Analysis of Dyes in Cosmetics: Challenges and Recent Developments
Abstract
:1. Introduction
2. Regulatory Overview
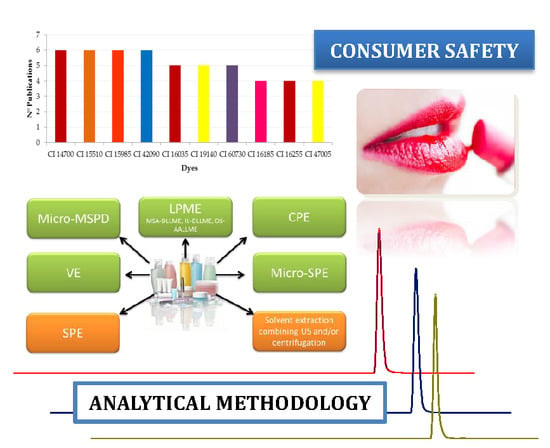
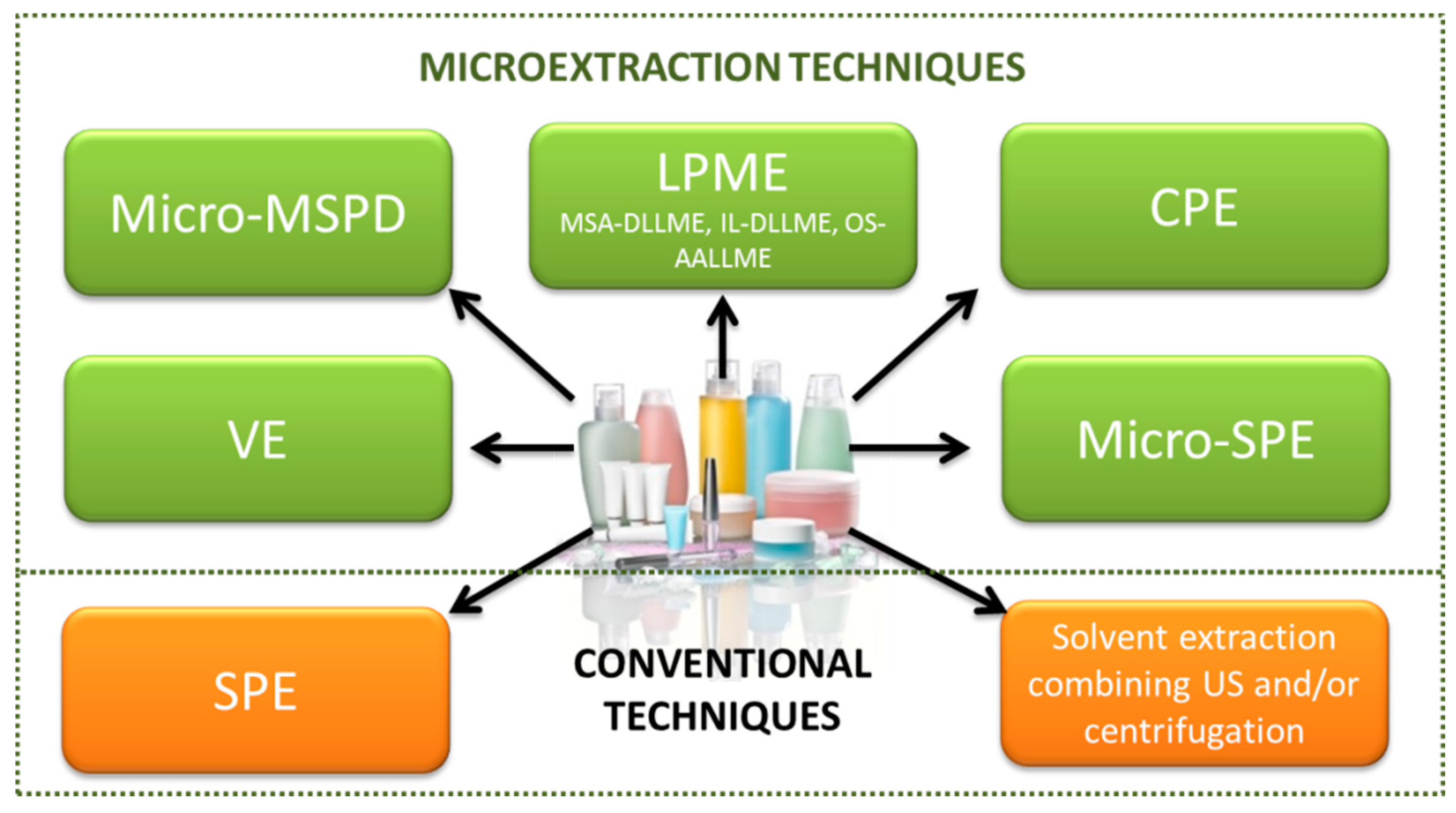
3. Analytical Methodology
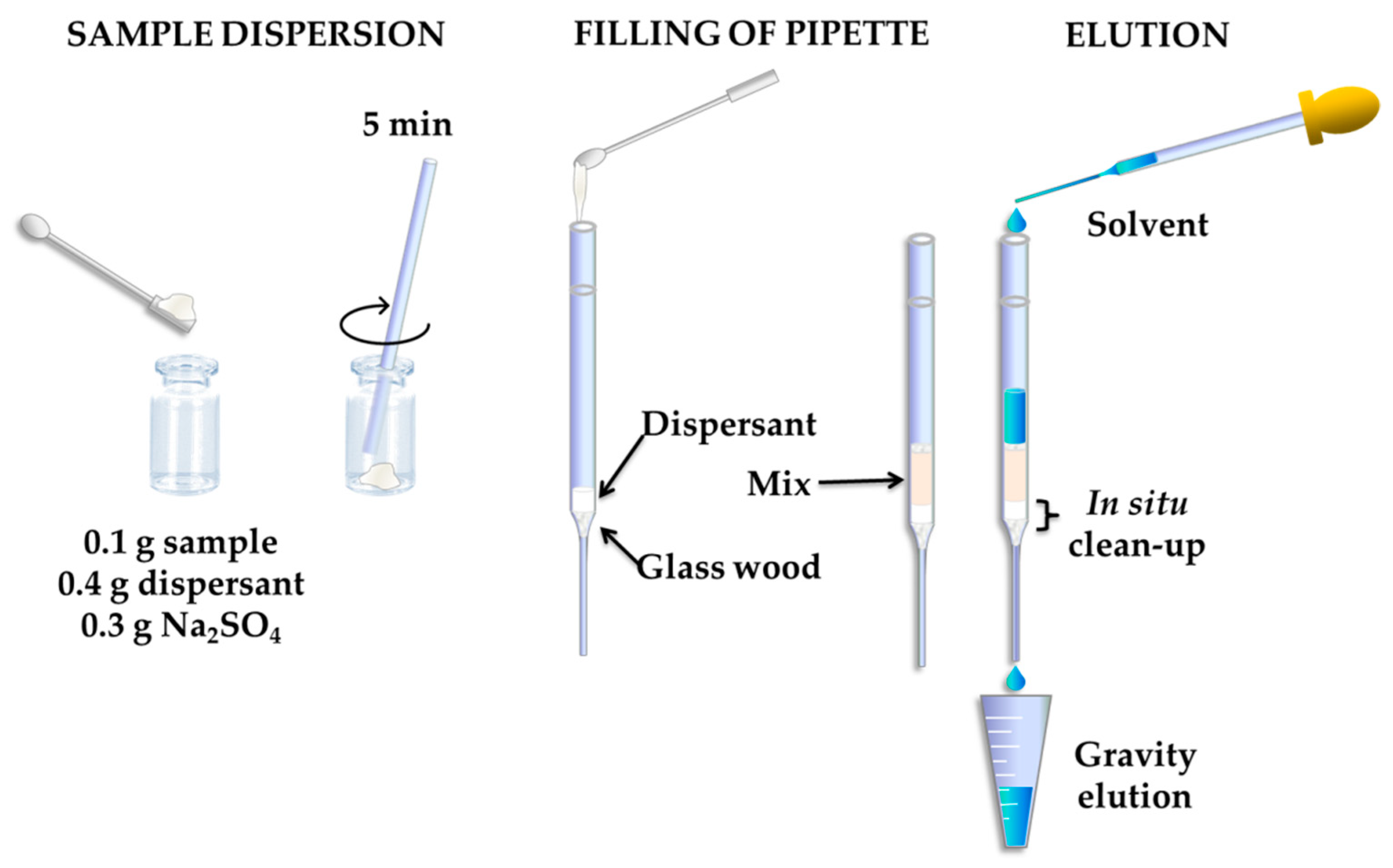
3.1. Sample Preparation
3.2. Analytical Techniques
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A: Abbreviations
| ACN | acetonitrile |
| CI | colour index |
| CPE | cloud point extraction |
| DAD | diodo-array detector |
| DCM | dichloromethane |
| DESI-MS | desorption electrospray ionization mass spectrometry |
| DLLME | dispersive liquid-liquid microextraction |
| DMF | dimethylformamide |
| ESI | electrospray ionization |
| FIA | flow injection analysis |
| HAc | acetic acid |
| IL | ionic liquid |
| IL-DLLME | ionic liquid independent disperse liquid-liquid microextraction |
| LLOQ | lower limit of quantification |
| LPME | liquid-phase microextraction |
| MeOH | methanol |
| MRM | multiple reaction monitoring |
| MSA-DLLME | magnetic stirring assisted dispersive liquid-liquid microextraction |
| MSPD | matrix solid-phase dispersion |
| OS-AALLME | one-step air-assisted liquid-liquid microextraction |
| RSD | relative standard deviation |
| SPE | solid phase extraction |
| SRM | selected reaction monitoring |
| THF | tetrahydrofuran |
| UPLC | ultra performance liquid chromatography |
| US | ultrasounds |
References
- Fardouly, J.; Vartanian, L.R. Social Media and Body Image Concerns: Current Research and Future Directions. Curr. Opin. Psychol. 2016, 9, 1–5. [Google Scholar] [CrossRef]
- Gürses, A.; Açikyildiz, M.; Güneş, K.; Sadi Gürses, M. Classification on Dyes and Pigments. In Dyes Pigments (SpringerBriefs in Molecular Science); Springer: Cham, Switzerland, 2016; pp. 31–45. [Google Scholar]
- Chisvert, A.; Salvador, A. Colouring Agents in Decorative and other Cosmetics. Analytical Methods. In Analysis of Cosmetic Products, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Ahlstrom, L.H.; Sparr Eskilsson, C.; Bjorklund, E. Determination of banned azo dyes in consumer goods. TrAC-Trend Anal. Chem. 2005, 24, 49–56. [Google Scholar] [CrossRef]
- Platzek, T. Overview on toxicity and exposure to azo dyes and aromatic amines. Toxicol. Lett. 2013, 221, S53. [Google Scholar] [CrossRef]
- Lucova, M.; Hojerova, J.; Pazourekova, S.; Klimova, Z. Absorption of triphenylmethane dyes Brilliant Blue and Patent Blue through intact skin, shaven skin and lingual mucosa from daily life products. Food Chem. Toxicol. 2013, 52, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Chequer, F.M.D.; de Paula Venâncio, V.; de Souza Prado, M.R.; Lizier, T.M.; Zanoni, M.V.B.; Burbano, R.R.; Bianchi, M.L.P.; Antunes, L.M.G. The cosmetic dye quinoline yellow causes DNA damage in vitro. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2015, 777, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Mizutani, T. Toxicity of xanthene food dyes by inhibition of human drug-metabolizing enzymes in a noncompetitive manner. J. Environ. Public Health 2009, 2009, 953952. [Google Scholar] [CrossRef] [PubMed]
- Soylak, M.; Unsal, Y.E.; Yilmaz, E.; Tuzen, M. Determination of rhodamine B in soft drink, waste water and lipstick samples after solid phase extraction. Food Chem. Toxicol. 2011, 49, 1796–1799. [Google Scholar] [CrossRef] [PubMed]
- Schuttelaar, M.L.; Vogel, T. Contact Allergy to Hair Dyes. Cosmetics 2016, 3, 21. [Google Scholar] [CrossRef]
- Goossens, A. Cosmetic Contact Allergens. Cosmetics 2016, 3, 5. [Google Scholar] [CrossRef]
- Guerra, E.; Lamas, J.P.; Llompart, M.; Garcia-Jares, C. Determination of oxidative hair dyes using miniaturized extraction techniques and gas chromatography-tandem mass spectrometry. Microchem. J. 2017, 132, 308–318. [Google Scholar] [CrossRef]
- Da Fransa, S.; Dario, M.; Esteves, V.; Baby, A.; Velasco, M. Types of Hair Dye and Their Mechanisms of Action. Cosmetics 2015, 2, 110. [Google Scholar] [CrossRef]
- Piccinini, P.; Pakalin, S.; Contor, L.; Bianchi, I.; Senaldi, C. Safety of Tattoos and Permanent Make-Up: Final Report; JRC Science for policy report EUR 27947; European Commission: Brussels, Belgium, 2016. [Google Scholar]
- Weisz, A.; Milstein, S.R.; Scher, A.L.; Hepp, N.M.; Salvador, A.; Chisvert, A. Colouring Agents in Cosmetics: Regulatory Aspects and Analytical Methods. Analysis of Cosmetic Products, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 123–157. [Google Scholar]
- Lores, M.; Llompart, M.; Alvarez-Rivera, G.; Guerra, E.; Vila, M.; Celeiro, M.; Lamas, J.P.; Garcia-Jares, C. Positive lists of cosmetic ingredients: Analytical methodology for regulatory and safety controls—A review. Anal. Chim. Acta 2016, 915, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Buzek, J.; Ask, B. Regulation (EC) No 1223/2009 of the European Parliament and of the Council of 30 November 2009 on cosmetic products. Off. J. Eur. Union L342 2009, 52, 59–209. [Google Scholar]
- CFR-Code of Federal Regulations Title 21. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=70-82 (accessed on 16 July 2018).
- Ordinance to Regulate Coal-Tar Colors Permitted for Use in Drugs, Quasi-drugs and Cosmetics (As Amended by MHLW-Ministry of Health, Labor, and Welfare Ordinances Nos. 55/1972 and 126/2003); MHW Ordinance No. 30/1966; MHW-Ministry of Health and Welfare: Tokyo, Japan, 1966.
- Kucharska, M.; Grabka, J. A review of chromatographic methods for determination of synthetic food dyes. Talanta 2010, 80, 1045–1051. [Google Scholar] [CrossRef] [PubMed]
- Yamjala, K.; Nainar, M.S.; Ramisetti, N.R. Methods for the analysis of azo dyes employed in food industry—A review. Food Chem. 2016, 192, 813–824. [Google Scholar] [CrossRef] [PubMed]
- Rebane, R.; Leito, I.; Yurchenko, S.; Herodes, K. A review of analytical techniques for determination of Sudan I-IV dyes in food matrixes. J. Chromatogr. A 2010, 1217, 2747–2757. [Google Scholar] [CrossRef] [PubMed]
- Rovina, K.; Siddiquee, S.; Shaarani, S.M. A Review of Extraction and Analytical Methods for the Determination of Tartrazine (E 102) in Foodstuffs. Crit. Rev. Anal. Chem. 2017, 47, 309–324. [Google Scholar] [CrossRef] [PubMed]
- Guerra, E.; Alvarez-Rivera, G.; Llompart, M.; Garcia-Jares, C. Simultaneous determination of preservatives and synthetic dyes in cosmetics by single-step vortex extraction and clean-up followed by liquid chromatography coupled to tandem mass spectrometry. Talanta 2018, 188, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Bisgin, A.T.; Surme, Y.; Ugan, M.; Narin, I. Separation, Preconcentration and Spectrophotometric Determination of Rhodamine B in Industrial, Cosmetic and Water Samples by Cloud Point and Solid Phase Extraction. J. Anal. Chem. 2018, 73, 452–458. [Google Scholar] [CrossRef]
- Guerra, E.; Llompart, M.; Garcia-Jares, C. Miniaturized matrix solid-phase dispersion followed by liquid chromatography-tandem mass spectrometry for the quantification of synthetic dyes in cosmetics and foodstuffs used or consumed by children. J. Chromatogr. A 2017, 1529, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Ozkantar, N.; Soylak, M.; Tuzen, M. Spectrophotometric detection of rhodamine B in tap water, lipstick, rouge, and nail polish samples after supramolecular solvent microextraction. Turkish J. Chem. 2017, 41, 987–994. [Google Scholar] [CrossRef]
- Ranjbari, E.; Hadjmohammadi, M.R. Optimization of magnetic stirring assisted dispersive liquid-liquid microextraction of rhodamine B and rhodamine 6G by response surface methodology: Application in water samples, soft drink, and cosmetic products. Talanta 2015, 139, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Guerra, E.; Celeiro, M.; Lamas, J.P.; Llompart, M.; Garcia-Jares, C. Determination of dyes in cosmetic products by micro-matrix solid phase dispersion and liquid chromatography coupled to tandem mass spectrometry. J. Chromatogr. A 2015, 1415, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Franco, J.H.; Silva, B.F.; Zanoni, M.V.B. Using ionic liquid combined with HPLC-DAD to analyze semi-permanent hair dyes in commercial formulations. Anal. Methods 2015, 7, 1115–1122. [Google Scholar] [CrossRef]
- Barfi, B.; Asghari, A.; Rajabi, M.; Sabzalian, S. Organic solvent-free air-assisted liquid-liquid microextraction for optimized extraction of illegal azo-based dyes and their main metabolite from spices, cosmetics and human bio-fluid samples in one step. J. Chromatogr. B: Anal. Technol. Biomed. Life Sci. 2015, 998, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Miranda-Bermudez, E.; Harp, B.P.; Barrows, J.N. Qualitative Identification of Permitted and Non-permitted Color Additives in Cosmetics. J. AOAC Int. 2014, 97, 1039–1047. [Google Scholar] [CrossRef] [PubMed]
- Xian, Y.; Wu, Y.; Guo, X.; Lu, Y.; Luo, H.; Luo, D.; Chen, Y. Simultaneous determination of 11 restricted dyes in cosmetics by ultra high-performance liquid chromatography/tandem mass spectrometry. Anal. Methods 2013, 5, 1965–1974. [Google Scholar] [CrossRef]
- Nizzia, J.L.; O'Leary, A.E.; Ton, A.T.; Mulligan, C.C. Screening of cosmetic ingredients from authentic formulations and environmental samples with desorption electrospray ionization mass spectrometry. Anal. Methods 2013, 5, 394–401. [Google Scholar] [CrossRef]
- Guo, J.; Wu, H.; Du, L.; Fu, Y. Determination of Brilliant Blue FCF in food and cosmetic samples by ionic liquid independent disperse liquid-liquid micro-extraction. Anal. Methods 2013, 5, 4021–4026. [Google Scholar] [CrossRef]
- Bagheri, H.; Daliri, R.; Roostaie, A. A novel magnetic poly(aniline-naphthylamine)-based nanocomposite for micro solid phase extraction of rhodamine B. Anal. Chim. Acta 2013, 794, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.H.; Huang, S.J. Studies on the voltammetric behavior of azo dyes and its determination in cosmetic products. Russ. J. Electrochem. 2010, 46, 1414–1418. [Google Scholar] [CrossRef]
- Wang, C.C.; Masi, A.N.; Fernandez, L. On-line micellar-enhanced spectrofluorometric determination of rhodamine dye in cosmetics. Talanta 2008, 75, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Pourreza, N.; Rastegarzadeh, S.; Larki, A. Micelle-mediated cloud point extraction and spectrophotometric determination of rhodamine B using Triton X-100. Talanta 2008, 77, 733–736. [Google Scholar] [CrossRef]
- Noguerol-Cal, R.; Lopez-Vilarino, J.M.; Fernandez-Martinez, G.; Barral-Losada, L.; Gonzalez-Rodriguez, M.V. High-performance liquid chromatography analysis of ten dyes for control of safety of commercial articles. J. Chromatogr. A 2008, 1179, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, S.C.; Barwick, V.J.; Carter, S.V. Identification of organic colourants in cosmetics by HPLC-diode array detection. Chromatographia 1997, 45, 215–228. [Google Scholar] [CrossRef]
- Perez-Gonzalez, M.; Vu, N.; Harp, B.P. Ultra-performance liquid chromatographic determination of manufacturing intermediates and subsidiary colors in D&C Red No. 6, D&C Red No. 7, and their lakes. J. AOAC Int. 2015, 98, 1752–1759. [Google Scholar] [CrossRef] [PubMed]
- Belai, N.; Harp, B.P.; Mazzola, E.P.; Lam, Y.F.; Abdeldayem, E.; Aziz, A.; Mossoba, M.M.; Barrows, J.N. Subsidiary colors in D&C Red No. 34 and its lakes: Synthesis, structural characterization, and analysis by ultra-performance liquid chromatography. Dyes Pigments 2012, 95, 304–312. [Google Scholar] [CrossRef]
- Harp, B.P.; Belai, N.; Barrows, J.N. Ultra-performance liquid chromatographic determination of manufacturing intermediates and subsidiary colors in D&C Red No. 34 and its lakes. J. AOAC Int. 2011, 94, 1548–1554. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.H.W.; Scher, A. Determination of components of the color additive FD&C Green No. 3 (Food Green 3) using high performance liquid chromatography. In Abstracts of Papers of the American Chemical Society; American Chemical Society: Washington, DC, USA, 2011. [Google Scholar]
- Jandera, P. Selection of Separation Conditions for HPLC and HPLC/MS of Aromatic Sulphonic Acids and Acid Azo Dyes. J. Liq. Chromatogr. Relat. Technol. 2007, 30, 2349–2367. [Google Scholar] [CrossRef]

| Category | CI Range | Example |
|---|---|---|
| Azo | 11XXX–35XXX |  CI 19140 Tartrazine |
| Triarylmethane | 42XXX–44XXX |  CI 42090 Brilliant Blue |
| Xanthene | 45XXX |  CI 45410 Acid Red 92 |
| Quinoline | 47XXX |  CI 47005 Quinoline Yellow |
| Indigoid | 73XXX |  CI 73015 Indigotine |
| Analyte | Sample Matrix | Sample Preparation | Analysis | Year | Ref. |
|---|---|---|---|---|---|
| CI 14700, CI 14720, CI 15510, CI 15985, CI 16035, CI 16185, CI 16255, CI 19140, CI 42051, CI 42090, CI 44090, CI 47005, CI 60730, CI 73015 | Lip gloss, lip lacquer, lipstick, body lotion, body butter, hand cream, age spot corrector mask, rough skin remover, shampoo, shower gel, products combining both, soap, facial and intimate gels, moisturizing and smoothing masks and toothpaste | Single-step vortex extraction and clean-up 1.5 mL of MeOH and 0.1 g of C18 for clean-up | LC-MS/MS | 2018 | Guerra et al. [24] |
| CI 45170 | Lipstick | SPE and CPE Using amberlite as adsorbent and surfactant Previous solution in CCl4 | UV-Vis spectroscopy | 2018 | Bisgin et al. [25] |
| CI 13065, CI 14700, CI 14720, CI 15510, CI 15985, CI 16035, CI 16185, CI 16255, CI 19140, CI 42051, CI 42090, CI 42640, CI 44090, CI 45170, CI 45410, CI 45430, CI 47005, CI 60730, CI 73015 | Children’s shampoo, children’s toothpaste, face painting, lip balm, coloured hairspray, eye shadow, soap, nail polish | Micro-MSPD Using C18 as sorbent and 2 mL of MeOH for elution | LC-MS/MS | 2017 | Guerra et al. [26] |
| CI 45170 | Lipstick, rouge and nail polish | DLLME THF and decanoic acid as dispersing solvents | UV-Vis spectroscopy | 2017 | Ozkantar et al. [27] |
| CI 45160, CI 45170 | Lipstick | MSA-DLLME 1-octanol and acetone as dispersing solvents | HPLC-Vis | 2015 | Ranjbari et al. [28] |
| CI 14700, CI 15510, CI 15985, CI 16035, CI 19140, CI 42090, CI 45410, CI 47005, CI 60730 | Lip balm, nail polish, eye shadow, toothpaste, shampoo, decorative makeup, perfume and mouthwash | Micro-MSPD Using Florisil as sorbent and 2 mL of MeOH for elution | LC-MS/MS | 2015 | Guerra et al. [29] |
| CI 12245, CI 12250, CI 12719, CI 56059, CI 60730 | Semi-permanent hair-dyeing formulations | Sample dilution and SPE Strata-X cartridges and ACN/H2O (1:1) for elution | HPLC-DAD with IL Comparison with LC-MS/MS | 2015 | Franco et al. [30] |
| CI 11920, CI 12055, CI 12140, CI 26100, CI 26105 | Lipstick | OS-AALLME ILs as extraction solvent | HPLC-UV | 2015 | Barfi et al. [31] |
| CI 10316, CI 14700, CI 14720, CI 15510, CI 15850, CI 15850:1, CI 15880:1, CI 15985, CI 16035, CI 16185, CI 16255, CI 17200, CI 19140, CI 42051, CI 42053, CI 42090, CI 45100, CI 45170, CI 45350, CI 45350:1, CI 45370, CI 45380, CI 45380:2, CI 45410, CI 45410:1, CI 45430, CI 47005, CI 59040, CI 60730, CI 61570, CI 73015, CI 75470 | Lip product, nail polish, eye product, blush, body glitter, face paint, cream and toothpaste | US and centrifugation Combining DCM, MeOH, HAc and H2O | LC-PDA | 2014 | Miranda-Bermudez et al. [32] |
| CI 14600, CI 14700, CI 15510, CI 15985, CI 16035, CI 16185, CI 16255, CI 18050, CI 19140, CI 20470, CI 42090 | Eye shadow, lipstick and lip gloss | US and centrifugation MeOH-H2O for eye shadow and CHCl3-H2O for lip products | UPLC-MS/MS | 2013 | Xian et al. [33] |
| CI 14700, CI 15510, CI 15985, CI 26100 | Blemish cream and hair dye | Without sample preparation | DESI-MS | 2013 | Nizzia et al. [34] |
| CI 40290 | Eau de toilette and shampoo | IL-DLLME Adjusted to pH 4 | UV-Vis spectroscopy | 2013 | Guo et al. [35] |
| CI 45170 | Shampoo, pencil and eye shadow | Micro SPE Novel nanocomposite as magnetic sorbent | Fluorescence spectroscopy | 2013 | Bagheri et al. [36] |
| CI 45170 | Lipstick | SPE Sepabeads SP 70 resin and ACN for elution | UV-Vis spectroscopy | 2011 | Soylak et al. [9] |
| CI 15850 | Nail preparation, lipstick and rouge | Solvent extraction and centrifugation DMF and MeOH (2:8) | Voltammetry Comparison with LC-UV | 2010 | Wang et al. [37] |
| CI 45170 | Lipstick | Solvent extraction in hot water (emulsion for micellar determination) | FIA Fluorescence spectroscopy | 2008 | Wang et al. [38] |
| CI 45170 | Hand washing liquid soap | CPE Aq solution with Triton X-100 in acidic media | UV-Vis spectroscopy | 2008 | Pourreza et al. [39] |
| CI 11020, CI 11285, CI 11920, CI 12055, CI 12140, CI 26100, CI 26105, CI 26150, CI 42000:1, CI 61554 | Commercial products | Not provided | HPLC-UV and HPLC-MS/MS | 2008 | Noguerol et al. [40] |
| Dye | Recovery (%) | LOD/LOQ | RSD (%) | Ref. |
|---|---|---|---|---|
| Liquid Chromatography Coupled to Mass Spectrometry | ||||
| CI 14700, CI 14720, CI 15510, CI 15985, CI 16035, CI 16185, CI 16255, CI 19140, CI 42051, CI 42090, CI 44090, CI 47005, CI 60730, CI 73015 | 70.3–117 | --/0.070–3.437 µg g−1 | <13 | Guerra et al. [24] |
| CI 13065, CI 14700, CI 14720, CI 15510, CI 15985, CI 16035, CI 16185, CI 16255, CI 19140, CI 42051, CI 42090, CI 42640, CI 44090, CI 45170, CI 45410, CI 45430, CI 47005, CI 60730 CI 73015 | 69.5–121 | 0.0142–0.476 µg·g−1/-- | <15 | Guerra et al. [26] |
| CI 14700, CI 15510, CI 15985, CI 16035, CI 19140, CI 42090, CI 45410, CI 47005, CI 60730 | 70–120 | 0.010–0.62/1–5 ng·mL−1 (LLOQ) | <15 | Guerra et al. [29] |
| CI 14600, CI 14700, CI 15510, CI 15985, CI 16035, CI 16185, CI 16255, CI 18050, CI 19140, CI 20470, CI 42090 | 81.6–118.2 | 1.2–30.3/4.1–100 µg·kg−1 | <8 | Xian et al. [33] |
| CI 11020, CI 11285, CI 11920, CI 12055, CI 12140, CI 26100, CI 26105, CI 26150, CI 42000:1, CI 61554 | Not provided | 4.54–14.3/15.0–47.6 µg·L−1 | -- | Noguerol et al. [40] |
| Liquid Chromatography Coupled to Absorbance Detector | ||||
| CI 45160, CI 45170 | 97–100 | 1.15–1.23/3.82–4.10 ng·mL−1 | <2 | Ranjbari et al. [28] |
| CI 12245, CI 12250, CI 12719, CI 56059, CI 60730 | Not provided | 0.53–2.98 × 10−7/ 1.08–3.66 × 10−7 mol·L−1 | <5 | Franco et al. [30] |
| CI 11920, CI 12055, CI 12140, CI 26100, CI 26105 | 86.8–102.3 | 3.9–84.8 ng·mL−1/-- | <6 | Barfi et al. [31] |
| CI 10316, CI 14700, CI 14720, CI 15510, CI 15850, CI 15850:1, CI 15880:1, CI 15985, CI 16035, CI 16185, CI 16255, CI 17200, CI 19140, CI 42051, CI 42053, CI 42090, CI 45100, CI 45170, CI 45350, CI 45350:1, CI 45370, CI 45380, CI 45380:2, CI 45410, CI 45410:1, CI 45430, CI 47005, CI 59040, CI 60730, CI 61570, CI 73015, CI 75470 | -- | 0.1–1.5 mg·L−1 (estimated, qualitative method)/-- | -- | Miranda- Bermudez et al. [32] |
| CI 11020, CI 11285, CI 11920, CI 12055, CI 12140, CI 26100, CI 26105, CI 26150, CI 42000:1, CI 61554 | Not provided | 60–890/200–2990 µg·L−1 | -- | Noguerol et al. [40] |
| DESI-MS | ||||
| CI 14700, CI 15510, CI 15985, CI 26100 | -- | 15–100 ng (<1 ng SRM mode) | 9–27 | Nizzia et al. [34] |
| Dye | Recovery (%) | LOD/LOQ | RSD (%) | Ref. |
|---|---|---|---|---|
| UV-Vis spectroscopy | ||||
| CI 45170 | 85–100 | 0.7/1.9 µg·L−1 for CPE, 1.2/3.2 µg·L−1 for SPE | <7 | Bisgin et al. [25] |
| CI 45170 | 99–104 | 0.49/1.47 µg·L−1/ | <6 | Ozkantar et al. [27] |
| CI 42090 | 99–103 | 0.34 µg·L−1 | <1 | Guo et al. [35] |
| CI 45170 | Not provided | Not provided | <5 | Soylak et al. [9] |
| CI 45170 | 97–102 | 1.3 ng·mL−1/-- | <3 | Pourreza et al. [39] |
| Fluorescence spectroscopy | ||||
| CI 45170 | 94–99 | 0.10/0.35 µg·L−1 | <8 | Bagheri et al. [36] |
| CI 45170 | 98–102.4 | 5 × 10−10/1.6 × 10−9 mol·L−1 | <3 | Wang et al. [38] |
| Voltammetry | ||||
| CI 15850 | Not provided | Not provided | <4 | Wang et al. [37] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guerra, E.; Llompart, M.; Garcia-Jares, C. Analysis of Dyes in Cosmetics: Challenges and Recent Developments. Cosmetics 2018, 5, 47. https://doi.org/10.3390/cosmetics5030047
Guerra E, Llompart M, Garcia-Jares C. Analysis of Dyes in Cosmetics: Challenges and Recent Developments. Cosmetics. 2018; 5(3):47. https://doi.org/10.3390/cosmetics5030047
Chicago/Turabian StyleGuerra, Eugenia, Maria Llompart, and Carmen Garcia-Jares. 2018. "Analysis of Dyes in Cosmetics: Challenges and Recent Developments" Cosmetics 5, no. 3: 47. https://doi.org/10.3390/cosmetics5030047
APA StyleGuerra, E., Llompart, M., & Garcia-Jares, C. (2018). Analysis of Dyes in Cosmetics: Challenges and Recent Developments. Cosmetics, 5(3), 47. https://doi.org/10.3390/cosmetics5030047