Safety Assessment of Nano-Hydroxyapatite as an Oral Care Ingredient according to the EU Cosmetics Regulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Physico-Chemical Characterization
2.1.1. Hydroxyapatite Nanoparticles
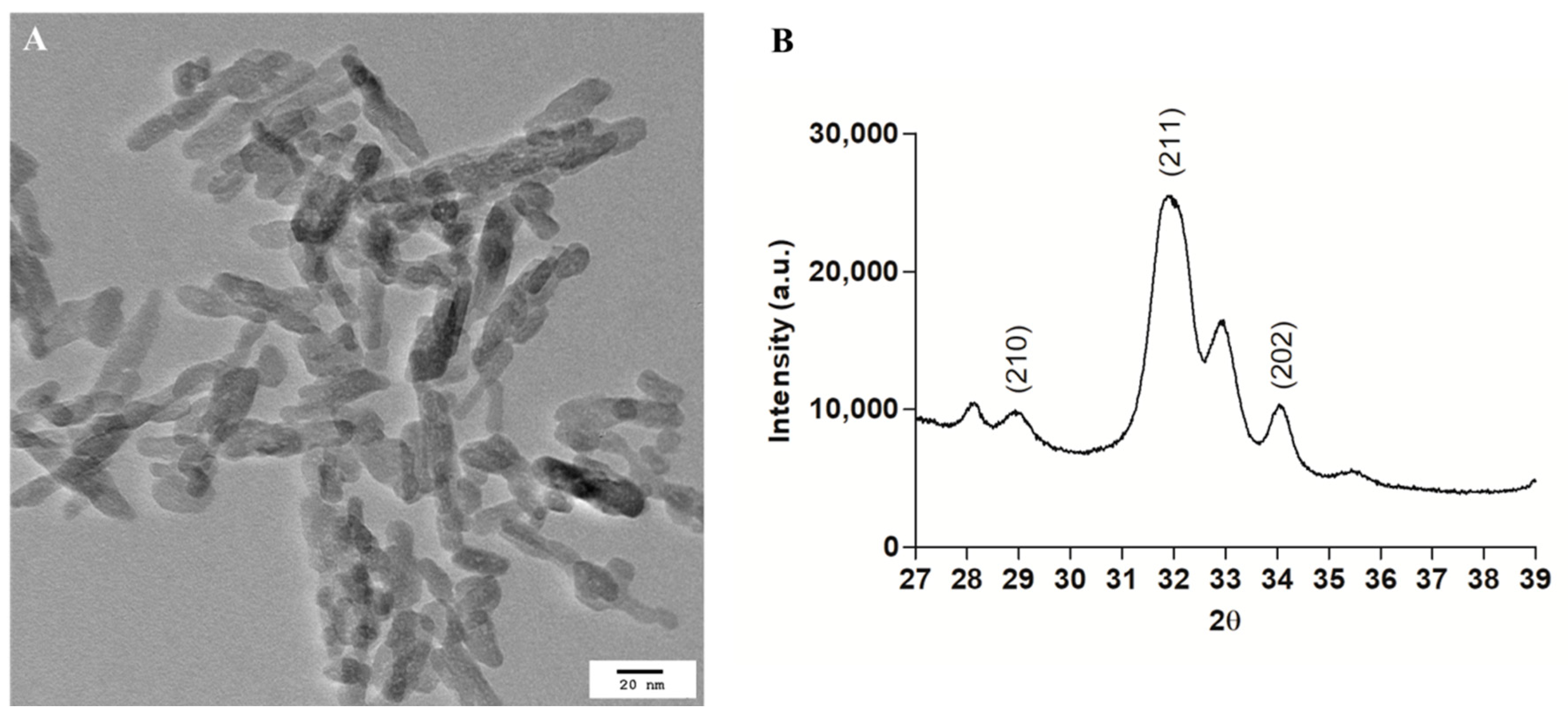
2.1.2. Transmission Electron Microscopy
2.1.3. X-ray Diffraction
2.1.4. Surface Area
2.2. In Vitro Biocompatibility
2.2.1. Reconstructed Human Gingival Epithelium
2.2.2. Treatment with the Hydroxyapatite Nanoparticles
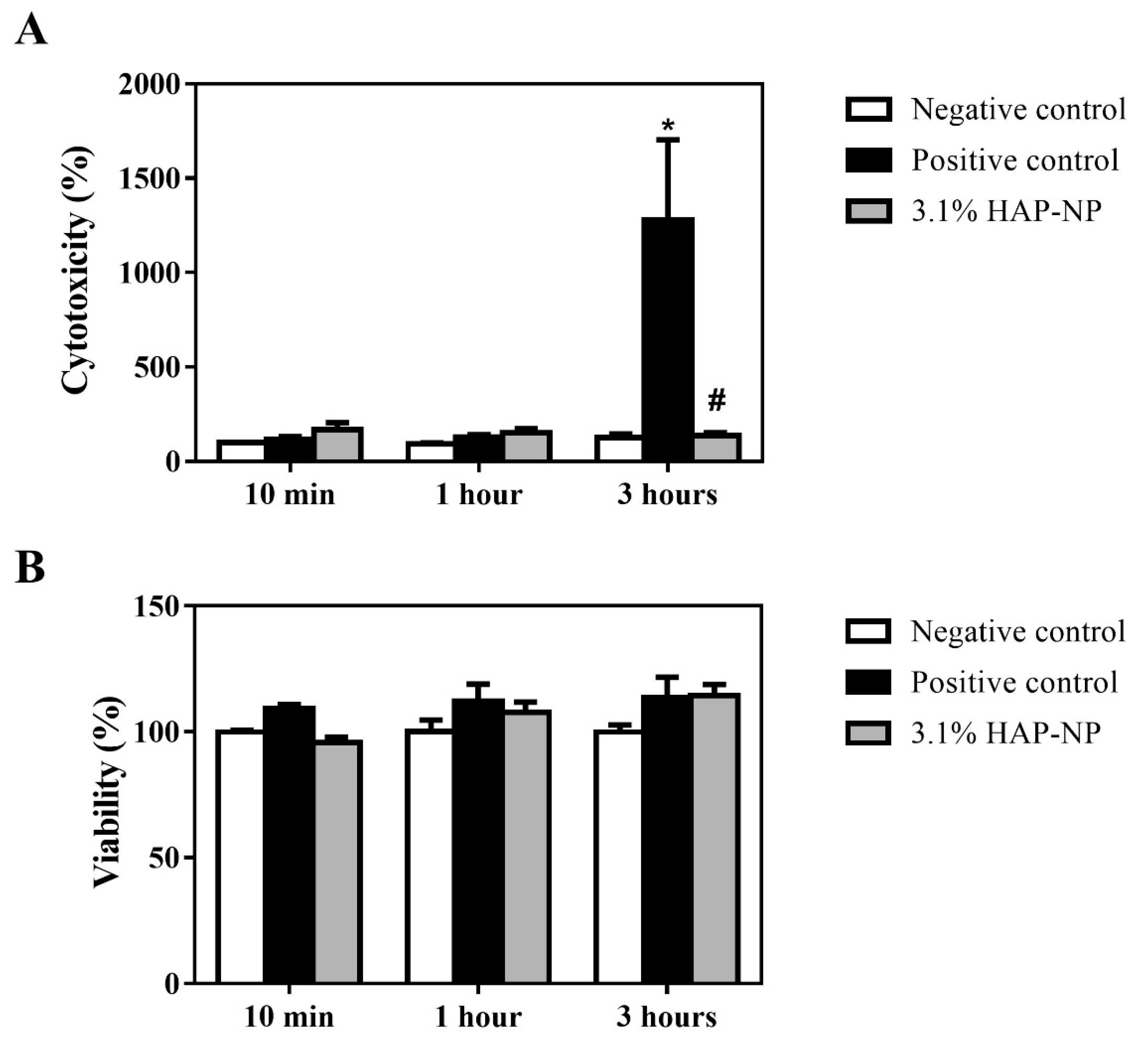
2.2.3. LDH Activity
2.2.4. MTT Test
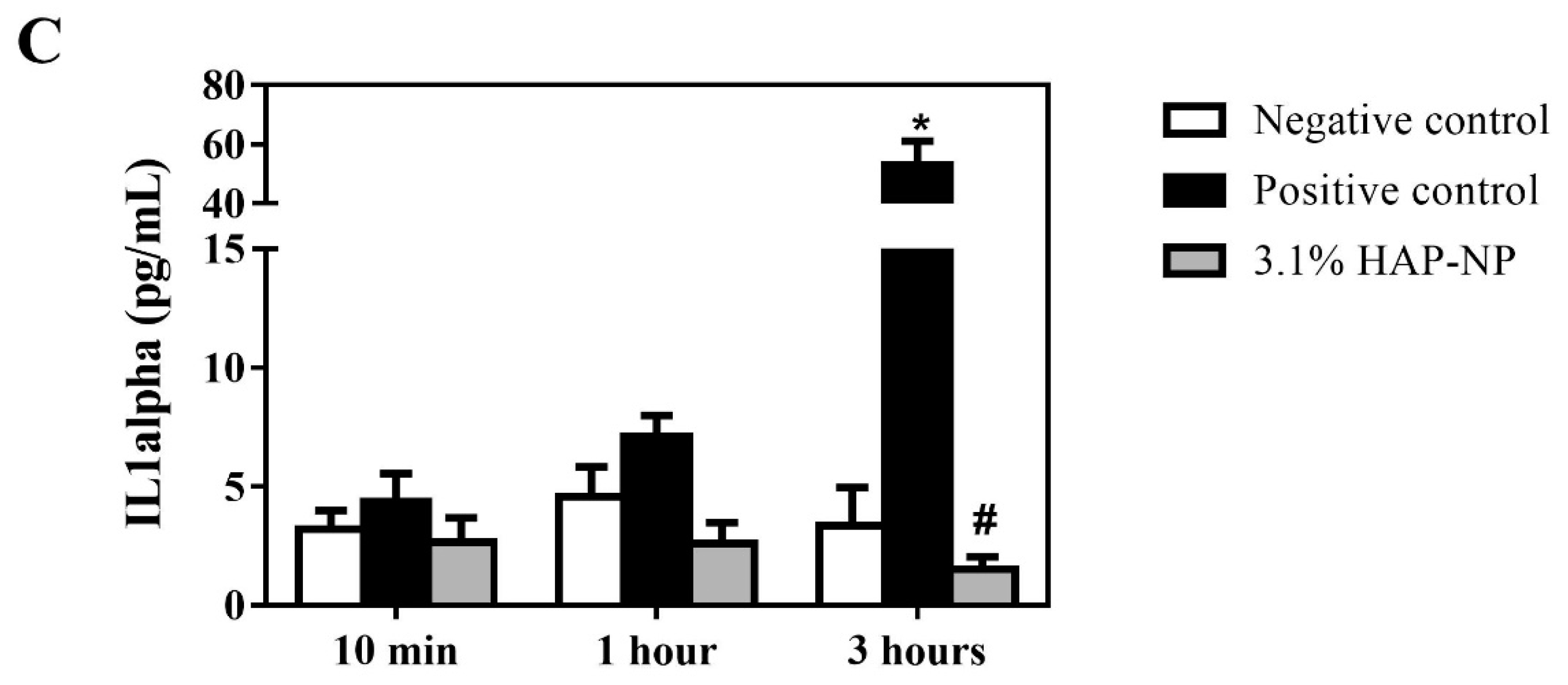
2.2.5. IL-1alpha Determination
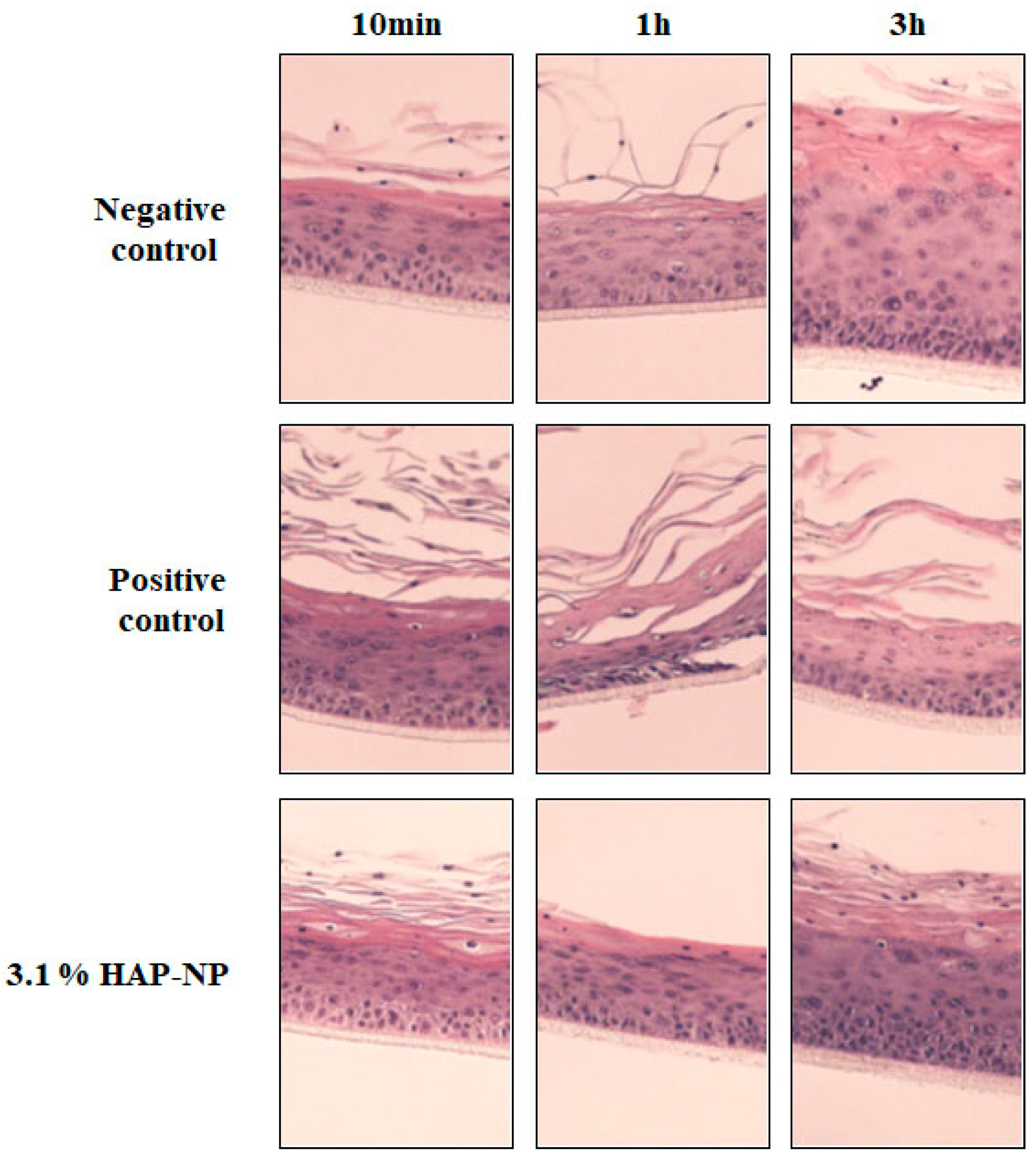
2.2.6. Histology
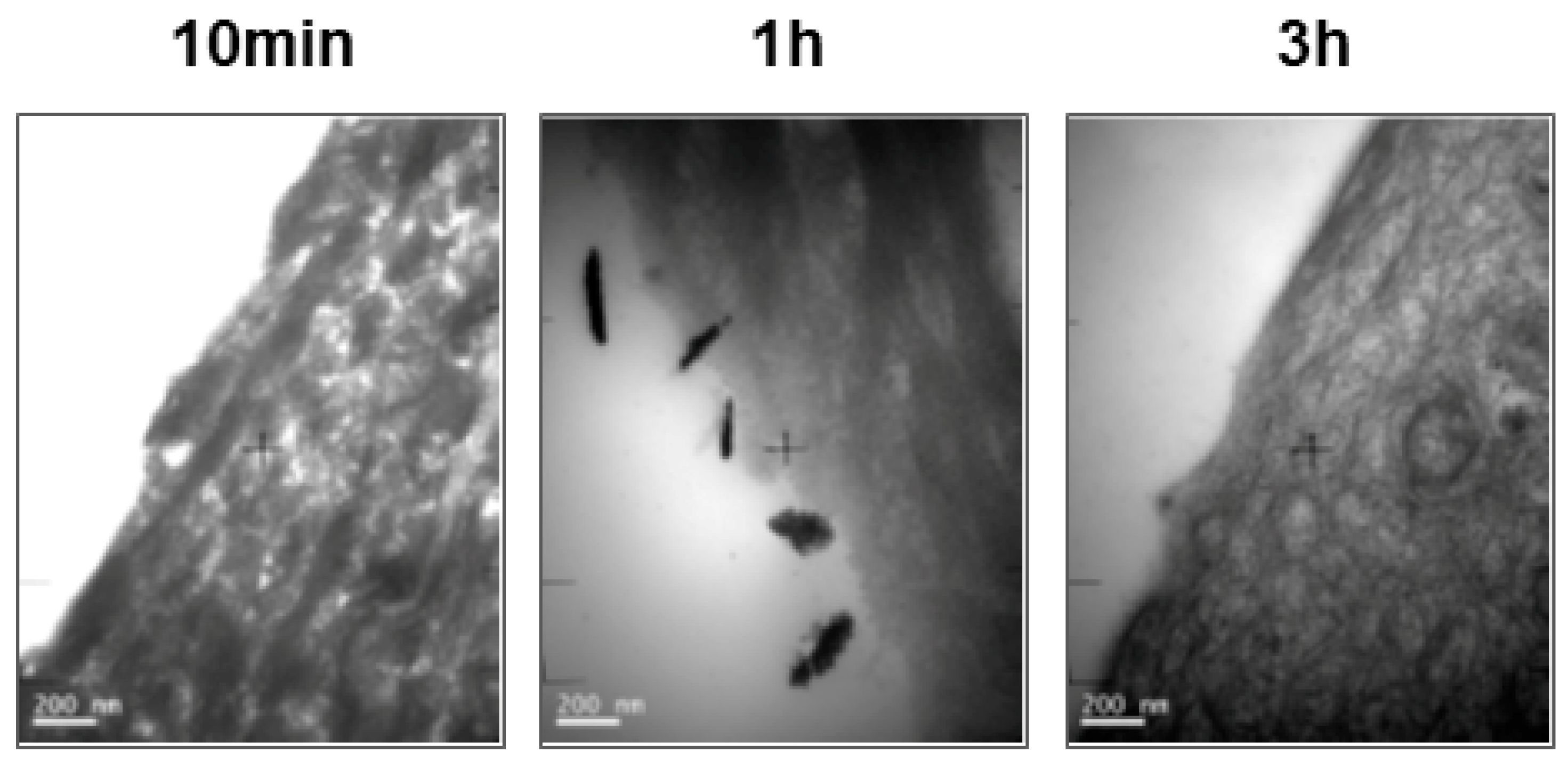
2.2.7. Observation with TEM
2.3. HAP-NP Dissolution in Simulated Gastric Fluid
2.3.1. Composition of Simulated Gastric Fluid (SGF)
2.3.2. Calcium Content in the Digestion Media
2.4. Statistics
3. Results
3.1. Hydroxyapatite Nanoparticles Physico-Chemical Characterization
3.2. Biocompatibility of Hydroxyapatite Nanoparticles
3.3. Histologycal Results and TEM Observation
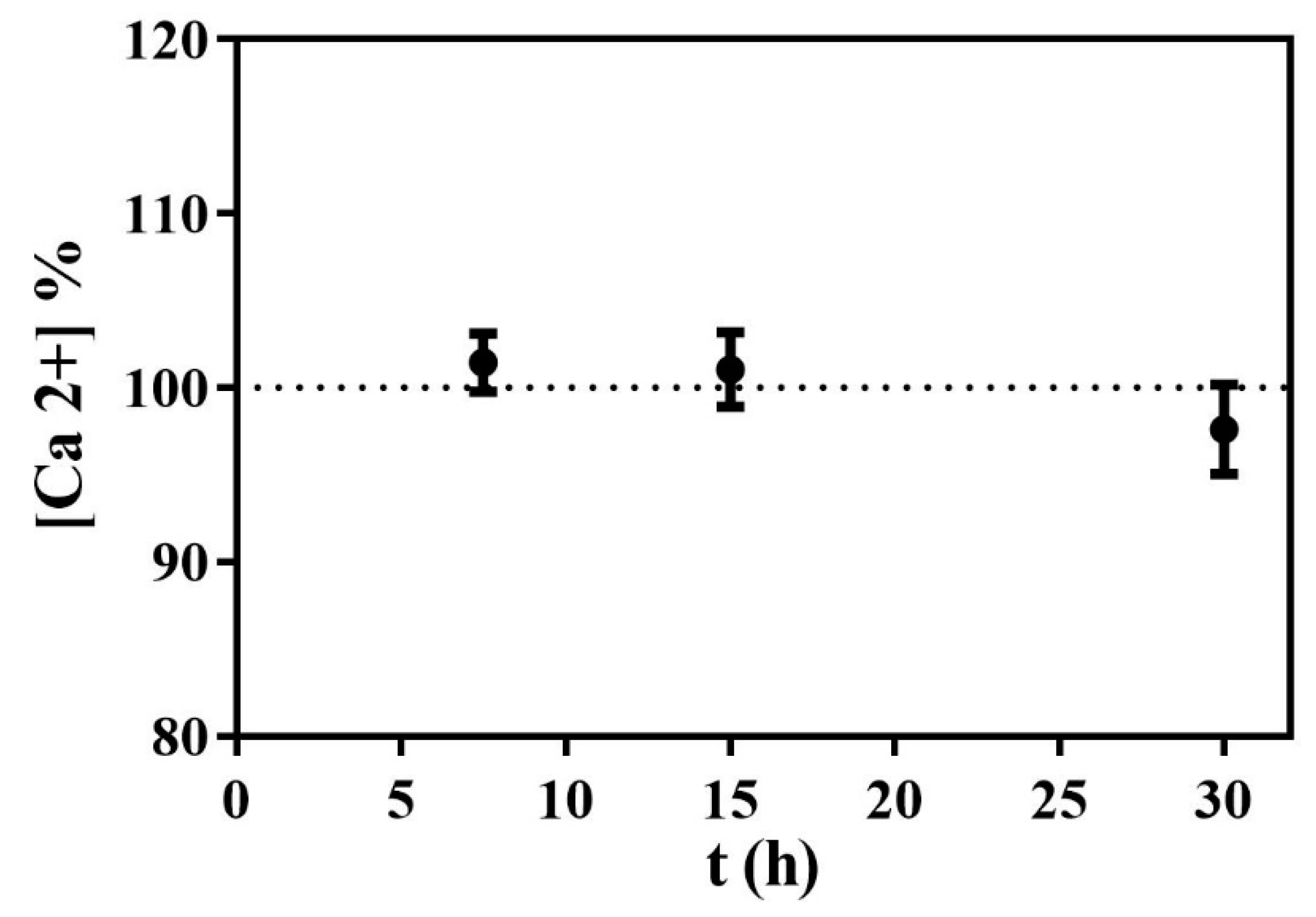
3.4. Calcium Content in the Digestion Media
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhou, H.; Lee, J. Nanoscale hydroxyapatite particles for bone tissue engineering. Acta Biomater. 2011, 7, 2769–2781. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.B.; Gao, S.S.; Yu, H.Y. Effect of nano-hydroxyapatite concentration on remineralization of initial enamel lesion in vitro. Biomed. Mater. 2009, 4, 34104. [Google Scholar] [CrossRef] [PubMed]
- Vano, M.; Derchi, G.; Barone, A.; Covani, U. Effectiveness of nano-hydroxyapatite toothpaste in reducing dentin hypersensitivity: A double-blind randomized controlled trial. Quintessence Int. 2014, 45, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Vano, M.; Derchi, G.; Barone, A.; Pinna, R.; Usai, P.; Covani, U. Reducing dentine hypersensitivity with nano-hydroxyapatite toothpaste: A double-blind randomized controlled trial. Clin. Oral Investig. 2018, 22, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Low, S.B.; Allen, E.P.; Kontogiorgos, E.D. Reduction in dental hypersensitivity with nano-hydroxyapatite, potassium nitrate, sodium monoflurophosphate and antioxidants. Open Dent. J. 2015, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Jena, A.; Kala, S.; Shashirekha, G. Comparing the effectiveness of four desensitizing toothpastes on dentinal tubule occlusion: A scanning electron microscope analysis. J. Conserv. Dent. 2017, 20, 269–272. [Google Scholar] [CrossRef] [PubMed]
- Tschoppe, P.; Zandim, D.L.; Martus, P.; Kielbassa, A.M. Enamel and dentine remineralization by nano-hydroxyapatite toothpastes. J. Dent. 2011, 39, 430–437. [Google Scholar] [CrossRef] [PubMed]
- European Comission Regulation (EC) No 1223/2009 of the European Parliament and of the Council of 30 November 2009 on cosmetic products. OJ L 2009, 342, 59. Available online: https://ec.europa.eu/health/sites/health/files/endocrine_disruptors/docs/cosmetic_1223_2009_regulation_en.pdf (accessed on 7 September 2018).
- SCCS (Scientific Committee on Consumer Safety) Guidance on safety assessment of nanomaterials in cosmetics, 26–27 June 2012. Eur. Comm. Available online: http://ec.europa.eu/health/scientific_committees/consumer_safety/docs/sccs_s_005.pdf (accessed on 7 September 2018).
- Wurzburger, L.; Kazmi, P.; Re, T.; Alonso, A.; Bertin, B.; Barnes, N.; de Brugerolle de Fraissinette, A.; Hilberer, A.; Raabe, H.; Wilt, N.; et al. Evaluation of an Oral Care Product Safety Screening Program Utilizing the In Vitro SkinEthic Human Gingival Epithelium (RHG) and Oral Buccal (RHO) Models. In Proceedings of the 50th SOT Annual Meeting, Washington, DC, USA, 6–10 March 2011. [Google Scholar]
- LLopis-Grimalt, M.; Munar-Bestard, M.; Ramis, J.M.; Monjo, M. Tissue-Engineered Oral Mucosa Constructs for in Vitro Research and Clinical Applications. Biomed. J. Sci. Tech. Res. 2018, 2, 3–5. [Google Scholar] [CrossRef]
- SCCS (Scientific Committee on Consumer Safety) The SCCS Notes of Guidance for the Testing of Cosmetic Ingredients and Their Safety E Valuation, 9th revision 2015. Available online: http://ec.europa.eu/health/scientific_committees/consumer_safety/docs/sccs_o_190.pdf (accessed on 7 September 2018).
- Pauwels, M.; Rogiers, V. Human health safety evaluation of cosmetics in the EU: A legally imposed challenge to science. Toxicol. Appl. Pharmacol. 2010, 243, 260–274. [Google Scholar] [CrossRef] [PubMed]
- SCCS (Scientific Committee on Consumer Safety) Opinion on Hydroxyapatite (nano), 16 October 2015, 2016, revision of 16 March 2016. Available online: https://ec.europa.eu/health/scientific_committees/consumer_safety/docs/sccs_o_191.pdf (accessed on 7 September 2018).
- de Brugerolle, A. SkinEthic Laboratories, a company devoted to develop and produce in vitro alternative methods to animal use. ALTEX 2007, 24, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration Guidance for Industry: Dissolution Testing of Immediate Release Solid Oral Dosage Forms 1997, 4. Available online: https://www.fda.gov/downloads/drugs/guidances/ucm070237.pdf (accessed on 7 September 2018).
- Casas, J.W.; Lewerenz, G.M.; Rankin, E.A.; Willoughby, J.A.; Blakeman, L.C.; McKim, J.M.; Coleman, K.P. In vitro human skin irritation test for evaluation of medical device extracts. Toxicol. In Vitro 2013, 27, 2175–2183. [Google Scholar] [CrossRef] [PubMed]
- Anand, S.; Rejula, F.; Sam, J.V.G.; Christaline, R.; Nair, M.G.; Dinakaran, S. Comparative Evaluation of Effect of Nano-hydroxyapatite and 8% Arginine Containing Toothpastes in Managing Dentin Hypersensitivity: Double Blind Randomized Clinical Trial. Acta Medica 2017, 60, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Vano, M.; Derchi, G.; Barone, A.; Genovesi, A.; Covani, U. Tooth bleaching with hydrogen peroxide and nano-hydroxyapatite: A 9-month follow-up randomized clinical trial. Int. J. Dent. Hyg. 2015, 13, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Winterfeld, T.; Schlueter, N.; Harnacke, D.; Illig, J.; Margraf-Stiksrud, J.; Deinzer, R.; Ganss, C. Toothbrushing and flossing behaviour in young adults—A video observation. Clin. Oral Investig. 2015, 19, 851–858. [Google Scholar] [CrossRef] [PubMed]
- Creeth, J.E.; Gallagher, A.; Sowinski, J.; Bowman, J.; Barrett, K.; Lowe, S.; Patel, K.; Bosma, M.L. The effect of brushing time and dentifrice on dental plaque removal in vivo. J. Dent. Hyg. 2009, 83, 111–116. [Google Scholar] [PubMed]
- Scheel, J.; Hermann, M. Integrated risk assessment of a hydroxyapatite-protein-composite for use in oral care products: A weight-of-evidence case study. Regul. Toxicol. Pharmacol. 2011, 59, 310–323. [Google Scholar] [CrossRef] [PubMed]
- Tay, C.Y.; Fang, W.; Setyawati, M.I.; Chia, S.L.; Tan, K.S.; Hong, C.H.L.; Leong, D.T. Nano-hydroxyapatite and nano-titanium dioxide exhibit different subcellular distribution and apoptotic profile in human oral epithelium. ACS Appl. Mater. Interfaces 2014, 6, 6248–6256. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Huang, X.; Cai, Y.; Tang, R.; Yang, D. Size effect of hydroxyapatite nanoparticles on proliferation and apoptosis of osteoblast-like cells. Acta Biomater. 2009, 5, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Epple, M. Review of potential health risks associated with nanoscopic calcium phosphate. Acta Biomater. 2018, 77, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Suwara, M.I.; Green, N.J.; Borthwick, L.A.; Mann, J.; Mayer-Barber, K.D.; Barron, L.; Corris, P.A.; Farrow, S.N.; Wynn, T.A.; Fisher, A.J.; et al. IL-1α released from damaged epithelial cells is sufficient and essential to trigger inflammatory responses in human lung fibroblasts. Mucosal Immunol. 2014, 7, 684–693. [Google Scholar] [CrossRef] [PubMed]
| Score | Description of observations |
|---|---|
| 0 | Absence of, or minor epithelium changes |
| 1 | Slight epithelium changes: Slight edema and/or slight cellular changes and/or upper cell layer disintegration |
| 2 | Moderate epithelium changes: Moderate edema and/or moderate cellular alterations and/or presence of significant number of necrotic cells and/or disintegration of superficial cell layers |
| 3 | Marked to severe epithelium changes: Marked edema and/or cellular alterations and/or partial tissue necrosis and/or disintegration of supra basal cell layers |
| 4 | Total tissue necrosis and/or tissue disintegration |
| Group | Score | ||
|---|---|---|---|
| Negative Control | Positive Control | 3.1 % HAP-NP | |
| 10 min | 0 | 0 | 0 |
| 1 h | 0 | 1 | 0 |
| 3 h | 0 | 1 | 0 |
| Time (min) | Ca2+ Concentration (mg/L) |
|---|---|
| 7.5 | 104 ± 3 |
| 15 | 104 ± 2 |
| 30 | 100 ± 4 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramis, J.M.; Coelho, C.C.; Córdoba, A.; Quadros, P.A.; Monjo, M. Safety Assessment of Nano-Hydroxyapatite as an Oral Care Ingredient according to the EU Cosmetics Regulation. Cosmetics 2018, 5, 53. https://doi.org/10.3390/cosmetics5030053
Ramis JM, Coelho CC, Córdoba A, Quadros PA, Monjo M. Safety Assessment of Nano-Hydroxyapatite as an Oral Care Ingredient according to the EU Cosmetics Regulation. Cosmetics. 2018; 5(3):53. https://doi.org/10.3390/cosmetics5030053
Chicago/Turabian StyleRamis, Joana M., Catarina C. Coelho, Alba Córdoba, Paulo A. Quadros, and Marta Monjo. 2018. "Safety Assessment of Nano-Hydroxyapatite as an Oral Care Ingredient according to the EU Cosmetics Regulation" Cosmetics 5, no. 3: 53. https://doi.org/10.3390/cosmetics5030053
APA StyleRamis, J. M., Coelho, C. C., Córdoba, A., Quadros, P. A., & Monjo, M. (2018). Safety Assessment of Nano-Hydroxyapatite as an Oral Care Ingredient according to the EU Cosmetics Regulation. Cosmetics, 5(3), 53. https://doi.org/10.3390/cosmetics5030053







