The Anti-Aging Potential of Extracts from Chaenomeles sinensis
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Reagents
2.3. Extracts Preparation
2.4. Isolation of Compounds from an Extract of C. sinensis sarcocarp
2.5. Elastase Inhibition Assay
2.6. Assay for SOD-Like Activity
2.7. Assay of Inhibition of Collagenase from Clostridium histolyticum
2.8. 3D Human Skin Model and Assay for Collagenase Activity
2.9. 3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide (MTT) Assay
2.10. Total Phenolic Content
2.11. Total Condensed Tannin Content
2.12. Sample Acetylation and Gel Permeation Chromatography (GPC)
2.13. Decomposition and Analysis of Phenolic Composition of Sample Using High Performance Liquid Chromatography (HPLC) Analysis
2.14. Statistical Analysis
3. Results
3.1. Extract Preparation
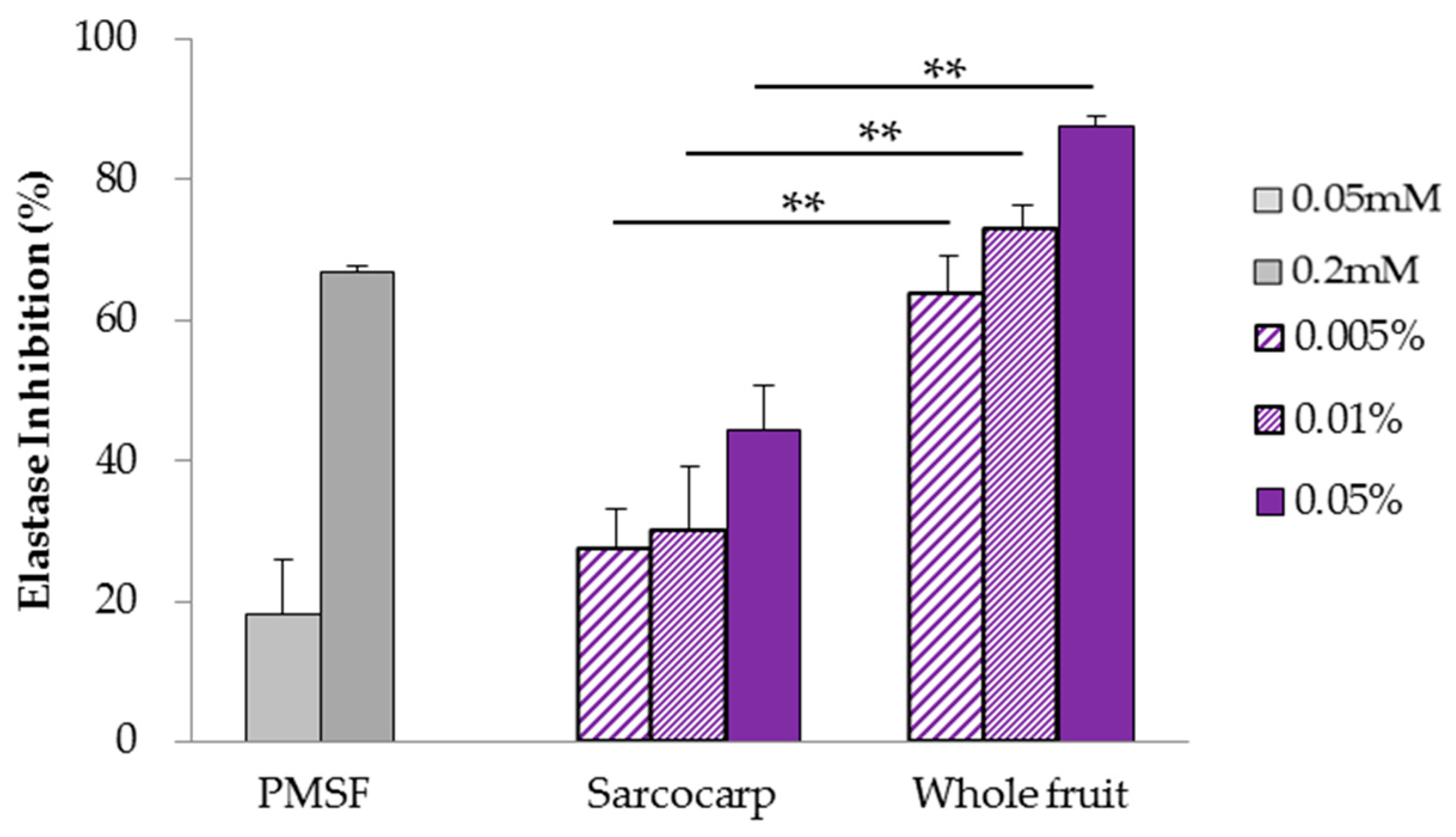
3.2. Analysis of Elastase Inhibition Effect Using Human Neutrophil Elastase
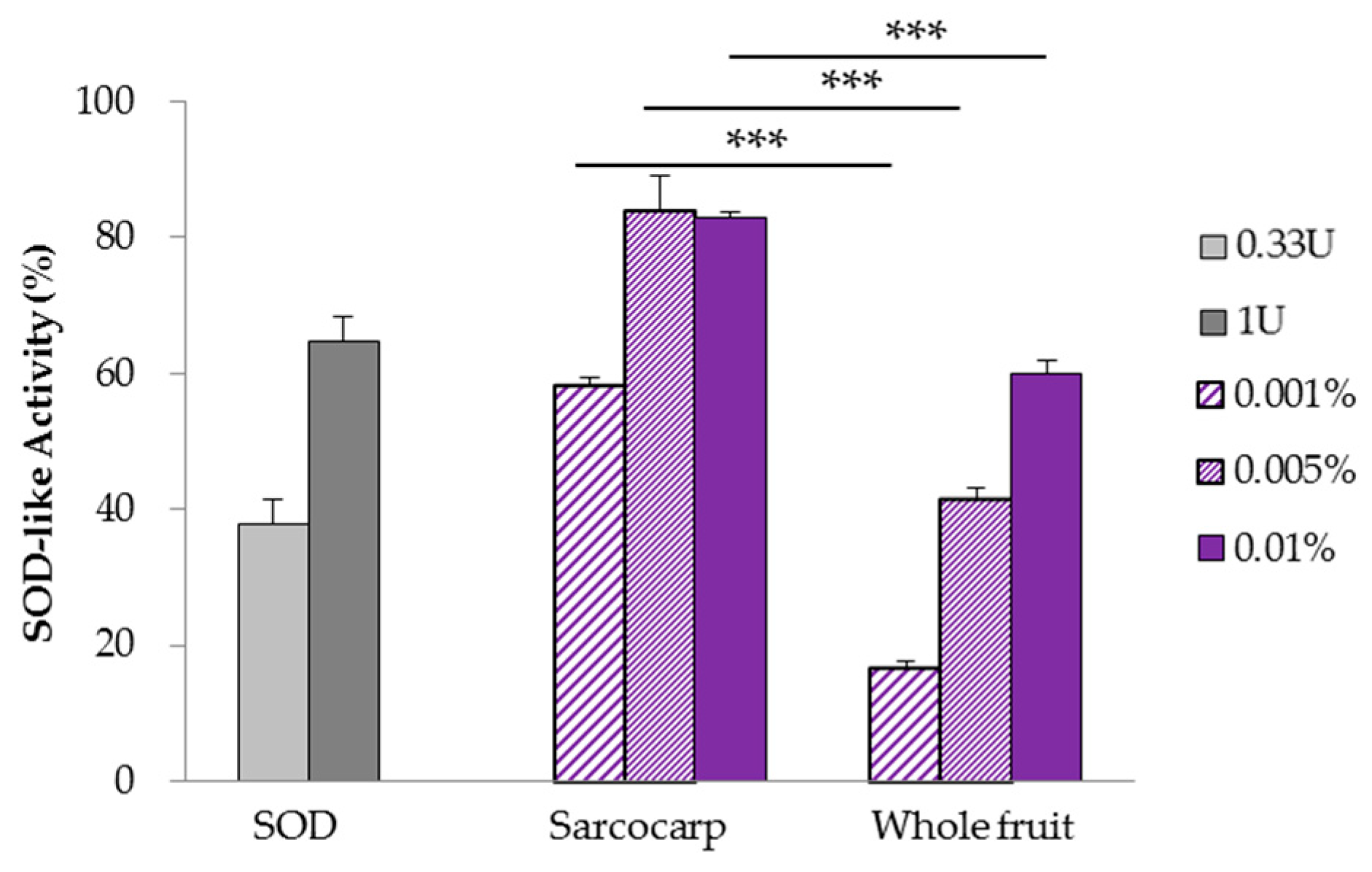
3.3. Analysis of SOD-Like Activity Using Xanthine Oxidase
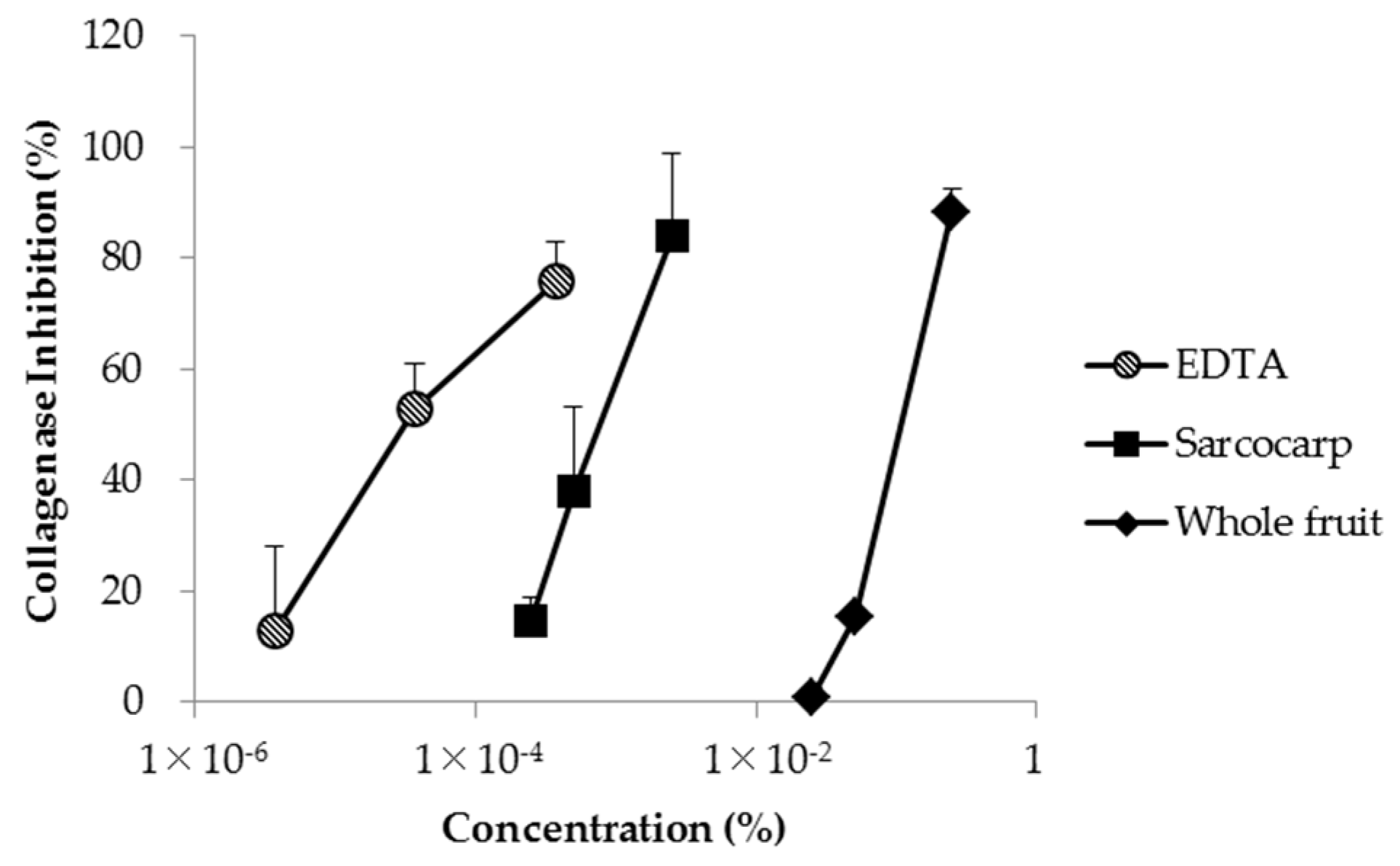
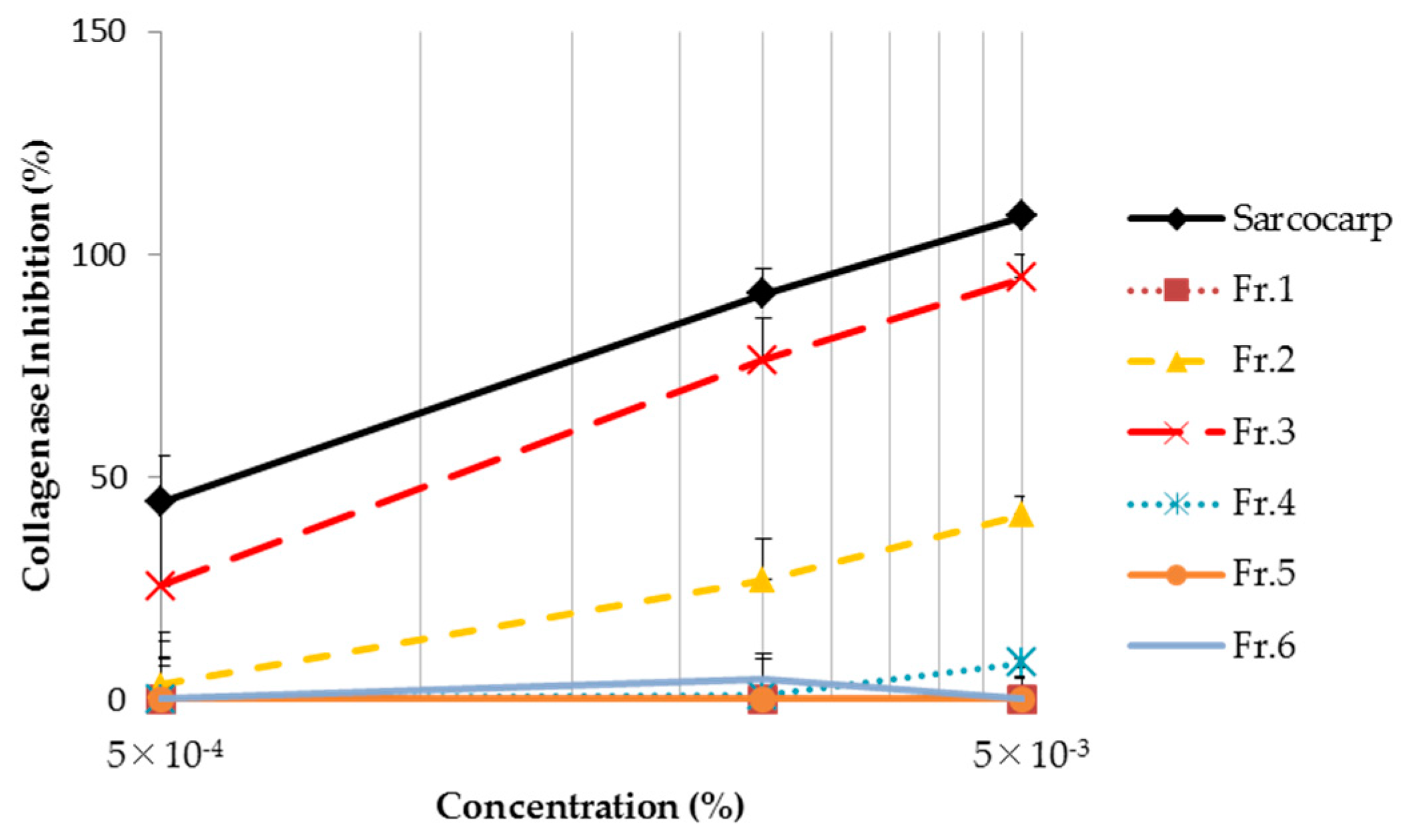
3.4. Analysis of Collagenase Inhibition Effect Using Clostridium histolyticum Collagenase
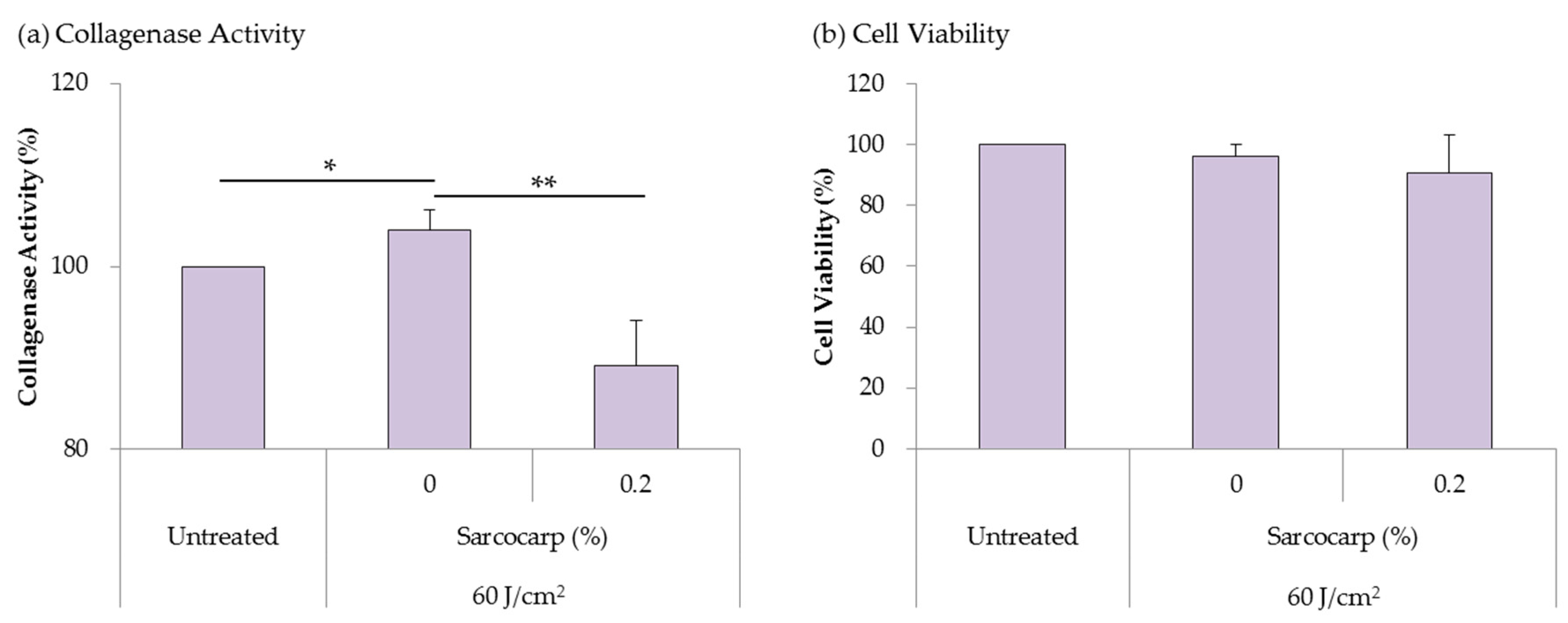
3.5. Analysis of Inhibition Effect on Collagenase Activity Induced by UV Irradiation
3.6. Total Phenolic and Condensed Tannin Content
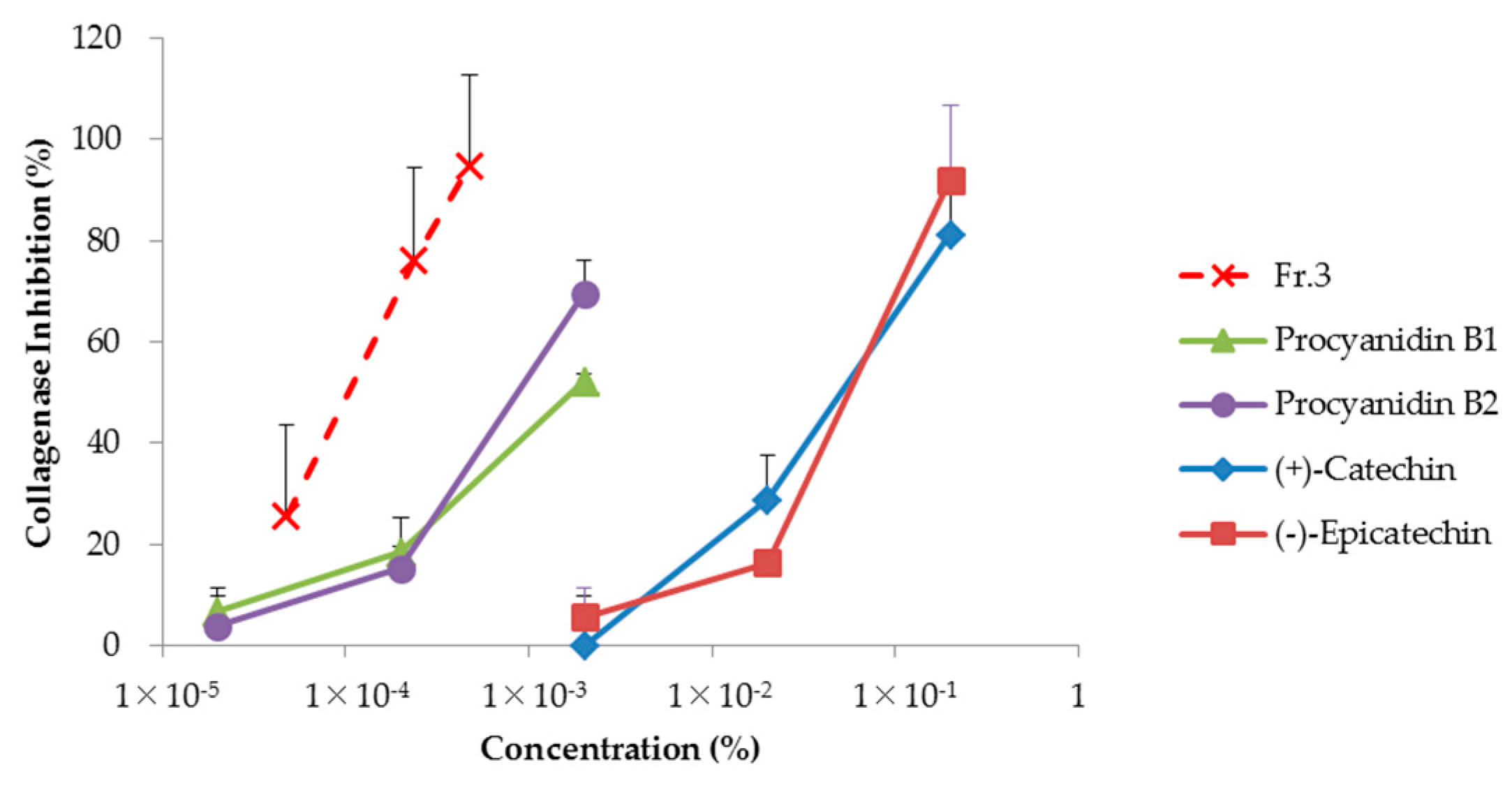
3.7. Analysis of Collagenase Inhibition Effect Using Clostridium histolyticum Collagenase
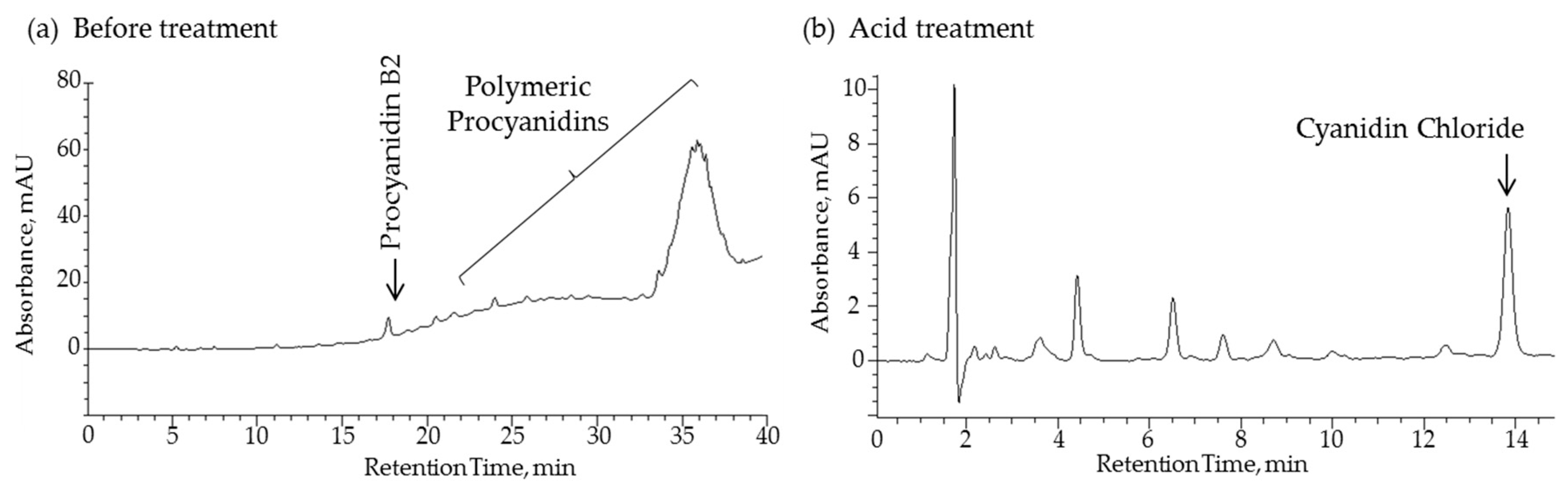
3.8. Analysis of Polymeric Polyphenols in Fr.3
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sveikata, K.; Balciuniene, I.; Tutkuviene, J. Factors influencing face aging. Literature review. Stomatologija 2011, 13, 113–116. [Google Scholar] [PubMed]
- Brem, R.; Li, F.; Montaner, B.; Reelfs, O.; Karran, P. DNA breakage and cell cycle checkpoint abrogation induced by a therapeutic thiopurine and UVA radiation. Oncogene 2010, 29, 3953–3963. [Google Scholar] [CrossRef] [PubMed]
- Rinnerthaler, M.; Bischof, J.; Streubel, M.K.; Trost, A.; Richter, K. Oxidative stress in aging human skin. Biomolecules 2015, 5, 545–589. [Google Scholar] [CrossRef] [PubMed]
- Cavinato, M.; Waltenberger, B.; Baraldo, G.; Grade, C.V.C.; Stuppner, H.; Jansen-Dürr, P. Plant extracts and natural compounds used against UVB-induced photoaging. Biogerontology 2017, 18, 499–516. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, H.; Gomi, T.; Shishido, M.; Watanabe, H.; Suenobu, N. Neutrophil elastase contributes to extracellular matrix damage induced by chronic low-dose UV irradiation in a hairless mouse photoaging model. J. Dermatol. Sci. 2010, 60, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Fisher, G.J.; Wang, Z.Q.; Datta, S.C.; Varani, J.; Kang, S.; Voorhees, J.J. Pathophysiology of premature skin aging induced by ultraviolet light. N. Engl. J. Med. 1997, 337, 1419–1428. [Google Scholar] [CrossRef]
- Miyamoto, K. Characterization of Skin Photo-Aging through Skin Longitudinal Research, and It’s Application to Skin-Aging Protection. J. JPN Cosmet. Sci. Soc. 2017, 41, 188–195. [Google Scholar]
- e-Stat (Portal Site of Official Statistics of Japan). Research on the Production Trends of Specialty Fruit Trees. 2015. Available online: http://www.webcitation.org/74e7cJhF6 (accessed on 14 December 2018).
- Osawa, K.; Yasuda, H.; Morita, H.; Takeya, K.; Itokawa, H. Antibacterial and antihemolytic activity of triterpenes and β-Sitosterol isolated from Chinese Quince (Chaenomeles sinensis). Nat. Med. 1997, 51, 365–367. [Google Scholar]
- Hamauzu, Y.; Yasui, H.; Inno, T.; Kume, C.; Omanyuda, M. Phenolic profile, antioxidant property, and anti-influenza viral activity of Chinese quince (Pseudocydonia sinensis Schneid.), quince (Cydonia oblonga Mill.), and apple (Malus domestica Mill.) fruits. J. Agric. Food Chem. 2005, 53, 928–934. [Google Scholar] [CrossRef]
- Nakata, S.; Kakuta, M.; Misaki, A. Structure and functional properties of seed surface mucilage of karin (Chaenomelese sinensis). Trace Nutr. Res. 2009, 26, 46–49. [Google Scholar]
- Matsuda, H.; Nakamura, S.; Kubo, M. Studies of cuticle drugs from natural sources. II. Inhibitory Effects of Prunus plants on melanin biosynthesis. Biol. Pharm. Bull. 1994, 17, 1417–1420. [Google Scholar] [CrossRef]
- Hamauzu, Y.; Inno, T.; Kume, C.; Irie, M.; Hiramatsu, K. Antioxidant and antiulcerative properties of phenolics from Chinese quince, quince, and apple fruits. J. Agric. Food Chem. 2006, 54, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Strek, M.; Gorlach, S.; Podsedek, A.; Sosnowska, D.; Koziolkiewicz, M.; Hrabec, Z.; Hrabec, E. Procyanidin oligomers from Japanese quince (Chaenomeles japonica) fruit inhibit activity of MMP-2 and MMP-9 metalloproteinases. J. Agric. Food Chem. 2007, 55, 6447–6452. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M.; Shigeyama, K.; Yoshikawa, M.; Matsumoto, T.; Yonezawa, Y.; Shiota, H.; Ueyama, T.; Sakaguchi, I. Effects of cultivation month and genetic background on phenolic content of Citrus tachibana peel. Biol. Pharm. Bull. 2018, 41, 1277–1281. [Google Scholar] [CrossRef] [PubMed]
- Dupont, E.; Gomez, J.; Bilodeau, D. Beyond UV radiation: A skin under challenge. Int. J. Cosmet. Sci. 2013, 35, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Krutmann, J.; Bouloc, A.; Sore, G.; Bernard, B.A.; Passeron, T. The skin aging exposome. J. Dermatol. Sci. 2017, 85, 152–161. [Google Scholar] [CrossRef]
- Verma, V.; Fang, T.; Guo, H.; King, L.; Bates, J.T.; Peltier, R.E.; Edgerton, E.; Russell, A.G.; Weber, R.J. Reactive oxygen species associated with water-soluble PM2.5 in the southeastern United States: Spatiotemporal trends and source apportionment. Atmos. Chem. Phys. 2014, 14, 12915–12930. [Google Scholar] [CrossRef]
- Chen, Y.L.; Hwang, T.L.; Fang, J.Y.; Lan, Y.H.; Chong, K.Y.; Hsieh, P.W. Polysaccharides from Kochia scoparia fruits protect mice from lipopolysaccharide-mediated acute lung injury by inhibiting neutrophil elastase. J. Funct. Foods 2017, 38, 582–590. [Google Scholar] [CrossRef]
- Kim, S.J.; Han, D.; Moon, K.D.; Rhee, J.S. Measurement of superoxide dismutase-like activity of natural antioxidants. Biosci. Biotechnol. Biochem. 1995, 59, 822–826. [Google Scholar] [CrossRef]
- Murakami, I.; Nakamura, T.; Ishibashi, Y.; Shibuya, R.; Ayano, E.; Morita-Murase, Y.; Nagata, Y.; Kanazawa, H. Simultaneous determination of catechins and procyanidins in bottled tea drinks by LC/MS. Chromatography 2006, 27, 27–33. [Google Scholar]
- Yamashita, Y.; Ashida, H. Functionality of procyanidin. Kagaku to Seibutsu 2016, 54, 747–752. [Google Scholar] [CrossRef]
- Hamauzu, Y.; Iijima, E. Polyphenolic composition and antioxidative activity of apple flesh extracts. J. JPN Soc. Food Sci. 1999, 46, 645–651. [Google Scholar] [CrossRef]
- Madhan, B.; Krishnamoorthy, G.; Rao, J.R.; Nair, B.U. Role of green tea polyphenols in the inhibition of collagenolytic activity by collagenase. Int. J. Biol. Macromol. 2007, 41, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, A.; Kayahara, H.; Inbe, T.; Tadasa, K. Inhibition of angiotensin I converting enzyme and collagenase by water extracts of wild and cultivated herbs. J. Fac. Agric. Shinshu Univ. 1994, 31, 97–107. [Google Scholar]
| Fraction | Total Phenolic Content (mg/g extract) | Total Condensed Tannin Content (mg/g Extract) |
|---|---|---|
| Sarcocarp | 168 ± 5 | 150 ± 10 |
| Fr.1 | 13 ± 2 | <25 |
| Fr.2 | 626 ± 14 | 397 ± 29 |
| Fr.3 | 1004 ± 38 | 688 ± 103 |
| Fr.4 | 505 ± 19 | 205 ± 10 |
| Fr.5 | 362 ± 38 | 64 ± 4 |
| Fr.6 | 17 ± 1 | <25 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Itoh, S.; Yamaguchi, M.; Shigeyama, K.; Sakaguchi, I. The Anti-Aging Potential of Extracts from Chaenomeles sinensis. Cosmetics 2019, 6, 21. https://doi.org/10.3390/cosmetics6010021
Itoh S, Yamaguchi M, Shigeyama K, Sakaguchi I. The Anti-Aging Potential of Extracts from Chaenomeles sinensis. Cosmetics. 2019; 6(1):21. https://doi.org/10.3390/cosmetics6010021
Chicago/Turabian StyleItoh, Shintaro, Manami Yamaguchi, Keita Shigeyama, and Ikuyo Sakaguchi. 2019. "The Anti-Aging Potential of Extracts from Chaenomeles sinensis" Cosmetics 6, no. 1: 21. https://doi.org/10.3390/cosmetics6010021
APA StyleItoh, S., Yamaguchi, M., Shigeyama, K., & Sakaguchi, I. (2019). The Anti-Aging Potential of Extracts from Chaenomeles sinensis. Cosmetics, 6(1), 21. https://doi.org/10.3390/cosmetics6010021




