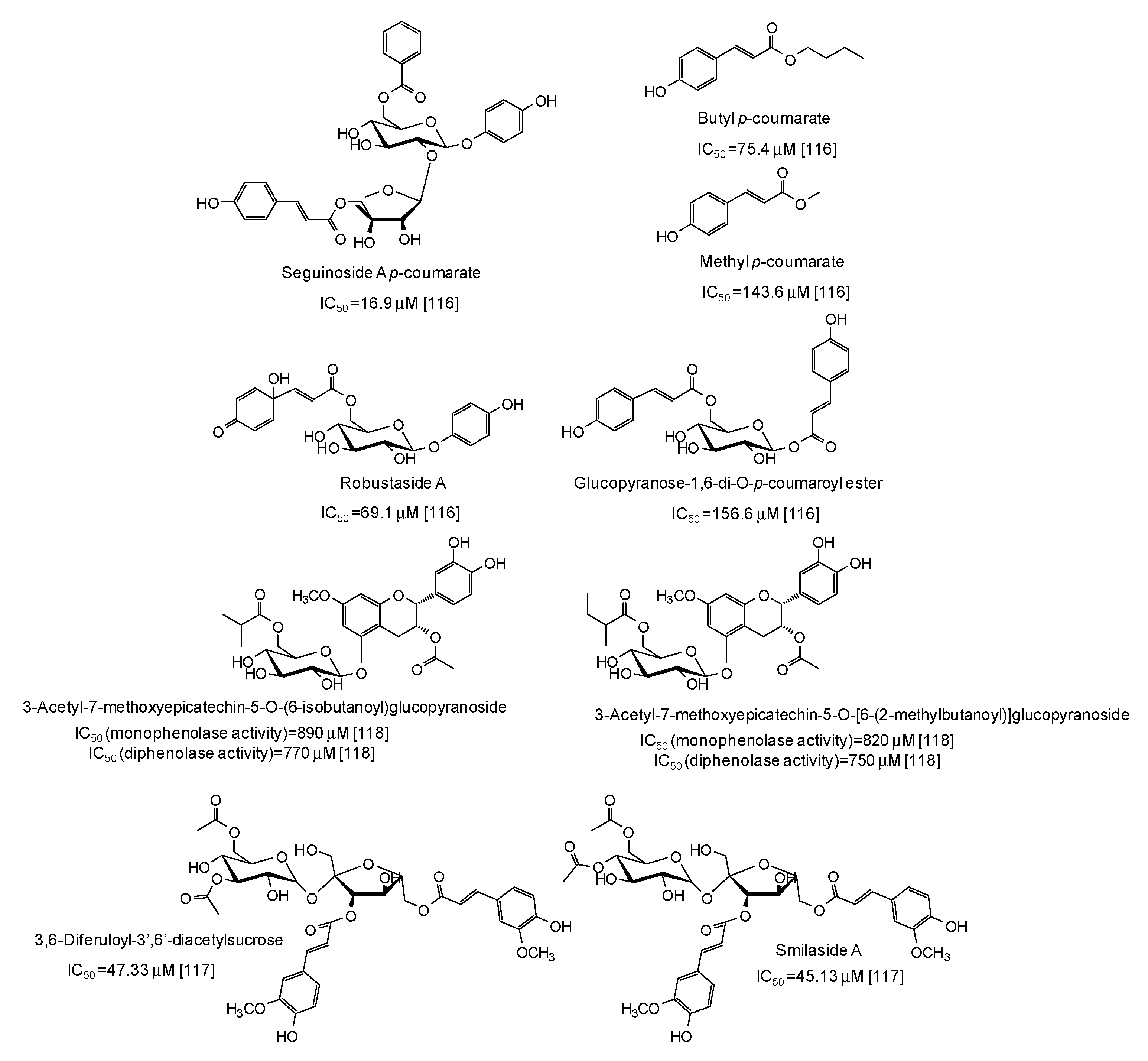
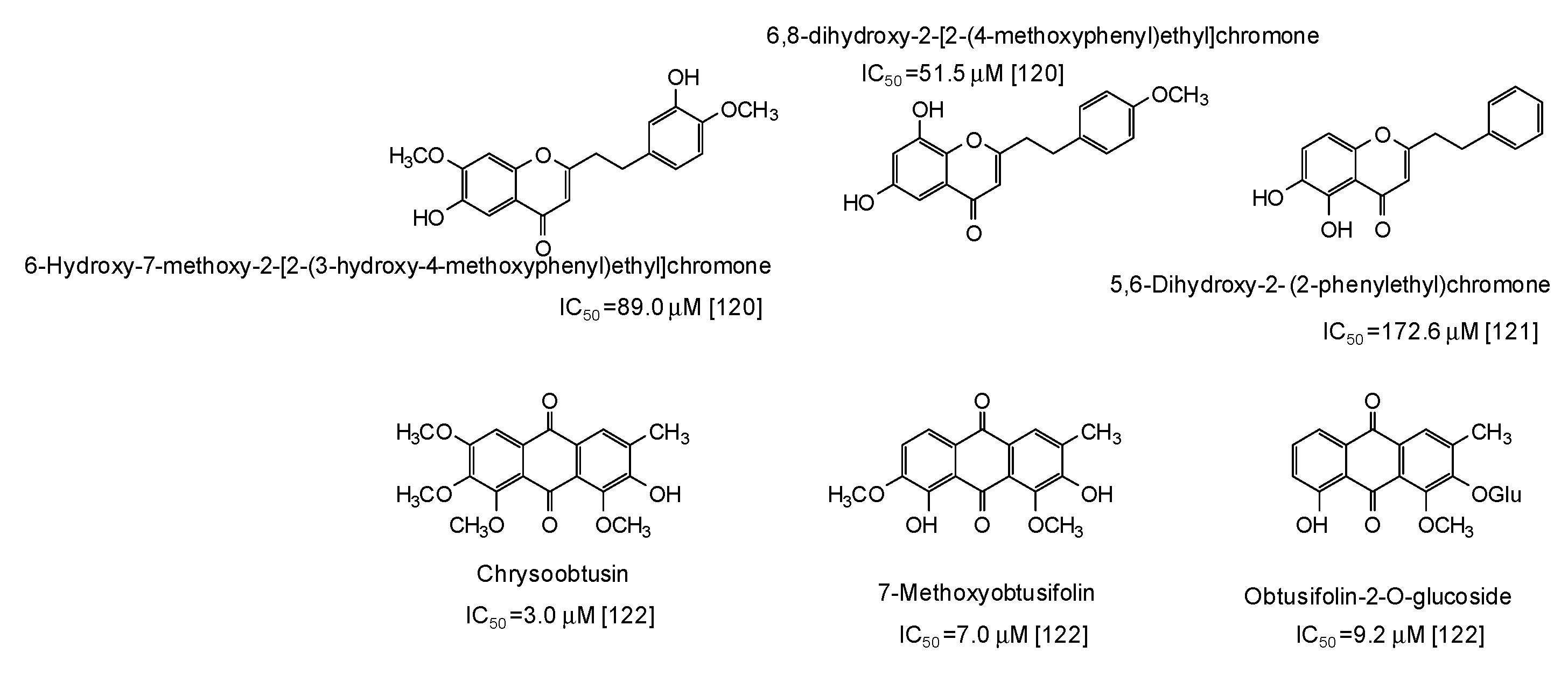

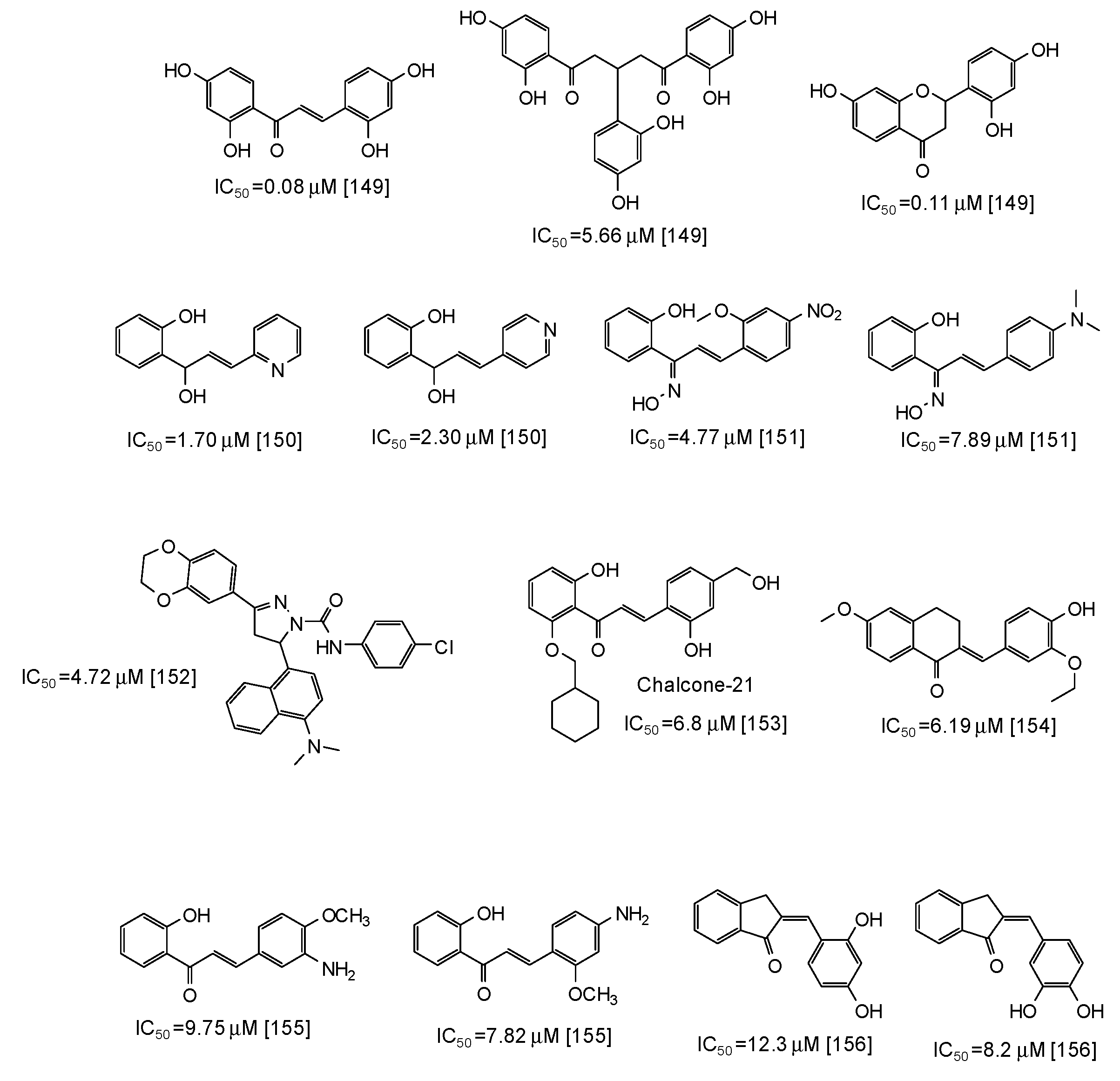
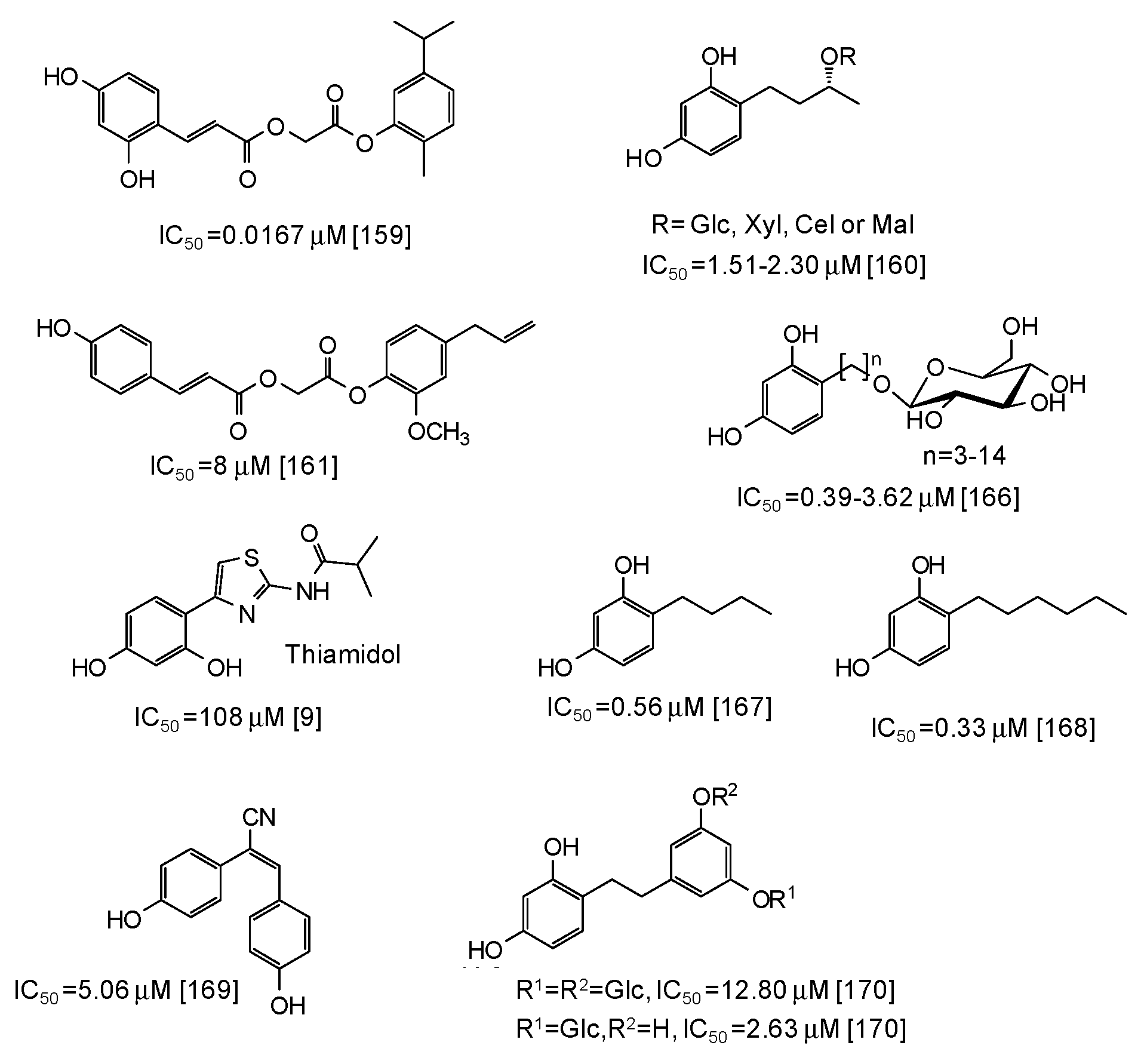
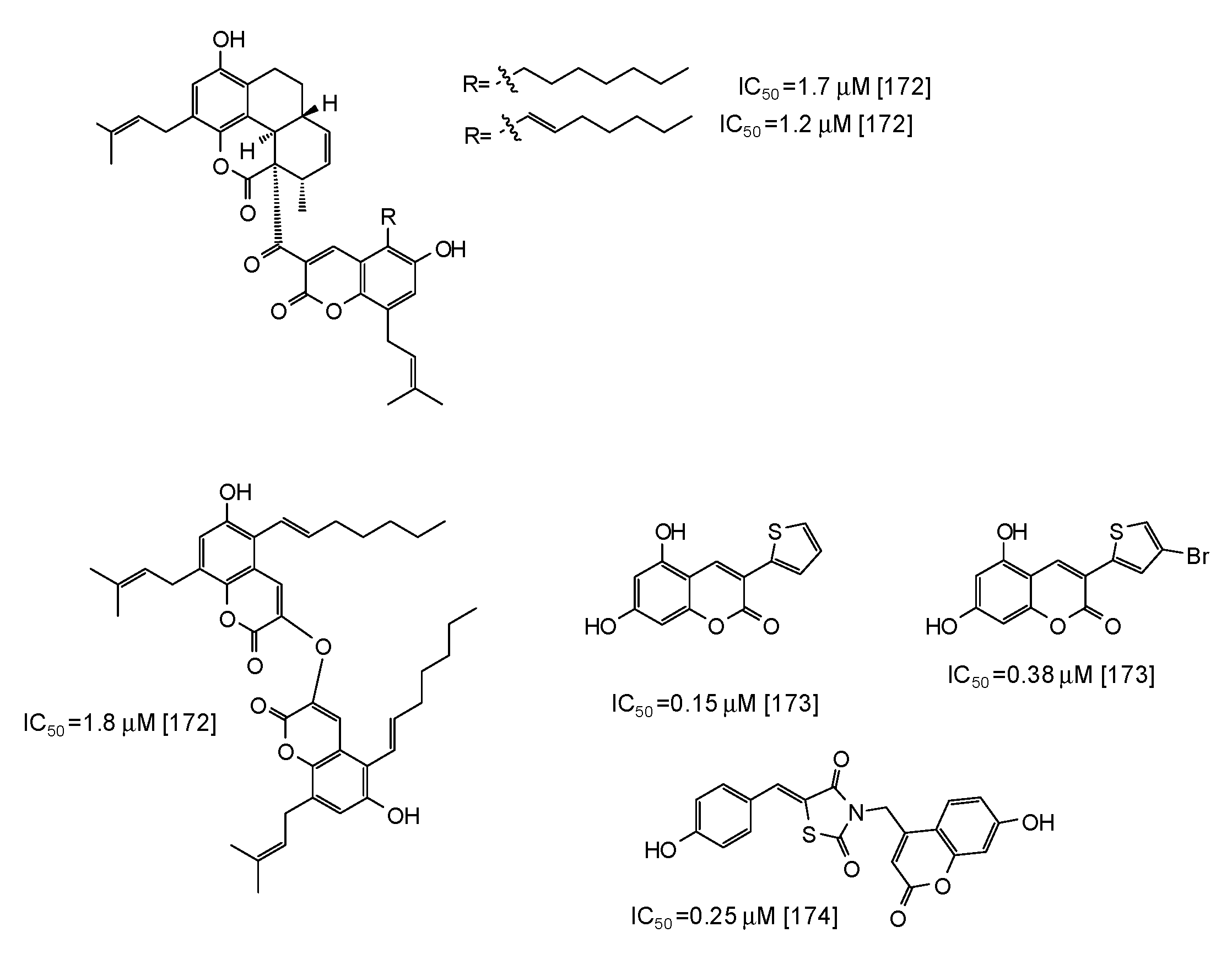
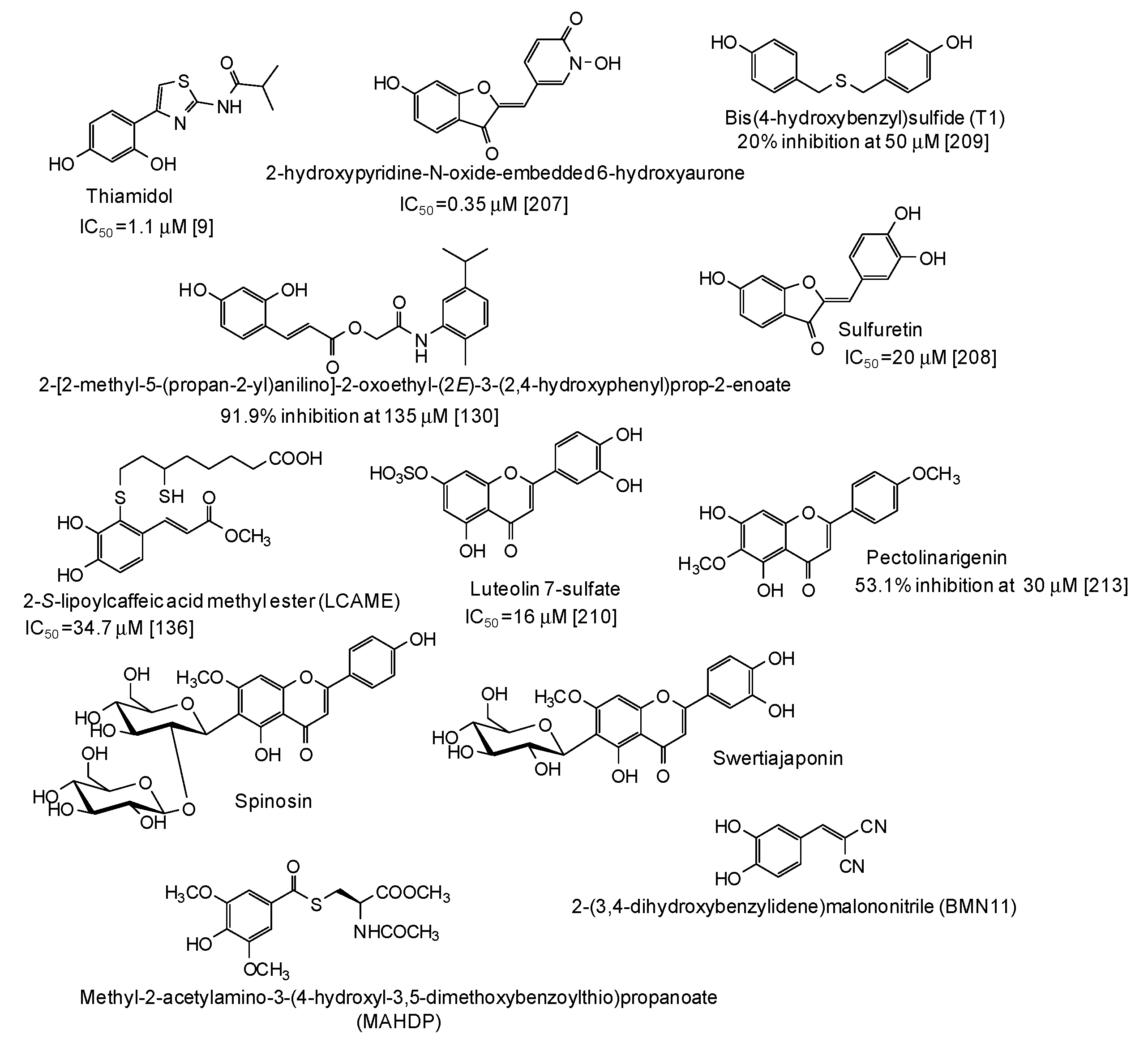
| Source | IC50 (μg/mL) (Monophenolase Activity) | IC50 (μg/mL) (Diphenolase Activity) |
|---|---|---|
| Vigna angularis [96] | 130.0 | 35.1 |
| Leucaena leucocephala [97] | 52.3 | 16.1 |
| Vigna radiata [98] | 80 | 20 |
| Longan [99] | 43.7 | 11.5 |
| Prunus cerasifera [100] | 738.37 | 137.69 |
| Annona squamosa [101] | 46.5 | 37.3 |
| Clausena lansium [102] | 23.6 | 7.0 |
| Ficus altissima [104] | 256.7 | 41.3 |
| Rhododendron pulchrum [105] | 200 | 200 |
| Persea americana [106] | 40 | 19.5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panzella, L.; Napolitano, A. Natural and Bioinspired Phenolic Compounds as Tyrosinase Inhibitors for the Treatment of Skin Hyperpigmentation: Recent Advances. Cosmetics 2019, 6, 57. https://doi.org/10.3390/cosmetics6040057
Panzella L, Napolitano A. Natural and Bioinspired Phenolic Compounds as Tyrosinase Inhibitors for the Treatment of Skin Hyperpigmentation: Recent Advances. Cosmetics. 2019; 6(4):57. https://doi.org/10.3390/cosmetics6040057
Chicago/Turabian StylePanzella, Lucia, and Alessandra Napolitano. 2019. "Natural and Bioinspired Phenolic Compounds as Tyrosinase Inhibitors for the Treatment of Skin Hyperpigmentation: Recent Advances" Cosmetics 6, no. 4: 57. https://doi.org/10.3390/cosmetics6040057
APA StylePanzella, L., & Napolitano, A. (2019). Natural and Bioinspired Phenolic Compounds as Tyrosinase Inhibitors for the Treatment of Skin Hyperpigmentation: Recent Advances. Cosmetics, 6(4), 57. https://doi.org/10.3390/cosmetics6040057






