Antioxidant and Moisturizing Effect of Camellia assamica Seed Oil and Its Development into Microemulsion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Determination of Fatty Acid Composition of Tea Seed Oils
2.3. Biological Activities Determination of Tea Seed Oils
2.3.1. Antioxidant Activity Determination
Inhibition of Lipid Peroxidation by the Ferric Thiocyanate Assay
Scavenging of 1,1-Diphenyl-2-picrylhydrazy Radical (DPPH Assay)
2.3.2. In Vitro Skin Moisturizing Effect Determination
2.4. Development of Microemulsion Containing Tea Seed Oils
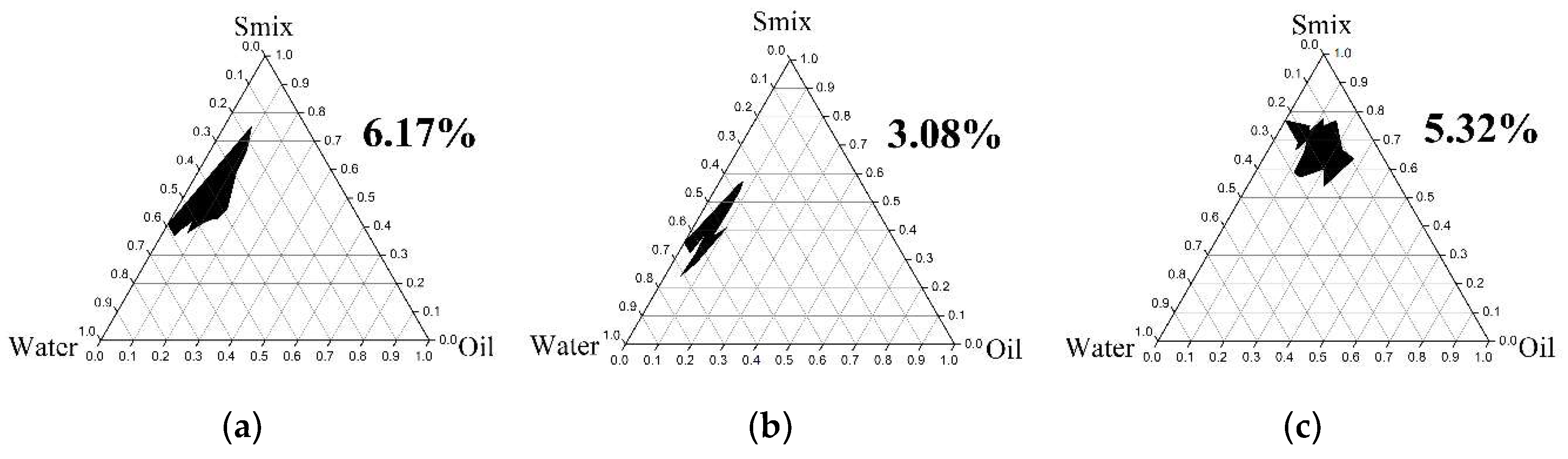
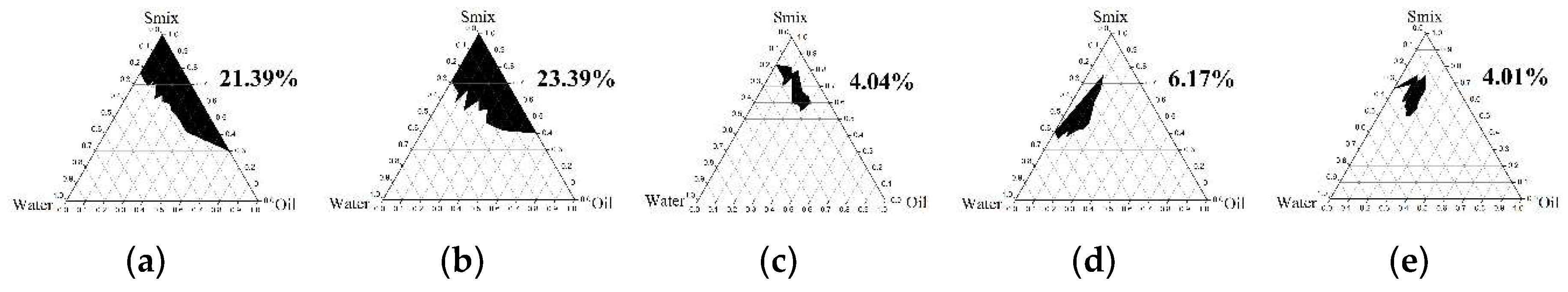
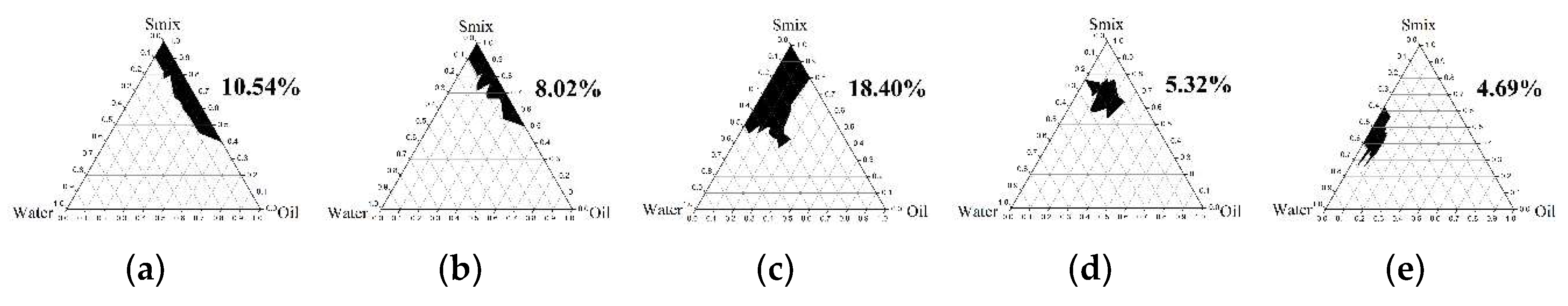
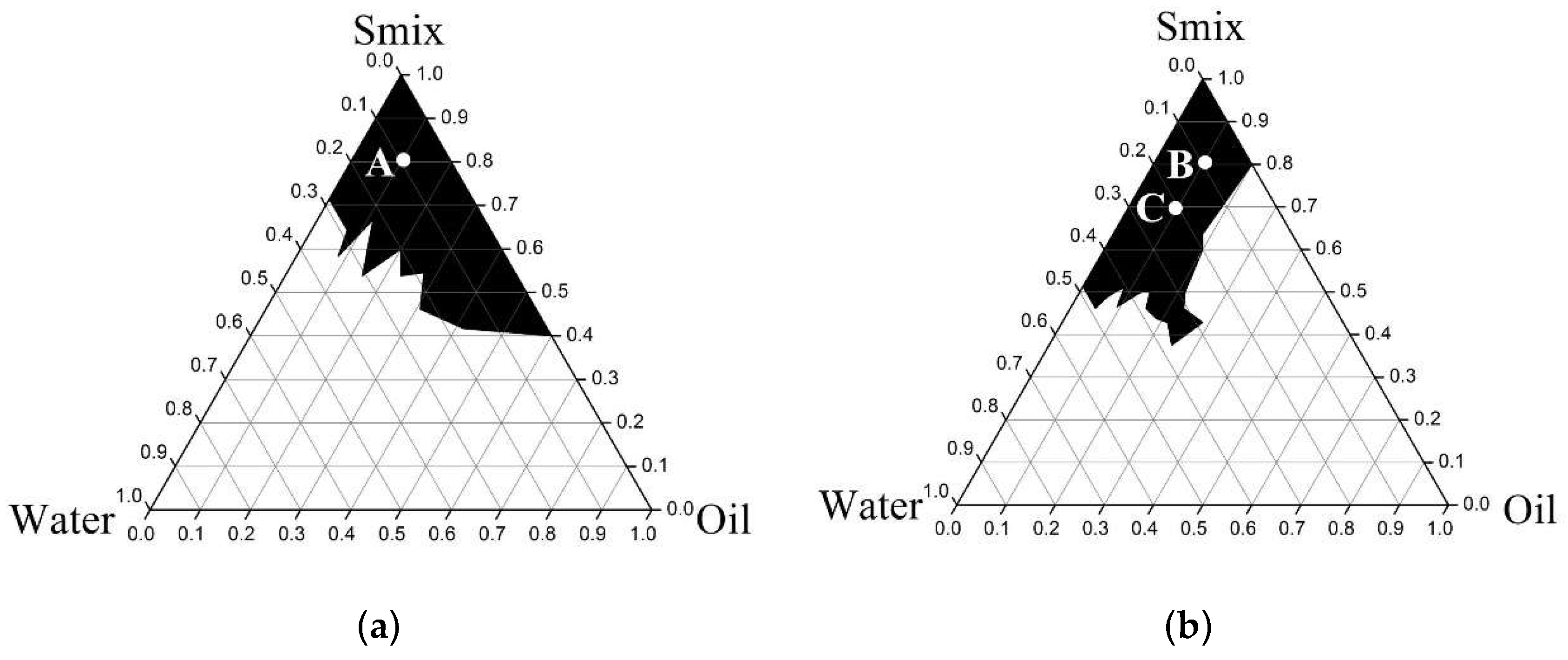
2.4.1. Pseudoternary Phase Diagram Construction
2.4.2. Microemulsion Development
2.4.3. Characterization of Microemulsion
Photon Correlation Spectroscopy
Rheology Study
Biological Activities Determination of Microemulsion Containing Tea Seed Oil
2.4.4. Stability Study
2.5. Statistical Analysis
3. Results
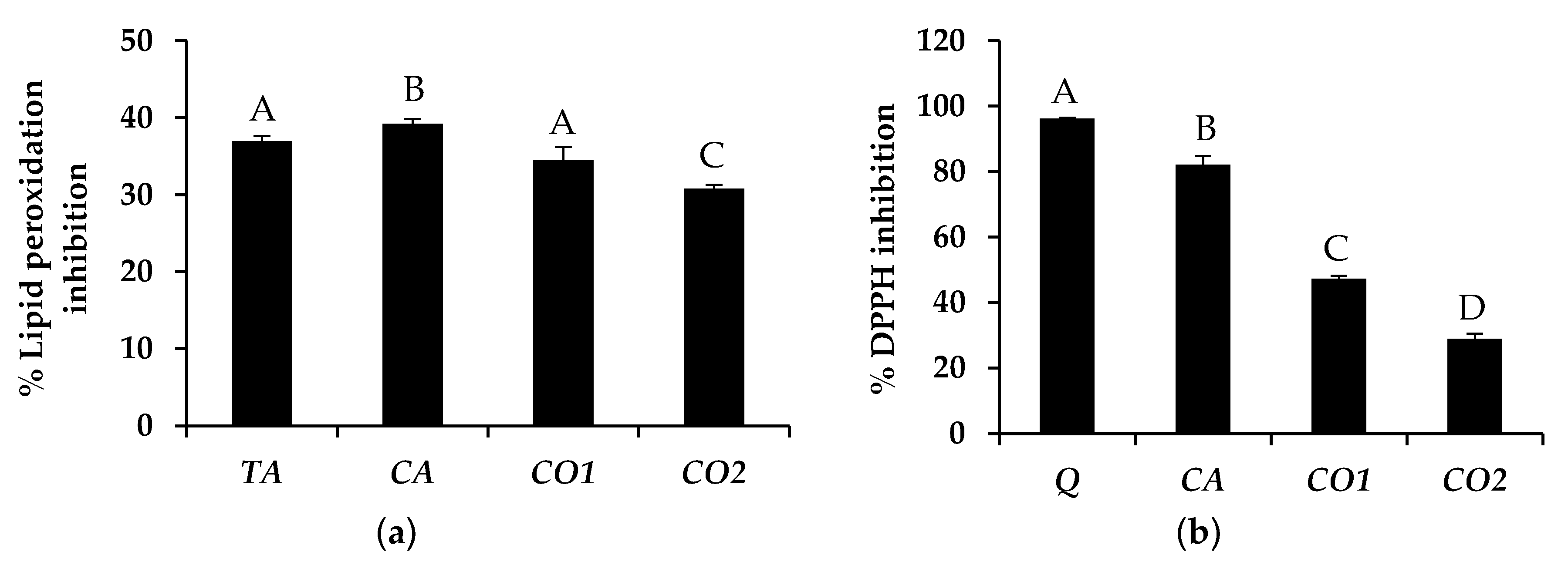
3.1. Fatty Acid Composition of Tea Seed Oils
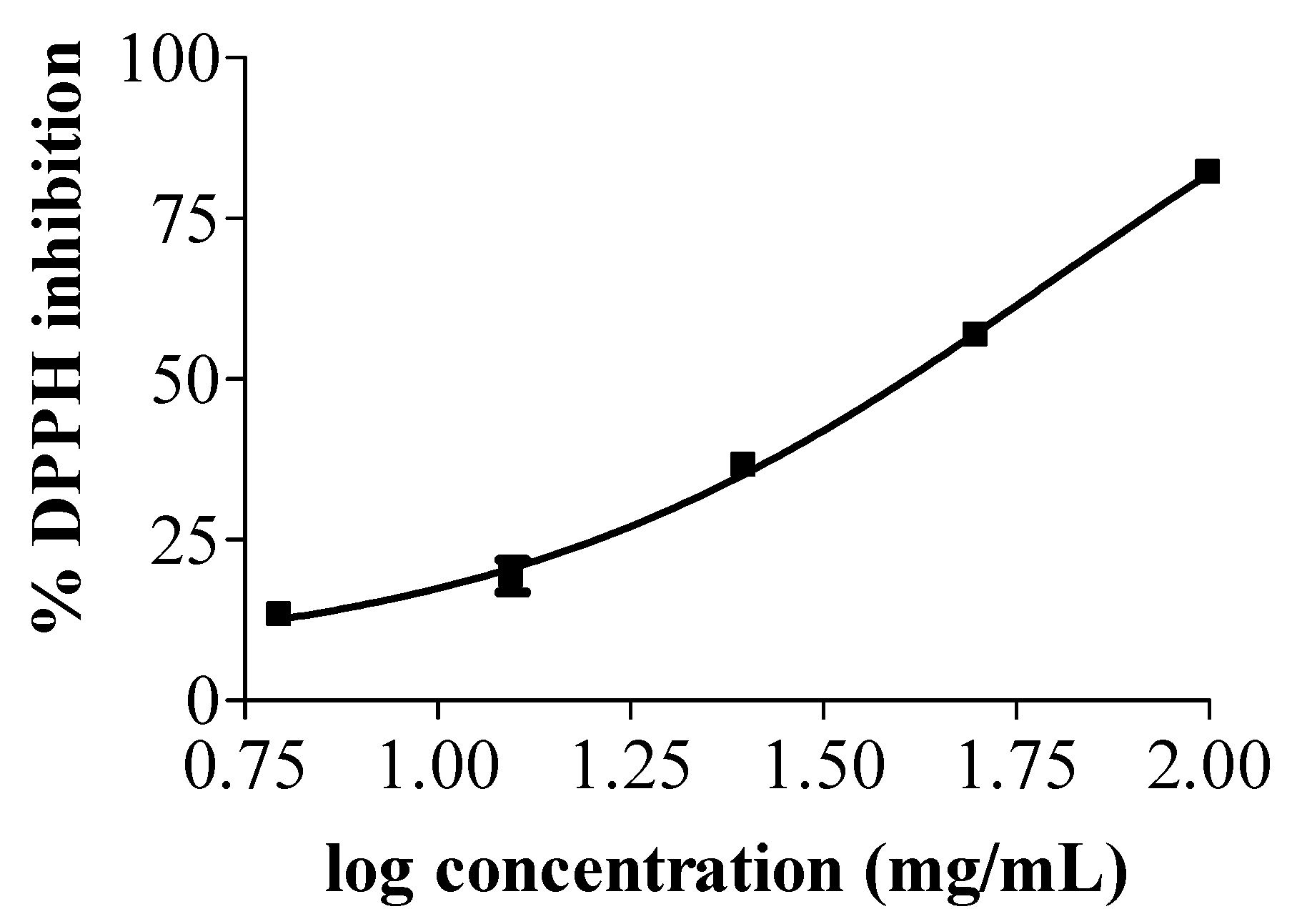
3.2. Antioxidant Activity of Tea Seed Oils
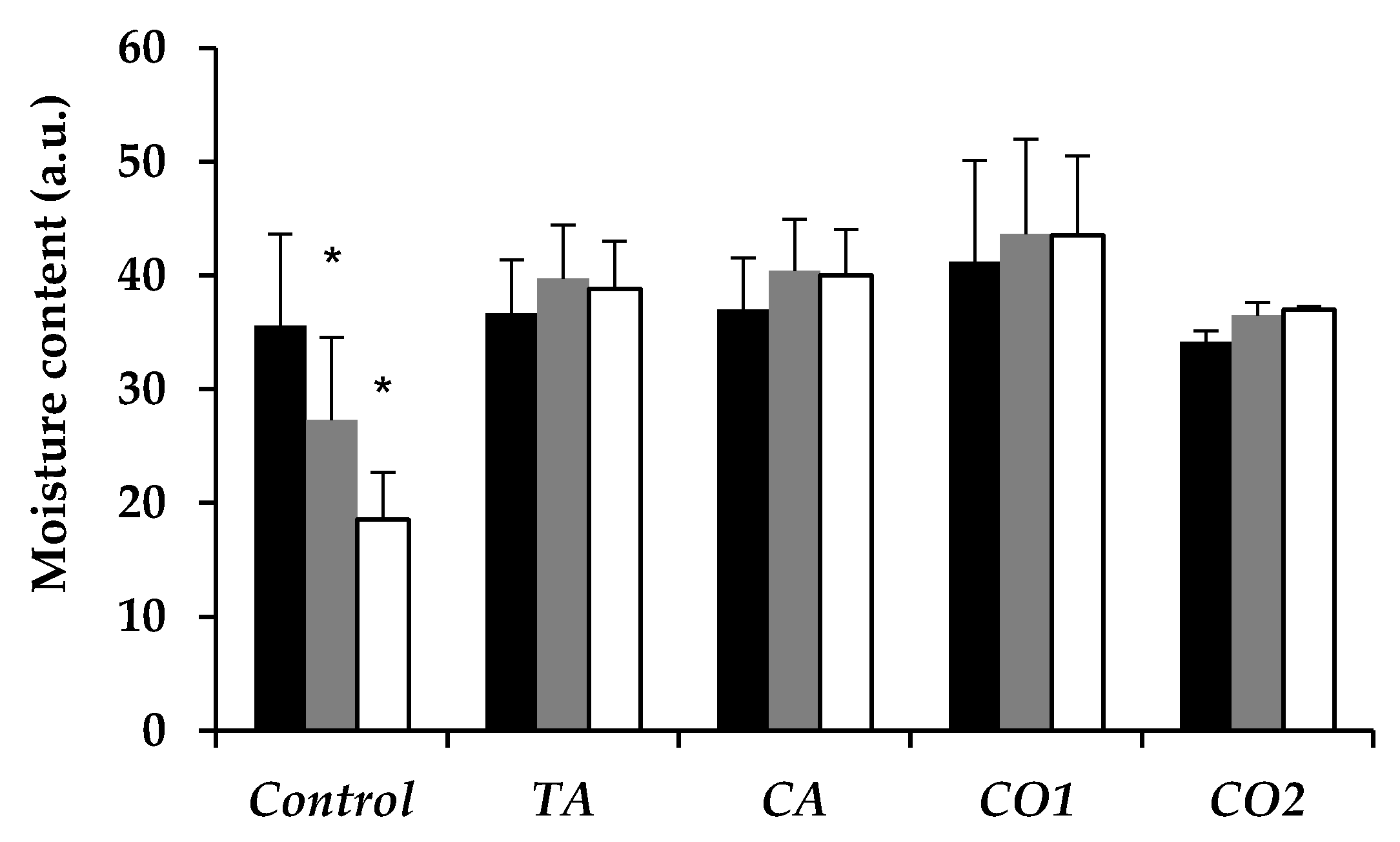
In Vitro Skin Moisturizing Effect of Tea Seed Oils
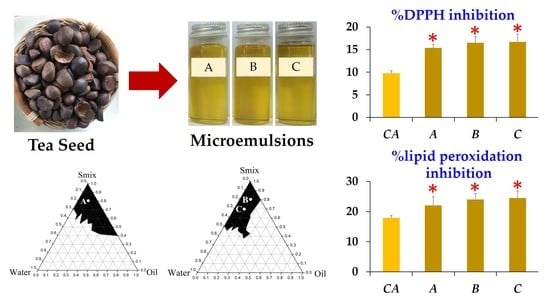
3.3. Development of Microemulsion Containing Tea Seed Oils
3.3.1. Pseudoternary Phase Diagram Construction
3.3.2. Microemulsion Formulations
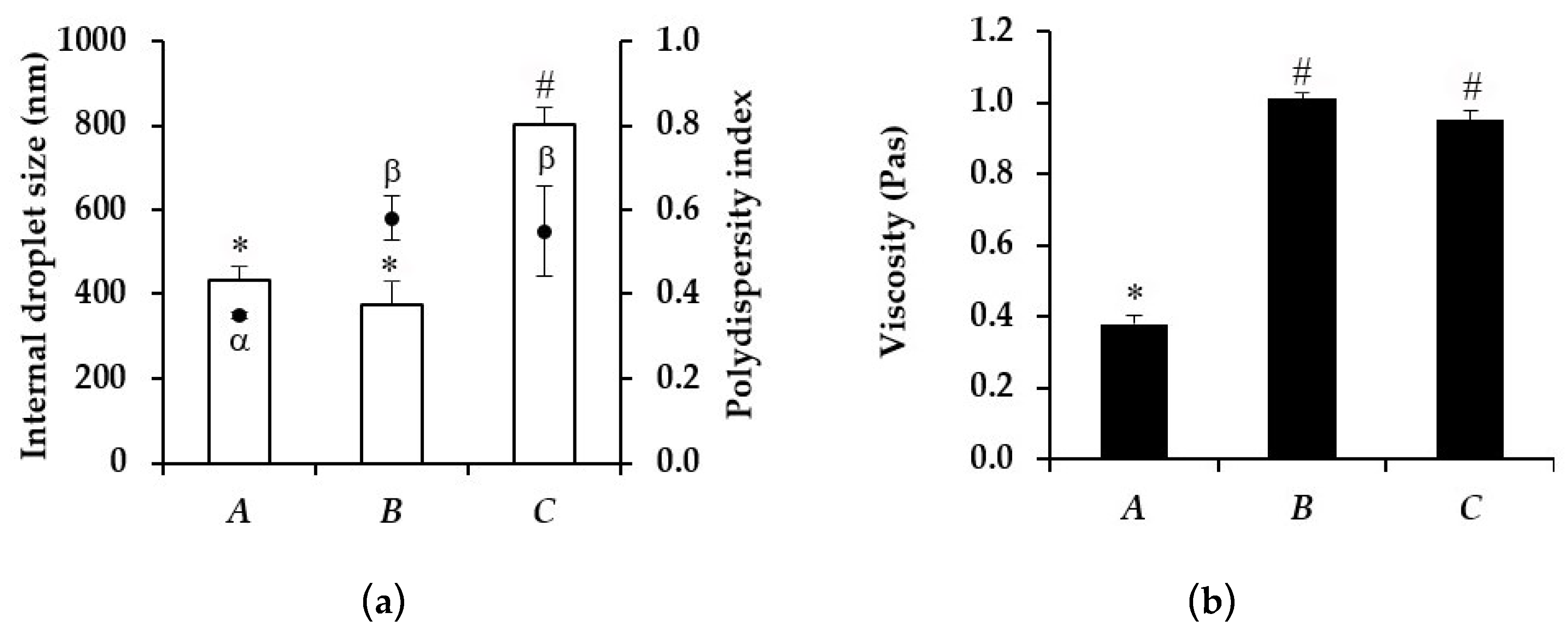
3.4. Characterization of Microemulsion
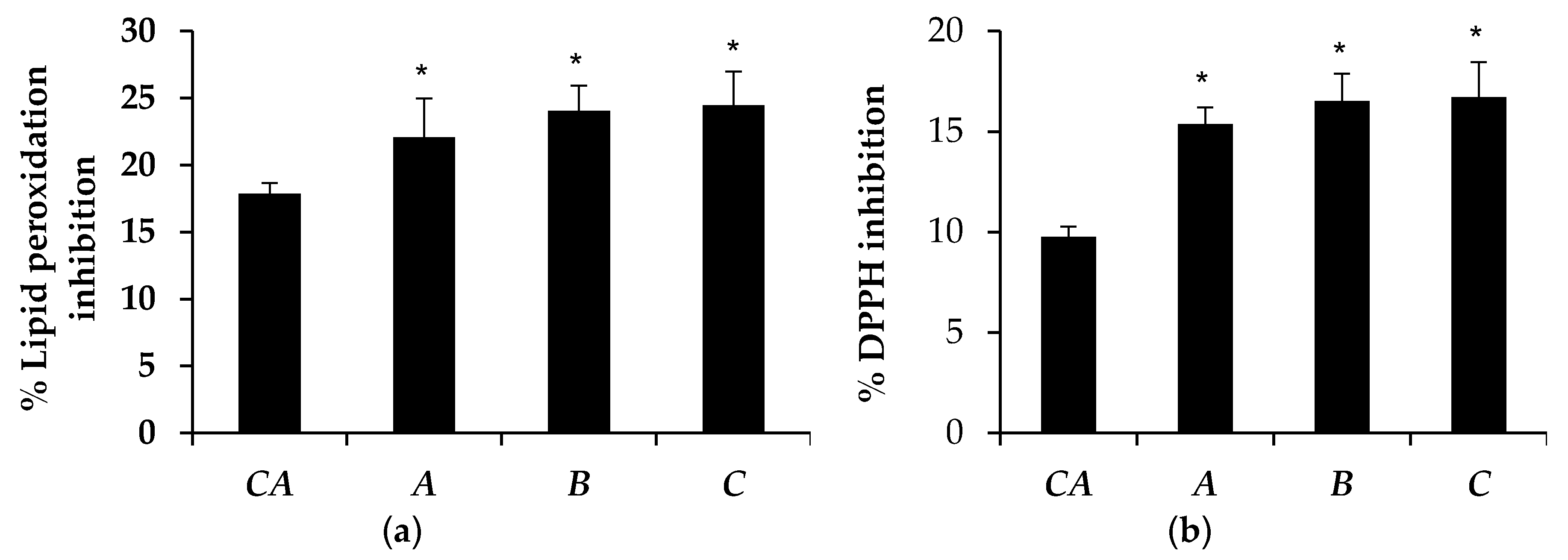
3.5. Antioxidant Activity of Microemulsion Containing Tea Seed Oils
3.6. Stability of Microemulsion Containing Tea Seed Oils
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Paul, S.; Wachira, F.N.; Powell, W.; Waugh, R. Diversity and genetic differentiation among populations of Indian and Kenyan tea (Camellia sinensis (L.) O. Kuntze) revealed by AFLP markers. Theor. Appl. Genet. 1997, 94, 255–263. [Google Scholar] [CrossRef]
- Li, S.; Zhu, X.; Zhang, J.; Li, G.; Su, D.; Shan, Y. Authentication of pure camellia oil by using near infrared spectroscopy and pattern recognition techniques. J. Food Sci. 2012, 77, C374–C380. [Google Scholar] [CrossRef] [PubMed]
- Richards, A.V. The breeding, selection and propagation of tea. Tea Res. Inst. 1966, 1966, 154–160. [Google Scholar]
- He, S.; Gu, Y. The comprehensive utilization of camellia fruits. In American Camellia Yearbook; CAB Direct: Oxfordshire, UK, 1982; pp. 104–107. [Google Scholar]
- Sahari, M.A.; Ataii, D.; Hamedi, M. Characteristics of tea seed oil in comparison with sunflower and olive oils and its effect as a natural antioxidant. J. Am. Oil Chem. Soc. 2004, 81, 585–588. [Google Scholar] [CrossRef]
- Zeb, A. Triacylglycerols composition, oxidation and oxidation compounds in camellia oil using liquid chromatography–mass spectrometry. Chem. Phys. Lett. 2012, 165, 608–614. [Google Scholar] [CrossRef] [PubMed]
- Baker, R.C.; Florence, A.T.; Ottewill, R.H.; Tadros, T.F. Investigations into the formation and characterization of micro-emulsions. II. Light scattering conductivity and viscosity studies of microemulsions. J. Colloid Interface Sci. 1984, 100, 332–349. [Google Scholar] [CrossRef]
- Pedro, A.S.; Cabral-Albuquerque, E.; Ferreira, D.; Sarmento, B. Chitosan: An option for development of essential oil delivery systems for oral cavity care? Carbohydr. Polym. 2009, 76, 501–508. [Google Scholar] [CrossRef]
- Sinico, C.; De Logu, A.; Lai, F.; Valenti, D.; Manconi, M.; Loy, G.; Fadda, A.M. Liposomal incorporation of Artemisia arborescens L. essential oil and in vitro antiviral activity. Eur. J. Pharm. Biopharm. 2005, 59, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, M.J.; Rees, G.D. Microemulsion-based media as novel drug delivery systems. Adv. Drug Deliv. Rev. 2012, 45, 98–121. [Google Scholar] [CrossRef]
- Lucchetti, S.; Ambra, R.; Pastore, G. Effects of peeling and/or toasting on the presence of tocopherols and phenolic compounds in four Italian hazelnut cultivars. Eur. Food Res. Technol. 2018, 244, 1057–1064. [Google Scholar] [CrossRef]
- Motamed, S.M.; Naghibi, F. Antioxidant activity of some edible plants of the Turkmen Sahra region in northern Iran. Food Chem. 2010, 119, 1637–1642. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant determination by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- O’Goshi, K.I.; Tabata, N.; Sato, Y.; Tagami, H. Comparative study of the efficacy of various moisturizers on the skin of the ASR miniature swine. Skin Pharmacol. Appl. Skin Physiol. 2000, 13, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Chaiyana, W.; Saeio, K.; Hennink, W.E.; Okonogi, S. Characterization of potent anticholinesterase plant oil based microemulsion. Int. J. Pharm. 2010, 401, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, J.; Amin, S.; Kohli, K.; Mir, S.R. Construction of pseudoternary phase diagram and its evaluation: Development of self-dispersible oral formulation. Int. J. Drug Dev. Res. 2013, 5, 84–90. [Google Scholar]
- Guan, L.E.I.; Chung, H.Y.; Chen, Z.Y. Comparison of hypocholesterolemic activity of tea seed oil with commonly used vegetable oils in hamsters. J. Food Biochem. 2011, 35, 859–876. [Google Scholar] [CrossRef]
- Lee, C.P.; Yen, G.C. Antioxidant activity and bioactive compounds of tea seed (Camellia oleifera Abel.) oil. J. Agric. Food Chem. 2006, 54, 779–784. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Sun, D.; Chen, H.; Qian, L.; Xu, P. Fatty acid composition and antioxidant activity of tea (Camellia sinensis L.) seed oil extracted by optimized supercritical carbon dioxide. Int. J. Mol. Sci. 2011, 12, 7708–7719. [Google Scholar] [CrossRef] [PubMed]
- Janaszewska, A.; Bartosz, G. Assay of total antioxidant capacity: Comparison of four methods as applied to human blood plasma. Scand. J. Clin. Lab. Investig. 2002, 62, 231–236. [Google Scholar] [CrossRef]
- Sullivan, P.T.; Eaglstein, H.W.; Davis, C.S.; Mertz, P. The pig as a model for human wound healing. Wound Repair Regen. 2001, 9, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Mehta, S.K.; Kaur, G. Microemulsions: Thermodynamic and Dynamic Properties; INTECH: London, UK, 2011. [Google Scholar]
- Yuan, Y.; Li, S.M.; Mo, F.K.; Zhong, D.F. Investigation of microemulsion system for transdermal delivery of meloxicam. Int. J. Pharm. 2006, 321, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Alany, R.G.; Rades, T.; Agatonovic-Kustrin, S.; Davies, N.M.; Tucker, I.G. Effects of alcohols and diols on the phase behaviour of quaternary systems. Int. J. Pharm. 2000, 196, 141–145. [Google Scholar] [CrossRef]
- Gao, Z.G.; Choi, H.G.; Shin, H.J.; Park, K.M.; Lim, S.J.; Hwang, K.J.; Kim, C.K. Physicochemical characterization and evaluation of a microemulsion system for oral delivery of cyclosporin A. Int. J. Pharm. 1998, 161, 75–86. [Google Scholar] [CrossRef]
- Moreno, M.A.; Ballesteros, M.P.; Frutos, P. Lecithin-based oil-in-water microemulsions for parenteral use: Pseudoternary phase diagrams, characterization and toxicity studies. J. Pharm. Sci. 2003, 92, 1428–1437. [Google Scholar] [CrossRef] [PubMed]
- Kale, N.J.; Allen, L.V. Studies on microemulsions using Brij 96 as surfactant and glycerin, ethylene glycol and propylene glycol as cosurfactants. Int. J. Pharm. 1989, 57, 87–93. [Google Scholar] [CrossRef]
- Friberg, S.; Lapczynska, I.; Gillberg, G. Microemulsions containing nonionic surfactants--the importance of the pit value. J. Colloid Interface Sci. 1976, 56, 19–32. [Google Scholar] [CrossRef]
- Clint, J.H. Microemulsions. In Surfactant Aggregation; Springer: Cham, The Netherlands, 1992. [Google Scholar]
- Buege, J.A.; Aust, S.D. Microsomal lipid peroxidation. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1978; pp. 302–310. [Google Scholar]
| Fatty Acid Composition | Amount (%) | |
|---|---|---|
| CA | CO1 | |
| Myristic acid (C14:0) | 0.09 | 0.04 |
| Pentadecanoic acid (C15:0) | 0.02 | ND |
| Palmitic acid (C16:0) | 17.32 | 7.00 |
| Heptadecanoic acid (C17:0) | 0.18 | 0.05 |
| Stearic acid (C18:0) | 3.7 | 3.02 |
| Arachidic acid (C20:0) | 0.11 | 0.16 |
| Behenic acid (C22:0) | 0.05 | 0.39 |
| Tricosanoic acid (C23:0) | 0.02 | ND |
| Lignoceric acid (C24:0) | 0.07 | 0.11 |
| Palmitoleic acid (C16:1n7) | 0.18 | 0.11 |
| Trans-9-Eladic acid (C18:1n9t) | ND | 0.26 |
| cis-9-Oleic acid (C18:1n9c) | 57.97 | 79.96 |
| cis-11-Eicosenoic acid (C20:1n11) | 0.85 | 0.44 |
| Erucic acid (C22:1n9) | 0.06 | 0.04 |
| Nervonic acid (C24:1n9) | 0.09 | 0.04 |
| cis-9,12-Linoleic acid (C18:2n6) | 18.57 | 8.32 |
| alpha-Linolenic acid (C18:3n3) | 0.66 | 0.06 |
| cis-11,14-Eicosadienoic acid (C20:2) | 0.04 | 0.02 |
| cis-13,16-Docosadienoic acid (C22:2) | 0.01 | ND |
| Total | 99.99 | 100.02 |
| Microemulsion | Viscosity (Pas) | Internal Droplet Size (nm) | ||
|---|---|---|---|---|
| Before Stability Test | After Stability Test | Before Stability Test | After Stability Test | |
| A | 0.38 ± 0.03 | 0.52 ± 0.03 * | 426.07 ± 56.96 | 351.43 ± 47.14 |
| B | 0.95 ± 0.07 | 1.24 ± 0.12 | 374.67 ± 43.05 | 1126.00 ± 125.04 * |
| C | 1.01 ± 0.03 | 0.95 ± 0.01 | 801.47 ± 40.13 | 1013.93 ± 140.90 |
| Microemulsion | Lipid Peroxidation Inhibition (%) | DPPH Inhibition (%) | ||
|---|---|---|---|---|
| Before Stability Test | After Stability Test | Before Stability Test | After Stability Test | |
| A | 22.07 ± 2.89 | 15.08 ± 0.93 * | 15.39 ± 0.82 | 15.08 ± 0.67 |
| B | 24.47 ± 2.50 | 15.80 ± 1.74 * | 16.72 ± 1.75 | 16.25 ± 2.89 |
| C | 24.05 ± 1.88 | 15.58 ± 1.02 * | 16.52 ± 1.35 | 15.16 ± 0.86 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaiyana, W.; Leelapornpisid, P.; Jakmunee, J.; Korsamphan, C. Antioxidant and Moisturizing Effect of Camellia assamica Seed Oil and Its Development into Microemulsion. Cosmetics 2018, 5, 40. https://doi.org/10.3390/cosmetics5030040
Chaiyana W, Leelapornpisid P, Jakmunee J, Korsamphan C. Antioxidant and Moisturizing Effect of Camellia assamica Seed Oil and Its Development into Microemulsion. Cosmetics. 2018; 5(3):40. https://doi.org/10.3390/cosmetics5030040
Chicago/Turabian StyleChaiyana, Wantida, Pimporn Leelapornpisid, Jaroon Jakmunee, and Chawalit Korsamphan. 2018. "Antioxidant and Moisturizing Effect of Camellia assamica Seed Oil and Its Development into Microemulsion" Cosmetics 5, no. 3: 40. https://doi.org/10.3390/cosmetics5030040
APA StyleChaiyana, W., Leelapornpisid, P., Jakmunee, J., & Korsamphan, C. (2018). Antioxidant and Moisturizing Effect of Camellia assamica Seed Oil and Its Development into Microemulsion. Cosmetics, 5(3), 40. https://doi.org/10.3390/cosmetics5030040