Noninvasive Skin Barrier Assessment: Multiparametric Approach and Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Subjects
2.2. Products
2.3. Study Procedures and Technical Device Specifications
2.4. Analysis
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| FTU | Finger-tip unit |
| NMF | Natural moisturizing factor |
| RCM | Reflectance confocal microscope |
| SC | Stratum corneum |
| TEWL | Transepidermal water loss |
References
- Yousef, H.; Sharma, S. Anatomy, Skin (Integument), Epidermis. In StatPearls. Treasure Island (FL); StatPearls Publishing LLC.: St. Petersburg, FA, USA, 2018. [Google Scholar]
- Loden, M. Role of topical emollients and moisturizers in the treatment of dry skin barrier disorders. Am. J. Clin. Dermatol. 2003, 4, 771–788. [Google Scholar] [CrossRef] [PubMed]
- Rawlings, A.V.; Harding, C.R. Moisturization and skin barrier function. Dermatol. Ther. 2004, 17 (Suppl. 1), 43–48. [Google Scholar] [CrossRef] [PubMed]
- Addor, F.A. Skin barrier in rosacea. Anais Brasileiros de Dermatologia 2016, 91, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Two, A.M.; Wu, W.; Gallo, R.L.; Hata, T.R. Rosacea: Part I. Introduction, categorization, histology, pathogenesis, and risk factors. J. Am. Acad. Dermatol. 2015, 72, 749–758. [Google Scholar] [CrossRef] [PubMed]
- Woo, Y.R.; Lim, J.H.; Cho, D.H.; Park, H.J. Rosacea: Molecular Mechanisms and Management of a Chronic Cutaneous Inflammatory Condition. Int. J. Mol. Sci. 2016, 17, 1562. [Google Scholar] [CrossRef] [PubMed]
- Addor, F.A.; Aoki, V. Skin barrier in atopic dermatitis. Anais Brasileiros de Dermatologia 2010, 85, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Dirschka, T.; Tronnier, H.; Folster-Holst, R. Epithelial barrier function and atopic diathesis in rosacea and perioral dermatitis. Br. J. Dermatol. 2004, 150, 1136–1141. [Google Scholar] [CrossRef] [PubMed]
- Tsakok, T.; Woolf, R.; Smith, C.H.; Weidinger, S.; Flohr, C. Atopic dermatitis: The skin barrier and beyond. Br. J. Dermatol. 2019, 180, 464–474. [Google Scholar] [CrossRef] [PubMed]
- Elias, P.M. Primary role of barrier dysfunction in the pathogenesis of atopic dermatitis. Exp. Dermatol. 2018, 27, 847–851. [Google Scholar] [CrossRef] [PubMed]
- Levin, J.; Miller, R. A Guide to the Ingredients and Potential Benefits of Over-the-Counter Cleansers and Moisturizers for Rosacea Patients. J. Clin. Aesthet. Dermatol. 2011, 4, 31–49. [Google Scholar] [PubMed]
- Hon, K.L.; Kung, J.S.C.; Ng, W.G.G.; Leung, T.F. Emollient treatment of atopic dermatitis: Latest evidence and clinical considerations. Drugs Context 2018, 7, 212530. [Google Scholar] [CrossRef] [PubMed]
- Moss, J. The effect of 3 moisturisers on skin surface hydration: Electrical conductance (Skicon-200), capacitance (Corneometer CM420), and transepidermal water loss (TEWL). Skin Res. Technol. 1996, 2, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Young, D.L.; Chakravarthy, D. A controlled laboratory comparison of 4 topical skin creams moisturizing capability on human subjects. J. Wound Ostomy Cont. Nurs. 2014, 41, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Loden, M. Effect of moisturizers on epidermal barrier function. Clin. Dermatol. 2012, 30, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Elias, P.M.; Wakefield, J.S.; Man, M.Q. Moisturizers versus Current and Next-Generation Barrier Repair Therapy for the Management of Atopic Dermatitis. Skin Pharmacol. Physiol. 2019, 32, 1–7. [Google Scholar] [CrossRef] [PubMed]
- van Erp, P.E.J.; Peppelman, M.; Falcone, D. Noninvasive analysis and minimally invasive in vivo experimental challenges of the skin barrier. Exp. Dermatol. 2018, 27, 867–875. [Google Scholar] [CrossRef] [PubMed]
- Fullerton, A.; Fischer, T.; Lahti, A.; Wilhelm, K.P.; Takiwaki, H.; Serup, J. Guidelines for measurement of skin colour and erythema. A report from the Standardization Group of the European Society of Contact Dermatitis. Contact Dermat. 1996, 35, 1–10. [Google Scholar] [CrossRef]
- Stamatas, G.N.; Zmudzka, B.Z.; Kollias, N.; Beer, J.Z. Non-invasive measurements of skin pigmentation in situ. Pigment Cell Res. 2004, 17, 618–626. [Google Scholar] [CrossRef] [PubMed]
- Imhof, R.E.; De Jesus, M.E.; Xiao, P.; Ciortea, L.I.; Berg, E.P. Closed-chamber transepidermal water loss measurement: Microclimate, calibration and performance. Int. J. Cosmet. Sci. 2009, 31, 97–118. [Google Scholar] [CrossRef] [PubMed]
- Alexander, H.; Brown, S.; Danby, S.; Flohr, C. Research Techniques Made Simple: Transepidermal Water Loss Measurement as a Research Tool. J. Investig. Dermatol. 2018, 138, 2295–3000.e1. [Google Scholar] [CrossRef] [PubMed]
- Pinnagoda, J.; Tupker, R.A.; Agner, T.; Serup, J. Guidelines for transepidermal water loss (TEWL) measurement. A report from the Standardization Group of the European Society of Contact Dermatitis. Contact Dermat. 1990, 22, 164–178. [Google Scholar] [CrossRef]
- Imhof, B. Stratum corneum hydration measurement using capacitance contact imaging. Presented at the COMET 2017, Cergy Pontoise, France, 6–7 June 2017. [Google Scholar]
- Zhang, X.; Bontozoglou, C.; Chirikhina, E.; Lane, M.; Xiao, P. Capacitive Imaging for Skin Characterizations and Solvent Penetration Measurements. Cosmetics 2018, 5, 52. [Google Scholar] [CrossRef]
- Logger, J.G.M.; Munchhoff, C.U.; Olydam, J.I.; Peppelman, M.; Van Erp, P.E.J. Anatomical site variation of water content in human skin measured by the Epsilon: A pilot study. Skin Res. Technol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Ardigo, M.; Longo, C.; Gonzalez, S. Multicentre study on inflammatory skin diseases from The International Confocal Working Group: Specific confocal microscopy features and an algorithmic method of diagnosis. Br. J. Dermatol. 2016, 175, 364–374. [Google Scholar] [CrossRef] [PubMed]
- Levine, A.; Markowitz, O. Introduction to reflectance confocal microscopy and its use in clinical practice. JAAD Case Rep. 2018, 4, 1014–1023. [Google Scholar] [CrossRef] [PubMed]
- Finlay, A.Y.; Edwards, P.H.; Harding, K.G. “Fingertip unit” in dermatology. Lancet 1989, 2, 155. [Google Scholar] [CrossRef]
- Farahmand, S.; Tien, L.; Hui, X.; Maibach, H.I. Measuring transepidermal water loss: A comparative in vivo study of condenser-chamber, unventilated-chamber and open-chamber systems. Skin Res. Technol. 2009, 15, 392–398. [Google Scholar] [CrossRef] [PubMed]
- Voegeli, R.; Rawlings, A.V.; Seroul, P.; Summers, B. A novel continuous colour mapping approach for visualization of facial skin hydration and transepidermal water loss for four ethnic groups. Int. J. Cosmet. Sci. 2015, 37, 595–605. [Google Scholar] [CrossRef] [PubMed]
- Marrakchi, S.; Maibach, H.I. Biophysical parameters of skin: Map of human face, regional, and age-related differences. Contact Dermat. 2007, 57, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Wa, C.V.; Maibach, H.I. Mapping the human face: Biophysical properties. Skin Res. Technol. 2010, 16, 38–54. [Google Scholar] [CrossRef] [PubMed]
- Tagami, H. Location-related differences in structure and function of the stratum corneum with special emphasis on those of the facial skin. Int. J. Cosmet. Sci. 2008, 30, 413–434. [Google Scholar] [CrossRef] [PubMed]
- Peppelman, M.; Wolberink, E.A.; Gerritsen, M.J.; van de Kerkhof, P.C.; van Erp, P.E. Application of leukotriene B4 and reflectance confocal microscopy as a noninvasive in vivo model to study the dynamics of skin inflammation. Skin Res. Technol. 2015, 21, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Robertson, K.; Rees, J.L. Variation in epidermal morphology in human skin at different body sites as measured by reflectance confocal microscopy. Acta Derm. Venereol. 2010, 90, 368–373. [Google Scholar] [PubMed]
- Puig, S.C.C.; Lovato, L. Reflectance Confocal Microscopy for Skin Diseases; Springer: Berlin/Hedelberg, Germany, 2012. [Google Scholar]
- Van Mulder, T.J.; de Koeijer, M.; Theeten, H.; Willems, D.; Van Damme, P.; Demolder, M.; De Meyer, G.; Beyers, K.C.; Vankerckhoven, V. High frequency ultrasound to assess skin thickness in healthy adults. Vaccine 2017, 35, 1810–1815. [Google Scholar] [CrossRef] [PubMed]
- Miyamae, Y.; Kawabata, M.; Yamakawa, Y.; Tsuchiya, J.; Ozaki, Y. Non-Invasive Estimation of Skin Thickness by near Infrared Diffuse Reflection Spectroscopy—Separate Determination of Epidermis and Dermis Thickness. J. Near Infrared Spectrosc. 2012, 20, 617–622. [Google Scholar] [CrossRef]
- Pena, A.; Arronte, M.; De Posada, E.; Ponce, L.; Flores, T. Non-invasive optical method for epidermal thickness estimation. OnLine J. Biol. Sci. 2014, 14, 163–166. [Google Scholar]
- Crowther, J.M.; Sieg, A.; Blenkiron, P.; Marcott, C.; Matts, P.J.; Kaczvinsky, J.R.; Rawlings, A.V. Measuring the effects of topical moisturizers on changes in stratum corneum thickness, water gradients and hydration in vivo. Br. J. Dermatol. 2008, 159, 567–577. [Google Scholar] [PubMed]
- Manfredini, M.; Mazzaglia, G.; Ciardo, S.; Simonazzi, S.; Farnetani, F.; Longo, C.; Pellacani, G. Does skin hydration influence keratinocyte biology? In vivo evaluation of microscopic skin changes induced by moisturizers by means of reflectance confocal microscopy. Skin Res. Technol. 2013, 19, 299–307. [Google Scholar] [PubMed]
- Nolan, K.; Marmur, E. Moisturizers: Reality and the skin benefits. Dermatol. Ther. 2012, 25, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Constantin, M.M.; Poenaru, E.; Poenaru, C.; Constantin, T. Skin Hydration Assessment through Modern Non-Invasive Bioengineering Technologies. Maedica (Buchar) 2014, 9, 33–38. [Google Scholar] [PubMed]
- Pierre, J.; Francois, G.; Benize, A.M.; Rubert, V.; Coutet, J.; Flament, F. Mapping, in vivo, the uniformity of two skin properties alongside the human face by a 3D virtual approach. Int. J. Cosmet. Sci. 2018, 40, 482–487. [Google Scholar] [CrossRef] [PubMed]
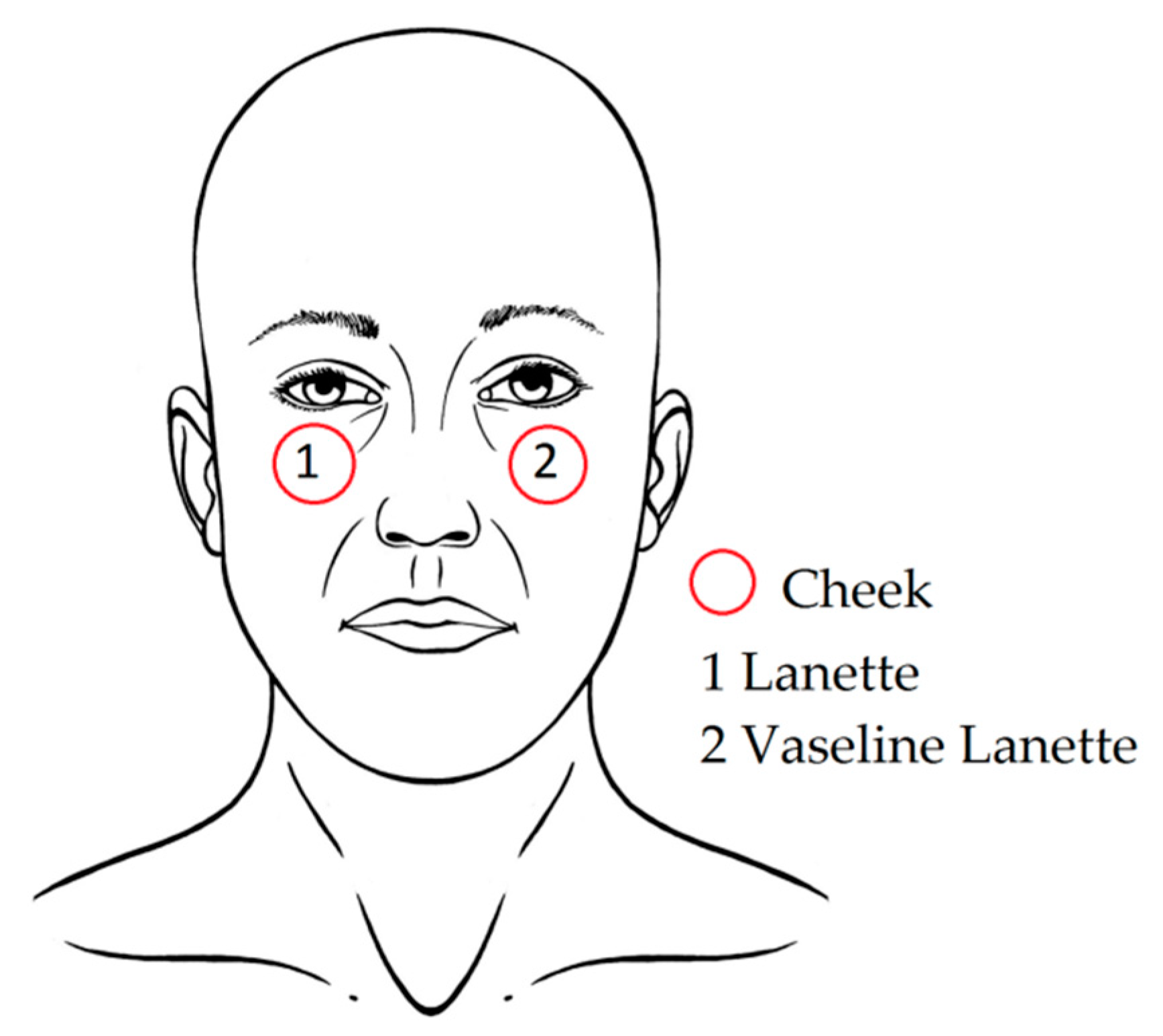
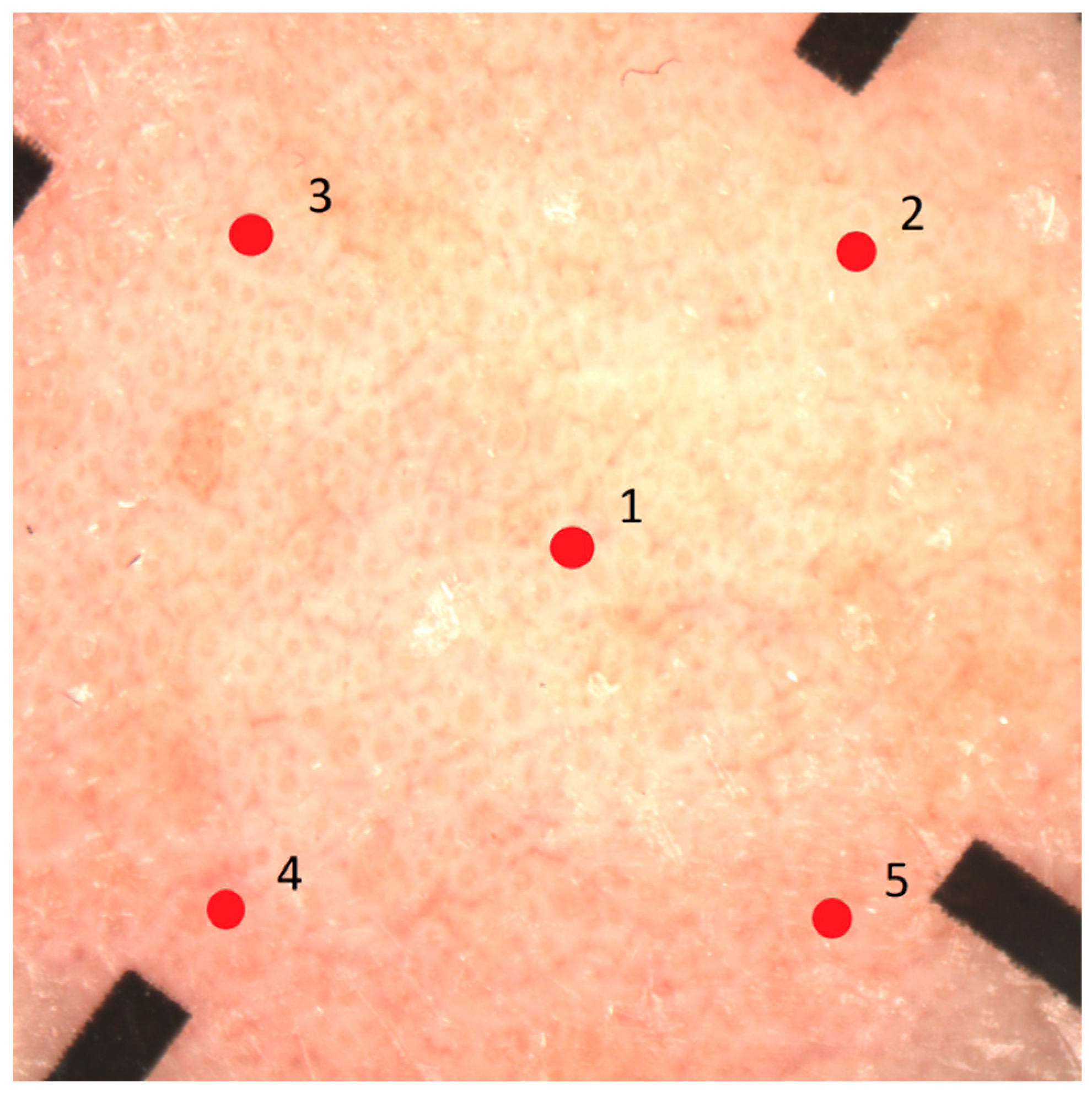
| Product | Ingredients (in Order of Percentage of Cream Content) |
|---|---|
| Lanette cream I FNA | Purified water, decyloleate, cetostearyl alcohol, B emulsifying, sorbitol solution, sorbic acid |
| Vaseline-Lanette cream FNA | Cetostearyl alcohol, B emulsifying, cetiol V, sorbic acid, sorbitol solution, white petrolatum (vaseline), purified water |
| Device Skin Parameter Assessed | Measurement Principle | Output | Measurement Time | References |
|---|---|---|---|---|
| Spectrophotometer (Konica CM-2600d) Erythema | Intense white light from a xenon lamp is emitted by a probe. The device is placed onto the skin. The color of the reflected light is analyzed by three photocells filtering the primary colors (blue: 450 nm, green: 550 nm, red: 610 nm). This allows measurement of the absorbance and reflectance spectrum in the 400–700-nm range. | Color expressed as L*a*b color space. This is a three-dimensional coordinate system with an L*axis (brightness) and two orthogonal axes representing chromaticity, namely a*axis (red-green) and b*axis (yellow-blue). The a* value from the measurement locations is obtained after repeated calibration on a white surface before each measuring session (0.00). | 1.5 s | [17,18,19] |
| Aquaflux (Biox) TEWL | A probe consisting of a closed chamber with a condenser and sensors for relative temperature and humidity is applied onto the skin surface. The flux of vapor is calculated due to increasing temperature and humidity rate. | Flux density of water vapor (g/m2/h). | Max. 180 s | [20,21,22] |
| Epsilon (Biox) Water content | A probe consisting of 76,800 sensors with a sensing area of 1.3 cm × 1.5 cm, a resolution of 50 µm, and a measurement depth of 20 µm is placed onto the skin. The electrical capacitance of the skin surface is calculated. | Calibrated dielectric permittivity (ε) through the SC. Moreover, capacitive contact images can be obtained (brighter color = higher dielectric constant; darker color = lower dielectric constant) for skin surface hydration mapping, taking skin relief and variable distribution of sweat glands into account. | 30 s | [23,24,25] |
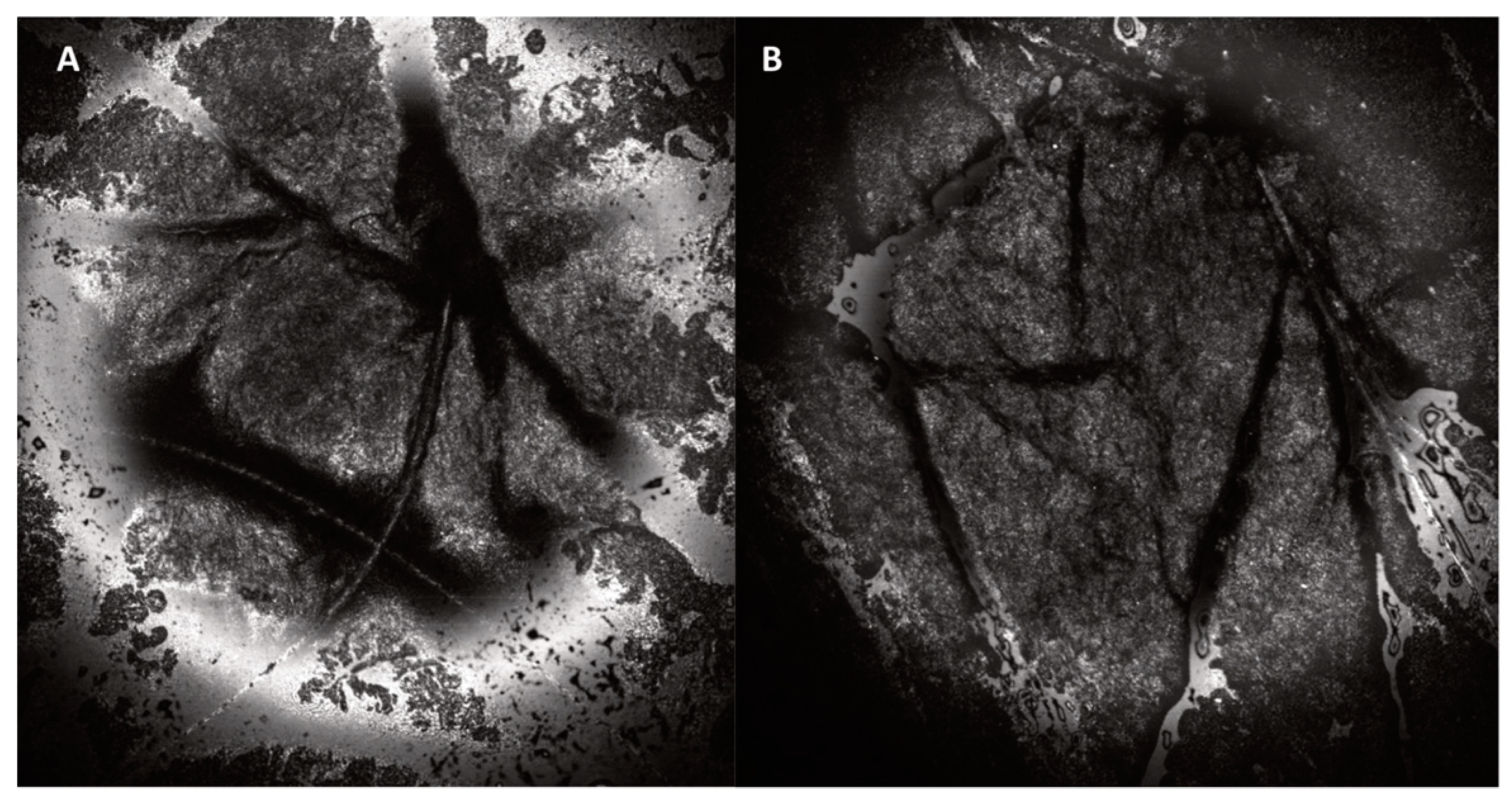
| Reflectance Confocal Microscope (VivaScope 1500) Thickness of SC and epidermis | Laser light at 830 nm is focused onto the skin with maximum imaging depth of 200 µm below skin surface (papillary dermis). Due to different refractive indexes between the cell structures and the surrounding tissue, en face images at 30× magnification of morphological and cellular resolution are obtained. Horizontal resolution: 0.5–1 µm, vertical resolution: 3–5 µm. | Black and white images showing skin morphology. Options: - VivaCam: dermoscopic image. - Confocal: basic image of 500 µm × 500 µm. - VivaBlock: multiple confocals acquired at the same level, stitched together to create one larger image (max. 8 mm × 8 mm). - VivaStack: multiple confocals along depth at a certain location, with interval steps of 3–5 µm. - Movie: e.g., to view blood flow in the superficial dermis. | 2–3 min | [17,26,27] |
| Skin Parameter | Lanette | Vaseline-Lanette | ||||
|---|---|---|---|---|---|---|
| Baseline | 30 min after cream application | p-Value | Baseline | 30 minafter cream application | p-Value | |
| Erythema (a*) | 48.40 (46.20–50.80) § | 48.00 (46.10–50.60) | 0.362 | 47.95 (44.60–49.80) | 48.05 (43.20–49.80) | 0.965 |
| TEWL (g/m2/h) | 19.28 (14.05–25.31) | 19.09 (15.87–26.71) | 0.363 | 18.81 (13.67–27.22) | 19.00 (14.61–24.54) | 0.300 |
| Water content (ε) | 20.16 (14.86–33.94) | 23.29 (15.89–28.33) | 0.730 | 21.11 (16.31–47.02) | 21.79 (14.03–40.02) | 0.363 |
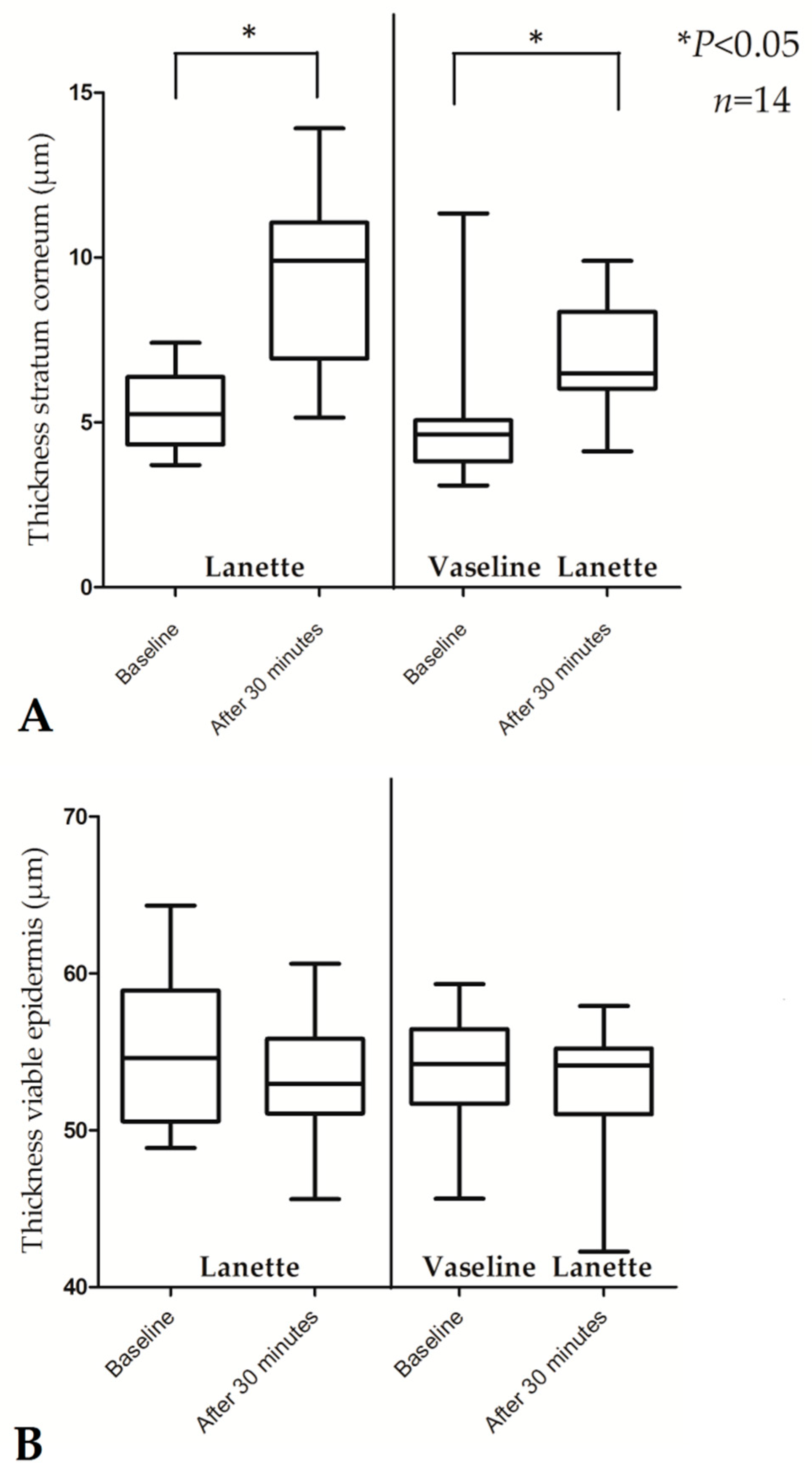
| Thickness SC (µm) | 5.25 (3.71–7.42) | 9.90 (5.15–13.92) | 0.001 * | 4.64 (3.09–11.34) | 6.49 (4.12–9.90) | 0.016 * |
| Thickness viable epidermis (µm) | 54.59 (48.86–64.32) | 52.96 (45.61–60.61) | 0.551 | 54.22 (45.64–59.31) | 54.12 (42.26–57.91) | 0.638 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Logger, J.G.M.; Olydam, J.I.; Woliner-van der Weg, W.; van Erp, P.E.J. Noninvasive Skin Barrier Assessment: Multiparametric Approach and Pilot Study. Cosmetics 2019, 6, 20. https://doi.org/10.3390/cosmetics6010020
Logger JGM, Olydam JI, Woliner-van der Weg W, van Erp PEJ. Noninvasive Skin Barrier Assessment: Multiparametric Approach and Pilot Study. Cosmetics. 2019; 6(1):20. https://doi.org/10.3390/cosmetics6010020
Chicago/Turabian StyleLogger, Jade G. M., Jill I. Olydam, Wietske Woliner-van der Weg, and Piet E. J. van Erp. 2019. "Noninvasive Skin Barrier Assessment: Multiparametric Approach and Pilot Study" Cosmetics 6, no. 1: 20. https://doi.org/10.3390/cosmetics6010020
APA StyleLogger, J. G. M., Olydam, J. I., Woliner-van der Weg, W., & van Erp, P. E. J. (2019). Noninvasive Skin Barrier Assessment: Multiparametric Approach and Pilot Study. Cosmetics, 6(1), 20. https://doi.org/10.3390/cosmetics6010020






