ES2 as a Novel Verbascoside-Derived Compound in the Treatment of Cutaneous Wound Healing
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Cell Treatments
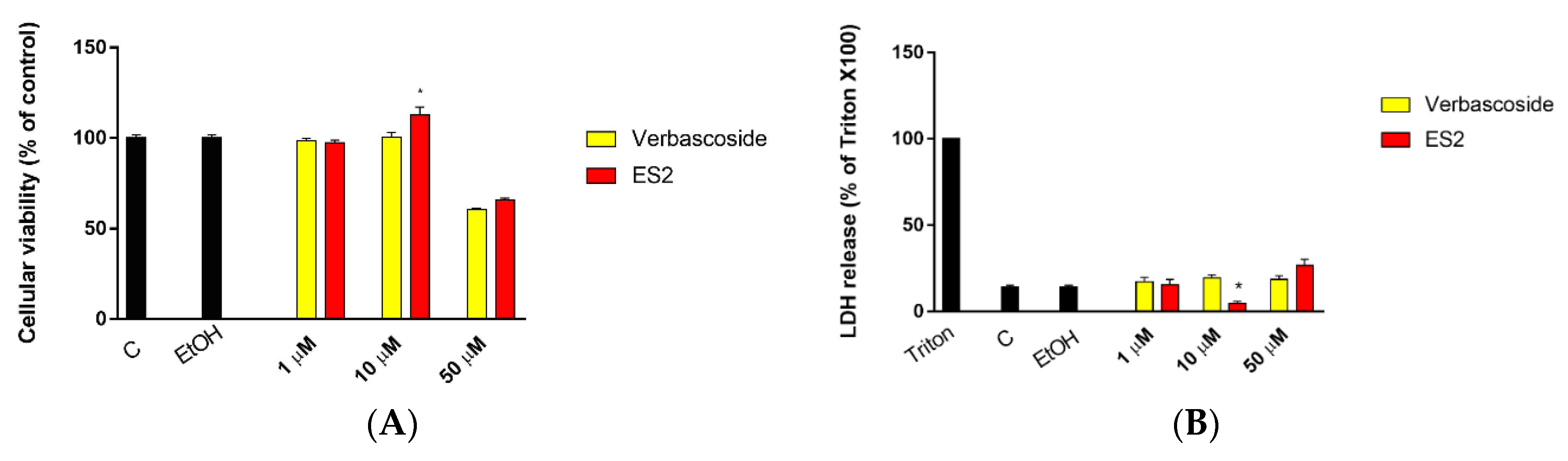
2.3. Cell Viability
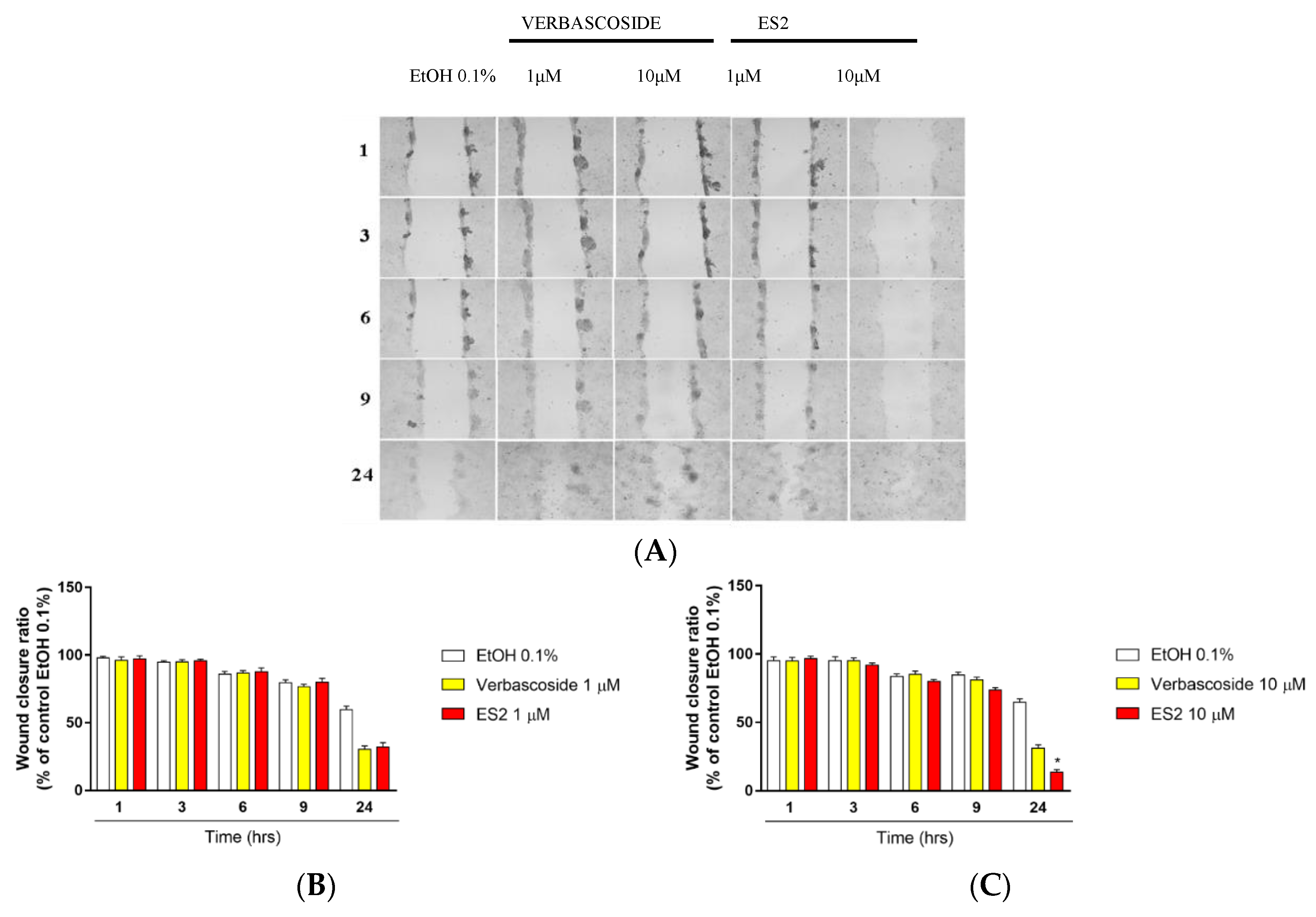
2.4. In Vitro Wound Healing Assay
2.5. Animals
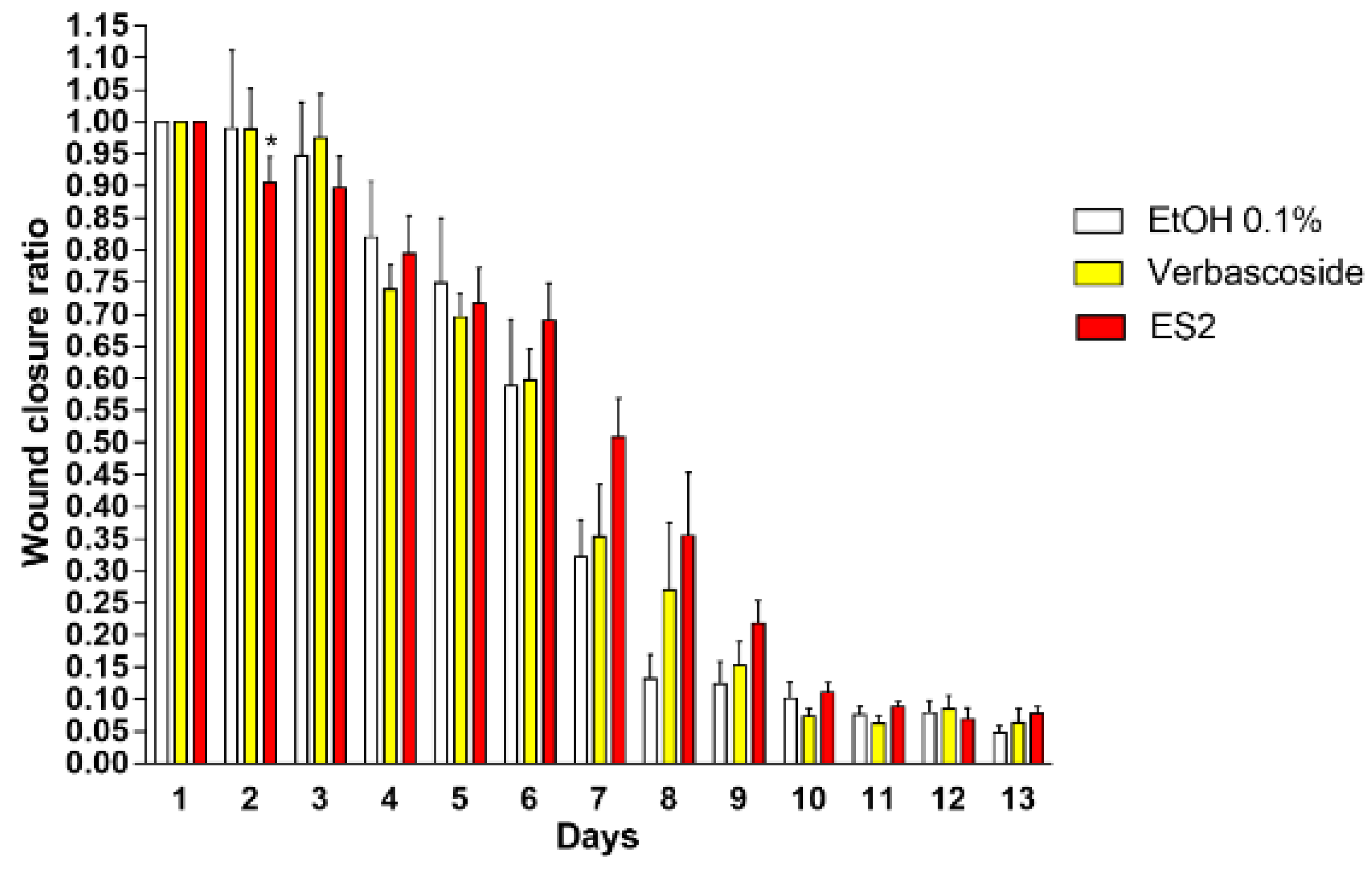
2.6. In Vivo Wound Healing Assay
2.7. Statistical Analysis
3. Results
3.1. Effect of Verbascoside and ES2 on Cell Viability
3.2. Effect of Verbascoside and ES2 on In Vitro Wound Closure
3.3. ES2 Induced an Higher Wound Closure after 2 Days of Topical Treatment
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound repair and regeneration. Nature 2008, 453, 14–321. [Google Scholar] [CrossRef] [PubMed]
- Kasuya, A.; Tokura, Y. Attempts to accelerate wound healing. J. Dermatol. Sci. 2014, 76, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Rousselle, P.; Braye, F.; Dayan, G. Re-epithelialization of adult skin wounds: Cellular mechanisms and therapeutic strategies. Adv. Drug Deliv. Rev. 2018. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.F.; Bártolo, P.J. Traditional Therapies for Skin Wound Healing. Adv. Wound Care 2016, 5, 208–229. [Google Scholar] [CrossRef] [PubMed]
- Eming, S.A.; Martin, P.; Tomic-canic, M.; Park, H.; Medicine, R. Wound repair and regeneration: Mechanism, signaling and translation. Sci. Transl. Med. 2014, 6. [Google Scholar] [CrossRef] [PubMed]
- Harding, K.G.; Morris, H.L.; Patel, G.K. Clinical review Healing chronic wounds. BMJ 2002, 324, 160–163. [Google Scholar] [CrossRef] [PubMed]
- Eaglstein, W.H.; Falanga, V. Chronic Wounds. Surg. Clin. N. Am. 1997, 77, 689–700. [Google Scholar] [CrossRef]
- Davis, S.C.; Perez, R. Cosmeceuticals and natural products: Wound healing. Clin. Dermatol. 2009, 27, 502–506. [Google Scholar] [CrossRef] [PubMed]
- Dembitsky, V.M. Astonishing diversity of natural surfactants: 5. Biologically active glycosides of aromatic metabolites. Lipids 2005, 40, 869–900. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, C.; Riguera, R. Phenylethanoid glycosides in plants: Structure and biological activity. Nat. Prod. Rep. 1994, 11, 591–606. [Google Scholar] [CrossRef] [PubMed]
- Speranza, L.; Franceschelli, S.; Pesce, M.; Reale, M.; Menghini, L.; Vinciguerra, I.; de Lutiis, M.A.; Felaco, M.; Grilli, A. Antiinflammatory effects in THP-1 cells treated with verbascoside. Phyther. Res. 2010, 24, 1398–1404. [Google Scholar] [CrossRef] [PubMed]
- Pastore, S.; Potapovich, A.; Kostyuk, V.; Mariani, V.; Lulli, D.; de Luca, C.; Korkina, L. Plant polyphenols effectively protect HaCaT cells from ultraviolet C-triggered necrosis and suppress inflammatory chemokine expression. Ann. N. Y. Acad. Sci. 2009, 1171, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Azaizeh, H.; Tafesh, A.; Najami, N.; Jadoun, J.; Halahlih, F.; Riepl, H. Synergistic antibacterial effects of polyphenolic compounds from olive mill wastewater. Evid. Based Complement. Altern. Med. 2011, 2011. [Google Scholar] [CrossRef]
- Sinico, C.; Caddeo, C.; Valenti, D.; Fadda, A.M.; Bilia, A.R.; Vincieri, F.F. Liposomes as carriers for verbascoside: Stability and skin permeation studies. J. Liposome Res. 2008, 18, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Vertuani, S.; Beghelli, E.; Scalambra, E.; Malisardi, G.; Copetti, S.; Toso, R.D.; Baldisserotto, A.; Manfredini, S. Activity and stability studies of verbascoside, a novel antioxidant, in dermo-cosmetic and pharmaceutical topical formulations. Molecules 2011, 16, 7068–7080. [Google Scholar] [CrossRef] [PubMed]
- Valacchi, G.; Rimbach, G.; Saliou, C.; Weber, S.U.; Packer, L. Effect of benzoyl peroxide on antioxidant status, NF-kappaB activity and interleukin-1alpha gene expression in human keratinocytes. Toxicology 2001, 165, 225–234. [Google Scholar] [CrossRef]
- Valacchi, G.; Sticozzi, C.; Belmonte, G.; Cervellati, F.; Demaude, J.; Chen, N.; Krol, Y.; Oresajo, C. Vitamin C Compound Mixtures Prevent Ozone-Induced Oxidative Damage in Human Keratinocytes as Initial Assessment of Pollution Protection. PLoS ONE 2015, 10, e0131097. [Google Scholar] [CrossRef] [PubMed]
- Valacchi, G.; Pecorelli, A.; Mencarelli, M.; Carbotti, P.; Fortino, V.; Muscettola, M.; Maioli, E. Rottlerin: A multifaced regulator of keratinocyte cell cycle. Exp. Dermatol. 2009, 18, 516–521. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.; Phung, A.D.; Corbacho, A.M.; Aung, H.H.; Maioli, E.; Reznick, A.Z.; Cross, C.E.; Davis, P.A.; Valacchi, G. Modulation of cutaneous wound healing by ozone: Differences between young and aged mice. Toxicol. Lett. 2006, 160, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Valacchi, G.; Zanardi, I.; Lim, Y.; Belmonte, G.; Miracco, C.; Sticozzi, C.; Bocci, V.; Travagli, V. Ozonated oils as functional dermatological matrices: Effects on the wound healing process using SKH1 mice. Int. J. Pharm. 2013, 458, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Brownrigg, J.R.W.; Apelqvist, J.; Bakker, K.; Schaper, N.C.; Hinchliffe, R.J. Evidence-based management of PAD & the diabetic foot. Eur. J. Vasc. Endovasc. Surg. 2013, 45, 673–681. [Google Scholar] [PubMed]
- Richmond, N.; Maderal, A.; Vivas, A. Evidence-based management of common chronic lower extremity ulcers. Dermatol. Ther. 2013, 26, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Atherley, G. Occupational disease prevention in Canada. A change of direction? Ann. N. Y. Acad. Sci. 1989, 572, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Rice, J.B.; Desai, U.; Cummings, A.K.G.; Birnbaum, H.G.; Skornicki, M.; Parsons, N. Burden of venous leg ulcers in the United States. J. Med. Econ. 2014, 17, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Woo, K.; Ayello, E.A.; Sibbald, R.G. The edge effect: Current therapeutic options to advance the wound edge. Adv. Skin Wound Care 2007, 20, 99–117. [Google Scholar] [CrossRef] [PubMed]
- Frykberg, R.G.; Banks, J. Challenges in the Treatment of Chronic Wounds. Adv. Wound Care 2015, 4, 560–582. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, K.P.; Wilhelm, D.; Bielfeldt, S. Models of wound healing: An emphasis on clinical studies. Skin Res. Technol. 2017, 23, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Valacchi, G.; Lim, Y.; Belmonte, G.; Miracco, C.; Zanardi, I.; Bocci, V.; Travagli, V. Ozonated sesame oil enhances cutaneous wound healing in SKH1 mice. Wound Repair Regen. 2011, 19, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Esposito, E.; Sticozzi, C.; Ravani, L.; Drechsler, M.; Muresan, X.M.; Cervellati, F.; Cortesi, R.; Valacchi, G. Effect of new curcumin-containing nanostructured lipid dispersions on human keratinocytes proliferative responses. Exp. Dermatol. 2015, 24, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Rosseto, H.C.; Toledo, L.A.S.; de Francisco, L.M.B.; Esposito, E.; Lim, Y.; Valacchi, G.; Cortesi, R.; Bruschi, M.L. Nanostructured lipid systems modified with waste material of propolis for wound healing: Design, in vitro and in vivo evaluation. Colloids Surf. B Biointerfaces 2017, 158, 441–452. [Google Scholar] [CrossRef] [PubMed]
- Alipieva, K.; Korkina, L.; Orhan, I.E.; Georgiev, M.I. Verbascoside—A review of its occurrence, (bio)synthesis and pharmacological significance. Biotechnol. Adv. 2014, 32, 1065–1076. [Google Scholar] [CrossRef] [PubMed]
- Mazzon, E.; Esposito, E.; di Paola, R.; Riccardi, L.; Caminiti, R.; Toso, R.D.; Pressi, G.; Cuzzocrea, S. Effects of verbascoside biotechnologically produced by Syringa vulgaris plant cell cultures in a rodent model of colitis. Naunyn. Schmiedebergs Arch. Pharmacol. 2009, 380, 79–94. [Google Scholar] [CrossRef] [PubMed]
- De Moura Sperotto, N.D.; Steffens, L.; Veríssimo, R.M.; Henn, J.G.; Péres, V.F.; Vianna, P.; Chies, J.A.B.; Roehe, A.; Saffi, J.; Moura, D.J. Wound healing and anti-inflammatory activities induced by a Plantago australis hydroethanolic extract standardized in verbascoside. J. Ethnopharmacol. 2018, 225, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Isacchi, B.; Bergonzi, M.C.; Iacopi, R.; Ghelardini, C.; Galeotti, N.; Bilia, A.R. Liposomal Formulation to Increase Stability and Prolong Antineuropathic Activity of Verbascoside. Planta Med. 2017, 83, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Ambrosone, L.; Guerra, G.; Cinelli, M.; Filippelli, M.; Mosca, M.; Vizzarri, F.; Giorgio, D.; Costagliola, C. Corneal epithelial wound healing promoted by verbascoside-based liposomal eyedrops. Biomed. Res. Int. 2014. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.Y.; Makino, K.; Xia, W.; Matin, A.; Wen, Y.; Kwong, K.Y.; Bourguignon, L.; Hung, M.C. Nuclear localization of EGF receptor and its potential new role as a transcription factor. Nat. Cell Biol. 2001, 3, 802–808. [Google Scholar] [CrossRef] [PubMed]
- Lo, H.W.; Hung, M.C. Nuclear EGFR signalling network in cancers: Linking EGFR pathway to cell cycle progression, nitric oxide pathway and patient survival. Br. J. Cancer 2006, 94, 184–188. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crivellari, I.; Vertuani, S.; Lim, Y.; Cervellati, F.; Baldisserotto, A.; Manfredini, S.; Valacchi, G. ES2 as a Novel Verbascoside-Derived Compound in the Treatment of Cutaneous Wound Healing. Cosmetics 2018, 5, 65. https://doi.org/10.3390/cosmetics5040065
Crivellari I, Vertuani S, Lim Y, Cervellati F, Baldisserotto A, Manfredini S, Valacchi G. ES2 as a Novel Verbascoside-Derived Compound in the Treatment of Cutaneous Wound Healing. Cosmetics. 2018; 5(4):65. https://doi.org/10.3390/cosmetics5040065
Chicago/Turabian StyleCrivellari, Ilaria, Silvia Vertuani, Yunsook Lim, Franco Cervellati, Anna Baldisserotto, Stefano Manfredini, and Giuseppe Valacchi. 2018. "ES2 as a Novel Verbascoside-Derived Compound in the Treatment of Cutaneous Wound Healing" Cosmetics 5, no. 4: 65. https://doi.org/10.3390/cosmetics5040065
APA StyleCrivellari, I., Vertuani, S., Lim, Y., Cervellati, F., Baldisserotto, A., Manfredini, S., & Valacchi, G. (2018). ES2 as a Novel Verbascoside-Derived Compound in the Treatment of Cutaneous Wound Healing. Cosmetics, 5(4), 65. https://doi.org/10.3390/cosmetics5040065







